Abstract
Obesity is an increasing health problem. Because drug treatments are limited, diets remain popular. High-protein diets (HPD) reduce body weight (BW), although the mechanisms are unclear. We investigated physiological mechanisms altered by switching diet induced obesity (DIO) rats from Western-type diet (WTD) to HPD. Male rats were fed standard (SD) or WTD (45% calories from fat). After developing DIO (50% of rats), they were switched to SD (15% calories from protein) or HPD (52% calories from protein) for up to 4 weeks. Food intake (FI), BW, body composition, glucose tolerance, insulin sensitivity, and intestinal hormone plasma levels were monitored. Rats fed WTD showed an increased FI and had a 25% greater BW gain after 9 wk compared with SD (P < 0.05). Diet-induced obese rats switched from WTD to HPD reduced daily FI by 30% on day 1, which lasted to day 9 (−9%) and decreased BW during the 2-wk period compared with SD/SD (P < 0.05). During these 2 wk, WTD/HPD rats lost 72% more fat mass than WTD/SD (P < 0.05), whereas lean mass was unaltered. WTD/HPD rats had lower blood glucose than WTD/SD at 30 min postglucose gavage (P < 0.05). The increase of pancreatic polypeptide and peptide YY during the 2-h dark-phase feeding was higher in WTD/HPD compared with WTD/SD (P < 0.05). These data indicate that HPD reduces BW in WTD rats, which may be related to decreased FI and the selective reduction of fat mass accompanied by improved glucose tolerance, suggesting relevant benefits of HPD in the treatment of obesity.
Keywords: diet-induced obesity, food intake, high-protein diet, gut hormones
obesity has become epidemic, and by the year 2030, half of the adult population is predicted to be obese in the United States (54). Its prevalence is expanding worldwide in industrialized countries and spreading also to less developed countries; therefore “globesity” is taking over many parts of the world (see World Health Organization homepage). The plethora of health consequences related to obesity involves increased risk of developing adult-onset Type 2 diabetes mellitus, dyslipidemia, arteriosclerosis, and certain forms of cancer, including colorectal and pancreatic cancer (25). Therefore, obesity represents a major medical problem in the United States, and 12% of the entire health care costs are spent taking care of obesity-related comorbidities (52). A modest 5 to 10% body weight loss in obese subjects has been shown to result in improvements of these metabolic disturbances (21, 53).
Although two antiobesity drugs have recently been approved, drug treatment options are still very limited (26), and therefore, dietary interventions remain popular. In particular, the use of a high-protein diet is considered a beneficial strategy to achieve a long-term reduction in body weight (8, 22, 47, 48). However, the means by which an isocaloric high-protein diet reduces body weight are not well understood. There is some evidence that protein decreases appetite by increasing anorexigenic signaling (16, 34, 55) and stimulates thermogenesis (13, 57, 58) in experimental animals and humans without inducing conditioned food aversion (5). The blunting of the reduction in resting energy expenditure associated with a decrease in body weight (20, 59) is another possible mechanism through which protein may facilitate long-term weight loss. It was shown that during energy restriction in human overweight and obese subjects with BMI 27–34 kg/m2, the 24-h energy expenditure was reduced following two low-protein diets (15% energy as protein, one high in carbohydrate, and the other one high in fat), an effect that was reduced by a high-protein diet (36% energy as protein) (59). However, the subsequent alterations of body composition exerted by a high-protein diet under conditions of obesity are not well characterized in rodents.
In the present study, we assessed changes in food intake, body weight, and composition in rats developing obesity in response to a Western-type diet (45% calories from fat, 20% from protein, and 35% from carbohydrates, including 17% from sucrose) that were switched to an isocaloric high-protein (9% calories from fat, 52% from protein, and 39% from carbohydrates) or standard diet (9% calories from fat, 15% from protein, and 76% from carbohydrates) for 14 days. In addition, because gut-derived hormones play an important role in the control of food intake and body weight (23), we also investigated whether these different dietary conditions alter postprandial plasma levels of several anorexigenic hormones, namely amylin, gastric inhibitory polypeptide (GIP), insulin, leptin, pancreatic polypeptide (PP), and peptide YY (PYY) in response to a 2-h dark-phase feeding period. Because a modest reduction of body weight was shown to exert beneficial actions on the metabolic comorbidities (21, 53), we also investigated the effects of high-protein diet on glycemic control using oral glucose tolerance and insulin sensitivity tests in diet-induced obese rats.
MATERIALS AND METHODS
Animals and diets.
Adult male Sprague-Dawley rats (Harlan, San Diego, CA) weighing 230–250 g were housed in pairs under controlled illumination (0600–1800) and temperature (21–23°C) during all experiments unless otherwise specified. Protocols were approved by the Institutional Animal Care and Use Committee of the Veterans Administration (no. 11047–09). Except otherwise stated, all experiments were started between 0900 and 1000 and during nonexperimental days, the maintenance was performed during that time. Animals had free access to water and different types of diet (Table 1): a standard diet (D10012M, calories: protein 15%, fat 9%, and carbohydrates 76%, 3.8 kcal/g), Western-type diet (D12451, calories: protein 20%, fat 45%, and carbohydrates 35%, 17% of calories from carbohydrates were derived from sucrose, 4.7 kcal/g) and a high-protein diet (D10011304, calories: protein 52%, fat 9%, and carbohydrates 39%, 3.8 kcal/g) purchased from Research Diets (New Brunswick, NJ). The main fat source of the Western-type diet was lard, shown to induce the most pronounced obesity compared with other fat sources, such as coconut fat and fish oil in rats (9, 24). Diets were stored at 4°C until use.
Table 1.
Composition of standard diet, Western-type diet, and high-protein diet
| Standard Diet |
Western-Type Diet |
High-Protein Diet |
||||
|---|---|---|---|---|---|---|
| Ingredient | g | kcal | g | kcal | g | kcal |
| Casein | 140 | 560 | 233 | 932 | 500 | 2000 |
| l-Cystine | 1.8 | 7.2 | 3.5 | 14 | 1.8 | 7.2 |
| Corn starch | 495.7 | 1982.8 | 84.8 | 339 | 310.5 | 1242 |
| Maltodextrin 10 | 125 | 500 | 116.5 | 466 | 0 | 0 |
| Sucrose | 100 | 400 | 201.3 | 805 | 50 | 200 |
| Cellulose | 50 | 0 | 58.3 | 0 | 50 | 0 |
| Soybean oil | 40 | 360 | 29.1 | 262 | 40 | 360 |
| Lard | 0 | 0 | 206.8 | 1862 | 0 | 0 |
| tert-Butylhydroquinone | 0.008 | 0 | 0 | 0 | 0.008 | 0 |
| Mineral mix | 35 | 0 | 11.7 | 0 | 35 | 0 |
| Vitamin mix | 10 | 40 | 11.7 | 47 | 10 | 40 |
| Choline bitartrate | 2.5 | 0 | 2.3 | 0 | 2.5 | 0 |
| Total | 1000 | 3850 | 1000 | 4727 | 999.9 | 3849 |
The composition of diets is given according to the manufacturer's information (Research Diets).
Measurements
Body composition.
Body composition was assessed in conscious lightly restrained (∼1 min) rats using a quantitative nuclear magnetic resonance analysis apparatus (EchoMRI-700 Composition Analyzer; Echo Medical Systems, Houston, TX), as described before (49). Changes in fat mass, lean mass, and total water were determined by assessing body composition before and 2 wk after switching to standard or high-protein diets.
Glucose tolerance.
Rats were food deprived overnight with free access to water and received an orogastric gavage of 2 g·kg−1·4 ml−1 glucose (Sigma-Aldrich, St. Louis, MO) in double-distilled H2O, as described before (27). Blood was obtained by tail prick before and at 15, 30, 60, and 120 min after gavage, and blood glucose was assessed using standard glucose test strips (One-Touch Ultra; LifeScan, Milpitas, CA). The total area under the curve (42) was calculated using the formula: blood glucose at [(0 min+15 min)·(15–0) + (15 min + 30 min)·(30–15) + (30 min + 60 min)·(60–30) + (60 min+120 min)·(120–60)]/2.
