Abstract
We previously demonstrated that, in nasal epithelial cells (NECs) from smokers, methylation of an antiviral gene was associated with impaired antiviral defense responses. To expand these findings and better understand biological mechanisms underlying cigarette smoke (CS)-induced modifications of host defense responses, we aimed to compare DNA methylation of genes that may play a role in antiviral response. We used a two-tiered analytical approach, where we first implemented a genome-wide strategy. NECs from smokers differed in the methylation levels of 390 genes, the majority (84%) of which showed decreased methylation in smokers. Secondly, we generated an a priori set of 161 antiviral response-related genes, of which five were differentially methylated in NEC from smokers (CCL2, FDPS, GSK3B, SOCS3, and ULBP3). Assessing these genes at the systems biology level revealed a protein interaction network associated with CS-induced epigenetic modifications involving SOCS3 and ULBP3 signaling, among others. Subsequent confirmation studies focused on SOCS3 and ULBP3, which were hypomethylated and hypermethylated, respectively. Expression of SOCS3 was increased, whereas ULBP3 expression was decreased in NECs from smokers. Addition of the demethylating agent 5-Aza-2-deoxycytidine enhanced ULBP3 expression in NECs from smokers. Furthermore, infection of differentiated NECs with influenza virus resulted in significantly lower levels of ULBP3 in cells from smokers. Taken together, our findings show that genomic DNA methylation profiles are altered in NECs from smokers and that these changes are associated with decreased antiviral host defense responses, indicating that epigenenic dysregulation of genes such as SOCS3 and ULBP3 likely impacts immune responses in the epithelium.
Keywords: cigarette smoke, epigenetic, antiviral defense response, influenza
individuals chronically exposed to cigarette smoke (CS), either as smokers or through passive secondhand smoke exposure, experience increased risk of developing asthma, chronic obstructive pulmonary disease (COPD), cardiovascular disease, and lung cancer (37). Smoking not only induces cellular damage and inflammation but also serves as an immunosuppressor (37). Although the underlying molecular mechanisms of smoking-induced disease remain largely unknown, one of the proposed mechanisms involves epigenetic modifications (e.g., DNA methylation) that potentially alter gene expression profiles of CS-exposed target cells (15, 19, 22, 39). Gene-specific epigenetic regulation can occur through loss or gain of cytosine methylation in promoter-associated cytosine-phosphate-guanine (CpG) islands (33). Increased methylation (i.e., hypermethylation) of promoter CpG islands commonly causes transcriptional silencing (15). Conversely, decreased methylation (i.e., hypomethylation) of promoter CpG islands is commonly associated with transcriptional activation (15). Because DNA methylation plays such a crucial role in gene expression regulation, it is imperative to study the epigenomic impact of CS exposure. Identifying DNA methylation profiles resulting from chronic CS exposure will increase the understanding of mechanisms underlying smoking-related diseases.
Exposure to CS is associated with immunosuppressive effects, including altered innate and antiviral immune responses at various levels. For example, virus-induced cytokine and chemokine expression is modified in CS-exposed epithelial cells (30). We and others have previously demonstrated that CS exposure significantly alters interferon-dependent antiviral responses at the level of the epithelium (19, 40). Exposure to CS also alters the ability of epithelial cells to communicate with resident immune cells, such as dendritic cells and Natural Killer (NK) cells in the context of viral infections (17, 18). Activation and maturation of NK cells can be modulated by soluble factors released by epithelial cells, such as cytokines/chemokines, as well as ligands expressed on epithelial cells directly interacting with NK cell surface receptors (5). Our previous reports have demonstrated that exposure to the oxidant pollutant ozone modifies NK cell function through enhanced expression of major histocompatibility complex class I-related chain A/B (MICA/B) and UL16 binding proteins (ULBPs) on nasal epithelial cells (NECs), which are ligands for the activating receptor NKG2D expressed on NK cells (24). However, the potential mechanisms by which exposure to CS modulates epithelial cell-NK cell interactions are not well understood.
NECs are among the first targets of both inhaled CS and respiratory pathogens, such as influenza, and therefore constitute an important first line of defense, yet mechanisms mediating immunosuppressive effects in the nasal mucosa are not completely understood. Recent investigations mostly conducted in cancer cells reveal DNA methylation as a potential regulator of immune processes, as genes associated with innate host defense responses, such as IRF7, IRF8, IFN-γ, and STAT1 have shown differential methylation (33). We have previously demonstrated that IRF7, which is an important transcription factor during type I interferon-related host defense responses, is hypermethylated in NECs from smokers and that this effect was associated with reduced expression of IRF7 in the context of viral infection (19). This initial study prompted us to conduct studies aimed at understanding whether there are additional DNA methylation changes in NECs from smokers that are associated with modified host defense responses.
In this study, we tested the hypothesis that smoking changes DNA methylation profiles in NECs, specifically impacting genes associated with influenza-induced defense responses and NK cell activation. Using NECs from human volunteers, genome-wide methylation levels were compared between cells from smokers and nonsmokers. From the genome-wide analysis, we demonstrate that there are 390 genes with differential methylation in NECs from smokers. Focusing on an a priori list of 161 antiviral response-related genes revealed five genes differentially methylated in NECs from smokers, namely CCL2, FDPS, GSK3B, SOCS3, and ULBP3. Confirmation studies uncovered a potentially important role for smoking-induced hypermethylation of ULBP3 and its suppressed expression in NECs from smokers.
MATERIALS AND METHODS
NEC collection and culture.
Primary human NECs were collected from healthy smoking and healthy nonsmoking adult volunteers by gently stroking the inferior surface of the turbinate several times with a Rhino-Probe curette (Arlington Scientific, Arlington, TX). The selection criteria for subject recruitment were similar to those described previously (8, 41). Briefly, all smokers recruited for the study were current smokers, and smoking status for all volunteers was confirmed by questionnaire and smoking history. Subjects were selected between the ages of 18–40 yr and identified themselves as generally healthy and without a diagnosis for any smoking-related disorder or history of asthma. A summary of the subjects used for this study is detailed in Table 1, along with their urine cotinine/creatine ratios, when available, as calculated in our previous study (8). This protocol was approved by the University of North Carolina School of Medicine Institutional Review Board for Biomedical Research. Primary human NECs were expanded to passage 2 in bronchial epithelial growth medium (Cambrex Bioscience Walkersville, Walkersville, MD), grown in tissue culture flasks, and subsequently used for isolation of DNA.
