Abstract
Premenopausal women have lower blood pressure and a reduced incidence of cardiovascular disease compared with age-matched men. Similar sex differences have been seen across species and in multiple animal models of hypertension. While important progress over the last decade has been made in elucidating some of the mechanisms underlying these differences, there are still significant gaps in our knowledge. Understanding the cellular and molecular mechanisms responsible for sex differences in hypertension will be important for developing sex-specific therapies targeted toward the prevention and treatment of hypertension. Female sex hormones, especially estrogen, have been demonstrated to modulate the renin-angiotensin-aldosterone system (RAAS) and to have beneficial effects on cardiovascular function through actions not only on the kidney, heart, and vasculature, but also on the central nervous system (CNS). This review primarily focuses on the central regulatory actions of estrogen on brain nuclei involved in blood pressure regulation and the interactions between estrogen and the RAAS in the CNS by which estrogen plays an important protective role against the development of hypertension.
Keywords: sex difference, renin-angiotensin-aldosterone system, estrogen/estrogen receptor, central nervous system, blood pressure
sex differences in the development of hypertension and female protection from hypertension have been well described in humans and in animal models (8, 49). Activation of the renin-angiotensin-aldosterone system (RAAS) is associated with the development and progression of hypertension. There is a wealth of evidence from human and animal studies that female sex hormones, especially estrogen, provide protective effects by directly modulating the RAAS. These protective effects of estrogen have been demonstrated through its actions not only on the kidney, heart, and vasculature, but also on the central nervous system (CNS). The interactions between estrogen and the RAAS in the periphery have been reviewed elsewhere (26, 31). This review will focus on the central protective effect of estrogen in the development of hypertension, including the sites of action, estrogen receptor (ER) subtypes in the brain, the central interaction between estrogen and the RAAS, as well as possible mechanisms.
Specific Sites and Receptor Subtypes of Estrogen Action in the Brain
The central regulation of blood pressure (BP) and heart rate (HR) involves complex interactions of several brain stem and forebrain nuclei. Both ERα and ERβ, the two classical ER subtypes, have been shown to be expressed in key regions of the brain involved in BP regulation (29), and there are no major differences in the levels of expression in these areas between the sexes (16). These include the nucleus of the solitary tract (NTS), nucleus ambiguus, area postrema (AP), caudal and rostral ventrolateral medulla (CVLM and RVLM), parabrachial nucleus (PBN), forebrain structures along the lamina terminalis (LT) [i.e., subfornical organ (SFO), median preoptic nucleus (MnPO), organum vasculosum of the lamina terminalis (OVLT)], and the paraventricular nucleus of hypothalamus (PVN). Immunohistochemical and molecular biological studies provide evidence for region-specific expression of ER subtypes in these nuclei. For example, ERα appears to predominate in the NTS and LT-related structures, while ERβ is more prevalent in the PVN (29).
An important question for consideration is whether these region-specific ERs may also be involved in the modulation of the central RAAS. Similar to the systemic RAAS, RAAS components that are synthesized de novo in the brain, including angiotensinogen, renin, angiotensin-converting enzyme (ACE1 and ACE2), and ANG II receptors (AT1R and AT2R), are located in the brain regions involved in BP regulation and can act within the same nuclei (17, 19). Together, these studies indicate that the brain stem nuclei and forebrain structures along the LT and PVN are possible sites where estrogen interacts with the RAAS to regulate BP in females.
Both ANG II- and aldosterone (Aldo)-induced hypertension in mice and rats have been shown to be attenuated by 17β-estradiol (E2) actions within the CNS, respectively. In these studies, central infusion of E2 attenuated ANG II- or Aldo-induced hypertension in both males and ovariectomized (OVX) females. Furthermore, central blockade of ERs by antagonists increased ANG II or Aldo pressor effects in intact females. These central protective effects of estrogen involve attenuated sympathetic outflow (39, 42).
