Abstract
Dysfunctional cystic fibrosis transmembrane conductance regulator (CFTR) leads to many cellular consequences, including perinuclear accumulation of free cholesterol due to impaired endosomal transport. The hypothesis being tested is that CF-related perinuclear cholesterol accumulation due to disrupted endocytic trafficking occurs as a result of reduced microtubule (MT) acetylation. Here, it is identified that acetylated-α-tubulin (Ac-tub) content is reduced by ∼40% compared with respective wild-type controls in both cultured CF cell models (IB3) and primary Cftr−/− mouse nasal epithelial tissue. Histone deacetylase 6 (HDAC6) has been shown to regulate MT acetylation, which provides reasonable grounds to test its impact on reduced Ac-tub content on CF cellular phenotypes. Inhibition of HDAC6, either through tubastatin treatment or HDAC6 knockdown in CF cells, increases Ac-tub content and results in redistributed free cholesterol and reduced stimulation of NF-κB activity. Mechanistically, endoplasmic reticulum stress, which is widely reported in CF and leads to aggresome formation, is identified as a regulator of MT acetylation. F508del CFTR correction with C18 in primary airway epithelial cells restores MT acetylation and cholesterol transport. A significant role for phosphatidyl inositol-3 kinase p110α is also identified as a regulator of MT acetylation.
Keywords: cholesterol, cystic fibrosis, histone deacetylase 6, microtubule, phosphatidyl inositol-3 kinase p110α
cystic fibrosis (CF) is caused by mutations in the gene encoding the CF transmembrane conductance regulator (CFTR). Consequences of mutated CFTR include decreased transepithelial chloride transport, hyperabsorption of sodium, dehydration of epithelial surfaces, and altered inflammatory responses (10, 20, 31). We have previously identified that impaired CFTR function leads to perinuclear free cholesterol accumulation (27). Interestingly, the perinuclear cholesterol accumulation is reversible, and recent evidence suggests that cholesterol accumulation in CF is a marker of impaired endosomal transport (27). Effective endosomal transport relies on a balanced network of microtubules and bidirectional movement among early and late endosomes and lysosomes (2, 3).
The microtubule network is a dynamic structure that undergoes a continual series of catastrophes and rescues and is subject to posttranslational modifications. Modifications include polyglutamylation, polyglycylation, carboxyterminal cleavage, and acetylation. Acetylation participates in microtubule dynamic regulation, including the recruitment of microtubule-associating proteins and the behavior of motor proteins. It is most likely that acetylation is linked to microtubule stability and increases the half-life of microtubules to more than a few hours, but no direct role has been described (14, 15, 29). Acetylation is added through the action of histone acetyl transferases and occurs on lysine-40 of α-tubulin (22, 41). This acetylation is reversible, where histone deacetylase (HDAC) enzymes deacetylate pertinent lysines. In particular, HDAC6 is a cytosolic enzyme responsible for tubulin deacetylation (15). HDAC6 also has a known role as a scaffold protein between dynein motor proteins and endosomal vesicles to aid in transport along microtubules (18).
HDAC6 function must be coordinated and balanced with its surroundings; otherwise, perturbations will disrupt the state of acetylation and lead to further alterations in regulation of tubulin function. The hypothesis of this study is that disrupted endocytic trafficking and cholesterol accumulation observed in CF cells is influenced by microtubule acetylation. Microtubule acetylation has been previously implicated in various pathologies such as Huntington's disease, human immunodeficiency virus type 1 (HIV-1), and Charcot-Marie-Tooth (CMT). In Huntington's disease, acetylation enhances vesicular transport in differentiated neurons and thus aids in the anterograde and retrograde transport of brain-derived neurotrophic factor (BDNF) in neurons expressing mutant Huntington disease protein (13, 30). In HIV-1, the binding of HIV-1 to CD4 enhances tubulin acetylation through HDAC6 inhibition to promote trafficking of viral capsids along acetylated microtubules (37). In CMT, expression of mutant heat shock protein gene 1 (HSPB1) decreases acetylated α-tubulin (Ac-tub) abundance and induces severe axonal transport deficits. Pharmacological inhibition of HDAC6 reverts the axonal transport defects caused by HSPB1 mutations and rescues the CMT phenotype in symptomatic mutant HSPB1 mice (9).
In this study, reduced Ac-tub content is identified in both CF cell models and tissues. The impact of this finding on cholesterol processing and related signaling events is explored. HDAC6 inhibition reverses these phenotypes toward wild-type (WT) characteristics, supporting a central role of microtubule regulation in the control of CF cell biology. Mechanistically, endoplasmic reticulum (ER) stress is identified as a source of the microtubule alterations in CF epithelium. These data provide new evidence for promoting HDAC6 inhibitors as a therapeutic strategy for treating CF.
MATERIALS AND METHODS
Cells and materials.
IB3-1 cells, human epithelial with the ΔF508 mutation (CF phenotype), and S9 cells, IB3-1 cells stably transfected with the full-length WT CFTR (control), were a generous gift from Pamela L. Zeitlin (Johns Hopkins University, Baltimore, MD). Cells were cared for as previously described (27). Human airway epithelial cells (HAECs) were isolated from human bronchi at the time of lung transplant and grown in culture using established techniques (33). F508del CFTR-expressing cells were seeded onto Vectabond-treated glass coverslips (Thermo Fisher Scientific, Pittsburgh, PA) and exposed to F508del CFTR corrector C18 (10 μM) for 72 h. Cells were then fixed with 2% paraformaldehyde and examined for their cellular cholesterol localization by fluorescent filipin staining. Cholesterol mobilization was quantified by subtracting perinuclear fluorescence from total cellular fluorescence. Increased cholesterol mobilization would increase the (total − perinuclear) value reflecting efficiency of intracellular cholesterol transport. Corrector compound C18 was obtained from the Cystic Fibrosis Foundation Therapeutics panel library (www.cftrfolding.org).
Mice.
Mice were cared for in accordance with Case Western Reserve University Institutional Animal Care and Use Committee guidelines by the CF Animal Core Facility. The Animal Care and Use Committee of Case Western Reserve University approved all animal procedures. Nasal scrapings of mouse epithelium were obtained from both WT and CF animals.
Western immunoblotting.
Antibodies against Ac-tub (mouse), glucose-regulated protein 78 (GRP78) (mouse), and HDAC6 (mouse) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). α-Tubulin (rabbit) antibodies were obtained from Abcam (Cambridge, MA). Actin (rabbit) antibodies were obtained from Sigma-Aldrich (St. Louis, MO).
Cells were grown to 95% confluency in 35-mm dishes and lysed with lysis buffer (50 mM Tris pH 7.5, 1% Triton X-100, 50 mM NaF, 200 μM Na3VO4, and 10 μg/ml pepstatin and leupeptin) for 30 min at 4°C. Lysates were microcentrifuged at 10,000 rpm for 5 min. Proteins were separated using SDS-PAGE containing 20–40 μg protein on either a 7.5% or 10% polyacrylamide gel. Samples were transferred to an Immobilon-P membrane (Millipore, Bedford, MA) at 15 V for 30 min. Blots were blocked in 10% nonfat dehydrated milk in PBS (138 mM NaCl, 15 mM Na2HPO4, 1.5 mM KCl, and 2.5 mM KH2PO4) with 0.1% Tween-20 (PBS-T) for 45 min at room temperature and then incubated with primary antibodies (1:500 dilution) in 10% nonfat dehydrated milk in PBS-T overnight at 4°C. Blots were washed three times for 10 min each with PBS-T, incubated with the respective secondary antibodies conjugated to horseradish peroxidase (1:3,000 dilution) in PBS-T, washed again with PBS-T, and visualized using SuperSignal chemiluminescent substrate (Pierce, Rockford, IL) and the VersaDoc Imaging System (Bio-Rad, Hercules, CA). Quantification of protein expression was performed using densitometry software on the VersaDoc (Quality One, Bio-Rad).
Immunostaining.
Antibodies against Ac-tub were obtained from Santa Cruz. Texas Red goat anti-mouse IgG antibodies were obtained from Invitrogen (Carlsbad, CA). Briefly, cells were grown on collagen-coated coverslips to 75–80% confluency. Cells were rinsed three times with PBS and fixed with acetone for 20 min at −20°C. Cells were rinsed three more times with PBS and then blocked with 5% goat serum in PBS for 30–60 min of shaking at room temperature. Primary antibodies in 5% goat serum in PBS were added for 1 h at room temperature. Cells were rinsed three times with PBS and then incubated with secondary antibodies at a final concentration of 10 μg/ml in 5% goat serum in PBS. Cells were mounted with SlowFade antifade (Invitrogen) on slides. Cells were visualized in the appropriate range using a wide-field microscope on a Zeiss Axiovert 200 microscope (×40 oil objective) with Metamorph software.
NBD-cholesterol stain.
Briefly, cells were grown on collagen-coated coverslips to 75–80% confluency. Twenty-four hours before mounting, 5 μg/ml of 1-palmitoyl-2-[12-(7-nitro-2–1,3-benzoxadiazol-4-yl) amino] dodecanoyl (NBD)-cholesterol (Invitrogen) was added to cell media. Three hours before mounting, cells were washed out by replacing NBD media with new media. Cells were fixed in 2% paraformaldehyde in water for 30 min. Cells were mounted with SlowFade antifade (Invitrogen) on slides.
Filipin staining.
Cells were grown on collagen-coated coverslips to 75–80% confluency. F508del HAEC cells were seeded onto Vectabond-treated glass coverslips (Thermo Fisher Scientific) and exposed to F508del CFTR corrector C18 (10 μM) for 72 h. Cells were then fixed with 2% paraformaldehyde and examined for their cellular cholesterol localization by fluorescent filipin staining. Cells were rinsed twice and then incubated with 0.05 mg/ml filipin (Sigma) in PBS for 1 h in the dark on the shaker at room temperature. Filipin stain was freshly dissolved in dimethylsulfoxide before dilution with PBS. Cells were then rinsed twice with PBS before coverslips were mounted with SlowFade antifade (Invitrogen) on slides. Cholesterol mobilization was quantified by subtracting perinuclear fluorescence from total cellular fluorescence. Increased cholesterol mobilization would increase the (total − perinuclear) value reflecting efficiency of intracellular cholesterol transport. Cells were visualized in the ultraviolet range using a wide-field microscope on a Zeiss Axiovert 200 microscope with Metamorph software. A ×40 objective was used for all images.
Transfections.