Table 1.
Human subject summary and smoking information
| Subject Number | Current Smoking Status | Cotinine/Creatinine Ratio | Age, yr | Sex | Race |
|---|---|---|---|---|---|
| 1 | Smoker | 22425 | 33 | M | E |
| 2 | Smoker | 25756 | 39 | M | AA |
| 3 | Smoker | 8531 | 36 | F | E |
| 4 | Smoker | 7948 | 41 | M | E |
| 5 | Smoker | NA | 49 | F | AA |
| 6 | Smoker | 13412 | 35 | F | AA |
| 7 | Nonsmoker | ND | 34 | M | E |
| 8 | Nonsmoker | ND | 27 | F | E |
| 9 | Nonsmoker | ND | 32 | F | NA |
| 10 | Nonsmoker | ND | 24 | F | E |
| 11 | Nonsmoker | ND | 47 | F | E |
| 12 | Nonsmoker | ND | 26 | F | HISP |
NA, not available; ND, not detected; AA, African American; E, of Western European decent; HISP, Hispanic white.
DNA extraction and hybridization to Illumina Infinium assay.
Cells were lysed with TRizol (Invitrogen, Carlsbad, CA), and DNA was extracted per the supplier's protocol (Invitrogen). The DNA was hybridized to the IlluminaHumanMethylation27 BeadChip (Illumina, San Diego, CA) at Expression Analysis (Durham, NC). A bisulfite conversion reaction was first performed using 500 ng of genomic DNA according to the manufacturer's protocol for the Zymo EZ DNA Methylation kit (Zymo Research, Irvine, CA). DNA was added to Zymo M-Dilution buffer and incubated for 15 min at 37°C. CT conversion reagent was then added, and the mixture was denatured by heating to 95°C for 30 s followed by incubation for 1 h at 50°C. This denature/incubation cycle was repeated for a total of 16 h. After bisulfite conversion, the DNA was bound to a Zymo spin column and desulfonated on the column using desulfonation reagent per the manufacturer's protocol. The bisulfite-converted DNA was eluted from the column in 10 μl of elution buffer.
The bisulfite-converted DNA was next amplified, fragmented, and hybridized to the Infinium Methylation Assay and scanned. Briefly, 4 μl of bisulfite-converted product was transferred to a new plate with an equal amount of 0.1 N NaOH and 20 μl of MA1 reagent (Illumina) and then allowed to incubate at room temperature for 10 min. Immediately following incubation, 68 μl of MA2 reagent (Illumina) and 75 μl of MSM reagent (Illumina) were added, and the plate was incubated at 37°C overnight for amplification. After amplification, the DNA was fragmented enzymatically, precipitated, and resuspended in RA1 hybridization buffer. Fragmented DNA was then dispensed onto the multichannel HumanMethylation BeadChips, and hybridization was performed in an Illumina Hybridization oven for 20 h. Samples were randomly assigned to positions on the array. BeadChips were washed, primer extended, and stained per manufacturer protocols. BeadChips were coated and then imaged on an Illumina iScan Reader, images were processed, and data were normalized with GenomeStudio software (version 3.2) methylation module.
Analysis of CpG site methylation.
Genomic DNA isolated from NECs from smokers and nonsmokers was analyzed for DNA methylation in promoter site regions using the Illumina Infinium array. This method quantifies methylation levels at 27,578 CpG dinucloeotides in the proximal promoter regions of 14,495 genes (3). The methylation status of the CpG sites are calculated as the ratio of fluorescent signal from one allele relative to the sum of both methylated and unmethylated alleles. The resulting average β-intensity signals, representing percentage of methylation, range from 0 (unmethylated) to 1 (fully methylated). Any records below detection limit were analyzed with β-values of 0.05, approximating the median β-value across all samples.
For the genome-wide analysis, differential methylation was defined as a significant difference in percentage of methylation between smoker samples vs. nonsmoker samples, where the following two statistical requirements were set: 1) a percentage change in β-intensity signal of ≥33.3% or ≤−33.3% (smokers vs. nonsmokers), and 2) ANOVA P value <0.05. ANOVA P values were calculated using Partek Genomics Suite software (St. Louis, MO). To test the potential influence of age, an additional statistical analysis was performed on a subset of genes. Here, a mixed model ANCOVA was carried out comparing methylation levels of smokers vs. nonsmokers while considering age as a covariate (Partek). Array data have been submitted to the National Center for Biotechnology Information Gene Expression Omnibus (GEO) repository (http://www.ncbi.nlm.nih.gov/geo/) (11) and are available under accession number GSE28368.
To test whether ULBP3 methylation levels were related to cotinine levels, a correlation analysis was performed comparing the methylation array β-values for ULBP3 to the cotinine/creatinine ratios. This correlation analysis was carried out across all subjects, excluding subject 5, as cotinine/creatinine levels were not available for this subject. A Spearman Rank test was carried out using Spotfire (version 5.0.0; TIBCO Software, Somerville, MA) to correlate these two measurements.
A priori list of antiviral response-related genes.
For the a priori list of antiviral response-related genes, a list was curated using the KEGG pathway database, release 65.0 (www.genome.jp/kegg/pathway.html). Specifically, genes encoded by proteins within the Influenza A Pathway and in the NK Cell-Mediated Cytotoxicity Pathway were curated. In the gene list related to the NK Cell-Mediated Cytotoxicity Pathway, we only focused on genes marked as involved in signaling of the “target cell” because those genes would likely be expressed in NECs. This list of genes was filtered to include only those represented by the Illumina methylation array. The resulting antiviral response-related genes were compared against genes identified as differentially methylated in smoker NECs to identify genes with altered methylation levels associated with CS that are involved in antiviral response signaling.
Network analysis.