Insulin sensitivity.
Rats were fasted overnight and received an intraperitoneal injection of 1 IU·kg−1·ml−1 insulin (Humulin, Eli Lilly, Indianapolis, IN), as detailed before (11). Blood was obtained by tail prick before and at 15, 30, 60, 120, 180, and 240 min postinjection, and blood glucose was assessed using standard glucose test strips (One-Touch Ultra). The area under the curve was calculated as described above.
Plasma hormone panel.
Blood (1 ml) was obtained by decapitation and collected in ice-cooled tubes containing EDTA (7.5%, 20 μl/1 ml blood; Sigma-Aldrich) and aprotinin (1, 2 trypsin inhibitory unit/1 ml blood; ICN Pharmaceuticals, Costa Mesa, CA) and centrifuged at 3,000 rpm for 10 min at 4°C. The supernatant was separated and plasma was stored at −80°C until further processing. The measurement of the gut peptide hormones, amylin, GIP, insulin, PP, and PYY, as well as the adipocyte-derived protein hormone leptin was performed using the Luminex xMAP technology for rat gut hormones (RGT-88K, Millipore, Billerica, MA) as used before (50). This panel allows simultaneous determination of multiple hormones without cross-reactivity between the antianalyte antibodies (manufacturer's information; Millipore). The assay accuracy for amylin, GIP, insulin, leptin, PYY, and PP is 92, 90, 93, 87, 88, and 86%, respectively (manufacturer's information; Millipore). All samples were processed in one batch and read via the Luminex 100 (Luminex, Austin, TX); the intra-assay variability was <10%. The multiplex assay technology has been validated against individual immunoassays for various biomarkers in several previous studies (36, 40, 45).
Experimental Design
Development of diet-induced obesity.
Rats were fed ad libitum either a standard (n = 20) or a Western-type diet (n = 40), and food intake and body weight were monitored daily for 9 wk. In the Western-type diet group, rats that gained the most body weight (>200 g, compared with ∼150–180 g in the nonresponder group) during the 9-wk observation period were designated as diet-induced obese rats and selected for further experiments (50% of initial number; n = 20). These data are consistent with other studies showing that among outbred Sprague-Dawley rats or mice, approximately one-half develop diet-induced obesity (DIO) when kept on a Western-type diet (14, 35).
Switching diets.
Rats that developed DIO on the Western-type diet or rats fed standard diet were switched to standard or high-protein diet (day 1), resulting in four experimental groups: standard diet/standard diet, standard diet/high-protein diet, Western-type diet/standard diet, and Western-type diet/high-protein diet (n = 10/group). Food intake (expressed as kcal/day or kcal/wk and also adjusted to body weight) and body weight were monitored daily for a period of 14 days. Body composition was assessed before and on day 15 after switching diets.
Assessment of glucose tolerance and insulin sensitivity.
On day 18 after switching diets to standard or high-protein diet, the four groups of rats were fasted overnight and underwent an orogastric gavage with glucose for the glucose tolerance test. Then, rats had a recovery period of 1 wk, during which they were maintained on the same diets (either standard or high-protein diet). Then (on day 25 after switching diets), the four groups of rats were fasted overnight and injected intraperitoneally with insulin to perform the insulin sensitivity test.
Measurement of hormonal changes between light and dark phase.
On day 32 after switching diets to standard or high-protein diet, the four groups of rats (n = 5/group; standard diet/standard diet, standard diet/high-protein diet, Western-type diet/standard diet, and Western-type diet/high-protein diet fed ad libitum) were euthanized by decapitation to obtain trunk blood between 3 and 4 PM during the light phase. Afterward, the remaining rats of the four groups were deprived of food for 2 h but had free access to water. At the beginning of the dark phase at 6 PM, preweighed rat chow (standard or high-protein diet) was provided, and rats were left undisturbed for 2 h. At the end of this period, food intake was determined, and rats (n = 5/group, 4 groups) were euthanized by decapitation to obtain trunk blood, and plasma levels of amylin, GIP, insulin, leptin, PP, and PYY were assessed by the rat Luminex panel assay.
Statistical analysis.
Data are expressed as means ± SE and analyzed by one-way ANOVA followed by Tukey post hoc test or two-way ANOVA followed by Holm-Sidak method. Correlations were determined by univariate linear regression. P < 0.05 was considered significant.
RESULTS
Western-Type Diet Induces Obesity
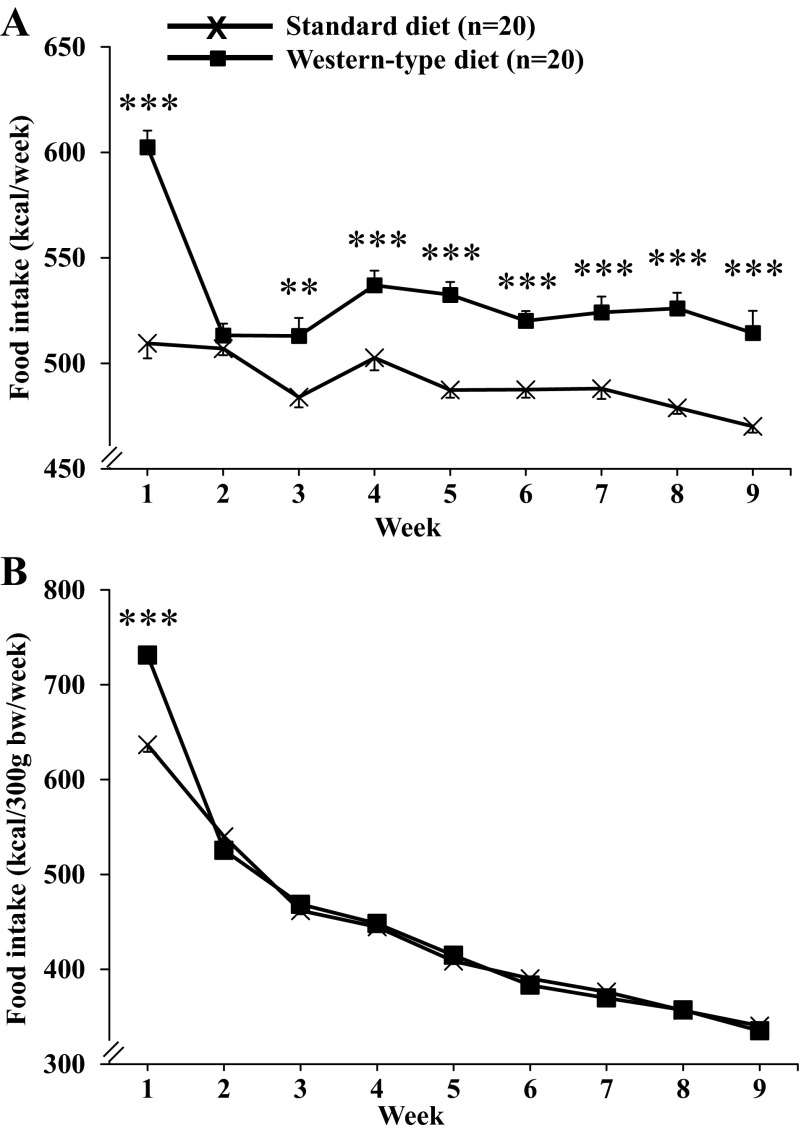
Rats fed a Western-type diet consumed significantly more calories during the first week of Western-type diet feeding compared with rats kept on a standard diet (+18%, 602.5 ± 5.0 vs. 509.5 ± 7.1 kcal/wk, P < 0.001; Fig. 1A). In the second week, there was a sharp decrease to reach similar food consumption as that of standard diet-fed rats. Thereafter, the food intake of Western-type diet-fed rats was increased during the remaining 7 wk (range: 6 to 10%), which was statistically significant compared with standard diet from weeks 3 to 9 (e.g., week 9: 514.4 ± 6.1 vs. 470.2 ± 3.3 kcal/wk, P < 0.001; Fig. 1A). Two-way ANOVA showed a significant influence of diet (F1,342 = 268.0, P < 0.001), time (F8,342 = 26.2, P < 0.001), and an interaction of diet and time (F8,342 = 10.1, P < 0.001). When adjusted for body weight, the food intake (expressed as kcal/300 g body wt/wk) did not differ between the dietary groups after week 2 (Fig. 1B).