HDAC6 shRNA was obtained from SABiosciences (Frederick, MD). Cells were seeded at 200,000 cells/well in a six-well dish 24 h before transfection. Transfections were performed with FuGene 6 (Roche, Indianapolis, IN), per manufacturer's protocol. Cells were selected by addition of hygromycin to media (80 mg/l).
NF-κB-luc was purchased from Clontech (Palo Alto, CA). Cells were seeded at a density of 50,000 cells/well in 24-well tissue culture dishes 24 h before transfection. For each transfection, 0.6 μl of FuGene 6 (Roche, Indianapolis, IN) was incubated for 5 min in 100 μl of OptiMEM (Gibco, Gaithersburg, MD). Then, 0.03 μg of DNA and 0.006 μg of pRL-TK (Promega, Madison, WI) were added to the FuGene-OptiMEM mix and incubated for another 15 min. Diluted transfection mix (100 μl) was added to each well, and cells were incubated at 37°C in 95% O2-5% CO2 for 24 h. Cells were treated with IL-1β/TNF-α (R&D Systems, Minneapolis, MN) for 18 h before we lysed them. Cells were then lysed in 1× Passive Lysis Buffer (Promega) at room temperature for 15 min and assayed for luciferase activity according to manufacturer's instructions (Promega). Results are expressed in relative light units and normalized to Renilla luciferase activity.
RESULTS
Ac-tub levels in CF cell models and tissues.
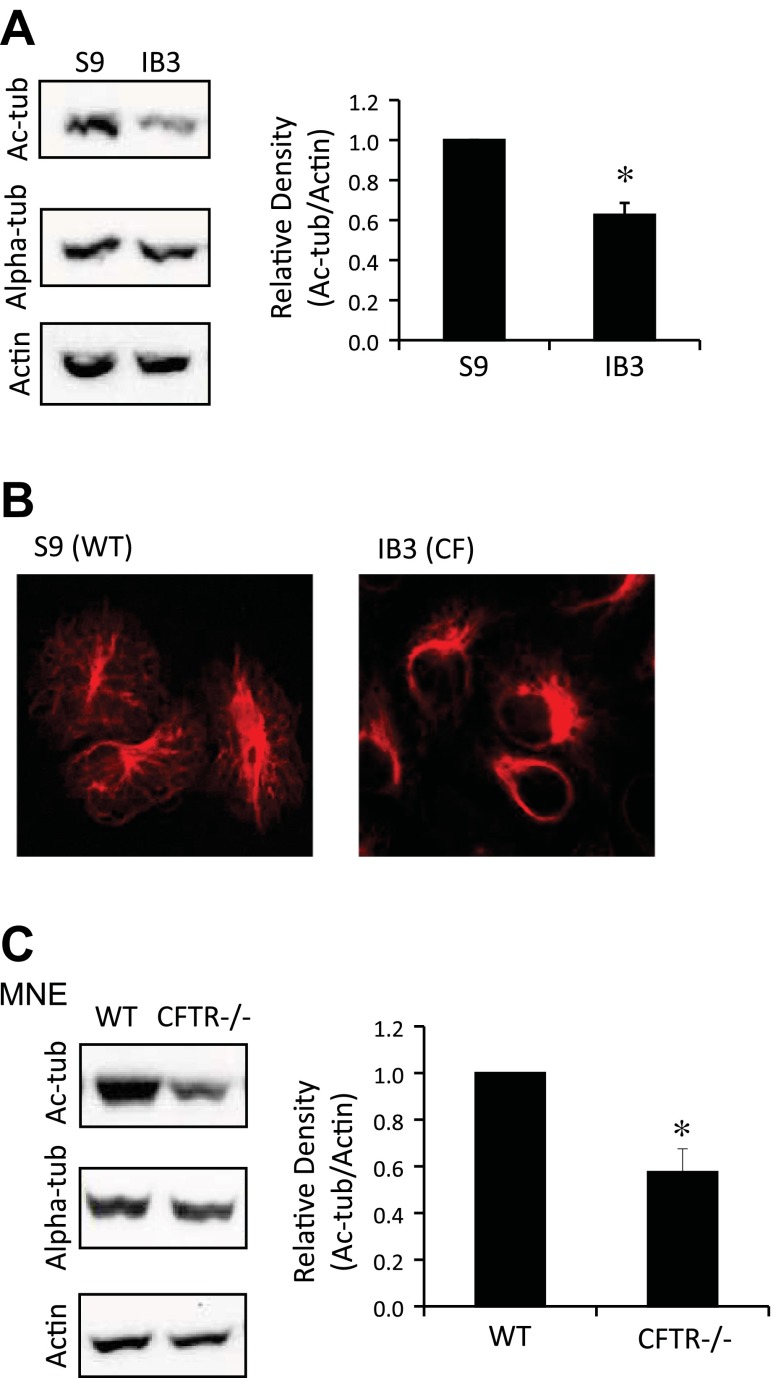
Previous findings demonstrated that perinuclear cholesterol accumulation in CF cells is likely a marker of reduced mobility of endosomes (27). To explore the underlying mechanism of decreased organelle trafficking in epithelial cells in the absence of CFTR function, cytoskeletal modifications were examined. IB3 (ΔF508/W1282X) cells and CFTR-corrected S9 cells were examined for Ac-tub content by Western blot (Fig. 1A). Densitometry analysis confirmed that IB3 cells had 62.6 ± 6.0% Ac-tub content compared with WT cells (Fig. 1A). S9 and IB3 cells were immunostained with anti-Ac-tub antibodies to reveal a gross difference in cellular localization (Fig. 1B). Although immortalized cell models draw concern in CF studies, IB3 cells exhibited perinuclear cholesterol accumulation that has been confirmed in primary CF tissue, thus validating the cell line as a viable model to explore microtubule changes associated with CFTR dysfunction (39).
Fig. 1.
Acetylated-α-tubulin (Ac-tub) protein expression and distribution in immortalized cystic fibrosis (CF) cell models. A: wild-type (WT) (S9) and CF (IB3) cells were lysed and analyzed through Western blot. Representative blots and densitometry data of Ac-tub expression normalized to actin protein content are shown. Densitometry was obtained using Quantity One software. Results are presented as relative density (Ac-tub/actin). Significance was determined by t-test (n = 7; *P < 0.001). B: S9 and IB3 cells were immunostained using Ac-tub antibodies. Images are representative of 24 images from 5 separate experiments. C: lysates of excised mouse nasal epithelium (MNE) were analyzed for Ac-tub through Western blotting. Representative blots and densitometry data of Ac-tub expression normalized to actin protein content are shown. Densitometry was obtained using Quantity One software. Results are presented as relative density (Ac-tub/actin). Significance was determined by t-test (n = 8; *P < 0.01). CFTR, cystic fibrosis transmembrane conductance regulator.
To further verify reduced Ac-tub protein content in a noncultured, primary tissue CF model, mouse nasal epithelium (MNE) was excised from WT and Cftr−/− mice and examined for Ac-tub content. Relative density (Ac-tub/actin) was reduced to 0.59 ± 0.06 in Cftr−/− MNE compared with WT MNE, a 40% decrease in CF Ac-tub content (P < 0.01; n = 8) (Fig. 1C). These data show that primary CF tissue not subject to any culture condition manipulations retain the reduced Ac-tub phenotype. This also supports the hypothesis that inherent dysregulation of microtubule modifications is a potential modulator of cell regulation in the absence of CFTR function.
Impact of HDAC6 inhibition on microtubule acetylation and cholesterol trafficking in CF cells.
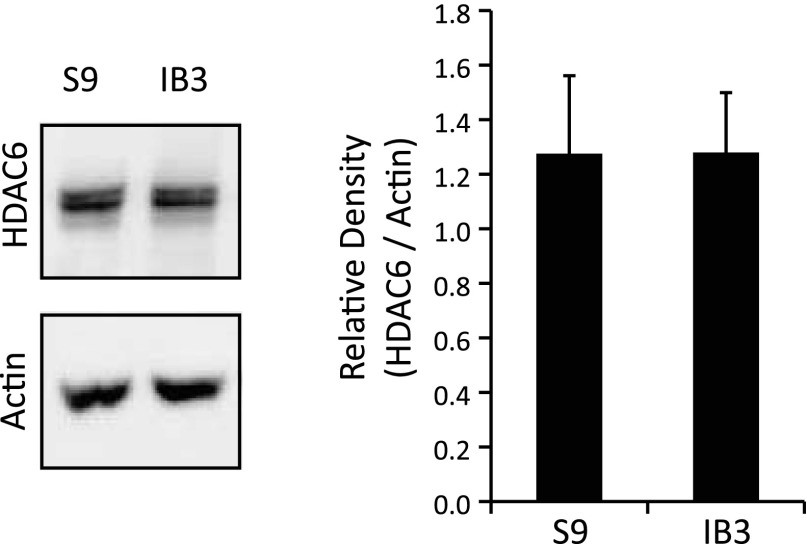
In both cell models and primary mouse tissue, we have shown that CF cells exhibit reduced Ac-tub content. Previous literature describes the importance of microtubule acetylation in regulating organelle transport (8, 12). HDAC6 regulates microtubule deacetylation and has been shown to recruit misfolded ubiqitinated proteins to dynein motors for intracellular transport to late endosomes and lysosomes (12). The decrease in Ac-tub content pointed to potentially altered regulation of HDAC6 in the absence of CFTR function. As seen in Fig. 2, HDAC6 was expressed equally in both CF and WT cells (IB3 and S9, respectively). Therefore, it was speculated that HDAC6 is a viable target and the inhibition of HDAC6 in CF epithelial cells may reduce the accumulation of cholesterol by modulating microtubule modifications.
Fig. 2.
Histone deacetylase 6 (HDAC6) expression in cultured WT and CF cells. S9 and IB3 cell lysates were examined for HDAC6 through Western blot analysis. A representative blot and densitometry data of HDAC6 expression normalized to actin protein content are shown. Densitometry was obtained using Quantity One software. Results are presented as relative density (HDAC6/actin). No significant difference in expression is found as determined by t-test (n = 3).