Acute injections of estrogen into the PBN, NTS, or the RVLM of OVX female or male rats decrease resting BP and augment baroreflex control of sympathetic nerve activity, while selective injection of estrogen into vagal nuclei, such as those in the nucleus ambiguus, augments baroreflex control of HR and increases parasympathetic activity, resulting in decreased HR without affecting BP (24, 25). In contrast, microinjection of estrogen into the hypothalamic PVN of male rats has no effect on resting BP and HR but attenuates the pressor response to l-glutamate injection into the PVN (9). These results suggest that estrogen regulation of BP, HR, and autonomic nerve activity in the CNS is region-specific. This notion is further supported by the early studies from Jonklaas and Buggy (13, 14), showing that central estrogen injection in OVX female rats depressed intracerebroventricular ANG II-induced increases in drinking and BP by decreasing AT1R binding in tissue blocks of the preoptic area and septum-thalamus, which includes LT-associated structures. The brain area implicated in this effect of estrogen was the medial preoptic area (13, 14).
The specific roles of ERα and ERβ in BP regulation have been studied in mice with targeted deletions of ERα and ERβ and in animals with acute treatments with ER-selective agonists (1, 23, 32, 52). Estrogen activation of central ERα in female mice protects against baroreflex dysfunction and hypertension induced by ANG II (22, 42). In ANG II-infused rats, the intensity of ERα-immunoreactivity increased exclusively in the commissural NTS, while neither the number nor intensity of ERβ-labeled cells changed, suggesting that ERα is important in the NTS (20). In other microinjection studies, ERβ appeared to mediate attenuated effects of estrogen on resting BP and voltage-gated calcium currents in the RVLM and the glutamate-induced increase in BP in the PVN (9, 28, 34). Recent studies from our laboratory showed that intracerebroventricular infusions of either diarylpropionitrile (DPN), a selective ERβ agonist, or propyl-pyrazole-triol (PPT), a selective ERα agonist, attenuated Aldo-induced hypertension in OVX rats (44). In contrast, in intact females, knockdown of ERα or ERβ by intracerebroventricular injections of siRNA-ERα or siRNA-ERβ augmented Aldo-induced hypertension. In these studies, site-specific PVN or RVLM injections of siRNA-ERβ augmented Aldo-induced hypertension. However, knockdown of ERα by siRNA-ERα injected into either the PVN or RVLM did not significantly increase BP induced by Aldo. These results indicate that ERβ in both the PVN and RVLM, but not ERα in these nuclei, contributes to the protective effects of estrogen against Aldo-induced hypertension (44). These studies suggest that specific ER subtypes mediate the regulatory effect of estrogen on BP in different nuclei.
Regulatory Effects of Estrogen on ANG II- or Aldo-Induced Intracellular Signals
More recently, it was demonstrated that either ANG II or Aldo can trigger the production of reactive oxygen species (ROS) via activation of the enzyme NADPH oxidase and that in the brain, excessive production of ROS within the SFO, PVN, and other central regions contributes to ANG II- or Aldo-induced increases in sympathetic activity and BP (40, 41, 51). Estrogen acting at the level of the SFO inhibited ANG II-induced hypertension and activation of SFO neurons via interactions with intracellular ROS production (48). Similar effects of ER inhibition of induced ROS production were also demonstrated within the PVN (44). In cultured PVN neurons, a significant increase in ROS production was evident after overnight incubation with Aldo. The Aldo-induced ROS production was further increased by genetic silencing of either ERα or ERβ with siRNAs (44). These results suggest that a potential mechanism involved in the attenuation of ANG II- or Aldo-induced hypertension by estrogen may involve ER inhibition of AT1- or mineralocorticoid receptor (MR)-induced increases in ROS (see Fig. 1). The study by Wang et al. (35) further demonstrated lower immunoreactivity of p47, a key NADPH oxidase subunit, on catecholamine neurons in the RVLM of female rats when compared with males. However, they also had the unexpected finding that immunoreactivity for AT1R in these neurons was higher in female than male rats. Within dissociated bulbospinal (sympathoexcitatory) neurons of the RVLM, ANG II induced similar increases in ROS production in male and female rats. The authors concluded that these observations may be due to the increased density of AT1R in the RVLM neurons in female rats and that this is counterbalanced by a reduced level of NADPH oxidase activity (35).