Fig. 1.
Food intake under conditions of Western-type diet feeding. Rats were fed either a standard or a Western-type diet (those selected as DIO responders) for 9 wk, and food intake was monitored daily and expressed as intake in kilocalories per week (A) or adjusted for body weight in kilocalories per 300 g body wt per week (B). Data are expressed as means ± SE of 20 rats/group. **P < 0.01, and ***P < 0.001 vs. standard diet.
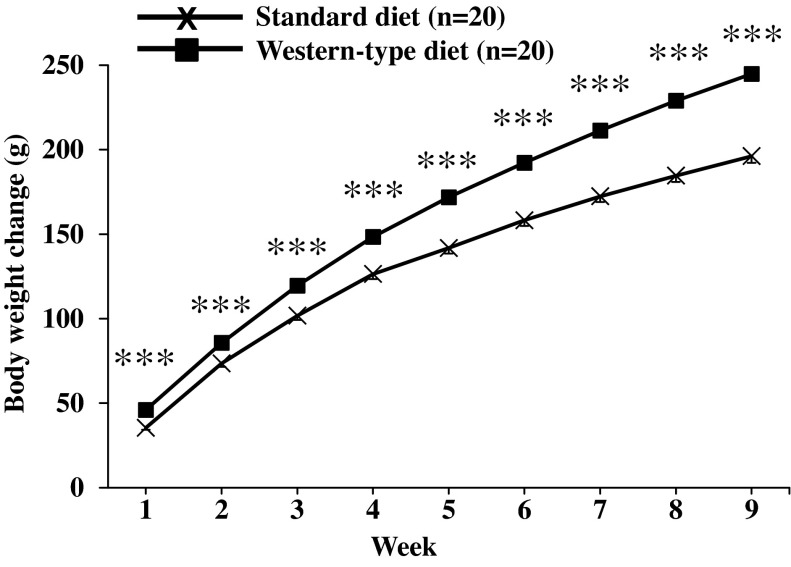
Rats (n = 20) fed a standard diet for 9 wk increased their body weight by 196.2 ± 2.9 g. In the Western-type diet group (n = 20), the body weight increase was significantly higher at all weekly measurement time points reaching a 25% higher body weight gain at 9 wk compared with standard diet (244.9 ± 3.1 g body wt change, P < 0.001; Fig. 2). Two-way ANOVA indicated a significant influence of diet (F1,342 = 524.0; P < 0.001), time (F8,342 = 1,045.5; P < 0.001), and an interaction of diet and time (F8,342 = 13.9; P < 0.001).
Fig. 2.
Body weight gain under conditions of Western-type diet feeding. Rats were fed either a standard or a Western-type diet (those selected as DIO responders) for 9 wk, and body weight was monitored daily and expressed as cumulative weekly body weight change. Data are expressed as means ± SE of 20 rats/group. ***P < 0.001 vs. standard diet.
High-protein diet for 14 days reduces body weight and fat mass in Western-type diet-induced obese rats.
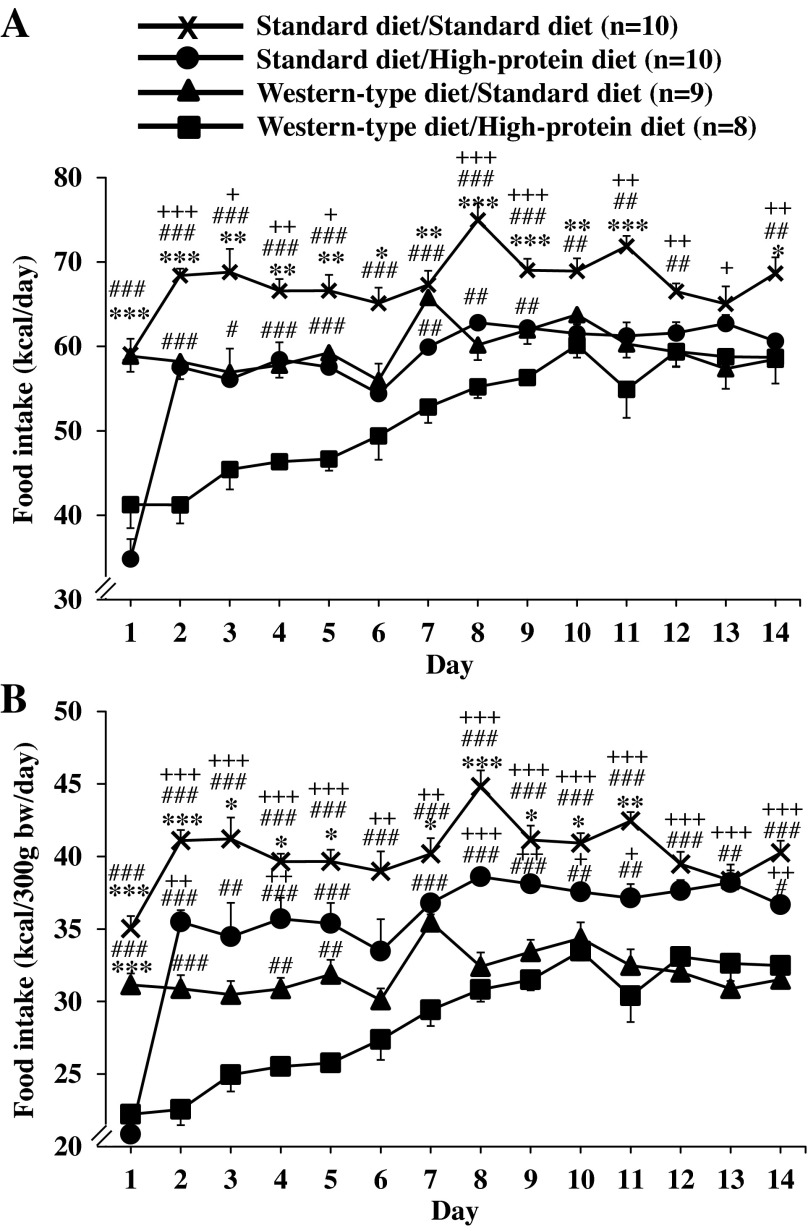
Rats fed a standard diet and remaining on this diet showed a stable food intake during the first day of the new measurement period (59.1 ± 1.8 kcal/day, Fig. 3A). The same was observed for rats fed a Western-type diet and switched to the standard diet (58.9 ± 1.9 kcal/day, Fig. 3A). In animals that received the standard diet before and were then switched to high-protein diet, the food intake was significantly decreased during the first day (34.8 ± 2.4 kcal/day), which was also observed in the rats switched from a Western-type diet to the high-protein diet (41.2 ± 2.8 kcal/day; P < 0.001; Fig. 3A). During the remaining observation period (days 2–14), rats in the standard diet/standard diet group displayed an overall stable food intake with an average of 67.6 ± 1.0 kcal/day (Fig. 3A). Animals fed Western-type diet before and switched to standard diet showed a 14% lower food intake than the standard diet/standard diet group throughout the remaining measurement period (days 2–14) with a mean daily food intake of 59.6 ± 0.7 kcal/day (P < 0.05; Fig. 3A). Similarly, rats that received standard diet before and were then switched to high-protein diet displayed a decreased caloric intake to the level of the Western-type diet/standard diet group throughout the remaining 13-day observation period (average: 58.0 ± 1.9 kcal/day, Fig. 3A). Lastly, rats fed Western-type diet before and then switched to high-protein diet displayed a markedly reduced food intake during days 2–5 of the measurement period (44.2 ± 1.2 kcal/day, −24% compared with standard diet/high-protein diet, P < 0.05) and then linearly increased until day 10 when it reached the level of the standard diet/high-protein diet group (Fig. 3A). Two-way ANOVA showed a significant influence of diet (F3,462 = 169.9, P < 0.001), time (F13,462 = 19.6, P < 0.001), and an interaction of diet and time (F39,462 = 4.9, P < 0.001). The same results were observed when food intake was adjusted for body weight (Fig. 3B).