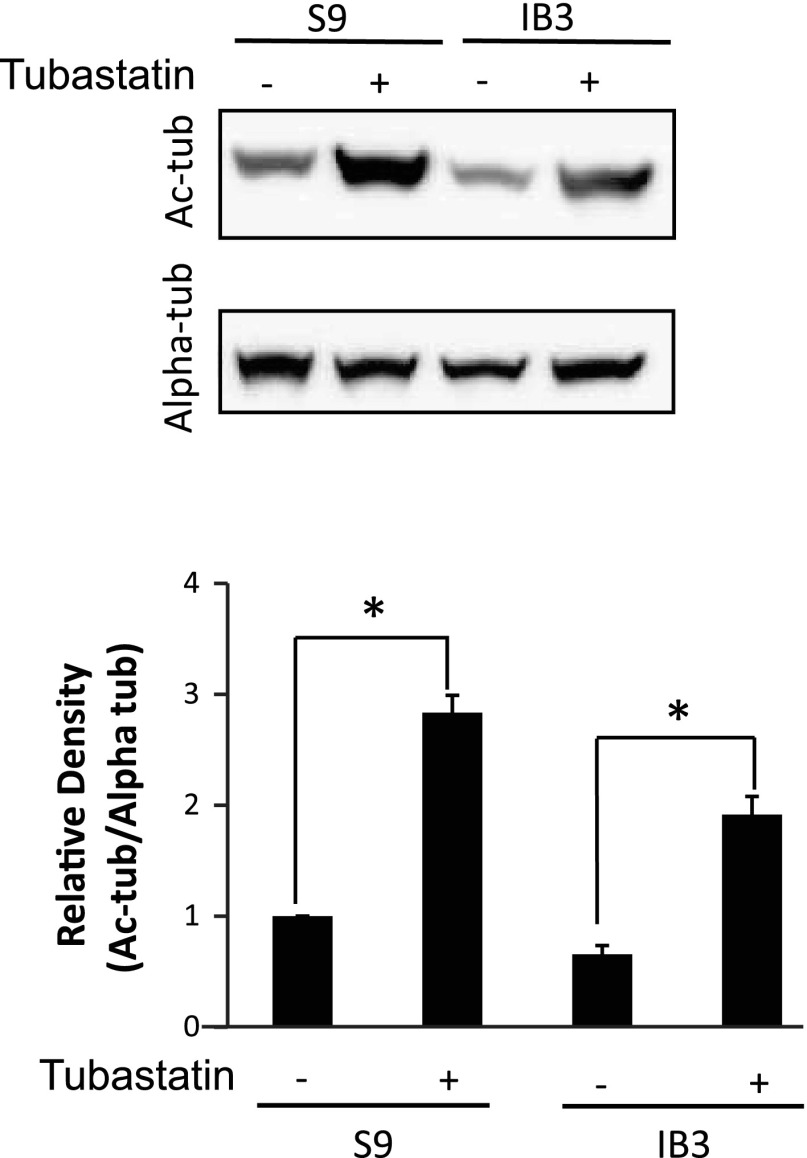
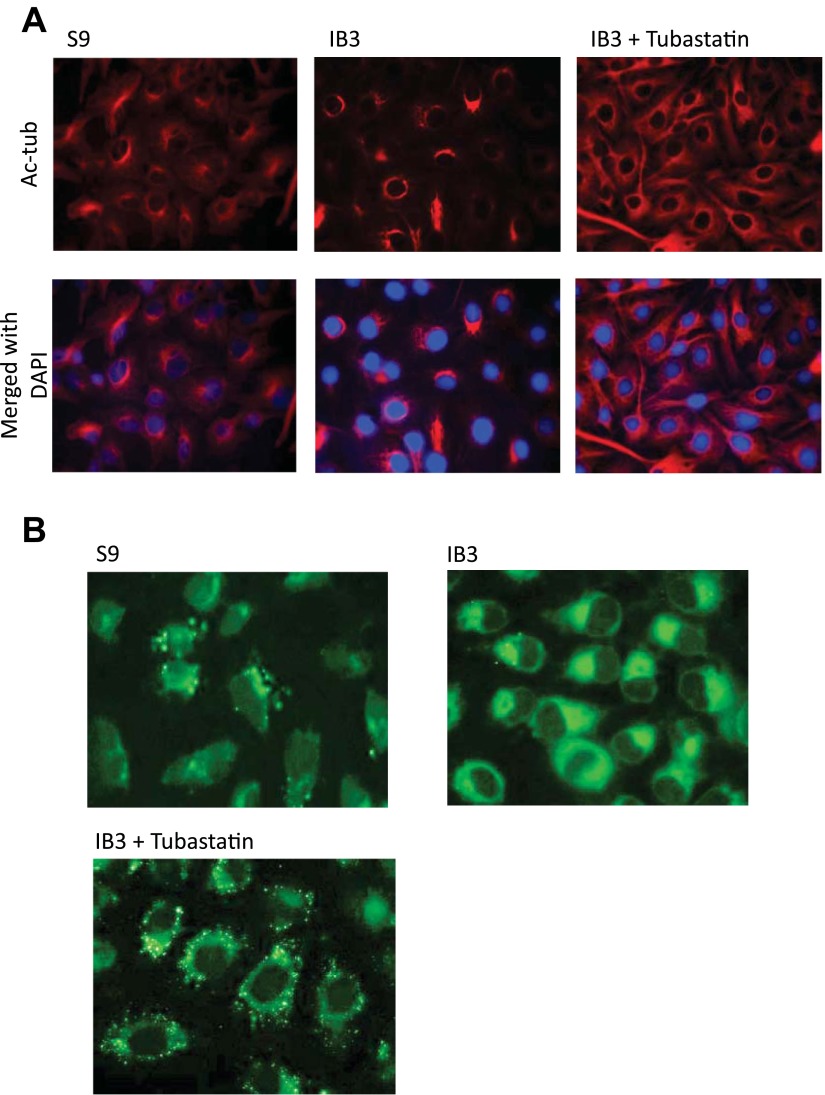
To test the hypothesis that increasing microtubule acetylation through HDAC6 inhibition would revert CF phenotypes toward WT profiles, S9 and IB3 cells were analyzed after treatment with the HDAC6-selective inhibitor tubastatin (10 μM) for 24 h. Initially, the impact of tubastatin treatment on Ac-tub content was examined to determine whether HDAC6 inhibition could increase CF microtubule acetylation. These data are shown in Fig. 3. Ac-tub content is increased approximately threefold in both S9 and IB3 cells in response to tubastatin. We then examined Ac-tub cellular distribution by immunostaining. In Fig. 4A, tubastatin-treated cells display extended Ac-tub staining to the cell periphery and increased protein content to produce a pattern similar to CFTR-corrected S9 cells. We have previously shown a relationship between microtubule transport and cholesterol distribution in CF IB3 cells (27); therefore, the impact of increasing microtubule acetylation on cholesterol processing was examined. HDAC6 inhibition with tubastatin resulted in a redistribution of accumulated perinuclear cholesterol determined by monitoring trafficking of fluorescent NBD-cholesterol (Fig. 4B). These data demonstrate that manipulation of HDAC6 activity can increase acetylated microtubule content in CF cells and restore WT levels of cholesterol movement.
Fig. 3.
Effect of tubastatin treatment on Ac-tub content in S9 and IB3 cells. A representative blot and densitometry data of Ac-tub expression normalized to total α-tubulin (Alpha-tub) protein content are shown. Densitometry was obtained using Quantity One software. Results are presented as relative density (Ac-tub/Alpha-tub). Error bars represent SE, and significance was determined by t-test (*P < 0.001, n = 6).
Fig. 4.
Effect of pharmacological HDAC6 inhibition on microtubule acetylation and cholesterol transport. A: S9 and IB3 cells were either treated with vehicle or 10 μM tubastatin for 24 h. Cells were fixed and immunostained using Ac-tub antibodies (red) and DAPI (blue). Representative images are shown from triplicate experiments. B: 1-palmitoyl-2-[12-(7-nitro-2–1,3-benzoxadiazol-4-yl) amino] dodecanoyl (NBD)-cholesterol accumulation and redistribution after HDAC6 inhibition in CF cells. S9 and IB3 cells were either treated with vehicle or with 10 μM tubastatin for 24 h, along with NBD-cholesterol, a fluorescent cholesterol probe for 24 h. NBD-cholesterol was washed out of cells with fresh media with either vehicle or tubastatin for 3 h before being fixed. Representative images are shown from triplicate experiments.
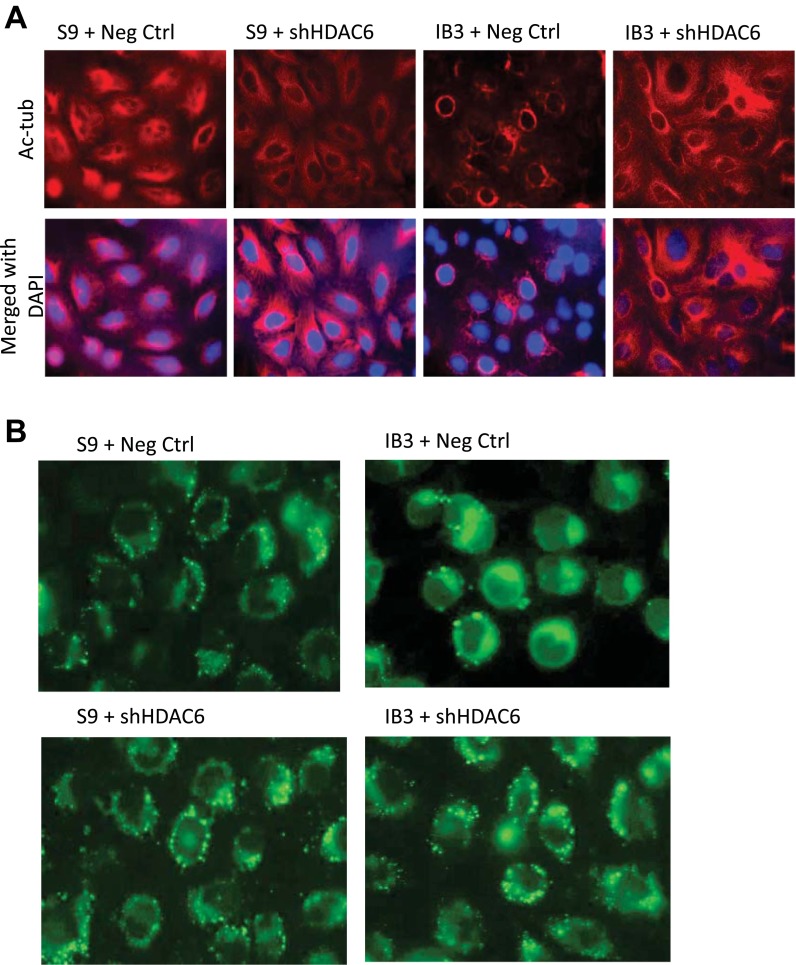
To ensure that the observed actions of tubastatin were specific to HDAC6 inhibition, HDAC6 expression was knocked down in S9 and IB3 cells through the use of shRNA against HDAC6 (S9+shHDAC6; IB3+shHDAC6). HDAC6 depletion in IB3 cells increased Ac-tub content (Fig. 5A) compared with control-transfected S9 and IB3 cells (S9+Neg Ctrl; IB3+Neg Ctrl). Depletion of HDAC6 expression (Fig. 6C) in IB3 cells resulted in reduced cholesterol accumulation similar to pharmacological inhibition of HDAC6 function (Fig. 5B). These data support that microtubule acetylation is a key regulator of cholesterol distribution in CF cells and manipulation of HDAC6 function is a viable method of reversing CF phenotypes.
