Abstract
Lifestyle intervention programs currently emphasize weight loss secondary to obesity as the primary determinant of phenotypic changes. We examined whether the effects of a short-term lifestyle intervention program differ in normal-weight versus overweight/obese children. Nineteen overweight/obese (O; BMI = 33.6 ± 1.9 kg/m2) and 14 normal-weight (N; BMI = 19.9 ± 1.5 kg/m2) children participated in a 2-wk program consisting of an ad libitum high-fiber, low-fat diet and daily exercise (2–2.5 h). Fasting serum samples were taken pre- and postintervention for determination of lipids, glucose homeostasis, inflammatory cytokines, and adipokines. Only the O group lost weight (3.9%) but remained overweight/obese (32.3 ± 1.9 kg/m2). Both groups exhibited significant intervention-induced decreases (P < 0.05) in serum insulin (N: 52.5% vs. O: 28.1%; between groups, P = 0.38), homeostatic model assessment for insulin resistance (N: 53.1% vs. O: 28.4%, P = 0.43), leptin (N: 69.3% vs. O: 44.1%, P = 0.10), amylin (N: 28.7% vs. O: 26.1%, P = 0.80), resistin (N: 40.0% vs. O: 35.1%, P = 0.99), plasminogen activator-inhibitor-1 (N: 30.8% vs. O: 25.6%, P = 0.59), IL-6 (N: 58.8% vs. O: 48.5%, P = 0.78), IL-8 (N: 46.0% vs. O: 42.2%, P = 0.49), and TNFα (N: 45.8% vs. O: 40.8%, P = 0.99). No associations between indices of weight change and phenotypic changes were noted. A short-term, intensive lifestyle modification program is effective in ameliorating metabolic risk factors in N and O children. These results suggest that obesity per se was not the primary driver of the phenotypes noted and that dietary intake and physical inactivity induce the phenotypic abnormalities. These data may have implications for the weight loss-independent management of cardiometabolic risk in pediatric populations.
Keywords: physical activity, nutrition, metabolic, cytokines
obesity is associated with a variety of chronic diseases, including but not limited to coronary artery disease, hypertension, diabetes, and certain forms of cancer. In the US, 34% of adolescents aged 12–19 yr are classified as “overweight” or “obese” (BMI > 85th percentile) (13). Unhealthy lifestyle factors such as physical inactivity/lack of exercise training and high-refined carbohydrate, high-fat diet consumption begin in childhood and contribute to both the development of obesity and other chronic diseases (17), and thus it is unclear whether obesity per se or the associated lifestyle factors are underlying causes of the cardiovascular and metabolic dysfunction and the related development of chronic disease.
The first line of defense against obesity-related disease has been weight loss, as the currently held view in lifestyle intervention programs emphasizes that weight loss secondary to obesity is the primary determinant of phenotypic changes (16, 23). However, it is unclear whether obesity and the subsequent weight loss are the primary drivers of the phenotypic modifications. Along these lines, we previously demonstrated reversal of the metabolic syndrome (4) and amelioration of a variety of atherosclerotic (18) and metabolic (10) risk factors, including markers of endothelial dysfunction, oxidative stress, inflammation, and fatty acid species in overweight/obese youth with lifestyle modification. Because the intervention was short-term, this allowed us to study lifestyle effects prior to reversal of obesity. The changes occurred despite small changes in weight, and correlation analysis indicated that changes in phenotypic markers were independent of weight loss. These findings suggested that lifestyle changes may be the underlying drivers of changes in metabolic and cardiovascular phenotypes. This led us to test the validity of overweight/obesity status as a primary cause of metabolic abnormalities by investigating the effects of an identical lifestyle intervention in normal-weight compared with overweight/obese children.
Thus, the present study was designed to examine the efficacy of a short-term daily exercise and plant-based ad libitum diet intervention program on serum endocrine and cytokine markers. We examined its effects on interleukin (IL)-8, IL-10, IL-1 receptor antagonist (IL-1ra), IL-6, tumor necrosis factor-α (TNFα), plasminogen activator-inhibitor (PAI)-1, resistin, amylin, and leptin in normal weight versus obese children. We hypothesized that normal-weight and obese children would respond similarly to the lifestyle intervention program irrespective of baseline obesity status, suggesting that dietary intake and lack of exercise/physical activity are the underlying causes of the phenotypic abnormalities noted.