Fig. 3.
Food intake after switching to standard diet or high-protein diet. After development of diet-induced obesity on a Western-type diet or feeding of standard diet, rats were fed a standard or high-protein diet resulting in four experimental groups: standard diet/standard diet, standard diet/high-protein diet, Western-type diet/standard diet, and Western-type diet/high-protein diet. Food intake was assessed daily for a period of 14 days and expressed as intake in kilocalories per day (A) or adjusted for body weight in kilocalories per 300 g body wt per day (B). Data are expressed as means ± SE of 8–10 rats/group. *P < 0.05, **P < 0.01 and ***P < 0.001 vs. standard diet/high-protein diet. +P < 0.05, ++P < 0.01 and +++P < 0.001 vs. Western-type diet/standard diet. #P < 0.05, ##P < 0.01, and ###P < 0.001 vs. Western-type diet/high-protein diet.
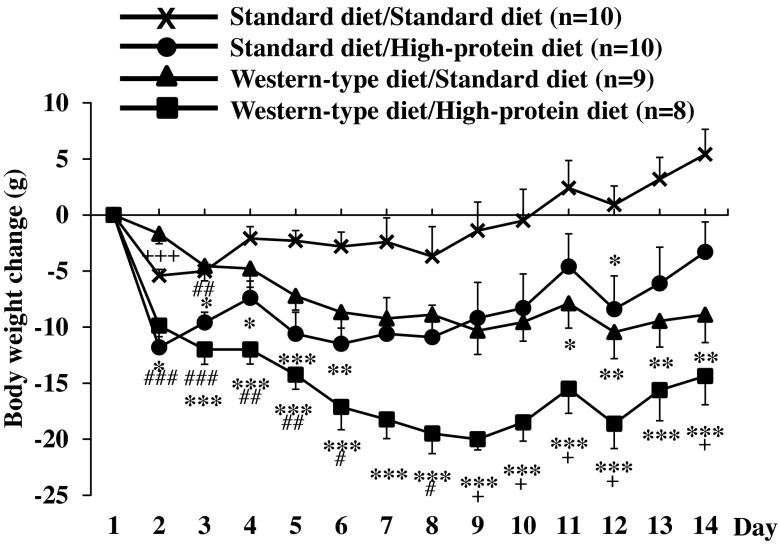
Similar to food intake, body weight remained stable in rats fed a standard diet before and kept on standard diet for 2 wk (Fig. 4). In rats fed Western-type diet before and switched to standard diet, a steady body weight decrease was observed reaching 8.9 ± 2.5 g after 14 days (P < 0.01 compared with standard diet/standard diet; Fig. 4). Rats fed standard diet before and switched to high-protein diet displayed the most pronounced body weight change during the first day and then remained body weight stable over the observation period with a slight increase during the last 2 days, resulting in a nonsignificant difference compared with the standard diet/standard diet group (body weight decrease of 3.3 ± 2.7 g after 14 days; Fig. 4). Lastly, rats fed Western-type diet before and then switched to high-protein diet displayed a steady body weight decrease over the first 9 days reaching a nadir body weight loss of 20.0 ± 1.0 g on day 9 and then showed a slight increase until the end of the 14-day measurement period but remained significantly lower than the standard diet/standard diet and the standard diet/high-protein diet group (P < 0.05; Fig. 4). Two-way ANOVA showed a significant influence of diet (F3,429 = 101.3; P < 0.001), time (F12,429 = 2.7; P < 0.01), and an interaction of diet and time (F36,429 = 1.7; P < 0.05).
Fig. 4.
Body weight change after switching to standard diet or high-protein diet. After development of diet-induced obesity on a Western-type diet or feeding of standard diet, rats were fed a standard or high-protein diet resulting in four experimental groups: standard diet/standard diet, standard diet/high-protein diet, Western-type diet/standard diet, and Western-type diet/high-protein diet. Body weight was assessed daily for a period of 14 days and expressed as daily body weight change. Data are expressed as means ± SE of 8–10 rats/group. *P < 0.05, **P < 0.01 and ***P < 0.001 vs. standard diet/standard diet; +P < 0.05 and +++P < 0.001 vs. standard diet/high-protein diet; #P < 0.05, ##P < 0.01 and ###P < 0.001 vs. Western-type diet/standard diet.
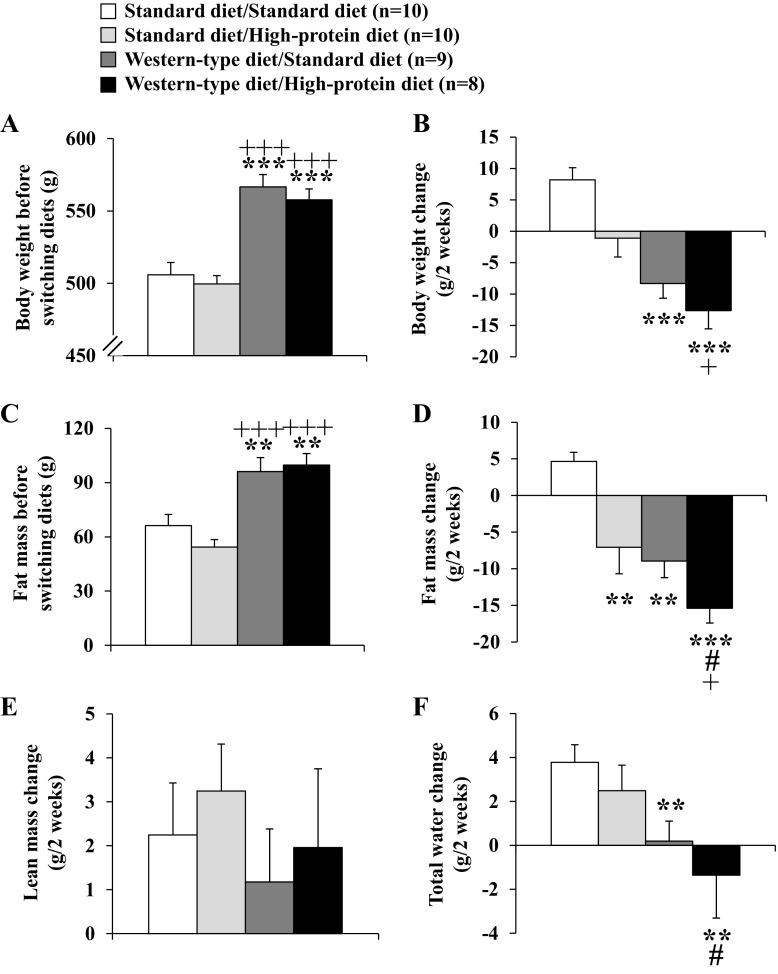
Before switching diets, the assessment of the body composition by automated magnetic resonance-based analysis showed that rats fed a Western-type diet displayed higher body weight (Fig. 5A) and fat mass (Fig. 5C) compared with the standard diet-fed groups (P < 0.01). Also, the ratio of lean-to-fat mass was different between the standard and Western-type diet groups with a higher ratio in the standard diet/to be assigned to standard diet (6.0 ± 0.6) and standard diet/to be assigned high-protein diet group (7.3 ± 0.6) compared with the Western-type diet/to be assigned standard diet (4.3 ± 0.4) and Western-type diet/to be assigned high-protein diet group (4.0 ± 0.3, P < 0.05).