Fig. 5.
Effects of HDAC6 knockdown on Ac-tub and cholesterol distribution. A: negative control and shHDAC6 knockdown S9 and IB3 cells were cultured for 3 days and then immunostained with Ac-tub antibodies (red). Nuclei were stained with DAPI (blue). Representative images are shown from triplicate experiments. B: negative control and shHDAC6 knockdown S9 and IB3 cells were cultured for 3 days and then stained with NBD-cholesterol for 18 h. Cells were washed out for 3 h with fresh media before being fixed. Representative images are shown from triplicate experiments.
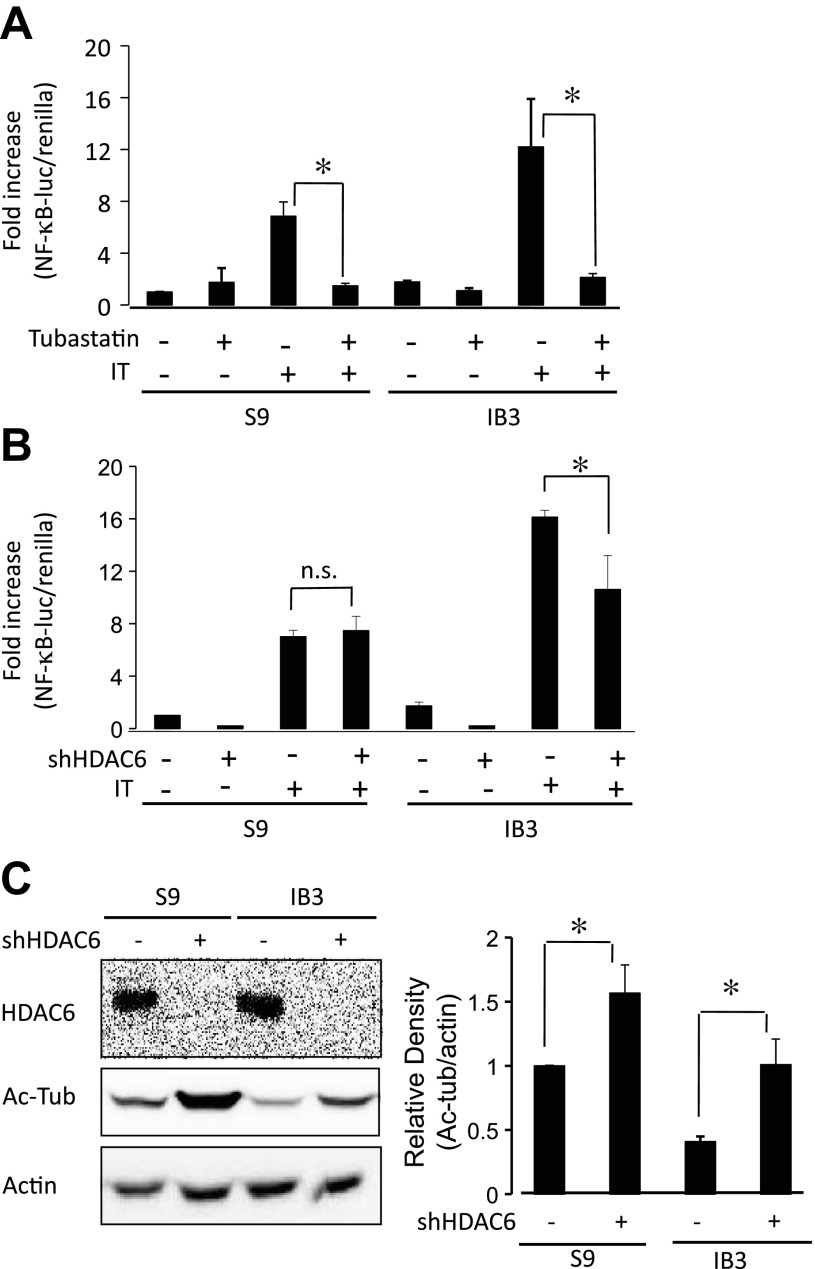
Fig. 6.
Effects of HDAC6 knockdown on NF-κB activation and Ac-tub protein expression. A: effect of tubastatin on NF-κB-luciferase activation in S9 and IB3 cells stimulated with IL-1β/TNF-α (IT). Data presented as a fold increase compared with untreated S9 cells using the ratio of reporter construct per Renilla luciferase control as relative light units (NF-κB-luciferase/Renilla). Error bars represent SE; n = 4 for each condition. Significance determined by ANOVA with a Newman-Keuls post hoc multiple-comparison test. *P < 0.05. B: effect of HDAC6 knockdown by shRNA (shHDAC6) on NF-κB-luciferase activation in S9 and IB3 cells stably expressing control shRNA or targeted HDAC6 shRNA stimulated with IT. Significance determined by ANOVA with a Newman-Keuls post hoc multiple-comparison test. *P < 0.05; n = 5 for each condition. C: cell lysates of negative control and HDAC6 shRNA (shHDAC6) knockdown S9 and IB3 cells were lysed and analyzed for HDAC6 and Ac-tub by Western blot analysis. Representative blots (left) and densitometry data of Ac-tub expression normalized to actin protein content (right) are shown. Densitometry was obtained using Quantity One software. Results are presented as relative density (Ac-tub/actin). Significance was determined by ANOVA. Comparisons between groups are performed with the Newman-Keuls post hoc test. (n = 6; *P < 0.05).
HDAC6-mediated control of NF-κB activation.
Previous work in our laboratory demonstrated that the cholesterol pathway is a key regulatory component of CF-related inflammatory signaling (21). Therefore, it was hypothesized that HDAC6 inhibition should reduce NF-κB activation, particularly in CF cells. Tubastatin treatment significantly reduced NF-κB activation in response to IL-1β/TNF-α treatment in both S9 and IB3 cells (Fig. 6A). Similarly, IB3+shHDAC6 cells exhibited reduced NF-κB activation stimulated with IL-1β/TNF-α compared with IB3+Neg ctrl cells (Fig. 6B). Depletion of HDAC6 by shRNA is shown in Fig. 6C along with the impact of depletion on Ac-tub content. Ac-tub content is increased ∼2.5-fold in IB3+shHDAC6 cells compared with control IB3 cells, whereas S9+shHDAC6 exhibit an approximate 1.5-fold increase. Although only a limited examination of inflammatory signaling, these results show that increased NF-κB activation characteristic of CF is reversed by HDAC6 inhibition. These findings are consistent with our previous work and suggest a more central role of HDAC6 signaling in CF cell biology. Interestingly, there is a discrepancy between S9 cells treated with tubastatin compared with S9+shHDAC6 cells. Tubastatin inhibits NF-κB activation in S9 cells, whereas depletion of HDAC6 expression had no impact on NF-κB stimulation. Residual HDAC6 expressed undetectable by Western blot in S9+shHDAC6 cells may be responsible for normal NF-κB activation. Acute HDAC6 inhibition results in an approximately threefold increase in Ac-tub content (Fig. 3) compared with only 1.5-fold increase in shHDAC6 cells, suggesting more complete inhibition of HDAC6 function by tubastatin.
Mechanism linking CFTR to reduced Ac-tub content.
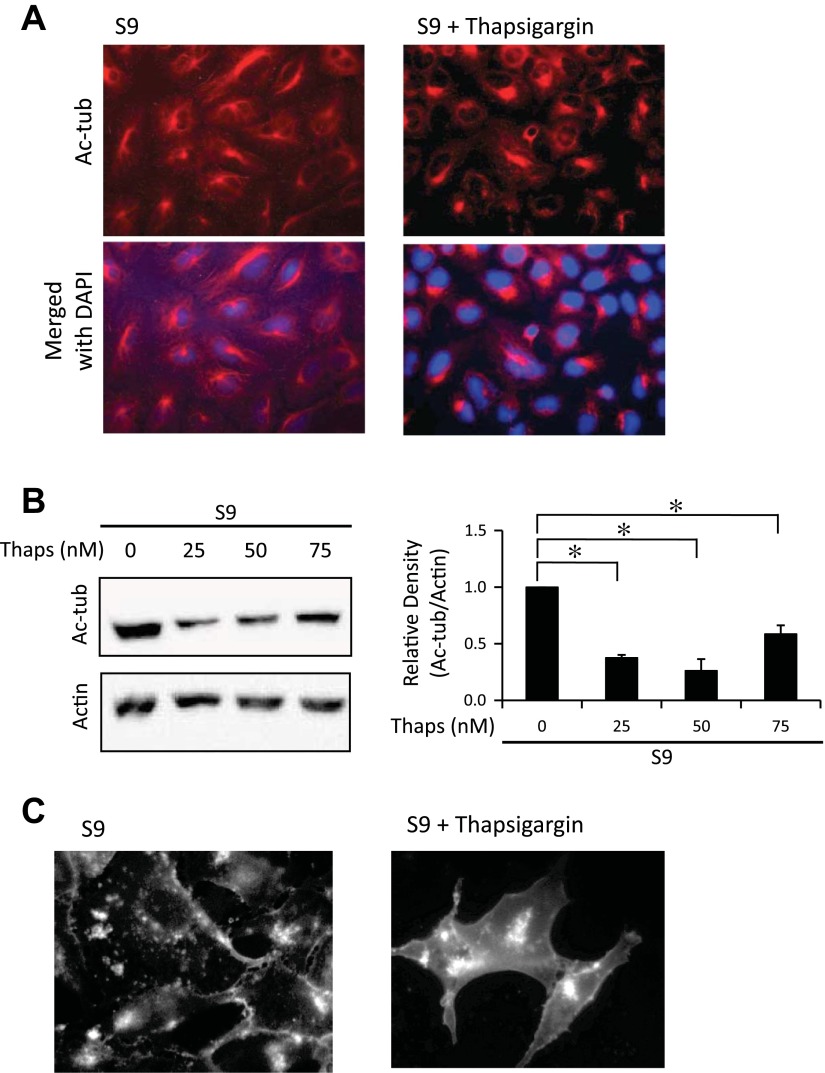
The above data demonstrate that CF epithelial cells have decreased Ac-tub content, leading to the CF cellular characteristic of increased perinuclear cholesterol accumulation and a role in influencing NF-κB activation. These findings are consistent with aggresome formation, another pertinent feature in CF models (17). ER stress has been widely predicted to occur in CF cells, usually attributed to misfolding of ΔF508 CFTR (17, 26). ER stress is also an effective trigger for aggresome formation (6). It was hypothesized that chronic ER stress in CF cells leads to reduced Ac-tub content and the subsequent sequelae. To evaluate the hypothesis, ER stress was induced in CFTR-corrected S9 cells with thapsigargin (100 nM). After 48-h exposure to thapsigargin, Ac-tub content was reduced 62.4 ± 2.5% relative to WT levels with 50 nM thapsigargin (Fig. 7, A and B). These data strongly suggest that ER stress is a key component in initiating cytoskeletal changes and related cellular events in CF cells. To test whether thapsigargin-induced decrease of Ac-tub content would replicate the cholesterol accumulation phenotype, filipin staining of thapsigargin-treated cells was performed to visualize free cholesterol. As shown in Fig. 7C, clear perinuclear accumulation of cholesterol is observed in WT-control S9 cells in the presence of thapsigargin.