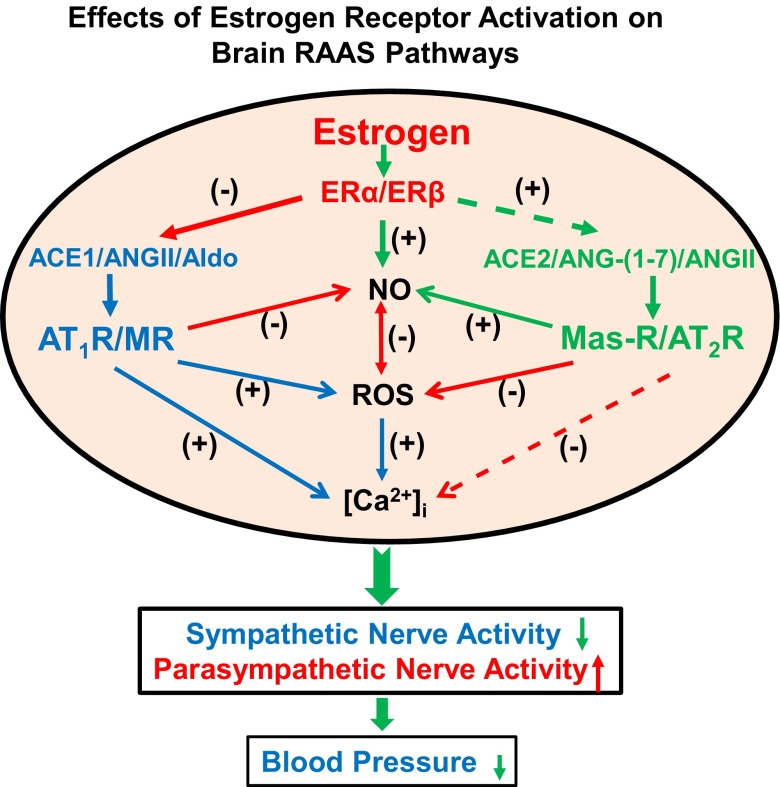
Fig. 1.
Schematic diagram showing possible mechanisms by which central estrogen plays a protective role against the development of hypertension. Estrogen downregulates the hypertensive axis and upregulates the antihypertensive axis of the renin-angiotensin-aldosterone system (RAAS) in the central nervous system (CNS), resulting in decreased intracellular calcium ([Ca2+]i) and reactive oxygen species (ROS) production, increased nitric oxide (NO) availability, and attenuated blood pressure mediated by sympathetic excitation during the development of hypertension. ER, estrogen receptor; ACE1, ACE2, angiotensin converting enzyme; ANG, angiotensin; AT1R and AT2R, ANG II receptor; Aldo, aldosterone; MR, mineralocorticoid receptor; Mas-R, ANG-(1–7) receptor. + stands for an enhanced effect; - stands for an attenuated effect; solid lines stand for regulatory pathways that have been demonstrated in the CNS; and dashed lines stand for possible regulatory pathways that need to be demonstrated in the CNS.
The effects of both estrogen and the RAAS within the CNS also involve kinase modulation (15, 50). In studies of intact and OVX spontaneously hypertensive female rats, depletion of endogenous estrogen by OVX resulted in greater increases in BP and an increase in RAAS via activation of the Rho/Rho-kinase pathway in the brain stem (12). While the specific mechanisms underlying the hypertensive effects of estrogen depletion in the spontaneously hypertensive rats are still unknown, these authors suggested that estrogen depletion may alter excitatory glutamate synapses within the brain stem via Rho/Rho-kinase modulation of dendritic spine postsynaptic contacts. This is an intriguing hypothesis that certainly warrants further investigation. Additional evidence in support of estrogen modulation of RAAS-related kinase function is found in studies in the rat anterior pituitary (15). In this study, infusion of ANG II increased PKC activity within the anterior pituitary in both males and females. Interestingly, treatment with E2 inhibited ANG II-induced increases in PKC activity in males and facilitated ANG II-induced increases in PKC activity in females.
In studies by Felder and colleagues (36), systemic infusion of ANG II in male rats increased phosphorylation of MAPK and increased expression of the AT1R in both the PVN and SFO. Further, the increased expression of AT1R was blocked by inhibiting p44/42 MAPK and JNK, suggesting that the central RAAS effects on PVN neurons require MAPK and JNK activation. The effects of MAPK and JNK within the PVN and SFO during ANG II-induced hypertension have not yet been studied in females. Given the known sex differences in neurogenic ANG II-induced hypertension, investigating the interactions between these kinase pathways and estrogen will be important for understanding their roles in the development of hypertension.