Fig. 5.
Body weight and body composition before and after switching to standard diet or high-protein diet. After development of diet-induced obesity on Western-type diet or feeding of standard diet for 9 wk (A), rats were fed a standard or high-protein diet, resulting in four experimental groups: standard diet/standard diet, standard diet/high-protein diet, Western-type diet/standard diet, and Western-type diet/high-protein diet. Body composition was assessed in conscious lightly restrained (∼1 min) rats directly before switching diets and after the 14-day measurement period on day 15 using quantitative nuclear magnetic resonance analysis. Changes of total body weight (B), fat mass (C and D), lean mass (E), and water (F) were assessed. Data are expressed as means ± SE of 8–10 rats/group. **P < 0.01 and ***P < 0.001 vs. standard diet/standard diet; +P < 0.05 and +++P < 0.001 vs. standard diet/high-protein diet; #P < 0.05 vs. Western-type diet/standard diet.
After switching diets, on day 15, the most pronounced reduction of body weight was observed in rats that were fed Western-type diet first and then switched to high-protein diet for 14 days (P < 0.05; Fig. 5B). When body composition was reassessed at day 15, fat mass was decreased (−15.4 ± 2.0 g) in the Western-type diet/high-protein diet group compared with −7.1 ± 3.6 g in the standard diet/high-protein diet group, and by −9.0 ± 2.3 g in the Western-type diet/standard diet group compared with an increase (+4.7 ± 1.2 g) in the standard diet/standard diet group (P < 0.05; Fig. 5D). The observed increase in lean mass (average +2.2 ± 0.4 g) did not differ between the four different dietary groups (P > 0.05; Fig. 5E). The ratio of lean to fat mass did not significantly change after the 14-day diet period, with a higher ratio remaining in the standard diet/standard diet (5.7 ± 0.6) and standard diet/high-protein diet group (8.3 ± 0.6) and a lower ratio remained in the Western-type diet/standard diet (4.8 ± 0.4) and Western-type diet/high-protein diet group (4.8 ± 0.5). Lastly, the total water content was decreased in rats fed Western-type diet and switched to high-protein diet (−1.4 ± 1.9 g), while it was unaltered in the Western-type diet/standard diet group (+0.2 ± 0.9 g) and increased in the standard diet/high-protein diet group (+2.5 ± 1.2 g) and in rats constantly fed with standard diet (standard diet/standard diet: +3.8 ± 0.8 g, P < 0.05; Fig. 5F).
High-protein diet improves glucose tolerance, whereas insulin sensitivity is not altered in diet-induced obese rats.
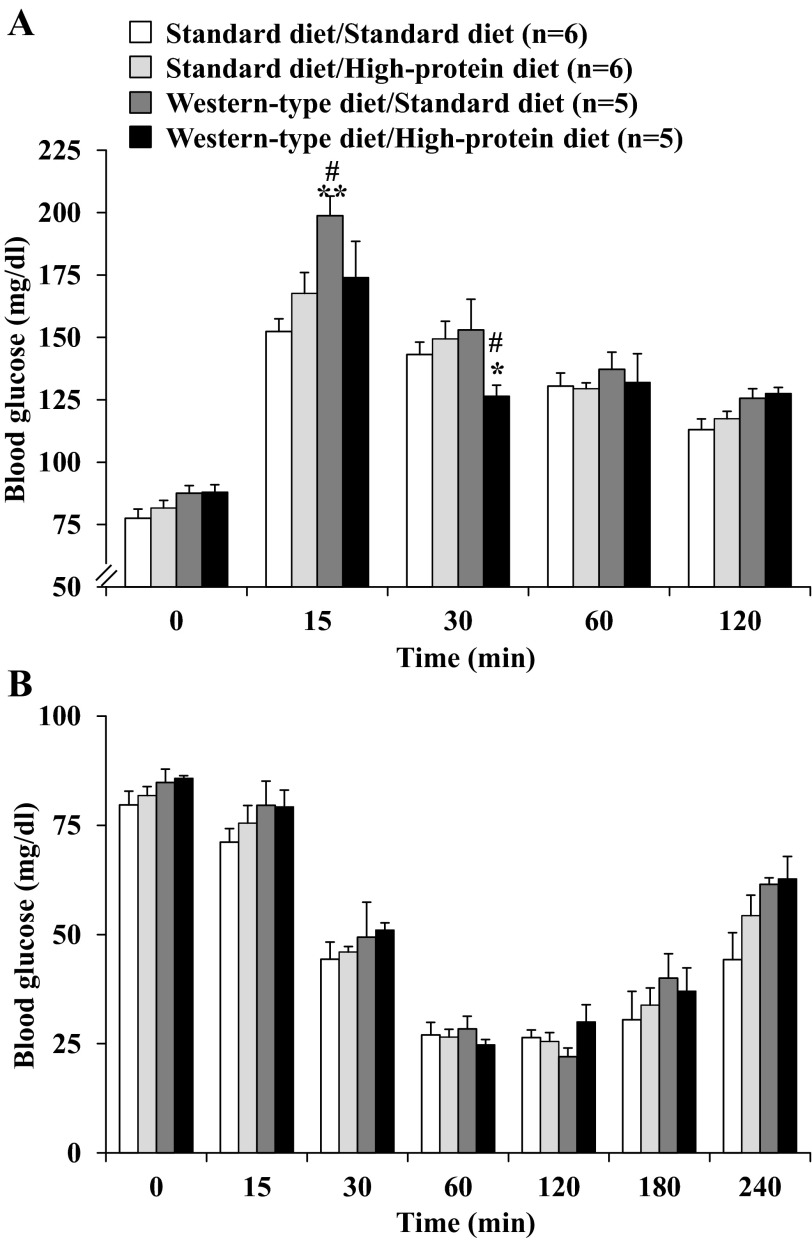
In the oral glucose tolerance test performed 18 days after switching diets, no differences were observed in the fasting blood glucose levels among the four dietary groups (P > 0.05; Fig. 6A). In the Western-type diet/standard diet group, rats displayed the largest increase of blood glucose reaching 198.9 ± 7.8 mg/dl at 15 min postgavage. Blood glucose levels were 12.5 and 17.3% lower in the Western-type diet/high-protein diet group at 15 min and 30 min, respectively (Fig. 4A). Afterward, no significant differences were observed (Fig. 6A). When calculated as area under the curve, two-way ANOVA indicated a significant influence of diet (F3,64 = 7.7, P < 0.001) and time (F3,64 = 1,529.1, P < 0.001).
Fig. 6.
Glucose tolerance and insulin sensitivity after switching to standard diet or high-protein diet. For assessment of glucose tolerance, rats were food-deprived overnight with free access to water and received an orogastric gavage with 2 g·kg−1·4 ml−1 glucose in ddH2O. Blood was obtained by tail prick before and at 15, 30, 60, and 120 min after gavage, and blood glucose was assessed using standard glucose test strips (A). In the insulin sensitivity test, rats were fasted overnight and received an intraperitoneal injection of 1 IU·kg−1·ml−1 insulin. The experiment started at 9 AM, and blood glucose was assessed using standard glucose test strips before and at 15, 30, 60, 120, 180, and 240 min postinjection (B). Data are expressed as means ± SE of 5 or 6 rats/group. *P < 0.05 and **P < 0.01 vs. standard diet/standard diet. #P < 0.05 vs. standard diet/high-protein diet.