METHODS
Subjects.
Normal-weight and overweight/obese children [classified by the Centers for Disease Control (CDC) as sex-specific BMI-for-age percentiles] participated voluntarily in the Pritikin Longevity Center 2-wk residential lifestyle modification program, where a plant-based diet was provided ad libitum and daily exercise (2–2.5 h) performed. Pre- and postintervention data were obtained from 19 overweight (O) children aged 8–17 yr (mean 13.1 ± 0.5 yr, 9 males and 10 females; mean BMI: 33.6 ± 1.9 kg/m2; BMI percentile: 95.2 ± 1.4%) and 14 normal-weight (N) children aged 9–15 yr (mean 11.2 ± 0.5 yr, 6 males and 8 females, 19.9 ± 1.5 kg/m2, 55.5 ± 7.9%) who participated in the 2-wk program. All subjects in the O group had a BMI >85th percentile, and 13 of the 19 were >95th percentile (obese) according to CDC BMI-for-age percentile standards. All 14 subjects in the N group were considered to be at a healthy weight (>5th and <85th percentiles) according to CDC guidelines. None of the subjects were using drugs or therapies for obesity, and none had prior histories of disease or injury that would prevent daily exercise. Consent to participate in a research program was obtained from the parents; all agreed to provide data for the study, and the project was approved by the University of California Los Angeles (UCLA) Institutional Review Board.
Diet and exercise intervention.
Participants in the program received a complete physical examination and underwent a 14-day diet and exercise intervention, as described previously (4, 10, 18). The plant-based ad libitum diet contained 12–15% of calories from fat (polyunsaturated/saturated fatty acid ratio = 2.4:1), 15–20% of calories from protein, and 65–70% of calories from primarily unrefined carbohydrate high in dietary fiber (>40 g/day). Carbohydrates were primarily in the form of high-fiber whole grains (≥ 5 servings/day), vegetables (≥4 servings/day), and fruits (≥3 servings/day). Protein was derived primarily from plant sources, along with nonfat dairy (≤2 servings/day) and lean animal protein (fish and fowl) served (in 3.5-oz. portions) 4 days/wk and in soups or casseroles (2 days/wk). All foods except animal-derived protein sources were served ad libitum. The exercise intervention consisted of 2–2.5 h/day of supervised activity, including gym-based exercises, swimming, tennis, and beach games, intended to encourage physical activity in the subjects. Blood samples were drawn after a 12-h overnight fast on days 1 and 12 of the intervention. The blood was separated by centrifugation, and serum was shipped on dry ice to UCLA, where it was stored at −80°C until analysis. Height and body weight were used to calculate BMI and were also assessed on these days using a stadiometer and calibrated scale. BMI was calculated as weight (kg)/height (m2).
Determination of serum lipids, glucose, insulin, homeostatic model assessment for insulin resistance, and quantitative insulin sensitivity check index.
Total cholesterol, triglycerides (TG), high-density lipoprotein (HDL), and glucose levels were measured at a national commercial laboratory (Quest Diagnostics, Miami, FL) using standardized techniques. Low-density lipoprotein (LDL) was calculated as described by the formula of Friedewald et al. (7). Total cholesterol/HDL and LDL/HDL ratios were also calculated. Insulin was quantified in duplicate using Luminex xMAP Multiplex (Millipore, Billerica, MA). The degree of insulin resistance was estimated with the use of the homeostatic model assessment for insulin resistance (HOMA-IR), calculated as the product of the fasting plasma insulin level (μU/ml) and the fasting plasma glucose level (mmol/l) divided by 22.5. Insulin sensitivity was also estimated by the quantitative insulin sensitivity check index (QUICKI) as defined by 1/{log[fasting insulin (μU/ml)] + log[fasting glucose (mg/dl)]}.
Determination of serum cytokines and metabolic markers.