Molecular network analysis was performed using the Ingenuity database (Ingenuity Systems, Redwood City, CA). The Ingenuity database provides a collection of gene to phenotype associations, molecular interactions, regulatory events, and chemical knowledge accumulated to develop a global molecular network. The list of differentially methylated genes related to antiviral response was overlaid onto this global molecular network, where cellular networks strongly associated with the targets were algorithmically constructed based on connectivity. Statistical significance of the constructed network was calculated using a modified Fisher's Exact Test, based on the following equation (6):
Here, N is the total number of molecules in the global network of the database, which contains D total proteins encoded by differentially methylated genes. Each individual network contains n molecules, d of which are proteins encoded by differentially methylated genes. C is the binomial coefficient (6). The resulting P value indicates the probability of finding the observed number of molecules in a given network by random chance.
Real-time PCR.
Total RNA was isolated using TRIzol (Invitrogen) according to the manufacturer instructions. First-strand cDNA synthesis and quantitative RT-PCR were performed as previously described (19, 20). Primers and probes were commercially available (Applied Biosystems, Foster City, CA). In some experiments, NECs were treated with 5 μM 5-Aza-2-deoxycytidine (5-Aza) (Sigma, St. Louis, MO) or DMSO vehicle each day for 3 days before isolation of total RNA.
Infection with Influenza A virus and analysis of ULBP3 expression.
NECs were obtained and expanded as described above, followed by seeding of the cells on Transwell inserts and culturing at air-liquid-interface conditions for 3–4 wk to induce differentiation (16–19). Differentiated NECs were infected with Influenza A virus as described by our laboratory before (16–19). At 24 h postinfection, differentiated NECs from smokers and nonsmokers were digested for 30 min at 37°C in 1 ml RPMI, 15 μg/ml DNAse I (Sigma), and 5 μg/ml PronaseE (Sigma), as described by our laboratory before (24). The digestion activity was stopped by adding 100 μl FBS, the cell suspension was centrifuged (500 g, 10 min, 4°C), and the cell pellet was resuspended in 1 ml flow-staining buffer [PBS (without Ca2+ and Mg2+; Gibco, Carlsbad, CA), 1% heat-inactivated FBS, and 0.09% Sodium Azide (Sigma)]. Cells were incubated 20 min at room temperature in the dark with antibodies against human ULBP3 or its respective isotype control (IgG2, both from R&D Systems, Minneapolis, MN). All samples were analyzed within 24 h on a BD LSRII flow cytometer (BD Biosciences, San Jose, CA). Results obtained from cells infected with Influenza A were normalized to matched noninfected samples using cells from the same subjects and presented as fold induction of noninfected controls.
Statistical analysis for the RT-PCR.
For the RT-PCR confirmation experiments, data were normalized to β-actin mRNA levels and analyzed using Student's t-test. For the flow cytometry data, results were analyzed using the Wilcoxon signed rank test to test the fold induction to a theoretical value of 1. For all experiments, a value of P < 0.05 was considered significant.
RESULTS
CS exposure impacts the DNA methylation profiles of hundreds of genes.
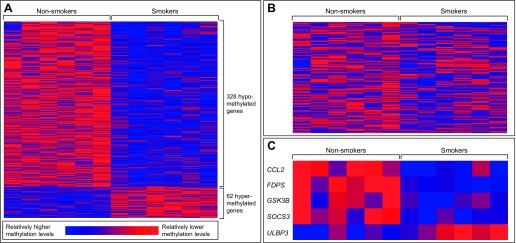
Genome-wide gene-specific DNA methylation profiles were analyzed using NECs collected from six healthy smokers and six healthy nonsmokers (Table 1). The original nasal scrape biopsies were expanded in vitro, and genomic DNA was extracted from the expanded nasal cells and analyzed for DNA methylation in gene promoter regions using the Illumina Infinium assay. This assay measures the methylation levels of 27,578 CpG sites across 14,495 genes. We employed a comparative statistical approach to identify genes with differential DNA methylation resulting from CS exposure. A total of 390 genes showed differential methylation (ANOVA P value <0.05, percentage change in methylation ≥±33.3%) between NECs from smokers and nonsmokers (Fig. 1A, Supplemental Table S1; supplemental material for this article is available online at the American Journal of Physiology Lung Cellular and Molecular Physiology website). Of the CS-associated genes, 328 showed decreased methylation levels (i.e., hypomethylation) and 62 showed increased methylation levels (i.e., hypermethylation) in NECs from smokers compared with nonsmokers.
Fig. 1.
DNA methylation levels in smokers and nonsmokers. The relative methylation levels of genes are displayed with heat maps for the 390 genes differentially methylated in smokers (A), 161 antiviral response genes represented in the methylation array (B), and 5 antiviral response genes identified as differentially methylated in smokers compared with nonsmokers (C). Methylation levels are mean standardized for each gene across all human subjects.
Antiviral response-related genes are differentially methylated in smokers.
To focus on genes related to epithelial cell-dependent host defense responses in the context of influenza infections, we generated an a priori list of genes to be examined for differences in DNA methylation patterns. Because our previous studies identified differences in influenza-induced antiviral defense responses and NK cell activation in the nasal mucosa of smokers (17, 19, 24, 25), we used the KEGG Pathway Maps related to the Influenza A Pathway and the NK Cell-Mediated Cytotoxicity Pathway to generate a list of 186 antiviral response-related genes, of which 161 were represented on the DNA methylation array (Fig. 1B; Supplemental Table S2). Based on this list, we identified five genes that were differentially methylated in NECs from smokers and nonsmokers (Fig. 1C), with four genes being hypomethylated (CCL2, FDPS, GSK3B, and SOCS3) and one gene being hypermethylated (ULBP3) in NECs from smokers. The methylation levels of ULBP3 were correlated, to an extent (P = 0.09), with cotinine/creatinine levels.
An additional statistical analysis was performed for these five antiviral response-related genes, where array-based methylation levels in smokers were compared against nonsmokers while adjusting for age. The P values of significance for CCL2, FDPS, and SOCS3 remained <0.05. The P values for GSK3B and ULBP3 increased slightly to 0.098 and 0.067, respectively. Nevertheless, the difference in methylation in ULBP3 between smokers and nonsmokers is still biologically relevant, as determined through additional in vitro and functional tests.
