Abstract
Respiratory muscle-associated stretch has been implicated in normal lung development (fetal breathing movements) and postpneumonectomy lung growth. To test the hypothesis that mechanical stretch from diaphragmatic contraction contributes to lung growth, we performed left phrenic nerve transections (PNT) in mice with and without ipsilateral pneumonectomy. PNT was demonstrated by asymmetric costal margin excursion and confirmed at autopsy. In mice with two lungs, PNT was associated with a decrease in ipsilateral lung volume (P < 0.05) and lung weight (P < 0.05). After pneumonectomy, PNT was not associated with a change in activity level, measureable hypoxemia, or altered minute ventilation; however, microCT scanning demonstrated altered displacement and underinflation of the cardiac lobe within the first week after pneumonectomy. Coincident with the altered structural realignment, lung impedance measurements, fitted to the constant-phase model, demonstrated elevated airway resistance (P < 0.05), but normal peripheral tissue resistance (P > 0.05). Most important, PNT appeared to abrogate compensatory lung growth after pneumonectomy; the weight of the lobes of the right lung was significantly less than pneumonectomy alone (P < 0.001) and indistinguishable from nonsurgical controls (P > 0.05). We conclude that the cyclic stretch associated with diaphragmatic muscle contraction is a controlling factor in postpneumonectomy compensatory lung growth.
Keywords: phrenic nerve, imaging, regeneration, pneumonectomy
in many mammalian species, removal of one lung (pneumonectomy, PNX) results in the compensatory growth of the remaining lung. Although the potential importance of oxygen concentration (23), blood flow (5), and growth factors (20) to post-PNX growth have been reported, parenchymal stretch (14) is generally considered the major factor controlling compensatory growth.
The evidence for mechanical stretch regulating post-PNX lung growth is based on several indirect observations. Foremost, a reproducible experimental observation is that preventing stretch of the post-PNX lung, by packing of the residual intrathoracic space with inert material, limits compensatory growth (2, 3, 6, 13). Conversely, increasing the post-PNX stretch appears to increase compensatory growth. Maneuvers such as increasing the amount of lung resected (right lung vs. left lung) (15) and ex vivo overexpansion of the isolated perfused lung (22) produce responses consistent with enhanced growth.
The importance of respiratory muscle-associated stretch to lung growth is also suggested by several observations in development. Fetal breathing movements stimulate cellular proliferation and growth (16). The absence of expansion, due to diaphragmatic hernia or oligohydramnios, causes pulmonary development to cease (12). Similarly, in both humans (4) and animal models (1, 25, 26), normal intrauterine lung development appears to depend upon intact innervation of the diaphragm. In postnatal animals, phrenic nerve transection (PNT) results in compromised lung growth (19, 21, 24). Despite these data suggesting the relevance of stretch in pre- and postnatal lung growth, little is known about the contribution of respiratory muscle function to adult lung growth.
To test the hypothesis that diaphragmatic activity contributes to post-PNX lung growth, we studied post-PNX lung growth in adult mice. Our results suggest that diaphragmatic activity directly contributes to compensatory lung growth.
METHODS
Mice.
C57/B6 mice (Jackson Laboratory, Bar Harbor, ME), 8–12 wk old and 22–30 g, were used in all experiments. The care of the animals was consistent with guidelines of the American Association for Accreditation of Laboratory Animal Care (Bethesda, MD) with the protocol reviewed and approved by the Harvard Medical School Institutional Animal Care and Use Committee.
Anesthesia and intubation.
The animals were anesthetized with an intraperitoneal injection of 100 mg/kg ketamine (Fort Dodge Animal Health, Fort Dodge, IA) and 6 mg/kg xylazine (Phoenix Scientific, St. Joseph, MO). The animals were intubated under direct vision with a standard 20-gauge Angiocatheter (BD Insyte, Sandy, UT) for the PNX procedure. An 18-gauge Angiocatheter (BD Insyte) was used for forced oscillation measurements. The general anesthesia was performed using a FlexiVent rodent ventilator (SciReq, Montreal, QC Canada). Ventilator rates of 200/min, tidal volume (VT) of 10 ml/kg with positive end-expiratory pressures of 3 cmH2O, and a pressure limit of 30 cmH2O were used.
Pulse oximetry.
The MouseOx (STARR Life Sciences, Oakmont, PA), a pulse oximeter with a proprietary plethysmographic waveform analysis algorithm optimized for rats and mice (9), was placed on the shaved skin overlying either the neck or proximal thigh. MouseOx data file exported to Excel for analysis demonstrated oxygen saturation in all surgical groups greater than 95% during and after perioperative oxygen supplementation.