Intracellular Ca2+ ([Ca2+]i) is a key messenger in ANG II signaling in neurons, and ROS is involved in an ANG II-stimulated influx of extracellular Ca2+ into neurons (53) (see Fig. 1). In anesthetized female rats, intravenous injection of E2 inhibits AP neuronal activity (18). The cellular mechanisms involved in this effect of estrogen on neuronal activity have been suggested to include both inhibition of calcium currents and facilitation of potassium currents (IK). In cultured AP neurons, acute application of E2 was shown to increase the activity of the big-conductance calcium-activated potassium current (MaxiK+). In these studies, E2 did not change rapid-activating and rapid-inactivating potassium currents or the delayed rectifier current. Thus, MaxiK+ was suggested to be the principal component responsible for the AP total IK increase observed with E2 (18). Moreover, preincubation of E2 attenuated the ANG II-induced increase in [Ca2+]i in cultured AP neurons (21). It has also been reported that E2 decreases L-type Ca2+ currents in RVLM bulbospinal neurons (34). These data suggest that increases in the neuronal MaxiK+ currents and decreases in L-type Ca2+ currents are both mechanisms by which E2 inhibits neuronal activity in the AP, RVLM, or other nuclei involved in sympathetic activity. Interestingly, a recent study from Wang et al. (35) showed that ANG II-evoked increases in L-type Ca2+ currents in RVLM neurons were larger in female than male rats. If this is the case, the acute ANG II-evoked increase in L-type Ca2+ currents would be expected to result in an increase in neuronal excitability of RVLM sympathoexcitatory neurons in female rats, which is in contrast to the above-mentioned studies. However, L-type Ca2+ channels also modulate K+ channels. If the elevated L-type Ca2+ currents increase [Ca2+]i, that would be expected to activate MaxiK+ in the presence of estrogen (18). The activated MaxiK+ would, in turn, buffer the increased neuronal activity induced by elevated [Ca2+]i in females. Because menopause is accompanied by an accelerated age-related rise in sympathetic nerve activity (SNA) (33), these studies might imply that increased SNA by loss of estrogen may be related to impaired central modulation of baroreflex function and an attenuated inhibitory effect on SNA (33). However, in these studies the acute effects of estrogen on the cultured neurons do not always mimic the actions of long-term estrogen treatment. Therefore, these acute beneficial effects of estrogen need to be confirmed in vivo in animals and humans.
Central nitric oxide (NO) is an important component of cellular transduction pathways in neural systems that regulate BP and sympathetic outflow. Sex differences have been observed in the SFO and PVN of the expression of neuronal NO synthase (nNOS) with female mice having higher levels of expression (43). Central infusion of a nNOS inhibitor augmented the pressor effects of systemic ANG II in females, but not in males (43). In addition, ANG II infusion increased nNOS expression in intact females, but not in OVX females or males, suggesting that increased nNOS protein expression is associated with the presence of female sex hormones and plays a protective role against ANG II-induced hypertension in female mice (43). Cherney et al. (5) also reported that estrogen replacement in OVX rats reduced BP responses to psychological stress and increased NO levels in the hypothalamus and brain stem. These studies suggest that inhibition of NO production in the brain augments the response in restrained OVX/estrogen-treated rats but not in OVX/vehicle-treated animals. Together, these data suggest that the antihypertensive effects of estrogen are mediated, at least in part, by NO (see Fig. 1). This conclusion is further supported by several microinjection studies showing that blockade of NO generation and phosphatidylinositol 3-kinase (Pl-3K)/serine/threonine kinase (Akt) signaling pathway in the RVLM and PVN significantly attenuated the cardiovascular depressive effects elicited by ERβ activation (9, 28, 37).