Fig. 7.
Ac-tub protein expression decreases in the presence of endoplasmic reticulum (ER) stress. A: S9 cells were treated with 100 nM thapsigargin for 18 h. Cells were immunostained with Ac-tub antibodies (red) and DAPI (blue). Representative images are shown from triplicate experiments. B: S9 cells were treated with varying concentrations (0–75 nm) of thapsigargin for 18 h. Cell lysates were examined for Ac-tub through Western blot. A representative blot is shown, along with Ac-tub expression normalized to actin densitometry. Densitometry was obtained using Quantity One software. Results are presented as relative density (Ac-tub/actin). Significance was determined by ANOVA. Comparisons between groups were performed with the Newman-Keuls post hoc test with results shown compared with S9 NT (n = 3; *P < 0.05). C: S9 cells were either treated with vehicle or with 50 nM thapsigargin for 48 h and stained for free cholesterol with filipin. Representative images are shown from triplicate experiments.
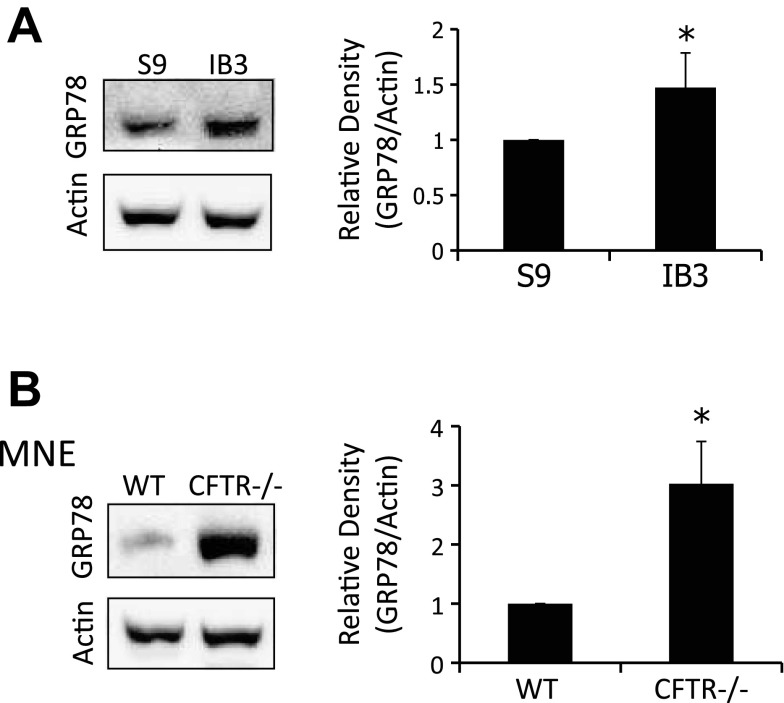
Because induction of ER stress is sufficient to mimic the CF cellular phenotypes of decreased microtubule acetylation and cholesterol accumulation, we examined our CF model systems for a marker of ER stress. We examined IB3 cells and MNE from Cftr−/− mice for the expression of GRP78, an established marker of ER stress (23). IB3 cells exhibited a 1.5 ± 0.3-fold increase in GRP78 protein content compared with S9 cells, and Cftr−/− MNE displayed a 3.0 ± 0.7-fold increase compared with Cftr+/+ MNE (Fig. 8). These data demonstrate baseline ER stress in both test models. Interestingly, data from Cftr−/− MNE demonstrate that loss of CFTR function, not mutant CFTR protein misfolding, is sufficient to trigger ER stress.
Fig. 8.
ER stress is present in CF cells. A: S9 and IB3 cell lysates were analyzed through Western blot for glucose-regulated protein 78 (GRP78). A representative blot and densitometry data of GRP78 expression normalized to actin protein content are shown. Densitometry was obtained using Quantity One software. Results are presented as relative density (GRP78/actin). Significance was determined by t-test (n = 5; *P < 0.05). B: WT and CFTR−/− MNE were lysed and examined for GRP78 protein expression. A representative blot and densitometry data of GRP78 expression normalized to actin protein content are shown. Densitometry was obtained using Quantity One software. Results are presented as relative density (GRP78/actin). Significance was determined by t-test (n = 4; *P < 0.01).
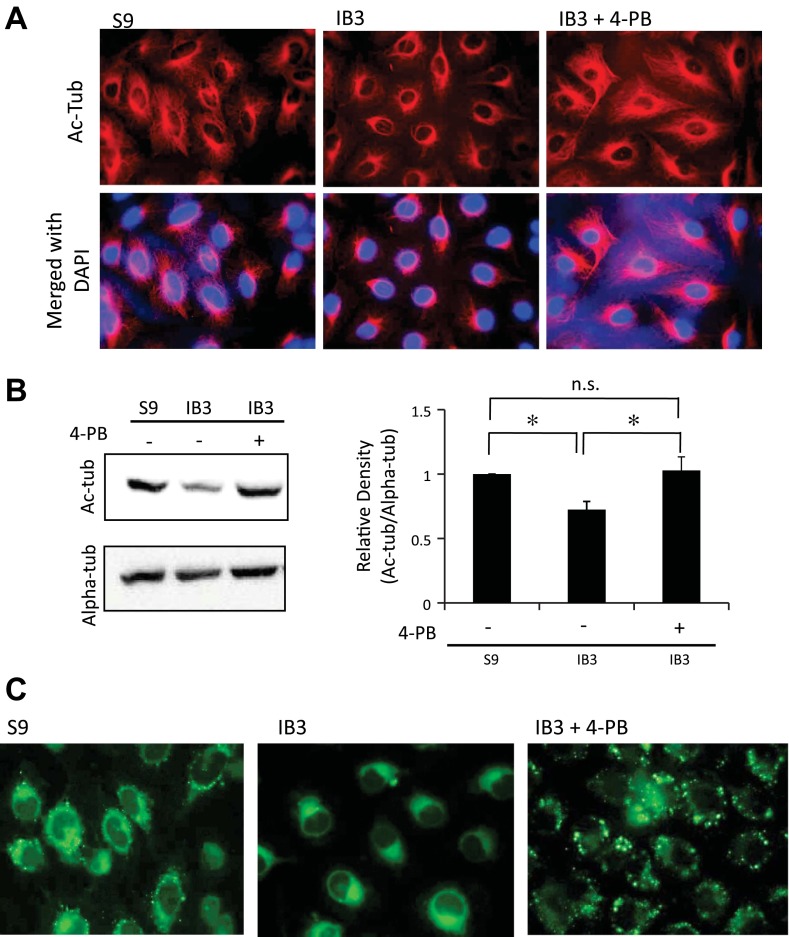
Given the CF-like reductions in microtubule acetylation induced by thapsigargin and that CF models exhibited ER stress through the expression of GRP78, it was hypothesized that relief of ER stress with 4-phenylbutyrate (4-PB) would reverse these phenotypes in CF cells. 4-PB has been widely studied in CF model systems for its ability to reverse ER stress by fostering trafficking of misfolded mutant CFTR (35, 36). IB3 cells were treated with 4-PB (1 mM) for 48 h and examined for Ac-tub content and cholesterol mobilization. 4-PB treatment resulted in a WT-like reorganization and extension of acetylated microtubules IB3 cells (Fig. 9A) and a quantifiable increase in Ac-tub content in IB3 cells to control S9 cell levels by Western blot (Fig. 9B). To determine whether the change in Ac-tub elicited by 4-PB treatment had a functional significance, NBD-cholesterol processing was assessed in IB3 cells (Fig. 9C). Consistent with the impact of increasing Ac-tub content with tubastatin, 4-PB treatment resulted in alleviating the perinuclear cholesterol accumulation in IB3 cells. These data are consistent with the hypothesis that ER stress is a key regulator of microtubule modifications in CF cells and the related phenotype of impaired cholesterol processing. However, there is a significant caveat to the use of 4-PB. In addition to relieving ER stress, 4-PB also acts as a pan HDAC inhibitor (1, 19). No studies specifically examine the impact of 4-PB on microtubule acetylation and HDAC6 inhibition, but the impact of 4-PB on HDAC function needs to be considered.
Fig. 9.
Ac-tub protein expression is reestablished after treatment with an ER stress reliever, 4-phenylbutyrate (4-PB). A: S9 and IB3 cells were either treated with vehicle or 1 mM 4-PB for 48 h. Cells were immunostained with Ac-tub antibodies (red) and DAPI (blue). Representative images are shown from 4 experiments. B: S9 and IB3 cells were either treated with vehicle or 1 mM 4-PB for 48 h. Lysates were analyzed for Ac-tub protein expression through Western blot. A representative blot is shown, along with Ac-tub expression normalized to α-tubulin (Alpha-tub) densitometry. Densitometry was obtained using Quantity One software. Results are presented as relative density (Ac-tub/Alpha-tub). Significance was determined by t-test (n = 6; *P < 0.05). C: NBD-cholesterol accumulation and redistribution after relieving ER stress. S9 and IB3 cells were either treated with vehicle or with 1 mM 4-PB for 48 h. All cells were simultaneously incubated with NBD-cholesterol for 24 h. Fresh media with either vehicle or 4-PB was placed on the cells 3 h before being fixed. Representative images are shown from 4 experiments.