PNX and PNT.
In all mice, the left lung was exposed with a fifth intercostal space thoracotomy, and the hilum was ligated with a 5–0 silk tie (Ethicon, Somerville, NJ). In PNT mice, the phrenic nerve was identified on the left lateral pericardium. The nerve was mobilized with gentle traction, producing diaphragmatic tenting, confirming its identity. The nerve was sharply transected. A recruitment maneuver (named “TLC” by SciReq) was performed while closing the thoracotomy. The animals were removed from the ventilator and extubated upon recovery of spontaneous ventilation. Bilateral ink markers were placed at the costal margins in the midclavicular line. Digital video of the marker movement was routinely recorded on emergence from anesthesia. The animals were recovered with supplemental oxygen and external warming; Subcutaneous 2.4 μg Buprenorphine (Hospira, Lake Forest, IL) was administered two times daily for 48 h.
Lung weights.
The blood-free lung wet weight was obtained after exsanguination. With a beating heart, a caval venotomy was performed, and the intravascular blood volume drained into an absorptive pad as previously described (17). The weight of the whole lung, as well as individual lobes, was expressed as a ratio of lung weight to animal weight. The lung weight index (LWI), grams per kilogram body weight, was calculated to correct for variability in animal size.
Lung volumes.
Lung volumes were measured using the Flexivent (SciReq) rodent ventilator (8). The animals were hyperventilated at a rate of 300/min to remove spontaneous respiratory drive. The animals were allowed to acclimate to the ventilator for 2 min before standardization of the volume history with three consecutive total lung capacity maneuvers using a 3-s ramp to a 30 cmH2O hold. The mean of three lung volume measurements was used for each data point. The variance between measures was uniformly less than 5%. The lung volume index, milliliters per kilogram body weight, was calculated to correct for variability in animal size.
Intubated pulmonary mechanics.
Before all measurements, the pressure transducers and ventilator tubing of the FlexiVent (Scireq) were calibrated by the two collected-point method. The volume and resistance of the endotracheal tube were then calibrated with open and closed measurements. After intubation, the mice were transferred to the FlexiVent system (SciReq) for pulmonary mechanics studies. Pulmonary mechanics were measured immediately post-PNX and on days 3, 7, 14, and 21 postprocedure. The animals were hyperventilated at a rate of 300/min and allowed to acclimate to the ventilator for 2 min before standardization of the volume history with three consecutive recruitment maneuvers (TLC by SciReq) followed by standard volume and compliance measurements. Subsequent to baseline measurements, ventilation was briefly stopped, and the animal passively exhaled to functional residual capacity; an 8-s multifrequency (0.5–19.5 Hz) oscillatory signal (Prime-8 by SciReq) was delivered with ventilation resumed at the completion of the maneuver.
Spontaneous ventilation mechanics.
The plethysmographic system (Buxco Electronics, Sharon, CT) consisted of six transparent experimental chambers equipped with pressure transducers connected to a preamplified pneumotachograph. The chambers were provided with a continuous airflow of 1 l/min via a flow pump-reservoir system. Before measurements, the chambers were calibrated with a 1-ml volume of ambient air introduced with a syringe. During the experiment, the airflow in all chambers was equalized using two multichannel bias flow regulators. The pressure signal from experimental and reference chambers was amplified and integrated by data analysis software (Biosystem XA for Windows; Buxco Electronics). From the amplitude of the plethysmographic signal, VT (ml) and frequency (f, breaths/min) were obtained by using the signal analysis software. Minute ventilation (MV, ml/min) was defined as the product of VT and f. All these data were collected in the breath-by-breath routine and stored for the off-line analysis.
Input impedance model.
Preliminary experiments in our laboratory demonstrated that the chest wall contributed less than 15% to absolute impedance measurements and did not influence the time course or reproducibility of the observed mechanical changes in the lung (8, 10). The constant-phase model (11) was fit to the input impedance to derive Newtonian resistance (Rn), tissue damping (G), tissue elastance (H), and hysteresivity (η) as described below:
where α = (2/π)tan−1 (1/η) and η = G/H.
In this study, measurements were obtained at low frequencies and functional residual capacity; therefore, inertance (I) was negligible. A minimum of three measures was taken per animal, with measurements accepted at a minimum coherence of 0.89; consistently high coherence suggested input impedance with a low signal-to-noise ratio.
CT scanning.