Serum IL-8, IL-10, IL-1ra, IL-6, TNFα, PAI-1, resistin, amylin, and leptin were measured in duplicate using specific Luminex xMAP Multiplex kits (Millipore) according to the manufacturer's instructions. Serum adiponectin (ACRP) was measured in duplicate using enzyme-linked immunosorbent assay kits (R & D Systems, Minneapolis, MN). For a particular assay, all subject samples were assayed on the same day, and according to the kit inserts recovery was generally 85–112% and the coefficient of variation <10%.
Statistical analyses.
Statistical analyses were performed with GraphPad Prism (GraphPad, San Diego, CA) and STATA (StataCorp, College Station, TX). Pearson correlations between changes in anthropometrics and metabolic markers were performed in Prism. To determine the effect of the intervention for each group and compare the changes between the N and O groups, we used a linear mixed model for repeated measurements in STATA. All data are expressed as means ± SE unless otherwise noted. A P value of <0.05 was considered statistically significant.
RESULTS
Anthropometric characteristics, blood pressure, lipids, glucose, and insulin.
Anthropometric and metabolic data are summarized in Table 1. Body weight, BMI, BMI percentile, and waist circumference were significantly different at baseline between the two groups (all P < 0.01). After the 2-wk program, there was a nonsignificant weight reduction in the N group, and O the group lost more weight (%decreases, N: 2.3% vs. O: 3.9%, P < 0.01) but remained obese (BMI percentile: 92.5 ± 2.4% post- vs. 95.2 ± 1.4% preintervention; BMI: 32.3 ± 1.9 vs. 33.6 ± 1.9 kg/m2). Waist circumference decreased similarly in both groups (N: 4.8% vs. O: 4.3%, P = 0.69), but the reduction was not significant in the N group (P = 0.07).
Table 1.
Anthropometric and metabolic phenotypes in normal-weight and obese children
| Obese Subjects (n = 19) |
Normal-Weight Subjects (n = 14) |
|||||
|---|---|---|---|---|---|---|
| Parameter | Pre | Post | %Change | Pre | Post | %Change |
| Body weight, kg | 94.0 ± 7.4 | 90.3 ± 7.1 | −3.9‡ | 47.4 ± 4.6† | 46.3 ± 4.3 | −2.3 |
| BMI, kg/m2 | 33.6 ± 1.9 | 32.3 ± 1.9 | −3.8‡ | 19.9 ± 1.5† | 19.5 ± 1.4 | −2.3* |
| BMI, percentile | 95.2 ± 1.4 | 92.5 ± 2.4 | −2.8* | 55.5 ± 7.9† | 51.6 ± 7.4 | −6.9‡ |
| WC, cm | 97.2 ± 5.7 | 93.0 ± 4.8 | −4.3‡ | 67.3 ± 5.4† | 64.1 ± 4.0 | −4.8 |
| RHR | 95 ± 4 | 80 ± 3 | −15.4‡ | 89 ± 3 | 82 ± 3 | −7.8 |
| SBP | 125 ± 4 | 115 ± 2 | −8.1‡ | 106 ± 5† | 105 ± 3 | −1.0 |
| DBP | 73 ± 3 | 68 ± 2 | −7.4* | 64 ± 1† | 59 ± 3 | −7.6 |
| Glucose, mg/dl | 81.3 ± 2.0 | 85.8 ± 1.3 | 5.5 | 84.7 ± 1.9 | 83.6 ± 2.6 | −1.3 |
| Insulin, μU/ml | 22.3 ± 3.5 | 16.1 ± 4.2 | −28.1* | 18.5 ± 4.1 | 8.8 ± 1.3 | −52.5‡ |
| HOMA-IR | 4.8 ± 0.8 | 3.4 ± 0.8 | −28.4* | 3.9 ± 0.9 | 1.9 ± 0.3 | −53.1‡ |
| QUICKI | 0.31 ± 0.01 | 0.33 ± 0.01 | 4.9‡ | 0.33 ± 0.01 | 0.36 ± 0.01 | 9.3‡ |
| TG, mg/dl | 137.7 ± 14.7 | 86.0 ± 7.9 | −37.5‡ | 105.3 ± 19.1 | 62.0 ± 8.1 | −41.1‡ |
| Total cholesterol, mg/dl | 166 ± 6.2 | 132.0 ± 5.8 | −20.8‡ | 157.3 ± 9.3 | 120.7 ± 5.1 | −23.3‡ |
| LDL cholesterol, mg/dl | 95.0 ± 6.1 | 71.8 ± 5.0 | −24.5‡ | 86.2 ± 8.4 | 60.8 ± 5.9 | −29.4‡ |
| HDL cholesterol, mg/dl | 44.0 ± 2.2 | 43.1 ± 2.7 | −2.1 | 50.1 ± 3.6 | 47.5 ± 3.0 | −5.0 |
| Total/HDL cholesterol | 4.0 ± 0.3 | 3.3 ± 0.3 | −18.2‡ | 3.3 ± 0.3 | 2.7 ± 0.2 | −19.0‡ |
| LDL/HDL | 2.3 ± 0.2 | 1.8 ± 0.2 | −20.7‡ | 1.8 ± 0.2 | 1.4 ± 0.2 | −24.0‡ |
All data are expressed as means ± SE.