In the insulin sensitivity test performed 25 days after switching diets, all four groups of rats showed a marked reduction of blood glucose with a nadir during the 60–120-min period after intraperitoneal injection of insulin followed by a linear increase thereafter (Fig. 6B). No differences were observed between the four diet groups when calculated either as individual concentrations (P > 0.05; Fig. 6B) or area under the curve (data not shown).
Circulating anorexigenic gut hormone response to a 2-h dark phase feeding.
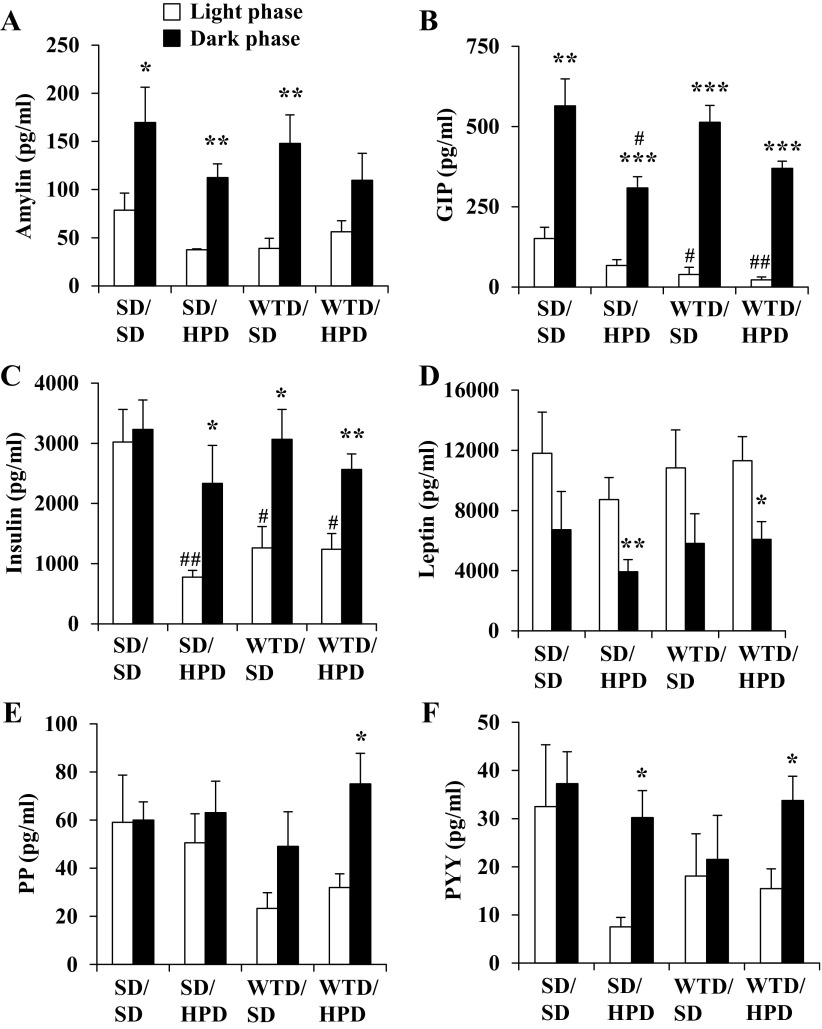
Food intake of standard or high-protein diet during the first 2 h of the dark phase did not differ between the four groups (average: 19.3 ± 0.6 kcal/2 h). In the light phase, plasma levels of GIP and insulin were significantly decreased in Western-type diet groups either switched to standard or high-protein diet compared with the standard diet/standard diet group, while the levels of the other gut hormones did not differ among groups during the light phase (Fig. 7). In the standard diet/standard diet group, plasma levels of anorexigenic hormones assessed near the end of the light phase compared with the first 2 h of the dark-phase food intake showed a significant increase of amylin and GIP (Fig. 7, A and B, Table 2). A similar response was maintained for amylin, GIP, and insulin in the other groups of rats switched to the high-protein diet (Fig. 7, A–C, Table 2). The increase of amylin was significantly lower in the Western-type diet/high-protein diet group compared with Western-type diet/standard diet rats (P < 0.05), whereas the increase of GIP was higher (P < 0.05; Table 2). While most gut hormones showed a postprandial increase, leptin levels decreased compared with the light phase (Fig. 7D, Table 2). Although no significant differences were observed for leptin between groups, leptin plasma levels showed a positive correlation with fat mass (r = 0.75, P = 0.001). Rats fed Western-type diet initially and then switched to high-protein diet for 32 days displayed a higher dark-phase food intake-related increase of PP (Fig. 7E) and a smaller increase of PYY (Fig. 7F) compared with the standard diet/high-protein diet group (P < 0.05; Table 2).
Fig. 7.
Changes in intestinal hormones after 2-h dark phase feeding compared with the preprandial light phase. After development of diet-induced obesity on Western-type diet or feeding of standard diet, rats were fed a standard or high-protein diet for 32 days, resulting in four experimental groups: standard diet/standard diet (SD/SD), standard diet/high-protein diet (SD/HPD), Western-type diet/standard diet (WTD/SD), and Western-type diet/high-protein diet (WTD/HPD). The four groups fed ad libitum with standard or high-protein diet, respectively, were euthanized by decapitation to obtain trunk blood between 3 and 4 PM during the light phase. The remaining rats were euthanized after a 2-h dark phase feeding period, trunk blood was collected, and the plasma levels of amylin (A), gastric inhibitory polypeptide (GIP; B), insulin (C), leptin (D), pancreatic polypeptide (PP; E), and peptide YY (PYY; F) were assessed. Data are expressed as mean ± SE of 5 rats/group. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. same diet group during light phase; #P < 0.05 and ##P < 0.01 vs. standard diet/standard diet group during same photoperiod.
Table 2.
Fold-changes in intestinal hormones after 2-h dark-phase feeding compared to the preprandial light phase
| Treatmenta |
||||
|---|---|---|---|---|
| Hormoneb | Standard Diet/Standard Diet | Standard Diet/high-Protein Diet | Western-Type Diet/Standard Diet | Western-Type Diet/High-Protein Diet |
| Amylin | 2.2 ± 0.5 | 3.0 ± 0.4 | 3.8 ± 0.8 | 2.0 ± 0.5# |
| GIP | 3.7 ± 0.6 | 4.6 ± 0.5 | 13.1 ± 1.3*+ | 16.3 ± 1.0*+# |
| Insulin | 1.1 ± 0.2 | 3.0 ± 0.8* | 2.4 ± 0.4* | 2.1 ± 0.2* |
| Leptin | 0.6 ± 0.2 | 0.5 ± 0.1 | 0.5 ± 0.2 | 0.5 ± 0.1 |
| PP | 1.0 ± 0.1 | 1.2 ± 0.3 | 2.1 ± 0.6 | 2.3 ± 0.4*+ |
| Peptide YY | 1.1 ± 0.2 | 4.0 ± 0.7# | 1.2 ± 0.5 | 2.2 ± 0.3*+ |
After development of diet-induced obesity on a Western-type diet or feeding of a standard diet, rats were fed a standard or high-protein diet for 32 days, resulting in four experimental groups: standard diet/standard diet, standard diet/high-protein diet, Western-type diet/standard diet, and Western-type diet/high-protein diet. The four groups fed ad libitum with standard or high-protein diet, respectively, were euthanized by decapitation to obtain trunk blood between 3 and 4 p.m. during the light phase (n = 5/group). Afterward, in the remaining rats, food was removed for 2 h. At the beginning of the dark phase at 6 p.m., preweighed rat chow (standard or high-protein diet, respectively) was provided, and rats were left undisturbed for 2 h. After this period, food intake was assessed and rats (n = 5/group) were euthanized to obtain trunk blood, and plasma levels of hormones were assessed.
Data are fold changes of plasma levels from light to the dark phase and expressed as means ± SE. Significant differences are indicated for the change in plasma levels from light to dark phase. GIP, gastric inhibitory polypeptide; PP, pancreatic polypeptide.