MicroCT scans were obtained with a GE eXplore 120 CT scanner at 50 μm/pixel resolution. A pressure-limited ventilator with a rate of 50/min was used during CT scanning; animals were anesthetized with an intraperitoneal injection of 100 mg/kg ketamine and 6 mg/kg xylazine. Cranial and caudal endpoints were defined, and the animal was imaged at two shots per gantry turn, gating on peak inspiration and expiration. Upon completion of imaging, the animal recovered in a warmed cage. Image analysis and three-dimensional reconstructions were performed with the OsiriX software (www.osirix-viewer.com).
Statistical analysis.
Data analysis was performed using XLstat (Addinsoft, New York, NY) add-in for Microsoft Excel. Results reflected the mean ± 1 SD unless otherwise noted. Statistical significance is determined using analysis of variance with a P value of 0.05. Pairwise comparisons were performed as needed using Tukey's test.
RESULTS
Ventilatory effects of PNT.
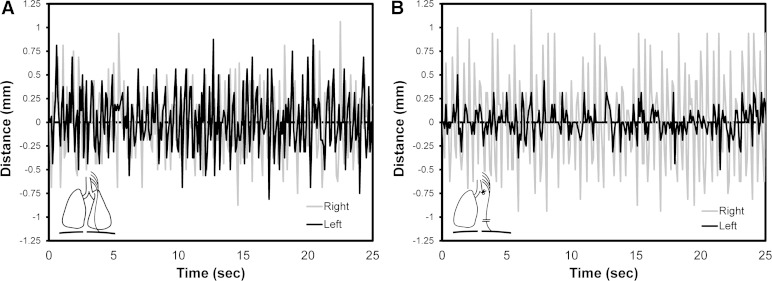
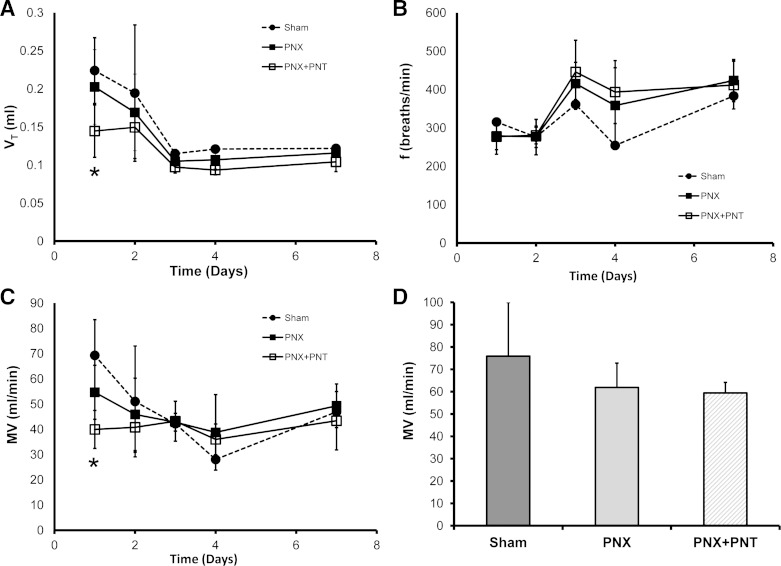
After PNT, the reduced spontaneous breathing rate associated with sedation enabled the visual tracking of costal margin excursion. Measured in the midclavicular line, ventilatory movement was markedly reduced after PNT (Fig. 1). The ipsilateral reduction in costal margin excursion was similar in all PNT groups; however, the contralateral (compensatory) excursion was greater in the PNX group (data not shown). Furthermore, the ventilatory asymmetry was persistent at all subsequent time points studied (1–21 days). To assess the physiological response to PNT, oximetry demonstrated oxygen saturations uniformly greater than 95%. To assess breathing mechanics after PNT, plethysmography demonstrated a decrease in VT and MV during the initial postoperative 48 h, the period of parenteral analgesia. From days 3 to 21, there was no significant difference between the PNX animals (PNX and PNX + PNT) in VT, frequency, and MV (P > 0.05) (Fig. 2).
Fig. 1.
Respiratory motion after phrenic nerve transection (PNT). The skin overlying the costal margins was marked in the midclavicular line with ink. Digital video of costal margin excursion was recorded during the induction of (A) and emergence from (B) general anesthesia after left pneumonectomy (PNX) and PNT. The digital video was analyzed using MetaMorph (Molecular Devices). The asymmetric costal margin excursion was associated with successful transection of the phrenic nerve.
Fig. 2.