Interactions Between Estrogen and RAAS Antihypertensive Axis in the CNS
Within the RAAS, ACE1/ANG II/AT1R and Aldo/MR are considered as a hypertensive axis, while ACE2/ANG-(1–7)/Mas-R and ANG II/AT2R are viewed as an antihypertensive axis (38) (see Fig. 1). Hypertension is associated with augmented activation of the classic RAAS hypertensive axis in the periphery and in the brain (6). Moreover, low doses of Aldo or ANG II enhance ANG II-induced increases in the expression of renin, AT1R, and ACE1 mRNA in forebrain nuclei (46, 47). In contrast to this type of enhancement of the classic RAAS hypertensive axis, estrogen directly interacts with the RAAS to downregulate renin and ACE1 activity and AT1R mRNA expression, as well as to upregulate AT2R mRNA expression and the level of ANG-(1–7) in the kidney (2–4, 27). Recent studies demonstrated that in male mice, brain overexpression of ACE2 increases ANG-(1–7) levels and prevents the development of hypertension induced by peripheral ANG II infusion (7). Further, in the deoxycorticosterone acetate-salt hypertensive male rats, central infusion of ANG-(1–7) reduces BP and improves baroreflex control of HR (10). These results highlight the importance of the antihypertensive axis of the RAAS in BP regulation by the CNS.
Recent studies have shown that the expression and activation of the ACE2/ANG-(1–7)/Mas-R/AT2R axis differ between the sexes. Studies from two groups reported that ACE2/ANG-(1–7) contributes to the sex differences in the development of ANG II- or obesity-induced hypertension. In both of these forms of hypertension, OVX and the Mas-R antagonist abolished the sex differences observed in BP (11, 30). Moreover, renal expression of AT2R was three-fold greater in female mice compared with males under basal conditions and after chronic ANG II, which was responsible for decreased BP induced by a low dose of ANG II in female rats (4). However, all of these sex differences in the RAAS antihypertensive axis were observed in peripheral tissues, such as kidney and adipose tissue. There is a limited amount of data available regarding the role of estrogen in regulating the components of brain RAAS antihypertensive axis that is involved in the protective effect of this female steroid in the development of hypertension. Preliminary data from our laboratory show that central blockade of the Mas-R or AT2R augmented Aldo-induced hypertension in intact female rats, while central infusion of ANG-(1–7) attenuated Aldo-induced hypertension in OVX females. These results suggest that brain endogenous ANG-(1–7) or AT2R in the female normally acts to buffer the increases in BP in the development of Aldo-induced hypertension (45). Further studies are needed to examine whether there are sex differences in central expression of ACE2, Mas-R or AT2R, and ANG-(1–7) levels in physiological and pathophysiological states, as well as the mechanisms underlying estrogen regulation of the brain RAAS antihypertensive axis.
Conclusions
The effects of estrogen on BP, HR, and neuronal activity in brain nuclei involved in BP regulation are regionally and receptor subtype-specific. As summarized in Fig. 1, estrogen inhibits the adverse effects of activation of the RAAS hypertensive axis through increased NO production, activation of neuronal MaxiK+ current and attenuation of ANG II- or Aldo-induced increases in ROS production and intracellular Ca2+ in neurons. Meanwhile, estrogen may also increase ANG-(1–7) levels and ACE2 and AT2R expression to enhance the beneficial effects of the RAAS antihypertensive axis in the brain, such that sympathetic outflow is decreased and parasympathetic activity and baroreflex sensitivity are increased. Thus, CNS actions of estrogen play an important protective effect against the development of hypertension. However, the molecular mechanisms underlying the central protective action of estrogen are far from being clarified, and the research regarding this is just beginning. Moreover, it should be noted that the acute effects of estrogen on the brain do not always mimic the actions of long-term estrogen treatment. Therefore, a comprehensive understanding of the molecular, cellular, and systemic mechanisms involved in the effects of estrogen on brain cardiovascular related nuclei to protect against hypertension will be essential to the development of sex-specific therapies to treat chronic high BP and related diseases in humans.
GRANTS
This work was supported by the National Institutes of Health Grants HL-14388, HL-98207, and MH-80241.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: B.X. drafted manuscript; B.X., A.K.J., and M.H. edited and revised manuscript; B.X., A.K.J., and M.H. approved final version of manuscript.