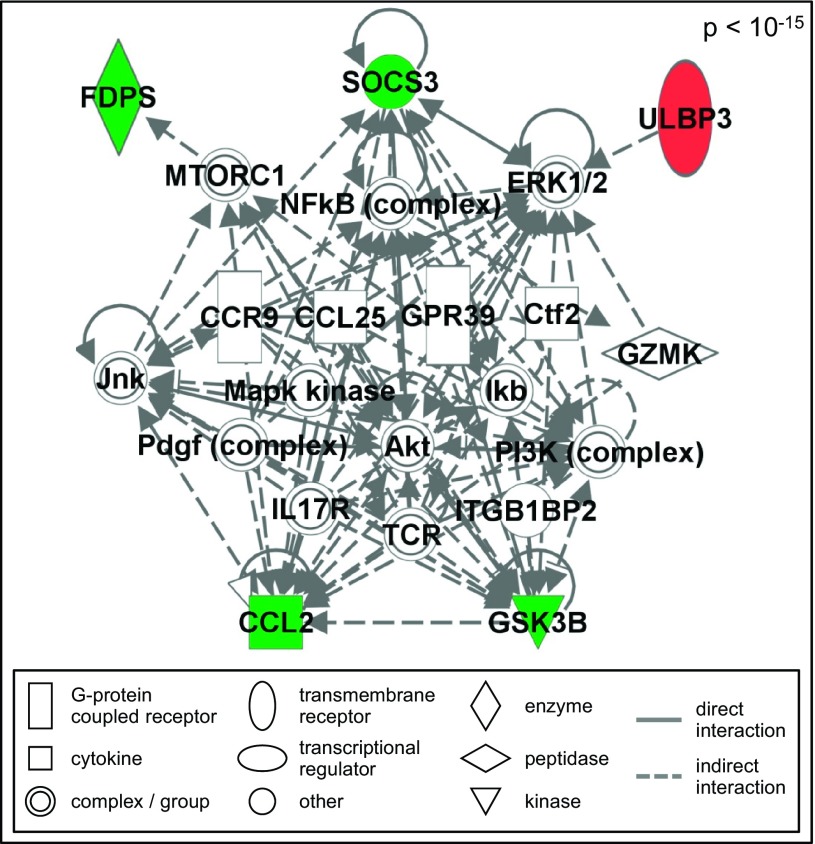
Network associated with DNA methylation contains SOCS3 and ULBP3 signaling.
To understand cellular interactions related to antiviral responses that may be modified by CS-associated epigenetic changes, network analysis of the five antiviral response-related genes that were differentially methylated in smokers was performed. One significant (P < 10−15) network was constructed containing interactions that may be epigenetically modified in NECs from smokers (Fig. 2). The overall network was not modified by the removal of T cell receptor and granzyme K (granzyme 3, tryptase II), known proteins involved in signaling within immune cells (data not shown). This network contained signaling related to SOCS3 and ULBP3, among others. SOCS3 negatively regulates type I interferon signaling and during viral infections increased expression of SOCS3 is associated with suppressed antiviral host defense responses (27). The gene ULBP3 is important for cell-cell communication during host defense responses, including activation of cytotoxic lymphocytes, such as NK cells. In addition, we have recently demonstrated that expression of ULBP3 on NECs modulates NK cell function (24). Therefore, based on their known function, our previous results, and our network-predicted interactions, we focused our subsequent analysis on SOCS3 and ULBP3.
Fig. 2.
Antiviral response-related network epigenetically modified in nasal epithelial cells (NECs) from smokers. The network is displayed with symbols representing products of the hypermethylated gene (red symbol), hypomethylated genes (green symbol), or gene products associated with differentially methylated genes (open symbols).
In vitro confirmation of CS-induced changes in SOCS3 and ULBP3 expression in NECs.
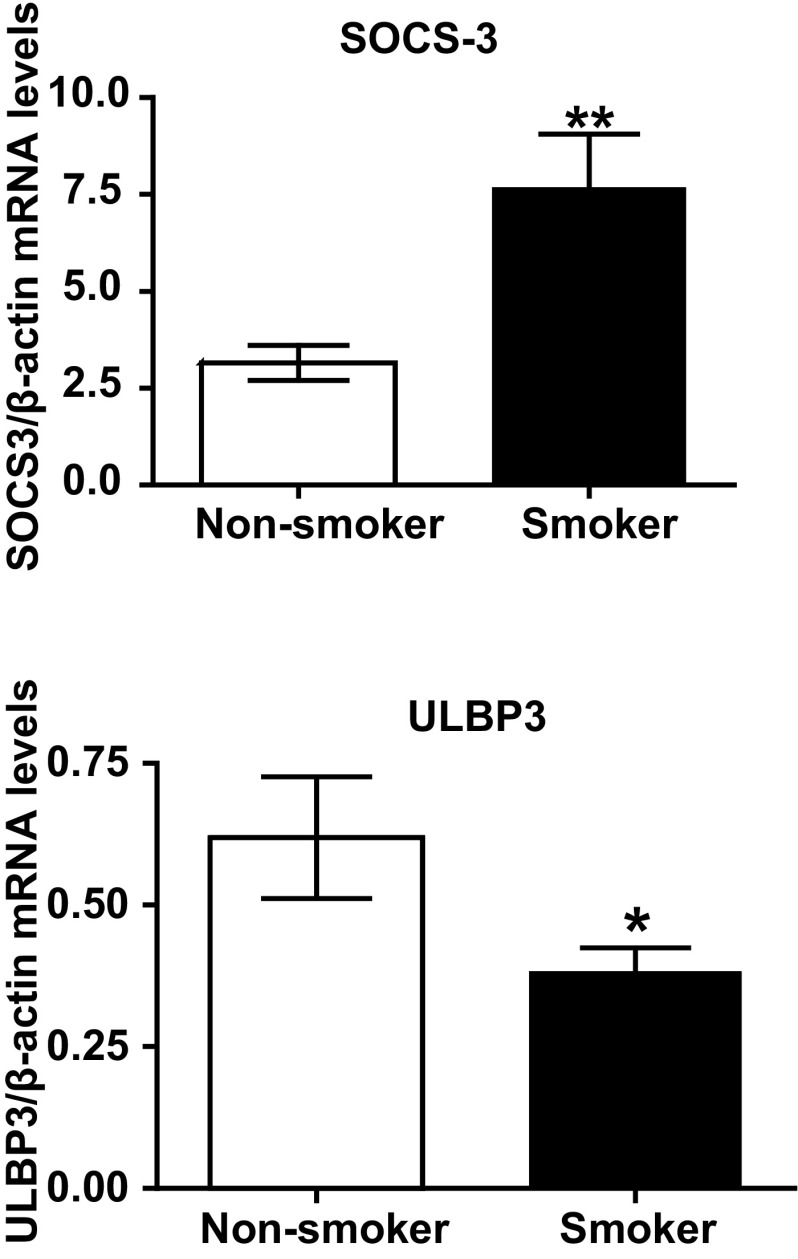
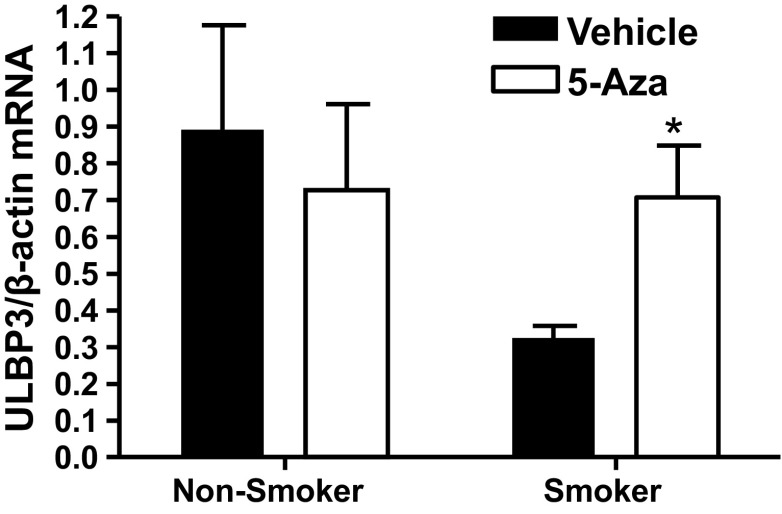