Spontaneous breathing mechanics in mice after sham thoracotomy, PNX, or PNX + PNT. Plethysmography measurements were obtained of tidal volume (VT, A), breathing frequency (f, B), and minute ventilation (MV, C) for 21 days after surgery. The only significant difference between experimental groups was the decrease in VT and MV of the PNX + PNT group compared with the sham thoracotomy group on day 1 after surgery (*P < 0.05). D: after 21 days, a trend toward an increase in the MV of the sham thoracotomy group relative to the PNX and PNX + PNT groups was not significant (P > 0.05). Each data point represents the mean of multiple measurements/mouse; n = 4–5 mice studied at each time point. Error bars reflect 1 SD.
Effect of PNT without PNX.
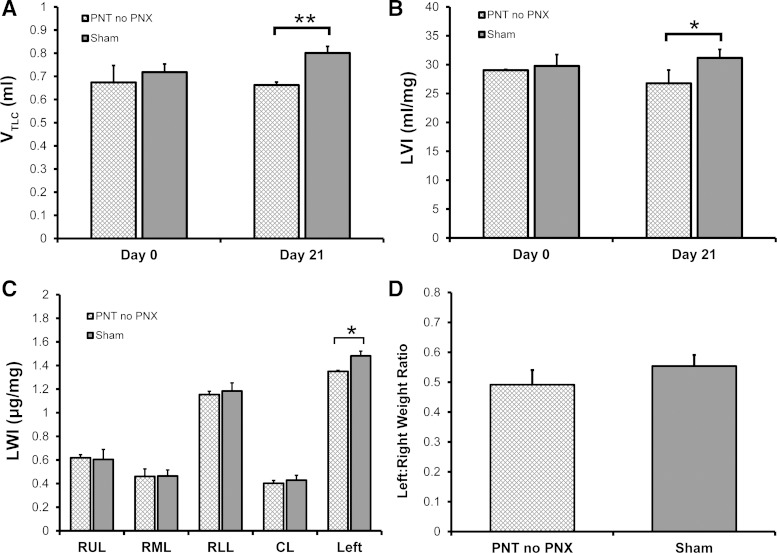
To establish the baseline effect of diaphragmatic paralysis on lung volume and lung weight, PNT was compared with sham thoracotomy alone. After 21 days, a Flexivent TLC maneuver (3-s ramp to 30 cmH2O hold) demonstrated that the volume of the lungs in the PNT mice was lower than the sham thoratocomy mice (P < 0.05) (Fig. 3). The lower lung volumes persisted despite additional recruitment maneuvers (data not shown). Similarly, the LWI was lower in the PNT mice compared with sham thoracotomy mice; most of the weight difference was associated with the ipsilateral left lung (P < 0.05) (Fig. 3C). The differentially lower weight index in the ipsilateral lung after PNT, however, was not significant when expressed as a ratio of LWIs (P > 0.05) (Fig. 3D).
Fig. 3.
Effect of PNT on lung volume and lung weight in normal mice 21 days after surgery. When compared with sham thoracotomy controls, PNT in mice with 2 lungs (no PNX) was associated with a decrease in lung volume as measured by a FlexiVent TLC maneuver (VTLC) (**P < 0.01) (A) or normalized to body weight using the lung volume index (LVI) (*P < 0.05) (B). C: a complementary measure of lung growth, the lung weight index (LWI), demonstrated no difference between the right upper lobe (RUL), right middle lobe (RML), right lower lobe (RLL), and cardiac lobe (CL) but a significant decrease in the LWI of the entire PNT left lung when compared with sham thoracotomy controls (*P < 0.05). D: when lung weight was expressed as a ratio of left to right lung, the difference between PNT and sham mice was not significant (P > 0.05).
Structural mechanics after PNT.