Pre, preintervention; post, postintervention; WC, waist circumference; RHR, resting heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; HOMA-IR, homeostatic model assessment for insulin resistance; QUICKI, quantitative insulin sensitivity check index; TG, triglyceride; LDL, low-density lipoprotein; HDL, high-density lipoprotein. Anthropometric and lipid measurements in normal-weight and obese children undergoing a 14-day diet and exercise intervention.
P < 0.05, baseline differences between the normal-weight and obese groups;
P < 0.01;
P < 0.05.
Resting heart rate (RHR) and systolic (SBP) and diastolic blood pressure (DBP) (N: SBP 0.9%, DBP 7.6%, RHR 7.8% vs. O: SBP 8.1%, DBP 7.4%, RHR 15.4%; all N vs. O, P > 0.10) had comparable decreases in both groups but only significantly in the O group (N: DBP P = 0.09, SBP P = 0.8, RHR P = 0.082).
Total cholesterol (N: 23.3% vs. O: 20.8%, P = 0.84), LDL (N: 29.4% vs. O: 24.5%, P = 0.81), and TG (N: 41.1% vs. O: 37.5%, P = 0.63) all had similar significant decreases in both groups (P < 0.01). Serum HDL (N: 5.0% vs. O: 2.1%, P = 0.38) decreased nonsignificantly in both N (P = 0.07) and O (P = 0.44).
Insulin (N: 52.5% vs. O: 28.1%, P = 0.38) decreased in both groups, whereas glucose did not change significantly in N (1.3% decrease, P = 0.66) or O (5.5% increase, P = 0.05), nor was there a difference between the N and O (P = 0.10). However, HOMA-IR (N: 53.1% vs. O: 28.4%, P = 0.43) had similar significant decreases in both groups (P < 0.05), and QUICKI (N: 9.3% vs. O: 4.9%, P = 0.12) increased significantly in both groups (P < 0.01), which was driven mainly by the decrease in insulin.
Cytokines, adipokines, and endocrine markers.
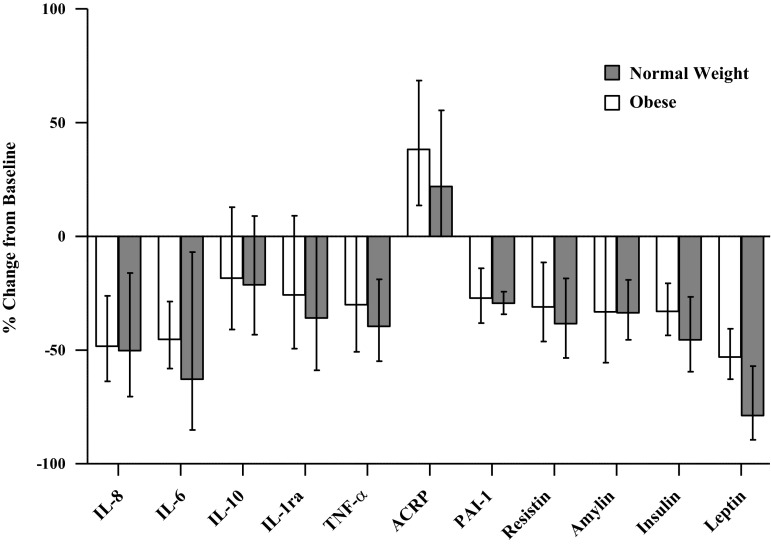
All biomarkers were similar at baseline between the two groups, except for PAI-1 (P < 0.01), leptin (P < 0.001), and IL-1ra (P < 0.05) (Figs. 1, 2, and 3). Serum PAI-1 (N: 30.8% vs. O: 25.6%, P = 0.59), resistin (N: 40.0% vs. O: 35.1%, P = 0.99), amylin (N: 28.7% vs. O: 26.1%, P = 0.80), and leptin (N: 69.3% vs. O: 44.1%, P = 0.10) decreased significantly (P < 0.01) and similarly in both groups. Adiponectin (N: 29.3% vs. O: 41.8%, P = 0.78) increased in both groups but was only statistically significant in O (O: P < 0.01; N: P = 0.12).