Using NECs from a separate cohort of smokers and nonsmokers, we confirmed DNA methylation changes using qRT-PCR. As expected, hypomethylation of SOCS3 was associated with increased expression of this gene in NECs from smokers (Fig. 3A). Similarly, hypermethylation of ULBP3 was associated with decreased mRNA levels in NECs from smokers (Fig. 3B). To determine whether the decreased ULBP3 expression in NECs from smokers was caused by methylation-dependent gene silencing, we treated NECs with the demethylating agent 5-Aza before analysis of ULBP3 expression. Treatment with 5-Aza enhanced ULBP3 expression in NECs from smokers, but not nonsmokers (Fig. 4), suggesting that pharmacological demethylation can reverse the effects of CS exposure on ULBP3 expression in NECs.
Fig. 3.
RT-PCR validation of SOCS3 and ULBP3 expression. A: SOCS3, identified as hypomethylated in NECs from smokers, is expressed at significantly higher levels in smokers compared with nonsmokers. B: ULBP3, identified as hypermethylated in NECs from smokers, is expressed at significantly lower levels in smokers compared with nonsmokers. Data are means ± SE (n = 3 for smokers and nonsmokers); *P < 0.05; **P < 0.005.
Fig. 4.
Demethylation reverses the effects of smoking on ULBP3 expression. NECs from smokers and nonsmokers were treated with fresh 5 μM 5-Aza-2-deoxycytidine (5-Aza) each day for 3 days. Total RNA was analyzed for ULBP3 mRNA levels 24 h after the last treatment. Data are expressed as means ± SE (n = 3 each for smokers and nonsmokers); *P < 0.05.
Confirmation of ULBP3 in the context of viral infection.
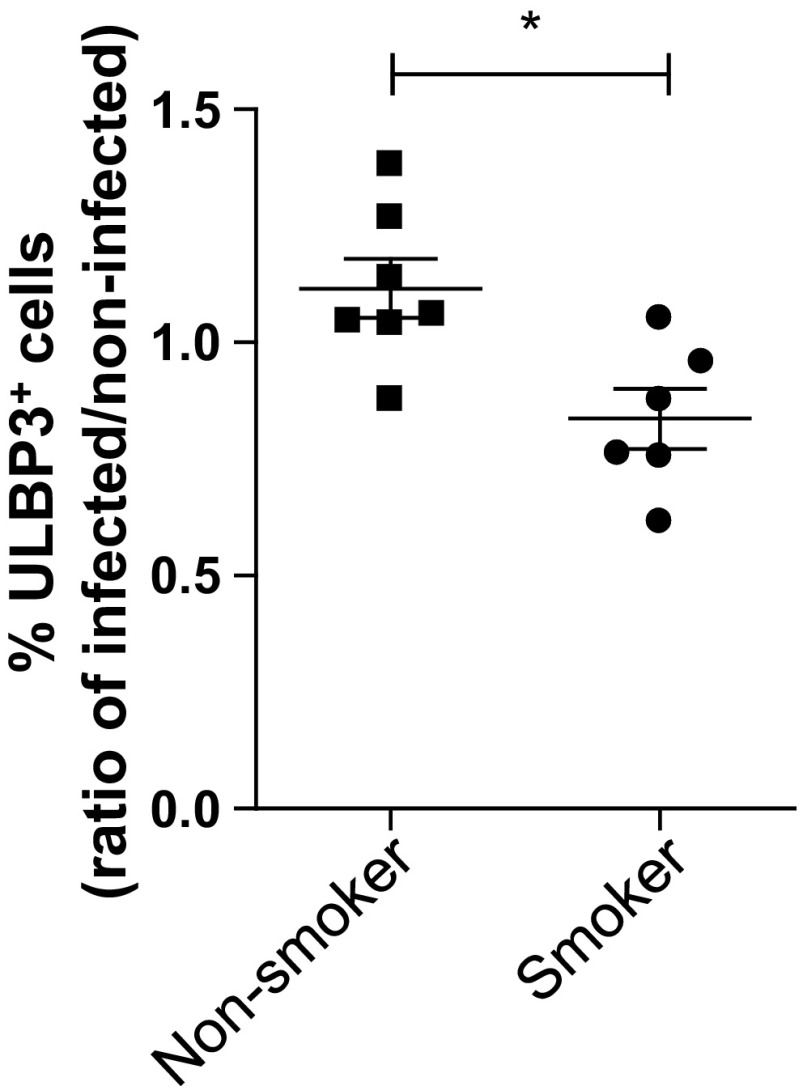
ULBP3 belongs to the family of UL16-binding proteins (ULBP1–6), which are ligands for the receptor NKG2D, found on cytotoxic lymphocytes, such as NK cells and CD8+ T lymphocytes (7). Expression of NKG2D ligands, such as ULBP3, is enhanced in stressed epithelial cells, cancer cells, and virus-infected cells (5, 26). Therefore, we determined whether DNA methylation of ULBP3 in NECs from smokers affects expression of this ligand in the context of viral infection. Differentiated NECs from smokers and nonsmokers were infected with Influenza A virus and subsequently analyzed for ULBP3 expression using flow cytometry. Figure 5 shows that Influenza A virus-induced expression of ULBP3 is significantly reduced in NECs from smokers, confirming the functional role of smoking-induced epigenetic modification of ULBP3 in the context of viral infection.