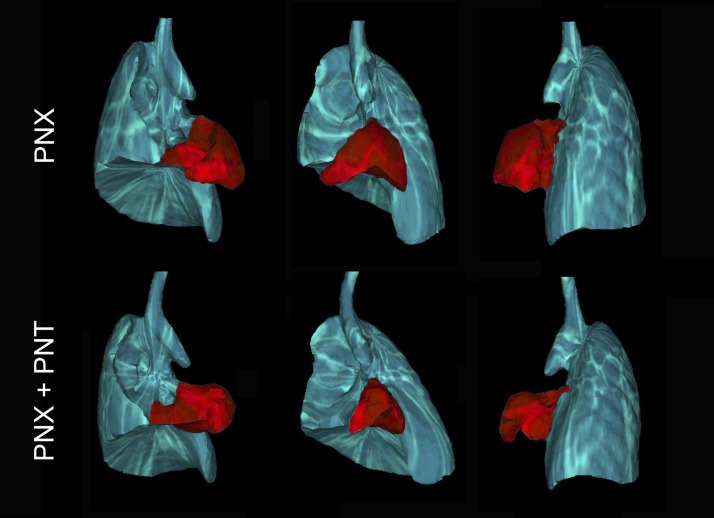
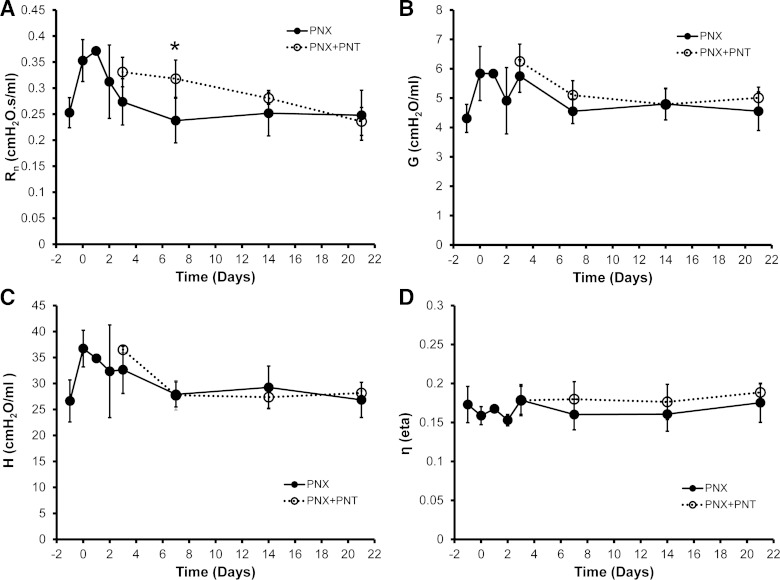
To investigate the structural changes of the mouse thorax post-PNT, respiratory-gated microCT imaging was performed 3 days after PNX. In mice with PNX alone (intact phrenic nerve), the cardiac lobe was displaced in the left hemithorax with a broad diaphragmatic interface (Fig. 4, top). In contrast, mice with PNX and PNT demonstrated incomplete displacement and significantly decreased volume of the cardiac lobe (Fig. 4, bottom); the microCT-derived volume of the cardiac lobe was 22% smaller after PNT (P < 0.05). To identify potential functional changes in the lung parenchyma associated with diaphragmatic paralysis, the lungs of post-PNX mice, with and without PNT, were probed using the forced oscillation technique. Fitting the constant-phase model to respiratory system impedance data produced measures of Newtonian resistance, tissue damping, elastance, and hysteresivity (Fig. 5). Newtonian resistance was elevated in the first week after PNT (P < 0.05), consistent with an altered structural alignment. Measures of tissue damping, tissue elastance, and hysteresivity, indicating the material properties of the peripheral lung, were unchanged after PNT (P > 0.05).
Fig. 4.
Respiratory-gated microCT scans demonstrating the anatomic configuration of the thorax at peak inspiration. Respiratory gating was used to obtain images at a comparable phase in the respiratory cycle. The lung is shown after PNX without (top) and with (bottom) PNT. The cardiac lobe is pseudocolored red for presentation. Cardiac lobe volumes, based on inspiratory-gated microCT scans, were 22% lower in mice after PNX + PNT (P < 0.05).
Fig. 5.
Flexivent input impedance measurements of the remaining right lung after left PNX or PNX + PNT. A FlexiVent “Prime8” perturbation and the constant-phase model were used for calculation of Newtonian resistance (Rn, A), tissue damping (G, B), tissue elastance (H, C), and hysteresivity (η, D). The only significant difference at any time point was the increase in Rn of the PNX + PNT group 7 days after PNX (*P < 0.01). Each data point represents the average of three measurements per mouse; n = 4–5 mice studied at each time point. Error bars reflect 1 SD.