Fig. 1.
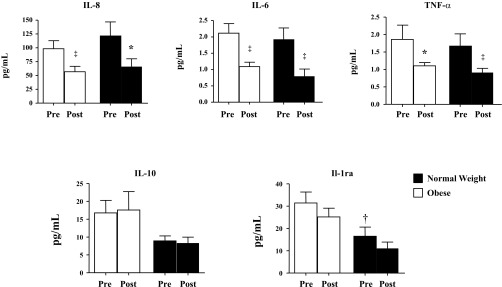
Effect of diet and exercise intervention on serum concentration of the cytokines IL-8, IL-6, TNFα, IL-10, and IL-1 receptor antagonist (IL-1ra) in normal-weight (filled bars) and obese children (open bars). All data are expressed as means ± SE. ‡P < 0.01 and *P < 0.05, postintervention (post) vs. preintervention (pre). †P < 0.05, baseline differences between the normal-weight and obese groups. The baseline difference between normal-weight and obese group for IL-10 was P = 0.12.
Fig. 2.
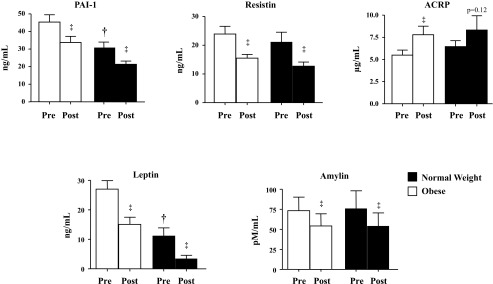
Effect of diet and exercise intervention on serum concentration of the metabolic risk markers plasminogen activator-inhibitor-1 (PAI-1), resistin, adiponectin (ACRP), leptin, and amylin in normal-weight (filled bars) and obese (open bars) children. All data are expressed as means ± SE. ‡P < 0.01, post- vs. preintervention; †P < 0.05, baseline differences between the normal-weight and obese groups.
Fig. 3.
Effect of diet and exercise intervention on %changes in concentration of cytokines and metabolic risk markers in normal-weight and obese children. No differences in changes between normal-weight and obese groups were noted post- vs. preintervention. %Changes from baseline were calculated based on the geometric mean. All data are expressed as means. Error bars represent the 95% confidence interval.
Cytokine changes also exhibited similar responses between the N and O groups. IL-6 (N: 58.8% vs. O: 48.5%, P = 0.78), IL-8 (N: 46.0% vs. O: 42.2%, P = 0.49), and TNFα (N: 45.8% vs. O: 40.8%, P = 0.99) decreased significantly in both groups (P < 0.05). IL-1ra decreased nonsignificantly in both groups (N: 32.8% vs. O: 19.9%, P = 0.90). IL-10 did not change in either group (N: 7.0% decrease vs. O: 4.9% increase, P = 0.77).
Additionally, we did not detect any significant correlations between changes in waist circumference, body weight, or BMI with changes serum cytokines, adipokines, or endocrine markers (all r values ranged between −0.34 and 0.41, all P > 0.12).