REFERENCES
- 1.Arias-Loza PA, Hu K, Dienesch C, Mehlich AM, König S, Jazbutyte V, Neyses L, Hegele-Hartung C, Heinrich Fritzemeier K, Pelzer T. Both estrogen receptor subtypes, alpha and beta, attenuate cardiovascular remodeling in aldosterone salt-treated rats. Hypertension 50: 432–438, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Bhatia K, Zimmerman MA, Sullivan JC. Sex differences in angiotensin-converting enzyme modulation of Ang (1–7) levels in normotensive WKY rats. Am J Hypertens 26: 591–598, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brosnihan KB, Hodgin JB, Smithies O, Maeda N, Gallagher P. Tissue-specific regulation of ACE/ACE2 and AT1/AT2 receptor gene expression by oestrogen in apolipoprotein E/oestrogen receptor-alpha knock-out mice. Exp Physiol 93: 658–664, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown RD, Hilliard LM, Head GA, Jones ES, Widdop RE, Denton KM. Sex differences in the pressor and tubuloglomerular feedback response to angiotensin II. Hypertension 59: 129–135, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Cherney A, Edgell H, Krukoff TL. NO mediates effects of estrogen on central regulation of blood pressure in restrained, ovariectomized rats. Am J Physiol Regul Integr Comp Physiol 285: R842–R849, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Crowley SD, Coffman TM. Recent advances involving the renin-angiotensin system. Exp Cell Res 318: 1049–1056, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng Y, Xia H, Cai Y, Halabi CM, Becker LK, Santos RA, Speth RC, Sigmund CD, Lazartigues E. Brain-selective overexpression of human angiotensin-converting enzyme type 2 attenuates neurogenic hypertension. Circ Res 106: 373–382, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer M, Baessler A, Schunkert H. Renin angiotensin system and gender differences in the cardiovascular system. Cardiovasc Res 53: 672–677, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Gingerich S, Krukoff TL. Estrogen in the paraventricular nucleus attenuates l-glutamate-induced increases in mean arterial pressure through estrogen receptor beta and NO. Hypertension 48: 1130–1136, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Guimaraes PS, Santiago NM, Xavier CH, Velloso EP, Fontes MA, Santos RA, Campagnole-Santos MJ. Chronic infusion of angiotensin-(1–7) into the lateral ventricle of the brain attenuates hypertension in DOCA-salt rats. Am J Physiol Heart Circ Physiol 303: H393–H400, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Gupte M, Thatcher SE, Boustany-Kari CM, Shoemaker R, Yiannikouris F, Zhang X, Karounos M, Cassis LA. Angiotensin converting enzyme 2 contributes to sex differences in the development of obesity hypertension in C57BL/6 mice. Arterioscler Thromb Vasc Biol 32: 1392–1399, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito K, Hirooka Y, Kimura Y, Sagara Y, Sunagawa K. Ovariectomy augments hypertension through rho-kinase activation in the brain stem in female spontaneously hypertensive rats. Hypertension 48: 651–657, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Jonklaas J, Buggy J. Angiotensin-estrogen central interaction: localization and mechanism. Brain Res 326: 239–249, 1985 [DOI] [PubMed] [Google Scholar]
- 14.Jonklaas J, Buggy J. Angiotensin-estrogen interaction in female brain reduces drinking and pressor responses. Am J Physiol Regul Integr Comp Physiol 247: R167–R172, 1984 [DOI] [PubMed] [Google Scholar]
- 15.Lachowicz A, Rebas E. Gender differences in steroid modulation of angiotensin II-induced protein kinase C activity in anterior pituitary of the rat. Biochem Biophys Res Commun 294: 95–100, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Laflamme N, Nappi RE, Drolet G, Labrie C, Rivest S. Expression and neuropeptidergic characterization of estrogen receptors (ERα and ERβ) throughout the rat brain: anatomical evidence of distinct roles of each subtype. J Neurobiol 36: 357–378, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Lavoie JL, Sigmund CD. Minireview: overview of the renin-angiotensin system- an endocrine and paracrine system. Endocrinology 144: 2179–2183, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Li Z, Hay M. 17-β-estradiol modulation of area postrema potassium currents. J Neurophysiol 84: 1385–1391, 2000 [DOI] [PubMed] [Google Scholar]