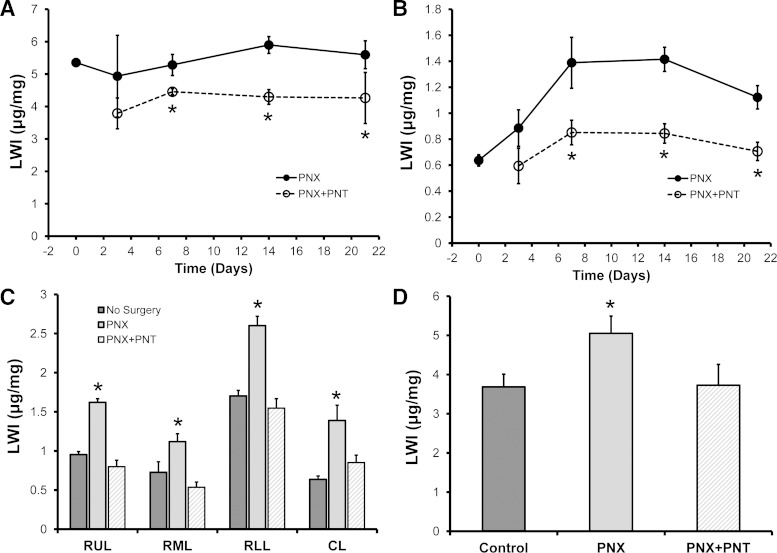
Effect of PNT on compensatory growth.
To determine the effect of PNT on lung growth after left PNX, age-matched mice were assigned to the following three groups: 1) no surgery, 2) left PNX, or 3) left PNX with PNT. As assessed by the LWI, compensatory growth was observed in the remaining right lung after left PNX (Fig. 6A). As expected, the cardiac lobe demonstrated the most relative growth (Fig. 6B). In contrast, compensatory growth was nearly completely abrogated by PNT (Fig. 6, A and B). The LWI of individual lobes, assessed on day 7 (Fig. 6C) after surgery, demonstrated that PNX + PNT lobes were statistically indistinguishable from nonsurgical controls (P > 0.05) and significantly smaller than lobes from PNX mice (P < 0.001). Similarly, the LWI on day 21 (Fig. 6D) after surgery demonstrated that PNX + PNT lungs were statistically indistinguishable from nonsurgical controls (P > 0.05) and significantly smaller than lungs from PNX mice (P < 0.001).
Fig. 6.
Effect of PNT on lung growth. Compensatory lung growth, reflected by the LWI, was examined in mice after either PNX or PNX + PNT. The LWI of the whole lung (A) and cardiac lobe (B) demonstrated no increase in lung growth (P > 0.05) and a significant decrease relative to PNX alone (*P < 0.01). C: examination of LWI in the specific lobes of age-matched mice at 14 days after surgery demonstrated a significant decrease relative to PNX alone (*P < 0.01) and no difference from no surgery control mice (P > 0.05). D: at 21 days after surgery, the difference between the PNX group and the other two groups (no surgery and PNX + PNT) remained highly significant (*P < 0.01); n = 4–5 mice/data point. Error bars reflect 1 SD.
DISCUSSION
PNT provided a unique test of the contribution of cyclic stretch to post-PNX lung growth. PNT selectively eliminated the ipsilateral cyclic stretch after PNX. In contrast to plombage, PNT did not alter the configuration of the left pleural space or entrap the remaining lung. The observation that PNT abrogated the expected increase in post-PNX lung weight indicates that cycle stretch is a controlling factor in compensatory lung growth.
The contribution of cyclic stretch to compensatory lung growth may be particularly relevant after murine PNX because of rodent anatomy. In contrast to the three lobes in the human right lung, rodents have an additional posterior mediastinal lobe, variously referred to as the cardiac, retrocaval, or median lobe. Prior studies have demonstrated that the cardiac lobe is displaced in the left hemithorax after PNX (8, 10). Intriguingly, the cardiac lobe demonstrates the greatest relative increase in compensatory lung growth (17).
In these studies, PNT was associated with altered structural realignment of the cardiac lobe after PNX. Within the first few days after surgery, microCT scanning demonstrated limited displacement and smaller volumes of the cardiac lobe. Consistent with underinflation of the lung parenchyma, and compromised tethering of the airways, airway resistance was elevated in the first week after surgery. These changes, in addition to blunted compensatory growth, were directly linked to the loss of diaphragmatic function.
In contrast to the apparent mechanical consequences of PNT, there were few detectable physiological effects. After the initial perioperative period, there were no observable differences between the sham thoracotomy, PNX, and PNX + PNT experimental groups; that is, the activity level and apparent respiratory pattern of the animals were indistinguishable. Similarly, the postoperative oxygen saturation and MV (except for the initial 48 h) were the same between PNX and PNX + PNT groups.
The potential impact of PNT on the material composition of the peripheral lung was assessed by ventilatory mechanics. Forced oscillation lung impedance measurements, fitted to the constant-phase model (11), provided a measure of central and peripheral lung mechanics. Peripheral tissue resistance, thought to be a measure of frictional losses occurring at the elemental level (7), was indistinguishable between PNX and PNX + PNT groups. These observations indicate that growth abrogation was less an untoward effect of PNT on the material composition of the peripheral lung than the absence of a compensatory growth signal.