Fig. 5.
Decreased ULBP3 expression in influenza-infected NECs from smokers. Differentiated NECs from smokers and nonsmokers were infected with Influenza A and analyzed for ULBP3 expression by flow cytometry 24 h postinfection. Data of percentage of cells staining positively for ULBP3 are expressed as fold change of infected over the respective noninfected control for each subject (n = 6 for smokers and n = 7 for nonsmokers); *P < 0.05.
DISCUSSION
NECs are major targets for CS exposure, both in smokers and those exposed passively through secondhand smoke exposure. Previous studies conducted by our group have demonstrated that smokers differ in their ability to respond to viral infections, as marked by increased viral replication, suppressed type I interferon responses and decreased activation of NK cells (17, 19, 25). These responses were associated with DNA methylation of specific antiviral defense response genes in NECs from smokers (19), encouraging us to systematically identify additional changes in DNA methylation that could mediate the impaired antiviral defense responses seen in smokers in vitro and in vivo. To do so, we first employed a genome-wide assessment to examine differences in DNA methylation patterns in NECs from smokers and nonsmokers. Secondly, we selected an a priori set of genes related to host defense responses potentially expressed in NECs. Using this approach, we identified a group of five genes differentially methylated in NECs from smokers (CCL2, FDPS, GSK3B, SOCS3, and ULBP3). Subsequent confirmation studies demonstrate that smoking-induced hypermethylation of ULBP3 leads to its suppressed expression in NECs from smokers in the context of viral infection. Thus smoking-induced effects on epithelial cells leading to gene silencing of ligands for cytotoxic lymphocytes, such as ULBP3, may contribute to the enhanced susceptibility to viral infections seen in smokers.
Our analysis identified ULBP3 as hypermethylated in NECs from smokers, resulting in decreased expression in these cells. This effect could be reversed by treatment with the demethylating agent 5-Aza, suggesting a direct link between smoking-induced DNA methylation of the gene and suppressed expression in NECs. ULBP3 belongs to the family of ULBPs, which were originally identified as ligands for the UL16 glycoprotein of the human cytomegalovirus (9). ULBPs are considered MHC class I-related molecules and are strong activating ligands for NKG2D, which is a surface receptor expressed on lymphocytes, including NK cells, cytotoxic T cells, as well as γ/δ T cells (2). ULBPs are expressed on stressed epithelial cells (5), and we have recently shown that their expression can be induced in NECs by the oxidant pollutant ozone, which we demonstrated affects lymphocyte function (24). In the context of viral infections, expression of ligands for NKG2D, such as ULBPs, by infected host cells targets them for detection and elimination by cytotoxic lymphocytes, such as NK cells (26, 32). Consequently, inability to enhance the expression of ULBPs by virus-infected cells, as shown by us here (Fig. 5), would result in decreased recognition by NK cells and T lymphocytes and impaired activation of cytotoxic lymphocytes through ULBP-NKG2D interactions. Differentiation of NECs in vitro takes several weeks, suggesting that the smoking-induced epigenetic changes of ULBP3 are not readily reversible. We have previously shown that virus-induced NK cell activation is reduced in the nasal mucosa of smokers (17). These studies demonstrated that smokers inoculated with live-attenuated influenza virus (LAIV) vaccine had reduced cytotoxic NK cells and markers of activated NK cells in their nasal lavage (17). In a similar study, we demonstrated that LAIV-induced recruitment of γ/δ T cells into the nasal mucosa is impaired in smokers (16). Thus smoking-induced gene silencing of ULBP3 in NECs could lead to decreased NK cell and γ/δ T cell function in the nasal mucosa in the context of viral infection and result in impaired ability to recognize and eliminate virus-infected host cells.
Smoking-induced suppression of ULBP3 expression could have implications beyond mucosal immune defense responses. Decreased ULBP3 expression has been shown in tumor cells, thus favoring evasion from recognition and killing by NK cells (38). Consequently, enhancing expression of ULBP3 has been suggested as a potential therapeutic target to increase recognition and killing of tumor cells by NK cells (10). Moreover, ULBP expression correlates with improved survival in patients with cancer (29). Thus, in addition to having important roles in cell-cell communication during host defense responses, gene silencing of ULBP genes could have significant implications for the development and progression of smoking-related cancers.
Our data demonstrate that SOCS3 was hypomethylated in NECs from smokers, which was associated with enhanced expression of SOCS3 in these cells. SOCS3 is a member of the SOCS or STAT-induced STAT inhibitor family, which includes SOCS 1–3 (21). These proteins are cytokine-inducible negative regulators of cytokine signaling. SOCS3 is a key regulator of JAK signaling, which is activated by interferon receptors, and is therefore considered a key negative regulator of interferon, particularly type I interferon-induced signaling (4). Several viruses, including influenza virus, have developed mechanisms to enhance SOCS3 expression in host cells to inhibit interferon signaling and therefore evade antiviral defense responses (27). Similar to our data shown here, chronic exposure to CS increased SOCS3 mRNA levels in the lungs of mice (14). However, these exposures did not induce any changes in SOCS3 protein levels in whole lung homogenates, indicating that either additional posttranscriptional regulation of SOCS3 or more cell type-specific changes, which are not reflected in whole lung homogenates, may play important roles.