- 19.McKinley MJ, Albiston AL, Allen AM, Mathai ML, May CN, McAllen RM, Oldfield BJ, Mendelsohn FA, Chai SY. The brain renin-angiotensin system: location and physiological roles. Int J Biochem Cell Biol 35: 901–918, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Milner TA, Drake CT, Lessard A, Waters EM, Torres-Reveron A, Graustein B, Mitterling K, Frys K, Iadecola C. Angiotensin II-induced hypertension differentially affects estrogen and progestin receptors in central autonomic regulatory areas of female rats. Exp Neurol 212: 393–406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pamidimukkala J, Hay M. 17β-estradiol inhibits angiotensin II activation of area postrema neurons. Am J Physiol Heart Circ Physiol 285: H1515–H1520, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Pamidimukkala J, Xue B, Newton LG, Lubahn DB, Hay M. Estrogen receptor-alpha mediates estrogen facilitation of baroreflex heart rate responses in conscious mice. Am J Physiol Heart Circ Physiol 288: H1063–H1070, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Pelzer T, Jazbutyte V, Hu K, Segerer S, Nahrendorf M, Nordbeck P, Bonz AW, Muck J, Fritzemeier KH, Hegele-Hartung C, Ertl G, Neyses L. The estrogen receptor-alpha agonist 16α-LE2 inhibits cardiac hypertrophy and improves hemodynamic function in estrogen-deficient spontaneously hypertensive rats. Cardiovasc Res 67: 604–612, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Saleh MC, Connell BJ, Saleh TM. Autonomic and cardiovascular reflex responses to central estrogen injection in ovariectomized female rats. Brain Res 879: 105–114, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Saleh MC, Connell BJ, Saleh TM. Medullary and intrathecal injections of 17β-estradiol in male rats. Brain Res 867: 200–209, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Sandberg K, Ji H. Sex differences in primary hypertension. Biol Sex Differ 3: 7, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shenoy V, Grobe JL, Qi Y, Ferreira AJ, Fraga-Silva RA, Collamat G, Bruce E, Katovich MJ. 17β-Estradiol modulates local cardiac renin-angiotensin system to prevent cardiac remodeling in the DOCA-salt model of hypertension in rats. Peptides 30: 2309–2315, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Shih CD. Activation of estrogen receptor β-dependent nitric oxide signaling mediates the hypotensive effects of estrogen in the rostral ventrolateral medulla of anesthetized rats. J Biomed Sci 16: 60, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spary EJ, Maqbool A, Batten TF. Oestrogen receptors in the central nervous system and evidence for their role in the control of cardiovascular function. J Chem Neuroanat 38: 185–196, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Sullivan JC, Bhatia K, Yamamoto T, Elmarakby AA. Angiotensin (1–7) receptor antagonism equalizes angiotensin II-induced hypertension in male and female spontaneously hypertensive rats. Hypertension 56: 658–666, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sullivan JC. Sex and the renin-angiotensin system: inequality between the sexes in response to RAS stimulation and inhibition. Am J Physiol Regul Integr Comp Physiol 294: R1220–R1226, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Tremblay AM, Dufour CR, Ghahremani M, Reudelhuber TL, Giguère V. Physiological genomics identifies estrogen-related receptor alpha as a regulator of renal sodium and potassium homeostasis and the renin-angiotensin pathway. Mol Endocrinol 24: 22–32, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vongpatanasin W. Autonomic regulation of blood pressure in menopause. Semin Reprod Med 27: 338–345, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Wang G, Drake CT, Rozenblit M, Zhou P, Alves SE, Herrick SP, Hayashi S, Warrier S, Iadecola C, Milner TA. Evidence that estrogen directly and indirectly modulates C1 adrenergic bulbospinal neurons in the rostral ventrolateral medulla. Brain Res 1094: 163–178, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Wang G, Milner TA, Speth RC, Gore AC, Wu D, Iadecola C, Pierce JP. Sex differences in angiotensin signaling in bulbospinal neurons in the rat rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 295: R1149–R1157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei SG, Yu Y, Zhang ZH, Felder RB. Angiotensin II upregulates hypothalamic AT1 receptor expression in rats via the