P < 0.05 vs. standard diet/standard diet;
P < 0.05 vs. standard diet/high-protein diet; and
P < 0.05 vs. Western-type diet/standard diet.
DISCUSSION
In this study evaluating the effects of a high-protein diet (52% protein containing 50% casein) for 2 wk on weight loss in rats fed either Western-type diet or a standard diet, we demonstrated that the high-protein diet induces a greater decrease in food intake and loss in body weight and fat mass in DIO than in lean rats with retention of body lean mass. This is associated with improved glucose tolerance in diet-induced obese rats only, while insulin sensitivity was not different between groups. Lastly, the high-protein diet fed to diet-induced obese rats induced a higher increase in the postprandial satiety hormone response to a 2-h dark phase feeding compared with the standard diet group.
Many studies have characterized the feeding-suppressive effect of a high-protein diet in rats starting the first day with a gradual adaptation during the following days (4, 6, 28, 32). However, less is known on the efficacy of a high-protein diet to curtail spontaneous energy intake in diet-induced obese rats, as studies were set mainly in the context of an additional 40% energy restriction (1, 12, 18), making it difficult to analyze the respective contributions of these interventions. In the current study, the shifting from a 20% to a 52% protein diet markedly reduced the daily energy intake on the first day by 41% and 31% in the standard diet and DIO group, respectively. On day 2, while the standard diet/high-protein diet rats resumed food consumption with a gradual return of caloric intake similar to the standard diet/standard diet group on day 12, the daily energy intake of the Western-type diet/high-protein diet group remained low for the first 5 days and then increased gradually to reach a plateau on days 10–14, with 5% lower daily energy intake compared with that observed in the standard diet/high-protein diet group. The enhanced magnitude and duration of food intake decrease in the Western-type diet/high-protein diet group may be indicative that diet-induced obese rats display an increased responsiveness to the food intake-reducing effect of the high-protein diet. Convergent functional and anatomical evidence in rats established that the high-protein diet effect is due to an increased satiety signaling rather than a low palatability of the diet or the induction of the conditioned taste aversion (20). Although an earlier study points to an initial orosensory preabsorptive poor palatability (37), subsequent results obtained using two choices and flavor testing, behavioral satiety sequence, taste reactivity in response to different percentages or sources of proteins support that the determinant of reduced daily energy intake in rats eating a high-protein diet is a protein-specific food intake-suppressive mechanism (4, 5, 19, 30, 31). Of relevance are also choice study experiments showing that rats select a casein-containing diet (40% protein) over other proteins (46), further supporting the enhanced acceptability of casein used in the present study (50% protein from casein, 52% protein in total).
The higher energy intake in rats fed a Western-type diet resulted in a 25% higher body weight increase compared with rats fed a standard diet over a period of 9 wk in agreement with other studies (35). This model is relevant in the context of common obesity, as it involves genetically unaltered, wild-type rats that become obese when exposed to a diet containing 32–45% fat, a proportion similar to that contained in the typical Western diet (56). Switching rats to the high-protein diet induced a marked reduction in body weight in the Western-type diet/high-protein diet group, reaching 14 g at day 14, the highest compared with all other diet groups (standard diet/standard diet, standard diet/high-protein diet, Western-type diet/standard diet). The reduction of body weight in rats that were fed the Western-type diet shifted to high-protein diet was accompanied by the reduction of fat mass (−15.4 g), likely accounting for the observed body weight loss. Likewise, the lower body weight of healthy rats fed a high-protein diet was mainly associated with a reduced body fat mass, in agreement with several previous studies during short (8 wk) (6) or long (6 mo) observation periods (33). In the present study, rats fed with a standard diet presented a higher lean-to-fat mass ratio (∼6–7) compared with diet-induced obese rats fed a Western-type diet (∼4). Despite the significant loss of fat mass, this ratio did not change significantly after the 14-day dietary period. This could be due to the duration of the period and may require a longer observation time. Of significance, the lean body mass is preserved as shown by the 2-g gain of lean mass, which was not different among the four groups. This is unlikely due to an increase in physical activity as a previous study in C57BL/6J mice indicated that nonexercise physical activity was not different between mice fed Western-type diet and mice fed high-protein diet for 3 mo (29). Similarly, in the same study, the respiratory quotient and energy expenditure did not differ between the Western-type and high-protein diet-fed groups (29). Consistent with our observation, other studies using dairy product diet or its components over 8 wk in diet-induced obese rats showed a similar improvement in body composition (10, 17).
After the moderate weight loss of 15 g and continued feeding with high-protein diet, we performed an oral glucose tolerance test. Rats fed with Western-type diet before and then kept on standard diet showed the largest increase of blood glucose following the orogastric gavage, which was blunted in the Western-type diet/high-protein diet group, indicating a beneficial effect. This improved glycemic control may be due to a stimulation of genes involved in gluconeogenesis, as reported in rats fed a high-protein diet (38). In the present study, the insulin sensitivity was not altered, while in a previous study using Wistar rats fed a high-fat diet (300 g fat/kg diet, 52% of calories from fat) before and switched to high whey protein diet, the insulin sensitivity was increased when calculated by the insulin-to-glucose ratio (3). Whether these differences are due to different measurements applied (insulin-to-glucose ratio vs. injection of insulin and measurement of the glucose response) or strain difference (Wistar vs. Sprague-Dawley rats) warrants further investigation.
The control of food intake and postprandial glycemia is tightly controlled by peptide hormones mainly derived from the gastrointestinal tract (23). Here, we investigated the physiological dark phase meal-stimulated release of different anorexigenic gut hormones among the four dietary conditions. In line with previous studies (44), most food intake-inhibitory hormones, namely amylin, GIP, insulin, PP, and PYY increased during the dark feeding phase to prevent further eating and overeating of the animals. However, leptin levels rather decreased after the dark-phase food intake. Previous studies did not detect alterations of leptin plasma levels between the light and dark photoperiod (2), and in a very recent study, leptin levels were not altered under conditions of fasting followed by 2 h of refeeding (62). These findings are in line with leptin being more involved in the long-term regulation of body weight (60). The increase of amylin was lower in the Western-type diet/high-protein diet group compared with the Western-type diet/standard diet group, which may point toward the restoration of amylin sensitivity reported to be reduced under conditions of chronic high-fat diet feeding (7). On the contrary, the increase of GIP (also known as glucose-dependent insulinotropic peptide) was higher in the Western-type diet/high-protein diet group compared with the Western-type diet/standard diet group. Because both hormones are involved in glucose homeostasis (41, 51) and affect insulin release and signaling, these changes may contribute to the observed beneficial effect of a high-protein diet on glucose tolerance in diet-induced obese rats. In addition, diet-induced obese rats switched to a high-protein diet displayed a higher dark feeding phase-related fold-change increase of PP and PYY compared with the standard diet/standard diet group, which may contribute to the anorexigenic effect of a high-protein diet. These results cannot be related to differences in food intake as the 2-h dark-phase caloric consumption of standard or high-protein diet did not differ between the four groups. Vagal afferent fibers are the major neuroanatomical pathway linking the alimentary tract and the brain through which nutrients or gut peptides influence food intake (15, 43, 61). However, a previous study indicates that vagal afferents are not the main pathway signaling the feeding-suppressive effects of a high-protein diet in lean rats (32). This was supported by the fact that subdiaphragmatic vagotomy dampened the daily food intake response to a high-protein diet by only 14% compared with sham-operated rats (32). Whether the contribution of the vagus nerve is more prominent under conditions of DIO warrants investigation, especially noting the observed dark phase-related changes in gut hormones and the influence of feeding status on gut hormone receptors in the nodose ganglion (15).