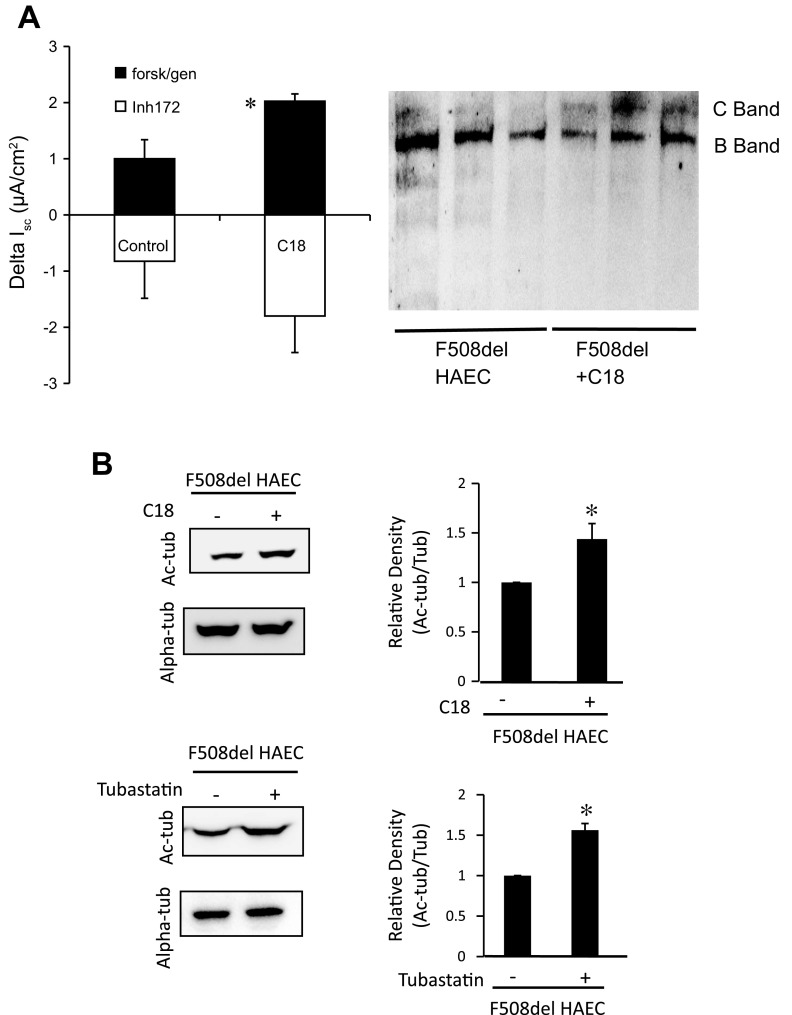
Because the efficacy of 4-PB can likely be attributed to its role as another HDAC6 inhibitor, a more specific corrector of F508del CFTR misfolding was examined. For this study, primary F508del/F508del HAECs were treated with F508del CFTR folding corrector C18. Efficacy of C18 was examined by monitoring an increase in Isc in response to cAMP/genistein treatment (2.03 ± 0.67-fold, P < 0.03) and by Western blot analysis of F508del CFTR band C formation (Fig. 10A). These data establish the efficacy of C18 in restoring F508del CFTR processing and show significant restoration of CFTR function. Efficacy of C18 treatment was also assessed by the impact of treatment on Ac-tub content compared with the HDAC6 inhibitor tubastatin. C18 treatment resulted in an increase in Ac-tub content normalized to total tubulin (1.44 ± 0.16-fold, P = 0.01). This increase was comparable to the increase in Ac-tub content achieved with tubastatin (1.56 ± 0.08, P = 0.001) (Fig. 10B).
Fig. 10.
Efficacy of C18 correction of F508del CFTR in primary F508del human airway epithelial cells (HAEC). A: short-circuit current (Isc) responses in F508del HAECs in response to C18 treatment. ΔIsc in response to forskolin (20 μM)/genistein (50 μM) is 2.03 ± 0.67 in C18-treated cells compared with 1.00 ± 0.35 for controls (n = 4; P = 0.03). CFTR-mediated current determined by sensitivity to CFTRinh172 (10 μM). Error bars are SD, and significance is determined by t-test. Representative gel showing F508del CFTR band C in C18-treated cells is also shown. B: either small-molecule CFTR corrector C18 or HDAC6 inhibition with tubastatin leads to increased Ac-Tub expression and cholesterol distribution in F508del HAECs. F508del HAECs were treated with vehicle, 10 μM C18 for 72 h, or 10 μM tubastatin for 24 h. Lysates were analyzed for Ac-tub protein expression through Western blot. A representative blot is shown, along with Ac-tub expression normalized to α-tubulin (tub). Densitometry was obtained using Quantity One software. Results are presented as relative density (Ac-tub/tub). Significance was determined by t-test (C18 treated, n = 4; *P = 0.02; tubastatin treated, n = 3; *P = 0.001).
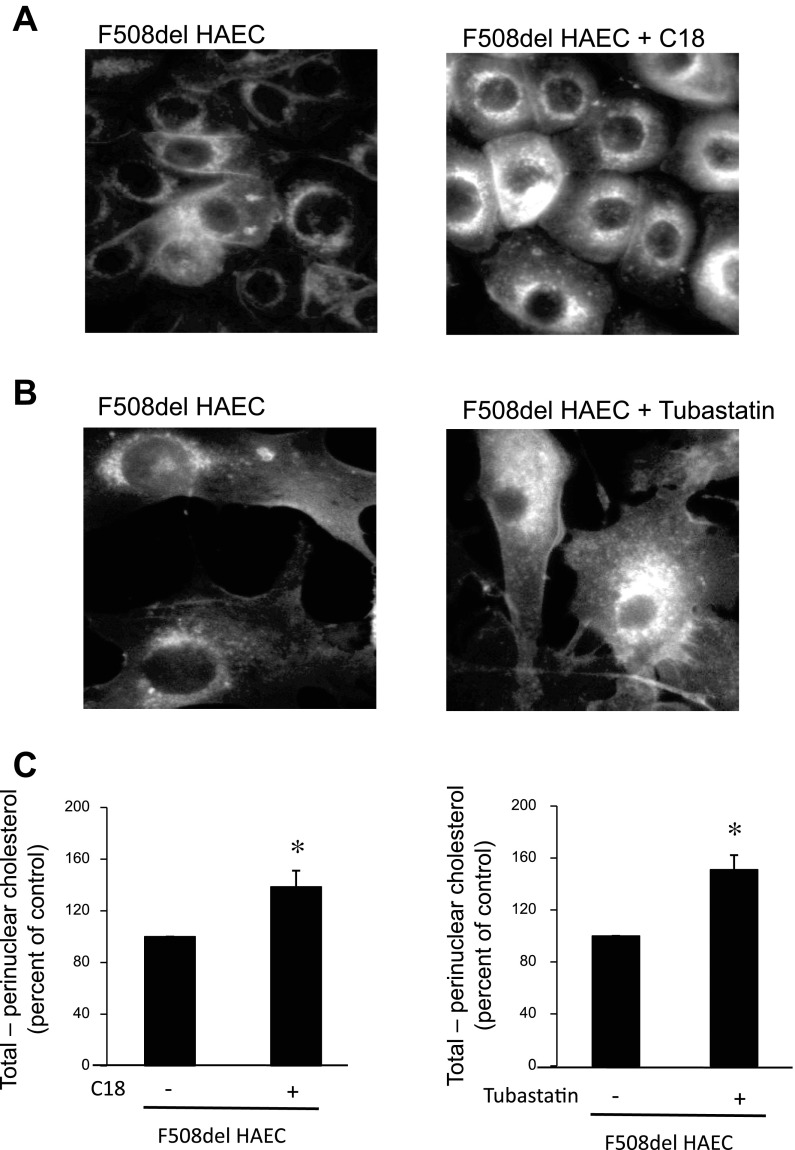
To determine whether these increases in Ac-tub content correlated with improved cholesterol mobilization, filipin staining of free cholesterol in F508del HAECs was performed. As shown in Fig. 11, A and B, C18 results in a modest increase in cholesterol mobilization, whereas tubastatin more effectively redistributes cholesterol. Mobilization was quantified by determining how much cholesterol was moved to the cytosolic region by subtracting perinuclear fluorescence from total cellular fluorescence (total − perinuclear). An increase in this difference would indicate increased outward movement of cholesterol. C18 treatment resulted in a 38.6 ± 12.3% increase in movement (total − perinuclear fluorescence) (control n = 35; C18-treated n = 40; P = 0.005). Tubastatin treatment of F508del HAEC cells resulted in a 51.0 ± 11.2% increase in movement (total − perinuclear fluorescence) (control n = 56; tubastatin-treated n = 46; P < 0.001) (Fig. 11C). These data demonstrate that correction of F508del CFTR folding at least partially corrects the phenotypes of reduced Ac-tub content and perinuclear cholesterol accumulation, results similar to direct HDAC6 inhibition. C18 and tubastatin experiments were performed separately on different populations of cells, so direct comparison of effectiveness is difficult.
Fig. 11.
Effect of C18 or tubastatin treatment on cholesterol movement in F508del HAECs. CFTR corrector C18 (A) and HDAC6 inhibitor tubastatin (B) were tested for efficacy in improving cholesterol distribution in F508del HAECs. F508del HAECs were treated with vehicle, 10 μM C18 for 72 h, or 10 μM tubastatin for 24 h and imaged for cholesterol by filipin staining. Representative images are shown. C: quantification of cholesterol movement in primary F508del HAECs by subtracting perinuclear fluorescence from total cellular fluorescence (total − perinuclear fluorescence). Individual cells were quantified on multiple images from separate experiments. Each replicate represents data from an individual cell. Data are presented as percentages of control. Error bars represent SE, and significance was determined by t-test (C18 control, n = 35; C18 treated, n = 40; *P = 0.005; tubastatin control, n = 56; tubastatin treated, n = 46; *P < 0.001).