mitogen-activated protein kinase pathway. Am J Physiol Heart Circ Physiol 296: H1425–H1433, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu KL, Chen CH, Shih CD. Nontranscriptional activation of PI3K/Akt signaling mediates hypotensive effect following activation of estrogen receptor β in the rostral ventrolateral medulla of rats. J Biomed Sci 19: 76, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu P, Sriramula S, Lazartigues E. ACE2/ANG-(1–7)/Mas pathway in the brain: the axis of good. Am J Physiol Regul Integr Comp Physiol 300: R804–R817, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xue B, Badaue-Passos D, Jr, Guo F, Gomez-Sanchez CE, Hay M, Johnson AK. Sex differences and central protective effect of 17β-estradiol in the development of aldosterone/NaCl-induced hypertension. Am J Physiol Heart Circ Physiol 296: H1577–H1585, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xue B, Beltz TG, Johnson RF, Guo F, Hay M, Johnson AK. PVN adenovirus-siRNA injections silencing either NOX2 or NOX4 attenuate aldosterone/NaCl-induced hypertension in mice. Am J Physiol Heart Circ Physiol 302: H733–H741, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xue B, Beltz TG, Yu Y, Guo F, Gomez-Sanchez CE, Hay M, Johnson AK. Central interactions of aldosterone and angiotensin II in aldosterone- and angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol 300: H555–H564, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xue B, Pamidimukkala J, Lubahn DB, Hay M. Estrogen receptor-α mediates estrogen protection from angiotensin II-induced hypertension in conscious female mice. Am J Physiol Heart Circ Physiol 292: H1770–H1776, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Xue B, Singh M, Guo F, Hay M, Johnson AK. Protective actions of estrogen on angiotensin II-induced hypertension: role of central nitric oxide. Am J Physiol Heart Circ Physiol 297: H1638–H1646, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xue B, Zhang Z, Beltz TG, Johnson RF, Guo F, Hay M, Johnson AK. Estrogen receptor-beta (ERβ) in the PVN and RVLM plays an essential protective role in aldosterone/salt-induced hypertension in female rats. Hypertension 61: 1255–1262, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xue B, Zhang Z, Guo F, Hay M, Johnson AK. Central blockade of angiotensin(1–7) or angiotensin II receptor type 2 (AT2) enhances aldosterone/salt-induced increases in blood pressure in female rats. The Physiologist 54: 14, 2011 [Google Scholar]
- 46.Xue B, Zhang Z, Johnson RF, Johnson AK. Sensitization of slow pressor angiotensin II (Ang II)-initiated hypertension: induction of sensitization by prior Ang II treatment. Hypertension 59: 459–466, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xue B, Zhang Z, Roncari CF, Guo F, Johnson AK. Aldosterone acting through the central nervous system sensitizes angiotensin II-induced hypertension. Hypertension 60: 1023–1030, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xue B, Zhao Y, Johnson AK, Hay M. Central estrogen inhibition of angiotensin II-induced hypertension in male mice and the role of reactive oxygen species. Am J Physiol Heart Circ Physiol 295: H1025–H1032, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang XP, Reckelhoff JF. Estrogen, hormonal replacement therapy and cardiovascular disease. Curr Opin Nephrol Hypertens 20: 133–138, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu Y, Xue BJ, Zhang ZH, Wei SG, Beltz TG, Guo F, Johnson AK, Felder RB. Early interference with p44/42 mitogen-activated protein kinase signaling in hypothalamic paraventricular nucleus attenuates angiotensin II-induced hypertension. Hypertension 61: 842–849, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang ZH, Yu Y, Kang YM, Wei SG, Felder RB. Aldosterone acts centrally to increase brain renin-angiotensin system activity and oxidative stress in normal rats. Am J Physiol Heart Circ Physiol 294: H1067–H1074, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, Hodgin J, Shaul PW, Thoren P, Smithies O, Gustafsson JA, Mendelsohn ME. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science 295: 505–508, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Zimmerman MC, Sharma RV, Davisson RL. Superoxide mediates angiotensin II-induced influx of extracellular calcium in neural cells. Hypertension 45: 717–723, 2005 [DOI] [PubMed] [Google Scholar]