An unexpected observation was the similar plethysmographic VT and respiratory rates in the PNX and PNX + PNT groups 2 days after PNX. We had anticipated a diminished VT and increased respiratory rate associated with PNT. The absence of a significant difference between groups suggests the relative insensitivity of plethysmographic data to compensatory mechanics after PNT. Our observation that contralateral chest wall excursion was greater after PNT suggests at least one mechanism for maintaining compensatory VT and respiratory rates. We suspect that future advances in dynamic imaging should provide useful insights into these compensatory mechanisms.
In a solitary study of phrenic nerve avulsion in adult rats, Cohn noted an 11–19% decrease in the expected weight of the lungs 14 days after surgery (3). Notably consistent with these findings, we observed an 8–11% decrease in the weight of the left lung after PNT alone (no PNX). These findings suggest that continued diaphragmatic function is important for the ongoing maintenance of proper lung structure. We suspect that these observations may provide evidence of morphostasis, that is, an ongoing process responsible for the maintenance of function and form (18). Based on these observations after PNT, we speculate that parenchymal lung stretch may participate in human lung repair. Similarly, patients with emphysema and pulmonary fibrosis may represent disordered or blunted morphostasis in humans.
Finally, an underappreciated challenge is the experimental confirmation of diaphragmatic paralysis. In contrast to humans, the rapid ventilation rate and sedation-related hypoxemia (9) precluded the use of conventional imaging to demonstrate paradoxical diaphragmatic movement. The most reliable indicator of diaphragmatic paralysis, in our studies, was the asymmetric excursion of the costal margin. Because of the rapid respiratory rate of the awake mouse, ventilatory asymmetry was most reliably assessed during the emergence from general anesthesia; typically, ventilatory asymmetry was assessed soon after extubation. We routinely obtained postoperative video recordings to document PNT. Although incomplete transection of the phrenic nerve was rare, these mice were readily identified during their emergence from anesthesia.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants HL-94567, HL-75426, and HL-07734.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.B.Y., J.M.B., B.C.G., A.V.F., and W.W. performed experiments; A.B.Y., J.M.B., B.C.G., A.V.F., W.W., A.T., M.A.K., and S.J.M. analyzed data; A.B.Y., J.M.B., A.T., and M.A.K. interpreted results of experiments; A.B.Y., J.M.B., B.C.G., A.V.F., W.W., A.T., M.A.K., and S.J.M. approved final version of manuscript; J.M.B., A.V.F., A.T., and M.A.K. edited and revised manuscript; S.J.M. conception and design of research; S.J.M. drafted manuscript.
REFERENCES
- 1.Alcorn D, Adamson TM, Maloney JE, Robinson PM. Morphological effects of chronic bilateral phrenectomy or vagotomy in the fetal lamb lung. J Anat 130: 683–695, 1980. [PMC free article] [PubMed] [Google Scholar]
- 2.Brody JS, Burki R, Kaplan N. Deoxyribonucleic-acid synthesis in lung-cells during compensatory lung growth after pneumonectomy. Am Rev Respir Dis 117: 307–316, 1978. [DOI] [PubMed] [Google Scholar]
- 3.Cohn R. Factors affecting the postnatal growth of the lung. Anat Rec 75: 195–205, 1939. [Google Scholar]
- 4.Cunningham M, Stocks J. Werdnig-hoffmann disease: effects of intra-uterine onset on lung growth. Arch Dis Child 53: 921–925, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez LG, Le Cras TD, Ruiz M, Glover DK, Kron IL, Laubach VE. Differential vascular growth in postpneumonectomy compensatory lung growth. J Thorac Cardiovasc Surg 133: 309–316, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Fisher JM, Simnett JD. Morphogenetic and proliferative changes in regenerating lung of rat. Anat Rec 176: 389–395, 1973. [DOI] [PubMed] [Google Scholar]
- 7.Fredberg JJ, Stamenovic D. On the imperfect elasticity of lung tissue. J Appl Physiol 67: 2408–2419, 1989. [DOI] [PubMed] [Google Scholar]