Linking ER stress to microtubule acetylation.
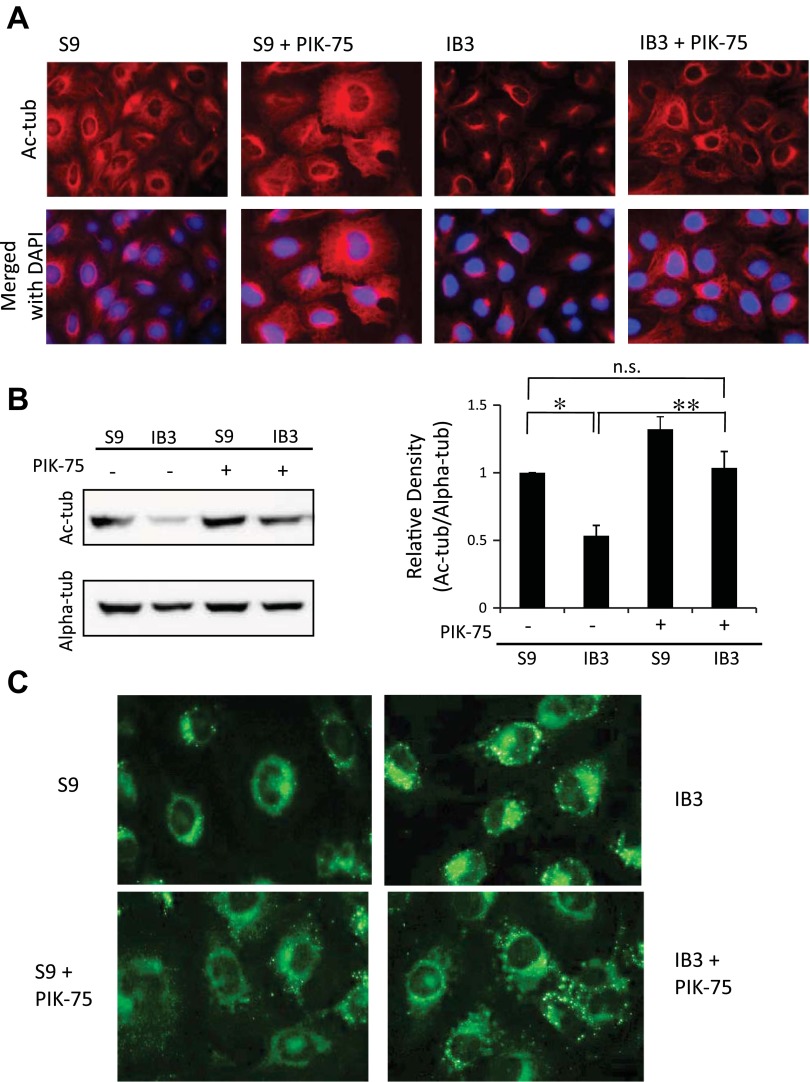
Because C18 correction of F508del CFTR trafficking was at least partially effective in resorting tubulin acetylation and cholesterol processing, ER stress is a likely contributing factor in these phenotypes. Therefore, we strove to elucidate more specific signaling interactions linking ER stress to the regulation of microtubule acetylation. We examined the role of phosphatidyl inositol-3 kinase p110α (PIK3CA), as it has been shown to associate directly with HDAC6 (24) and responds to various cellular stresses (7). Inhibition of PIK3CA with PIK-75 (0.5 μM, 24 h) resulted in a reelongation of acetylated microtubules and a significant increase in Ac-tub content in IB3 cells (Fig. 12, A and B). Cholesterol processing was also assessed using NBD-cholesterol. As shown in Fig. 12C, PIK-75 treatment restored cholesterol distribution in IB3 cells to a more WT profile. Although further characterization is needed, these data identify a strong candidate pathway linking ER stress in CF cells to the observed manifestations of reduced microtubule acetylation and cholesterol accumulation.
Fig. 12.
Mechanism of reestablishing Ac-tub involves p110α subunit of phosphatidyl inositol-3 kinase (PIK3CA). A: S9 and IB3 cells were either treated with vehicle or 0.5 μM PIK-75 for 24 h. Cells were immunostained with Ac-tub antibodies (red) and DAPI (blue). Representative images are shown from 4 experiments. B: S9 and IB3 cells were either treated with vehicle or 0.5 μM PIK-75 for 24 h. Lysates were analyzed for Ac-tub protein expression through Western blot. A representative blot is shown, along with Ac-tub expression normalized to α-tubulin (Alpha-tub) densitometry. Densitometry was obtained using Quantity One software. Results are presented as relative density (Ac-tub/Alpha-tub). Significance was determined by ANOVA. Comparisons between groups were performed with the Newman-Keuls post hoc test (n = 4; *P < 0.05). C: NBD-cholesterol accumulation and redistribution after PIK3CA inhibition. S9 and IB3 cells were either treated with vehicle (top) or with 0.5 μM PIK-75 for 24 h (bottom). All cells were simultaneously incubated with NBD-cholesterol for 24 h. Cells were washed out with fresh media with either vehicle or PIK-75 for 3 h before being fixed. Representative images are shown from 4 experiments.
DISCUSSION
Acetylation of α-tubulin is a common posttranslational modification of microtubules. Some studies have shown that fully acetylated microtubules enhance stability (15, 29), whereas others have shown that, once microtubules are not dynamically changing, acetylation occurs, but the modification itself does not signify stability (14). Regardless of stability, what is clear is that acetylation does enhance motor recruitment and therefore intracellular transport. It has been shown that there is an increase in binding in vitro of dynein and kinesin-1 to acetylated microtubules (11, 34). Acetylation and the enhancement in binding allows for the coordination between vesicular trafficking and related cellular events. Without precise homeostasis, vesicular transport and cell signaling mechanisms are altered, as exemplified in Huntington's disease (11). In Huntington's disease, there is a decrease in microtubule acetylation levels in tissue (11), which is consistent with the observations in CF presented here. The decrease of Ac-tub found in Huntington's correlates to a reduction in the transport of cargo proteins such as c-Jun N-terminal kinase-interacting protein-1, BDNF-containing vesicles, and lysosomes (11), similar to what has been shown in CF.
In CF, we have previously shown that endosomal trafficking is dysregulated, resulting in perinuclear cholesterol accumulation (27). The above data furthers the mechanistic evidence supporting the importance of autophagy in CF, which has been shown to lack effective autophagic clearance (25, 28). Autophagy is the essential cellular process that manages the removal of cellular debris. This process is highly regulated and requires acetylated microtubules for the formation of autophagosomes, where unwanted proteins are sequestered and degraded (21, 40). Altered endosomal trafficking due to reduced microtubule acetylation is consistent with previous studies and links present data to impaired autophagy in CF cells (38).
In this study, evidence is presented that Ac-tub levels are reduced in CF epithelial cells and primary mouse tissue. This change to microtubule structure impacts vesicular trafficking and leads to perinuclear endosomal accumulation. It demonstrates that manipulation of Ac-tub levels by inhibition of the microtubule deacetylase HDAC6 reverses cholesterol accumulation and NF-κB activation to more WT levels, supporting the hypothesis that microtubule acetylation is a key factor in CF cell regulation. These results establish HDAC6 as a therapeutic target because many CF phenotypes can be alleviated through its inhibition. The importance of HDAC function in relation to CF has been reported previously. Hutt et al. (16) demonstrated that inhibition of HDAC7 leads to the restoration of ΔF508 function. HDAC2 has also been implicated in CF inflammatory regulation. Bartling and Drumm (4) demonstrate a reduction of HDAC2 expression in response to the loss of CFTR function and found that the lack of HDAC2 activity resulted in increased IL-8 expression.
Mechanistically, we propose that ER stress leads to reduced Ac-tub content and related CF consequences. The most common mutation in CF, ΔF508, leads to misfolded CFTR proteins, which are retained in the ER. Literature supports that the accumulation of misfolded proteins is typically associated with the induction of ER stress and its initiation of the unfolded protein response (5). Once activated, unfolded protein response feedbacks to the transcriptional level, which decreases the endogenous level of CFTR (32). Here, we further this understanding by showing that IB3 cells and MNE from Cftr−/− mice exhibit both decreased Ac-tub and increased levels of GRP78 as indication of inherent ER stress in CF. Interestingly, GRP78 expression is increased in Cftr−/− nasal epithelium, suggesting loss of CFTR function is sufficient to induce ER stress. ER stress has also been shown to be due to altered ion transport, which may be the case seen in CFTR−/− mice. However, correction of F508del CFTR misfolding with C18 does improve microtubule acetylation and cholesterol processing, indicating that CFTR misfolding induces ER stress that impacts these pathways. We concluded that altered Ac-tub expression patterns occur as a result of chronically inducing ER stress in CF cells. The disruption of ER calcium homeostasis was sufficient to simulate the CF phenotypes of reduced Ac-tub and perinuclear cholesterol accumulation. Relief of ER stress through treatment with 4-PB reverses cholesterol accumulation and increases Ac-tub content in IB3 cells, which further establishes the importance of ER stress in these pathways. One caveat to the 4-PB experiments is that 4-PB is also a pan-HDAC inhibitor, which could also explain the increased Ac-tub expression. No studies to our knowledge have examined the impact of 4-PB on HDAC6 specifically, but the interaction needs to be considered. We were able to identify that PIK3CA activity is an important regulator of HDAC6 function in CF cells, further linking cellular stress to microtubule regulation.
Further investigation is needed to delineate the link between ER stress in CF cells and the regulation of organelle trafficking, but these findings establish ER stress as a viable target as a mechanism to explain these observations. CFTR trafficking has been shown to be influenced by HDAC6 function, and the link between aggresome formation and HDAC6 is well established (18). HDAC6 is also known to interact with and be regulated by PIK3CA. Consistent with this knowledge, we have found that PIK3CA is a likely link between ER stress and microtubule regulation with regard to acetylation and cholesterol transport. Glucose deprivation and oxidative stress are known to activate PIK3CA (7), but the direct relationship to ER stress will need to be further defined.
Our findings conclusively show that CF epithelial cells and tissue present decreased Ac-tub. Increasing tubulin acetylation in cellular models by HDAC6 inhibition reverses cholesterol accumulation through increasing organelle transport along microtubules. Combined, these findings open new avenues for understanding how the cell biology of CF cells can be manipulated to decrease the severity of disease progression and can be useful in the introduction of novel therapies targeting HDAC6 in patients with CF.