DISCUSSION
Currently, there is the perception that obesity is causally implicated in the pathogenesis of dyslipidemia, insulin resistance, inflammation, and other features related to metabolic health. It is certainly the case that adipose tissue, depending on location and characteristics, may exacerbate various risk factors. For example, it is well-established that visceral fat contributes to worsening of metabolic health (6, 25). However, if obesity is the primary cause, then 1) reversal of obesity would be required to reverse phenotypic abnormalities, and 2) subjects who were not obese would not respond similarly to obese subjects. Regarding the first point, in previous seminal studies, we demonstrated that short-term lifestyle modification could ameliorate metabolic syndrome phenotypes in both men (19) and children (10). In addition, a variety of cardiovascular disease risk factors were improved in men (19, 20), women (28), and children (4, 18) despite modest weight loss and subjects remaining overweight/obese by BMI classification. Furthermore, when we correlated changes in body weight or BMI with changes in phenotypic outcomes, no significant associations were noted. However, we did find significant correlations between various fatty acid species and inflammatory factors (10).
Regarding the second point, the present study was designed to test the hypothesis that responsiveness to a short-term daily physical activity and plant-based ad libitum diet intervention program would be similar between normal-weight and overweight/obese children. An advantage of using a short-term intervention is that lifestyle changes can be assessed independently of obesity reversal. The findings of the current study indicated that effects were similar in both overweight/obese and normal-weight subjects. This occurred even for metabolic outcomes that exhibited significant differences at baseline (PAI-1, leptin, and IL-1ra). Furthermore, the normal-weight subjects had responses in measured phenotypes/metabolic markers that were similar to the overweight/obese subjects despite not being overweight/obese at baseline or exhibiting weight loss. The potential contributing lifestyle factors involved in the changes seen in the metabolic profile have been discussed previously (10) and likely include the decrease in saturated trans fats and refined sugar consumption, the increase in ω-3 fatty acids and nutrients (vitamins, minerals, phytochemicals, and fiber) from the diet, and the increase in physical activity, all of which can alter inflammatory and oxidative processes. Interestingly, the changes in serum cytokines, adipokines, and endocrine markers were not associated with changes in body weight, BMI, or waist circumference in either group. One explanation for the similar effect is that both the normal-weight and obese subjects improve phenotypes related to lipid levels, insulin resistance, and adipokines, etc., but the obese subjects have a genetic predisposition (1) to gain weight more readily compared with normal-weight subjects. It is known that many obese subjects are not metabolically unhealthy (24), whereas many normal-weight individuals (by BMI) are metabolically unhealthy (29). Thus, long-term, it is possible that with continued lifestyle modification obesity may be reversed, but even if not, a metabolically healthy obese phenotype can develop, as was noted in the short term with the obese subjects enrolled in the current study. Furthermore, given that weight loss attempts are typically associated with a high degree of recidivism in adult and pediatric populations (5, 8), lifestyle modification that focuses on the normalization of metabolic phenotypes may be of significant value in the pediatric population.
The noted benefits on metabolic, cardiovascular, and inflammatory biomarkers independent of weight loss are not surprising, and it is apparent that the relationships between body weight and lifestyle are complex. For example, Phillips et al. (15) and Varady et al. (27) provided different diets to obese young adults and noted that, despite both groups exhibiting significant weight loss and decreased blood pressure, a “low-fat” diet improved whereas a “low-carbohydrate” diet worsened endothelial function. In addition, Bradley et al. (3) noted that a low-fat diet improved whereas a low-carbohydrate diet worsened augmentation index. Krogh-Madsen et al. (11) and Olsen et al. (14) noted that with a short-term decrease in daily physical activity there was decreased insulin sensitivity and aerobic fitness despite modest weight loss. Interestingly, in the Diabetes Prevention Program, those who met only the physical activity goal and not the weight loss goal had a 44% decrease in diabetes incidence (9), whereas in the Finnish Diabetes Trial, achieving 4 h/wk of physical activity led to a reduction in diabetes risk in subjects who did not lose weight (26).
Also of interest in our cohort is the fact that baseline and percent change in several of the biomarkers were similar in normal-weight and obese children, suggesting similar cardiovascular disease risk profiles. The Pathobiological Determinants of Atherosclerosis in Youth study demonstrated coronary atherosclerosis in both normal-weight and obese males, but it was more severe in the obese males (12). It is possible that the mechanisms by which normal-weight and obese subjects improve in an intervention of this type differ; however, this would require further investigation, using molecular techniques to establish potential differences.