- 8.Gibney B, Houdek J, Lee GS, Ackermann M, Lin M, Simpson DC, Chamoto K, Konerding MA, Tsuda A, Mentzer SJ. Mechanostructural adaptations preceding post-pneumonectomy lung growth. Exp Lung Res 38: 396–405, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibney B, Lee GS, Houdek J, Lin M, Chamoto K, Konerding MA, Tsuda A, Mentzer SJ. Dynamic determination of oxygenation and lung compliance in murine pneumonectomy. Exp Lung Res 37: 301–309, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibney BC, Park MA, Chamoto K, Ysasi AB, Konerding MA, Tsuda A, Mentzer SJ. Detection of murine post-pneumonectomy lung regeneration by 18-FDG PET imaging (Abstract). EJNMMI 2: 48, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hantos Z, Daroczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol 72: 168–178, 1992. [DOI] [PubMed] [Google Scholar]
- 12.Hedrick MH, Estes JM, Sullivan KM, Bealer JF, Kitterman JA, Flake AW, Adzick NS, Harrison MR. Plug the lung until it grows (PLUG): a new method to treat congenital diaphragmatic-hernia in-utero. J Pediatr Surg 29: 612–617, 1994. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman AM, Shifren A, Mazan MR, Gruntman AM, Lascola KM, Nolen-Walston RD, Kim CF, Tsai L, Pierce RA, Mecham RP, Ingenito EP. Matrix modulation of compensatory lung regrowth and progenitor cell proliferation in mice. Am J Physiol Lung Cell Mol Physiol 298: L158–L168, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsia CCW, Berberich MA, Driscoll B, Laubach VE, Lillehei CW, Massaro C, Perkett EA, Pierce RA, Rannels DE, Ryan RM, Tepper RS, Townsley MI, Veness-Meehan KA, Wang N, Warburton D. Mechanisms and limits of induced postnatal lung growth. Am J Respir Crit Care Med 170: 319–343, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Hsia CCW, Fryderdoffey F, Staldernavarro V, Johnson RL, Reynolds RC, Weibel ER. Structural changes underlying compensatory increase of diffusing capacity after left pneumonectomy in adult dogs. J Clin Invest 92: 758–764, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jani JC, Flemmer AW, Bergmann F, Gallot D, Roubliova X, Muensterer OJ, Hajek K, Deprest JA. The effect of fetal tracheal occlusion on lung tissue mechanics and tissue composition. Pediatric Pulmonol 44: 112–121, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Konerding MA, Gibney BC, Houdek J, Chamoto K, Ackermann M, Lee G, Lin M, Tsuda A, Mentzer SJ. Spatial dependence of alveolar angiogenesis in post-pneumonectomy lung growth. Angiogenesis 15: 23–32, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levin M. Morphogenetic fields in embryogenesis, regeneration, and cancer: non-local control of complex patterning. Biosystems 109: 243–261, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mansell AL, Rojas JV, Sillos EM, Stolar CJ, Collins MH, Rozovski SJ. Diaphragmatic activity is a determinant of postnatal lung growth. J Appl Physiol 61: 1098–1103, 1986. [DOI] [PubMed] [Google Scholar]
- 20.McAnulty RJ, Guerreiro D, Cambrey AD, Laurent GJ. Growth factor activity in the lung during compensatory growth after pneumonectomy: evidence of a role for IGF-1. Eur Respir J 5: 739–747, 1992. [PubMed] [Google Scholar]
- 21.Price MR, Galantowicz ME, Stolar CJH. Mechanical forces contribute to neonatal lung growth - the influence of altered diaphragm function in piglets. J Pediatr Surg 27: 376–381, 1992. [DOI] [PubMed] [Google Scholar]
- 22.Russo LA, Rannels SR, Laslow KS, Rannels DE. Stretch-related changes in lung cAMP after partial pneumonectomy. Am J Physiol Endocrinol Metab 257: E261–E268, 1989. [DOI] [PubMed] [Google Scholar]
- 23.Sekhon HS, Smith C, Thurlbeck WM. Effect of hypoxia and hyperoxia on postpneumonectomy compensatory lung growth. Exp Lung Res 19: 519–532, 1993. [DOI] [PubMed] [Google Scholar]
- 24.Sillos EM, Donnelly DF, Mansell AL. Unilateral diaphragmatic paralysis inhibits postnatal lung growth in the piglet. Pediatr Res 23: 463–465, 1988. [DOI] [PubMed] [Google Scholar]
- 25.Wigglesworth JS, Desai R. Effects on lung growth of cervical cord section in the rabbit fetus. Early Hum Dev 3: 51–65, 1979. [DOI] [PubMed] [Google Scholar]
- 26.Wigglesworth JS, Winston RML, Bartlett K. Influence of central nervous-system on fetal lung development: experimental-study. Arch Dis Child 52: 965–967, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]