Using genomic DNA from NECs from six healthy smoking and six healthy nonsmoking adult volunteers, our study analyzed DNA methylation in specific gene promoter regions. The Illumina Infinium assay was used to measure methylation levels of more than 27,000 CpG sites across the promoters of over 14,000 genes (3). We recognize that this technology does not measure potential changes in DNA methylation levels across the gene, or intragenic methylation, which can also play a role in regulating transcription (34). Still, the current understanding of transcriptional regulation via DNA methylation centers around CpG methylation within gene promoter regions (34). Of the 390 genes identified to have differential DNA methylation in NECs from smokers, 84% were hypomethylated. Smoking has been associated with global hypomethylation in patients at risk of developing squamous cell carcinoma (28). Smoking has also been associated with altered methylation in specific oncogenes or tumor suppressor genes (31). DNA methylation is in part regulated by the activity of DNA methyltransferases (DNMTs) and the availability of methyl donors in the cell. We have previously demonstrated that DNMT1 expression is enhanced in NECs from smokers (19). S-adenosyl-methionine (SAM) is the primary methyl donor used by DNMTs, and previous studies have shown that in guinea pigs chronic nicotine exposure decreases the levels of SAM in the lung (12). Thus the genome-wide tendency for hypomethylation of genes could be derived from limited methyl donors available in NECs from smokers.
In conclusion, our data demonstrate that smoking induces differential DNA methylation patterns in NECs and that these changes can be functionally integrated with smoking-induced alterations in host defense responses. It is well known that smoking impairs responses against microbial infections, and our data propose that epigenetic modification of immune genes is a potential mechanism mediating this effect. Similar to the NECs studied here, it is likely that host defense responses in lower airway epithelial cells are modulated by epigenetic effects. The nasal epithelium resembles the airway epithelium morphologically, and smoking-related gene expression as well as histological changes, such as mucus production, are similar in nasal and airway epithelial cells (13, 19, 36). In airway diseases such as asthma, previous studies have demonstrated that DNA methylation changes in NECs from asthmatic children were associated with disease parameters in this population (1), suggesting that epigenetic modification in NECs can reflect changes in the lower airways. In addition, whether and to what extent smoking cessation would be able to revert these epigenetic effects are unclear. Analyzing gene expression profiles in never, current, and former smokers have shown that some, but not all, smoking-induced gene expression changes are reversible and return to the levels of never smokers after smoking cessation (35). Thus reversal of smoking-induced changes in DNA methylation will likely be gene specific. Future studies will be able to determine whether and to what extent smoking-induced DNA methylation patterns are reversible.
GRANTS
This work was supported by grants from the National Institutes of Health (ES019315, ES007018, HL095163, P30ES010126), and the Flight Attendant Medical Research Institute. The project described was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Award Number UL1TR000083. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Although the research described in this article has been funded in part by the U.S. Environmental Protection Agency through cooperative agreement CR83346301 with the Center for Environmental Medicine, Asthma and Lung Biology at the University of North Carolina-Chapel Hill, it has not been subjected to the agency's required peer and policy review and therefore does not necessarily reflect the views of the agency, and no official endorsement should be inferred. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.E.R., R.N.B., L.L.M., L.S., J.L.C., and L.E.B. performed experiments; J.E.R., R.N.B., L.L.M., L.S., and I.J. analyzed data; J.E.R., L.L.M., J.L.C., R.C.F., and I.J. interpreted results of experiments; J.E.R., R.N.B., L.L.M., and L.S. prepared figures; J.E.R., R.N.B., R.C.F., and I.J. drafted manuscript; J.E.R., R.N.B., R.C.F., and I.J. edited and revised manuscript; R.C.F. and I.J. conception and design of research; R.C.F. and I.J. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Martha Almond, Carole Robinette, and Margret Herbst for subject recruitment.
REFERENCES
- 1.Baccarelli A, Rusconi F, Bollati V, Catelan D, Accetta G, Hou L, Barbone F, Bertazzi PA, Biggeri A. Nasal cell DNA methylation, inflammation, lung function and wheezing in children with asthma. Epigenomics 4: 91–100, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 285: 727–729, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Bibikova M, Le J, Barnes B, Saedinia-Melnyk S, Zhou L, Shen R, Gunderson KL. Genome-wide DNA methylation profiling using Infinium(R) assay. Epigenomics 1: 177–200, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Bonjardim CA, Ferreira PC, Kroon EG. Interferons: signaling, antiviral and viral evasion. Immunol Lett 122: 1–11, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borchers MT, Harris NL, Wesselkamper SC, Vitucci M, Cosman D. NKG2D ligands are expressed on stressed human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 291: L222–L231, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, Chen RO, Brownstein BH, Cobb JP, Tschoeke SK, Miller-Graziano C, Moldawer LL, Mindrinos MN, Davis RW, Tompkins RG, Lowry SF. A network-based analysis of systemic inflammation in humans. Nature 437: 1032–1037, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Cao W, He W. UL16 binding proteins. Immunobiology 209: 283–290, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Carson J, Lu TS, Brighton L, Hazucha M, Jaspers I, Zhou H. Phenotypic and physiologic variability in nasal epithelium cultured from smokers and non-smokers exposed to secondhand tobacco smoke. In Vitro Cell Dev Biol Anim 46: 606–612, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cosman D, Mullberg J, Sutherland CL, Chin W, Armitage R, Fanslow W, Kubin M, Chalupny NJ. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity 14: 123–133, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Coudert JD, Held W. The role of the NKG2D receptor for tumor immunity. Semin Cancer Biol 16: 333–343, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30: 207–210, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godin CS, Crooks PA. In vivo depletion of S-adenosyl-L-homocysteine and S-adenosyl-L-methionine in guinea pig lung after chronic S-(-)-nicotine administration. Toxicol Lett 31: 23–29, 1986. [DOI] [PubMed] [Google Scholar]