GRANTS
This work is supported by NIH grants R01EB009481 and 2P30DK027651, and CFF RDP R447-CR11 and CFF biomarker consortium grant CLANCY11CS0.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.M.R., A.H., D.A.C., and H.S. performed experiments; S.M.R., A.H., D.A.C., J.D.B., J.P.C., and T.J.K. analyzed data; S.M.R., J.D.B., J.P.C., and T.J.K. interpreted results of experiments; S.M.R. prepared figures; S.M.R. drafted manuscript; S.M.R. and T.J.K. edited and revised manuscript; H.S., J.P.C., and T.J.K. approved final version of manuscript; T.J.K. conception and design of research.
ACKNOWLEDGMENTS
The authors thank P. Bead for technical assistance.
REFERENCES
- 1.Ammerpohl O, Trauzold A, Schniewind B, Griep U, Pilarsky C, Grutzmann R, Saeger HD, Janssen O, Sipos B, Kloppel G, Kalthoff H. Complementary effects of HDAC inhibitor 4-PB on gap junction communication and cellular export mechanisms support restoration of chemosensitivity of PDAC cells. Br J Cancer 96: 73–81, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aniento F, Emans N, Griffiths G, Gruenberg J. Cytoplasmic dynein-dependent vesicular transport from early to late endosomes. J Cell Biol 123: 1373–1387, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bananis E, Nath S, Gordon K, Satir P, Stockert RJ, Murray JW, Wolkoff AW. Microtubule-dependent movement of late endocytic vesicles in vitro: requirements for Dynein and Kinesin. Mol Biol Cell 15: 3688–3697, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartling TR, Drumm ML. Loss of CFTR results in reduction of histone deacetylase 2 in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 297: L35–L43, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartoszewski R, Rab A, Jurkuvenaite A, Mazur M, Wakefield J, Collawn JF, Bebok Z. Activation of the unfolded protein response by deltaF508 CFTR. Am J Respir Cell Mol Biol 39: 448–457, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchberger A, Bukau B, Sommer T. Protein quality control in the cytosol and the endoplasmic reticulum: brothers in arms. Mol Cell 40: 238–252, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Cardone L, Bardelli A, Avvedimento VE. Activation of beta-catenin by oncogenic PIK3CA and EGFR promotes resistance to glucose deprivation by inducing a strong antioxidant response. PloS One 7: e37526, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen S, Owens GC, Makarenkova H, Edelman DB. HDAC6 regulates mitochondrial transport in hippocampal neurons. PloS One 5: e10848, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.d'Ydewalle C, Krishnan J, Chiheb DM, Van Damme P, Irobi J, Kozikowski AP, Vanden Berghe P, Timmerman V, Robberecht W, Van Den Bosch L. HDAC6 inhibitors reverse axonal loss in a mouse model of mutant HSPB1-induced Charcot-Marie-Tooth disease. Nat Med 17: 968–974, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Di Sant'Agnese P, Darling RC, Perara GA, Shea E. Abnormal electrolyte composition of sweat in cystic fibrosis of the pancreas. AMA Am J Dis Child 86: 618–619; discussion, 619, 1953 [PubMed] [Google Scholar]
- 11.Dompierre JP, Godin JD, Charrin BC, Cordelieres FP, King SJ, Humbert S, Saudou F. Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington's disease by increasing tubulin acetylation. J Neurosci 27: 3571–3583, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao YS, Hubbert CC, Yao TP. The microtubule-associated histone deacetylase 6 (HDAC6) regulates epidermal growth factor receptor (EGFR) endocytic trafficking and degradation. J Biol Chem 285: 11219–11226, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gauthier LR, Charrin BC, Borrell-Pages M, Dompierre JP, Rangone H, Cordelieres FP, De Mey J, MacDonald ME, Lessmann V, Humbert S, Saudou F. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell 118: 127–138, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Haggarty SJ, Koeller KM, Wong JC, Grozinger CM, Schreiber SL. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc Natl Acad Sci USA 100: 4389–4394, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP. HDAC6 is a microtubule-associated deacetylase. Nature 417: 455–458, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Hutt DM, Herman D, Rodrigues AP, Noel S, Pilewski JM, Matteson J, Hoch B, Kellner W, Kelly JW, Schmidt A, Thomas PJ, Matsumura Y, Skach WR, Gentzsch M, Riordan JR, Sorscher EJ, Okiyoneda T, Yates JR, 3rd, Lukacs GL, Frizzell RA, Manning G, Gottesfeld JM, Balch WE. Reduced histone deacetylase 7 activity restores function to misfolded CFTR in cystic fibrosis. Nat Chem Biol 6: 25–33, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnston JA, Ward CL, Kopito RR. Aggresomes: a cellular response to misfolded proteins. J Cell Biol 143: 1883–1898, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao TP. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell 115: 727–738, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Khan Z, Akhtar M, Ekstrom TJ. HDAC inhibitor 4-phenylbutyrate preserves immature phenotype of human embryonic midbrain stem cells: implications for the involvement of DNA methyltransferase. Int J Mol Med 28: 977–983, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Knowles M, Gatzy J, Boucher R. Increased bioelectric potential difference across respiratory epithelia in cystic fibrosis. N Engl J Med 305: 1489–1495, 1981 [DOI] [PubMed] [Google Scholar]
- 21.Kochl R, Hu XW, Chan EY, Tooze SA. Microtubules facilitate autophagosome formation and fusion of autophagosomes with endosomes. Traffic 7: 129–145, 2006 [DOI] [PubMed] [Google Scholar]
- 22.LeDizet M, Piperno G. Identification of an acetylation site of Chlamydomonas alpha-tubulin. Proc Natl Acad Sci USA 84: 5720–5724, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee AS. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods 35: 373–381, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Li J, Song J, Cassidy MG, Rychahou P, Starr ME, Liu J, Li X, Epperly G, Weiss HL, Townsend CM, Jr, Gao T, Evers BM. PI3K p110alpha/Akt signaling negatively regulates secretion of the intestinal peptide neurotensin through interference of granule transport. Mol Endocrinol 26: 1380–1393, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luciani A, Villella VR, Esposito S, Brunetti-Pierri N, Medina D, Settembre C, Gavina M, Pulze L, Giardino I, Pettoello-Mantovani M, D'Apolito M, Guido S, Masliah E, Spencer B, Quaratino S, Raia V, Ballabio A, Maiuri L. Defective CFTR induces aggresome formation and lung inflammation in cystic fibrosis through ROS-mediated autophagy inhibition. Nat Cell Biol 12: 863–875, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Lukacs GL, Mohamed A, Kartner N, Chang XB, Riordan JR, Grinstein S. Conformational maturation of CFTR but not its mutant counterpart (delta F508) occurs in the endoplasmic reticulum and requires ATP. EMBO J 13: 6076–6086, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manson ME, Corey DA, Bederman I, Burgess JD, Kelley TJ. Regulatory role of beta-arrestin-2 in cholesterol processing in cystic fibrosis epithelial cells. J Lipid Res 53: 1268–1276, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez-Vicente M, Talloczy Z, Wong E, Tang G, Koga H, Kaushik S, de Vries R, Arias E, Harris S, Sulzer D, Cuervo AM. Cargo recognition failure is responsible for inefficient autophagy in Huntington's disease. Nat Neurosci 13: 567–576, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuyama A, Shimazu T, Sumida Y, Saito A, Yoshimatsu Y, Seigneurin-Berny D, Osada H, Komatsu Y, Nishino N, Khochbin S, Horinouchi S, Yoshida M. In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J 21: 6820–6831, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGuire JR, Rong J, Li SH, Li XJ. Interaction of Huntingtin-associated protein-1 with kinesin light chain: implications in intracellular trafficking in neurons. J Biol Chem 281: 3552–3559, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Nichols D, Chmiel J, Berger M. Chronic inflammation in the cystic fibrosis lung: alterations in inter- and intracellular signaling. Clin Rev Allergy Immunol 34: 146–162, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Rab A, Bartoszewski R, Jurkuvenaite A, Wakefield J, Collawn JF, Bebok Z. Endoplasmic reticulum stress and the unfolded protein response regulate genomic cystic fibrosis transmembrane conductance regulator expression. Am J Physiol Cell Physiol 292: C756–C766, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Randell SH, Walstad L, Schwab UE, Grubb BR, Yankaskas JR. Isolation and culture of airway epithelial cells from chronically infected human lungs. In Vitro Cell Dev Biol Anim 37: 480–489, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Reed NA, Cai D, Blasius TL, Jih GT, Meyhofer E, Gaertig J, Verhey KJ. Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol 16: 2166–2172, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Rubenstein RC, Egan ME, Zeitlin PL. In vitro pharmacologic restoration of CFTR-mediated chloride transport with sodium 4-phenylbutyrate in cystic fibrosis epithelial cells containing delta F508-CFTR. J Clin Invest 100: 2457–2465, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubenstein RC, Zeitlin PL. A pilot clinical trial of oral sodium 4-phenylbutyrate (Buphenyl) in deltaF508-homozygous cystic fibrosis patients: partial restoration of nasal epithelial CFTR function. Am J Respir Crit Care Med 157: 484–490, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Valenzuela-Fernandez A, Alvarez S, Gordon-Alonso M, Barrero M, Ursa A, Cabrero JR, Fernandez G, Naranjo-Suarez S, Yanez-Mo M, Serrador JM, Munoz-Fernandez MA, Sanchez-Madrid F. Histone deacetylase 6 regulates human immunodeficiency virus type 1 infection. Mol Biol Cell 16: 5445–5454, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ward CL, Kopito RR. Intracellular turnover of cystic fibrosis transmembrane conductance regulator. Inefficient processing and rapid degradation of wild-type and mutant proteins. J Biol Chem 269: 25710–25718, 1994 [PubMed] [Google Scholar]
- 39.White NM, Jiang D, Burgess JD, Bederman IR, Previs SF, Kelley TJ. Altered cholesterol homeostasis in cultured and in vivo models of cystic fibrosis. Am J Physiol Lung Cell Mol Physiol 292: L476–L486, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Xie R, Nguyen S, McKeehan WL, Liu L. Acetylated microtubules are required for fusion of autophagosomes with lysosomes. BMC Cell Biol 11: 89, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang XJ, Seto E. HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention. Oncogene 26: 5310–5318, 2007 [DOI] [PubMed] [Google Scholar]