The current study has important strengths and limitations to consider. The major strength of the study is the monitoring permitted by the study. Monitoring food intake and physical activity reduces the need to query subjects about their compliance or to rely on food and activity questionnaires. Furthermore, all exercise sessions were supervised, facilitating adherence to the diet and activities. Additionally, the diet was ad libitum, a major advantage in cases where overeating is an issue, and thus, this is a more realistic program to implement into the daily lives of children rather than intentional caloric restriction. A limitation of the present study is the lack of body composition data to determine the fat mass and lean mass of both groups. Additionally, we did not look at diet and exercise independently, and therefore, we cannot attribute the changes directly to either aspect of the intervention.
Overall, short-term intensive lifestyle modification is effective in ameliorating several metabolic risk factors similarly in both normal-weight and overweight/obese children. Furthermore, because the normal-weight children were not obese and we did not find any associations between changes in indices of obesity and our serum measurements, baseline obesity and weight loss per se were not the primary drivers leading to the phenotypic changes noted. These findings suggest that dietary intake and exercise/physical activity changes may be the underlying causes of the phenotypic changes noted. Additionally, the normal-weight subjects exhibited metabolic abnormalities that were likely due to their ongoing diet and lifestyle habits. Even though current public health recommendations are centered on overweight/obesity and the metabolic abnormalities associated with the same, we have demonstrated that there may be room for improvement in the metabolic profiles of individuals who are not overweight/obese. Given that body weight and weight change are poor surrogates for lifestyle (2), these findings reinforce the need to remind even normal-weight individuals about healthy lifestyle choices. Furthermore, therapies driven by BMI classification and weight loss in young patients may lead to missed opportunities to counsel them on the benefits of weight loss-independent effects of lifestyle modification, including proper diet and physical activity (21, 22). Overall, the results support the need for larger, randomized, long-term studies to investigate the impact of lifestyle modification on disease outcomes independent of weight loss.
GRANTS
This work was supported by the American Heart Association (BGIA no. 0765139Y to C. K. Roberts), the National Heart, Lung, and Blood Institute (P50-HL-105188 to C. K. Roberts), and the National Institute of Diabetes and Digestive and Kidney Diseases (DK-090406 to C. K. Roberts).
DISCLOSURES
R.J. Barnard received a consulting honorarium from the Pritikin Longevity Center.
AUTHOR CONTRIBUTIONS
C.K.R., A.I., and R.J.B. contributed to the conception and design of the research; C.K.R., A.I., and R.J.B. interpreted the results of the experiments; C.K.R. and A.I. drafted the manuscript; C.K.R., A.I., S.S.A., and R.J.B. edited and revised the manuscript; C.K.R., A.I., S.S.A., and R.J.B. approved the final version of the manuscript; A.I. performed the experiments; A.I. analyzed the data; A.I. prepared the figures.