- 13.Hadar T, Yaniv E, Shvili Y, Koren R, Shvero J. Histopathological changes of the nasal mucosa induced by smoking. Inhal Toxicol 21: 1119–1122, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Halappanavar S, Russell M, Stampfli MR, Williams A, Yauk CL. Induction of the interleukin 6/signal transducer and activator of transcription pathway in the lungs of mice sub-chronically exposed to mainstream tobacco smoke. BMC Med Genomics 2: 56, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho SM. Environmental epigenetics of asthma: An update. J Allergy Clin Immunol 126: 453–465, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horvath KM, Brighton LE, Herbst M, Noah TL, Jaspers I. Live Attenuated Influenza Virus (LAIV) induces different mucosal T cell function in nonsmokers and smokers. Clin Immunol 142: 232–236, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horvath KM, Brighton LE, Zhang W, Carson JL, Jaspers I. Epithelial cells from smokers modify dendritic cell responses in the context of influenza infection. Am J Respir Cell Mol Biol 45: 237–245, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horvath KM, Herbst M, Zhou H, Zhang H, Noah TL, Jaspers I. Nasal lavage natural killer cell function is suppressed in smokers after live attenuated influenza virus. Respir Res 12: 102, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaspers I, Horvath KM, Zhang W, Brighton LE, Carson JL, Noah TL. Reduced expression of IRF7 in nasal epithelial cells from smokers after infection with influenza. Am J Respir Cell Mol Biol 43: 368–375, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaspers I, Zhang W, Brighton LE, Carson JL, Styblo M, Beck MA. Selenium deficiency alters epithelial cell morphology and responses to influenza. Free Radic Biol Med 42: 1826–1837, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kubo M, Hanada T, Yoshimura A. Suppressors of cytokine signaling and immunity. Nat Immunol 4: 1169–1176, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Liu F, Killian JK, Yang M, Walker RL, Hong JA, Zhang M, Davis S, Zhang Y, Hussain M, Xi S, Rao M, Meltzer PA, Schrump DS. Epigenomic alterations and gene expression profiles in respiratory epithelia exposed to cigarette smoke condensate. Oncogene 29: 3650–3664, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Müller L, Brighton LE, Jaspers I. Ozone exposed epithelial cells modify cocultured natural killer cells. Am J Physiol Lung Cell Mol Physiol 304: L332–L341, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noah TL, Zhou H, Monaco J, Horvath K, Herbst M, Jaspers I. Tobacco smoke exposure and altered nasal responses to live attenuated influenza virus. Environ Health Perspect 119: 78–83, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Connor GM, Hart OM, Gardiner CM. Putting the natural killer cell in its place. Immunology 117: 1–10, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pauli EK, Schmolke M, Wolff T, Viemann D, Roth J, Bode JG, Ludwig S. Influenza A Virus inhibits type I IFN signaling via NF-κB-Dependent induction of SOCS-3 expression. PLoS Pathog 4: e1000196, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piyathilake CJ, Frost AR, Bell WC, Oelschlager D, Weiss H, Johanning GL, Niveleau A, Heimburger DC, Grizzle WE. Altered global methylation of DNA: an epigenetic difference in susceptibility for lung cancer is associated with its progression. Hum Pathol 32: 856–862, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Poggi A, Venturino C, Catellani S, Clavio M, Miglino M, Gobbi M, Steinle A, Ghia P, Stella S, Caligaris-Cappio F, Zocchi MR. Vdelta1 T lymphocytes from B-CLL patients recognize ULBP3 expressed on leukemic B cells and up-regulated by trans-retinoic acid. Cancer Res 64: 9172–9179, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Proud D, Hudy MH, Wiehler S, Zaheer RS, Amin MA, Pelikan JB, Tacon CE, Tonsaker TO, Walker BL, Kooi C, Traves SL, Leigh R. Cigarette smoke modulates expression of human rhinovirus-induced airway epithelial host defense genes. PLoS One 7: e40762, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pulling LC, Vuillemenot BR, Hutt JA, Devereux TR, Belinsky SA. Aberrant promoter hypermethylation of the death-associated protein kinase gene is early and frequent in murine lung tumors induced by cigarette smoke and tobacco carcinogens. Cancer Res 64: 3844–3848, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Richard J, Sindhu S, Pham TN, Belzile JP, Cohen EA. HIV-1 Vpr up-regulates expression of ligands for the activating NKG2D receptor and promotes NK cell-mediated killing. Blood 115: 1354–1363, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shames D, Minna J, Gazdar A. DNA Methylation in Health, Disease, and Cancer. Curr Mol Med 7: 85–102, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Shenker N, Flanagan JM. Intragenic DNA methylation: implications of this epigenetic mechanism for cancer research. Br J Cancer 106: 248–253, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spira A, Beane J, Shah V, Liu G, Schembri F, Yang X, Palma J, Brody JS. Effects of cigarette smoke on the human airway epithelial cell transcriptome. Proc Natl Acad Sci USA 101: 10143–10148, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sridhar S, Schembri F, Zeskind J, Shah V, Gustafson A, Steiling K, Liu G, Dumas YM, Zhang X, Brody J, Lenburg M, Spira A. Smoking-induced gene expression changes in the bronchial airway are reflected in nasal and buccal epithelium. BMC Genomics 9: 259, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stämpfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol 9: 377–384, 2009. [DOI] [PubMed] [Google Scholar]
- 38.Sutherland CL, Rabinovich B, Chalupny NJ, Brawand P, Miller R, Cosman D. ULBPs, human ligands of the NKG2D receptor, stimulate tumor immunity with enhancement by IL-15. Blood 108: 1313–1319, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Veljkovic E, Jiricny J, Menigatti M, Rehrauer H, Han W. Chronic exposure to cigarette smoke condensate in vitro induces epithelial to mesenchymal transition-like changes in human bronchial epithelial cells, BEAS-2B. Toxicol In Vitro 25: 446–453, 2010. [DOI] [PubMed] [Google Scholar]
- 40.Wu W, Patel KB, Booth JL, Zhang W, Metcalf JP. Cigarette smoke extract suppresses the RIG-I-initiated innate immune response to influenza virus in the human lung. Am J Physiol Lung Cell Mol Physiol 300: L821–L830, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou H, Wang X, Brighton L, Hazucha M, Jaspers I, Carson JL. Increased nasal epithelial ciliary beat frequency associated with lifestyle tobacco smoke exposure. Inhal Toxicol 21: 875–881, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.