REFERENCES
- 1.Bouchard C, Tremblay A. Genetic influences on the response of body fat and fat distribution to positive and negative energy balances in human identical twins. J Nutr 127: 943S–947S, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Bouchard C, Tremblay A, Despres JP, Theriault G, Nadeau A, Lupien PJ, Moorjani S, Prudhomme D, Fournier G. The response to exercise with constant energy intake in identical twins. Obes Res 2: 400–410, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Bradley U, Spence M, Courtney CH, McKinley MC, Ennis CN, McCance DR, McEneny J, Bell PM, Young IS, Hunter SJ. Low-fat versus low-carbohydrate weight reduction diets: effects on weight loss, insulin resistance, and cardiovascular risk: a randomized control trial. Diabetes 58: 2741–2748, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen AK, Roberts CK, Barnard RJ. Effect of a short-term diet and exercise intervention on metabolic syndrome in overweight children. Metabolism 55: 871–878, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Dansinger ML, Tatsioni A, Wong JB, Chung M, Balk EM. Meta-analysis: the effect of dietary counseling for weight loss. Ann Intern Med 147: 41–50, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Despres JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, Rodes-Cabau J, Bertrand OF, Poirier P. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol 28: 1039–1049, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 499–502, 1972 [PubMed] [Google Scholar]
- 8.Gibson LJ, Peto J, Warren JM, dos Santos Silva I. Lack of evidence on diets for obesity for children: a systematic review. Int J Epidemiol 35: 1544–1552, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, Hoskin M, Kriska AM, Mayer-Davis EJ, Pi-Sunyer X, Regensteiner J, Venditti B, Wylie-Rosett J. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 29: 2102–2107, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Izadpanah A, Barnard RJ, Almeda AJ, Baldwin GC, Bridges SA, Shellman ER, Burant CF, Roberts CK. A short-term diet and exercise intervention ameliorates inflammation and markers of metabolic health in overweight/obese children. Am J Physiol Endocrinol Metab 303: E542–E550, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krogh-Madsen R, Thyfault JP, Broholm C, Mortensen OH, Olsen RH, Mounier R, Plomgaard P, van Hall G, Booth FW, Pedersen BK. A 2-wk reduction of ambulatory activity attenuates peripheral insulin sensitivity. J Appl Physiol 108: 1034–1040, 2010 [DOI] [PubMed] [Google Scholar]
- 12.McGill HC, Jr, McMahan CA, Herderick EE, Zieske AW, Malcom GT, Tracy RE, Strong JP; Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group Obesity accelerates the progression of coronary atherosclerosis in young men. Circulation 105: 2712–2718, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among us children and adolescents, 1999–2010. JAMA 307: 483–490, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olsen RH, Krogh-Madsen R, Thomsen C, Booth FW, Pedersen BK. Metabolic responses to reduced daily steps in healthy nonexercising men. JAMA 299: 1261–1263, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Phillips SA, Jurva JW, Syed AQ, Kulinski JP, Pleuss J, Hoffmann RG, Gutterman DD. Benefit of low-fat over low-carbohydrate diet on endothelial health in obesity. Hypertension 51: 376–382, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pierce GL, Beske SD, Lawson BR, Southall KL, Benay FJ, Donato AJ, Seals DR. Weight loss alone improves conduit and resistance artery endothelial function in young and older overweight/obese adults. Hypertension 52: 72–79, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts CK, Barnard RJ. Effects of exercise and diet on chronic disease. J Appl Physiol 98: 3–30, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Roberts CK, Chen AK, Barnard RJ. Effect of a short-term diet and exercise intervention in youth on atherosclerotic risk factors. Atherosclerosis 191: 98–106, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Roberts CK, Ng C, Hama S, Eliseo AJ, Barnard RJ. Effect of a short-term diet and exercise intervention on inflammatory/anti-inflammatory properties of HDL in overweight/obese men with cardiovascular risk factors. J Appl Physiol 101: 1727–1732, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Roberts CK, Vaziri ND, Barnard RJ. Effect of diet and exercise intervention on blood pressure, insulin, oxidative stress, and nitric oxide availability. Circulation 106: 2530–2532, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Ross R, Bradshaw AJ. The future of obesity reduction: beyond weight loss. Nat Rev Endocrinol 5: 319–325, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Ross R, Janiszewski PM. Is weight loss the optimal target for obesity-related cardiovascular disease risk reduction? Can J Cardiol 24, Suppl D: 25D–31D, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shankar SS, Steinberg HO. Weight loss and vascular function: the good and the unknown. Hypertension 52: 57–58, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, Balletshofer B, Machicao F, Fritsche A, Häring HU. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med 168: 1609–1616, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab 7: 410–420, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344: 1343–1350, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Varady KA, Bhutani S, Klempel MC, Phillips SA. Improvements in vascular health by a low-fat diet, but not a high-fat diet, are mediated by changes in adipocyte biology. Nutr J 10: 8, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wegge JK, Roberts CK, Ngo TH, Barnard RJ. Effect of diet and exercise intervention on inflammatory and adhesion molecules in postmenopausal women on hormone replacement therapy and at risk for coronary artery disease. Metabolism 53: 377–381, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch Intern Med 168: 1617–1624, 2008 [DOI] [PubMed] [Google Scholar]