Perspectives and Significance
We showed that a high-protein diet for 2 wk reduces body weight in Western-type diet-induced obese rats by selectively reducing fat, while sparing lean mass. Similar findings were observed in healthy lean rats fed a standard diet and switched to high-protein diet, although less pronounced. Concomitantly, the selective reduction of fat mass is accompanied by improved glucose tolerance in the diet-induced obese rats, which may be related to an altered meal-induced release of gastrointestinal peptides, in particular, the enhanced response of GIP. In view of the paucity of studies on the levels of postprandial circulating gut hormones in response to a high-protein diet in nonobese or diet-induced obese rats, additional investigations on their variations and contributions under these conditions will provide more insight into the role of hormonal mechanisms in the high-protein diet-induced curtailing of food intake. Recent evidence that the melanocortin pathway in the arcuate nucleus, a hypothalamic region with weaker blood-brain barrier and, therefore, sensitive to circulating nutrients and/or hormones (39), is activated by a 50% protein diet (19), supports such as possibility. Future studies will be needed to elucidate the full extent to which alterations in circulating gut hormones and signaling pathways within the arcuate nucleus contribute to the suppression of feeding in diet-induced obese rats fed a high-protein diet. Still, many questions remain unanswered regarding the peripheral and central mechanisms of a high-protein diet underlying its beneficial effect to improve body composition with loss of fat and retention of lean body mass in diet-induced obese rats (20). Taken together, these results support an important role for high-protein diet as an additional column in the multimodal treatment of obesity in addition to dietary coaching (regular eating schedule, slow eating, reduction of fat, and carbohydrate intake), exercise, psychotherapy, drug treatment, and bariatric surgery.
GRANTS
This article was supported by VA Merit award (to Y. Taché and J. R. Pisegna), National Institute of Health center grant DK-41301 (Animal Core, to Y. Taché), VA Career Scientist Award (to Y. Taché) and 5 RC1 DK-086150-02 (S. B., C.P.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.S., L.W., J.R.P., and Y.T. conception and design of research; A.S., M.G.-S., E.H., and H.K. performed experiments; A.S. analyzed data; A.S. interpreted results of experiments; A.S. prepared figures; A.S. drafted manuscript; A.S., M.G.-S., L.W., J.R.P., and Y.T. edited and revised manuscript; A.S., M.G.-S., L.W., H.K., J.R.P., and Y.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Sylvie Bradesi and Dr. C. Pothoulakis for enabling us to use their quantitative nuclear magnetic resonance equipment.
REFERENCES
- 1.Aoyama T, Fukui K, Takamatsu K, Hashimoto Y, Yamamoto T. Soy protein isolate and its hydrolysate reduce body fat of dietary obese rats and genetically obese mice (yellow KK). Nutrition 16: 349–354, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Asakuma S, Hiraku O, Kurose Y, Kobayashi S, Terashima Y. Diurnal rhythm of cerebrospinal fluid and plasma leptin levels related to feeding in non-lactating and lactating rats. J Endocrinol 180: 283–286, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Belobrajdic DP, McIntosh GH, Owens JA. A high-whey-protein diet reduces body weight gain and alters insulin sensitivity relative to red meat in wistar rats. J Nutr 134: 1454–1458, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Bensaid A, Tome D, Gietzen D, Even P, Morens C, Gausseres N, Fromentin G. Protein is more potent than carbohydrate for reducing appetite in rats. Physiol Behav 75: 577–582, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Bensaid A, Tome D, L'Heureux-Bourdon D, Even P, Gietzen D, Morens C, Gaudichon C, Larue-Achagiotis C, Fromentin G. A high-protein diet enhances satiety without conditioned taste aversion in the rat. Physiol Behav 78: 311–320, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Blouet C, Mariotti F, Azzout-Marniche D, Bos C, Mathe V, Tome D, Huneau JF. The reduced energy intake of rats fed a high-protein low-carbohydrate diet explains the lower fat deposition, but macronutrient substitution accounts for the improved glycemic control. J Nutr 136: 1849–1854, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Boyle CN, Rossier MM, Lutz TA. Influence of high-fat feeding, diet-induced obesity, and hyperamylinemia on the sensitivity to acute amylin. Physiol Behav 104: 20–28, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Brehm BJ, D'Alessio DA. Benefits of high-protein weight loss diets: enough evidence for practice? Curr Opin Endocrinol Diabetes Obes 15: 416–421, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Buettner R, Parhofer KG, Woenckhaus M, Wrede CE, Kunz-Schughart LA, Scholmerich J, Bollheimer LC. Defining high-fat-diet rat models: metabolic and molecular effects of different fat types. J Mol Endocrinol 36: 485–501, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Chen H, Wang Y, Ma L, Zhao J, Li Y, Li M. Long-term high animal protein diet reduces body weight gain and insulin secretion in diet-induced obese rats. J Sci Food Agric 92: 2638–2643, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Zhang T, Nyomba BL. Insulin resistance of gluconeogenic pathways in neonatal rats after prenatal ethanol exposure. Am J Physiol Regul Integr Comp Physiol 286: R554–R559, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Chevalier L, Bos C, Azzout-Marniche D, Fromentin G, Mosoni L, Hafnaoui N, Piedcoq J, Tome D, Gaudichon C. Energy restriction only slightly influences protein metabolism in obese rats, whatever the level of protein and its source in the diet. Int J Obes (Lond) 37: 263–271, 2013 [DOI] [PubMed] [Google Scholar]
- 13.D'Alessio DA, Kavle EC, Mozzoli MA, Smalley KJ, Polansky M, Kendrick ZV, Owen LR, Bushman MC, Boden G, Owen OE. Thermic effect of food in lean and obese men. J Clin Invest 81: 1781–1789, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demigne C, Bloch-Faure M, Picard N, Sabboh H, Besson C, Remesy C, Geoffroy V, Gaston AT, Nicoletti A, Hagege A, Menard J, Meneton P. Mice chronically fed a westernized experimental diet as a model of obesity, metabolic syndrome and osteoporosis. Eur J Nutr 45: 298–306, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Dockray GJ. The versatility of the vagus. Physiol Behav 97: 531–536, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Eisenstein J, Roberts SB, Dallal G, Saltzman E. High-protein weight-loss diets: are they safe and do they work? A review of the experimental and epidemiologic data. Nutr Rev 60: 189–200, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Eller LK, Reimer RA. Dairy protein attenuates weight gain in obese rats better than whey or casein alone. Obesity (Silver Spring) 18: 704–711, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Eller LK, Reimer RA. A high calcium, skim milk powder diet results in a lower fat mass in male, energy-restricted, obese rats more than a low calcium, casein, or soy protein diet. J Nutr 140: 1234–1241, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Faipoux R, Tome D, Gougis S, Darcel N, Fromentin G. Proteins activate satiety-related neuronal pathways in the brainstem and hypothalamus of rats. J Nutr 138: 1172–1178, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Fromentin G, Darcel N, Chaumontet C, Marsset-Baglieri A, Nadkarni N, Tome D. Peripheral and central mechanisms involved in the control of food intake by dietary amino acids and proteins. Nutr Res Rev 25: 29–39, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord 16: 397–415, 1992 [PubMed] [Google Scholar]
- 22.Halton TL, Hu FB. The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review. J Am Coll Nutr 23: 373–385, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Hameed S, Dhillo WS, Bloom SR. Gut hormones and appetite control. Oral Dis 15: 18–26, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Hariri N, Thibault L. High-fat diet-induced obesity in animal models. Nutr Res Rev 23: 270–299, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Hevener AL, Febbraio MA. The 2009 stock conference report: inflammation, obesity and metabolic disease. Obes Rev 11: 635–644, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Holes-Lewis KA, Malcolm R, O'Neil PM. Pharmacotherapy of obesity: clinical treatments and considerations. Am J Med Sci 345: 284–288, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Homma T, Fujisawa M, Arai K, Ishii M, Sada T, Ikeda M. Spironolactone, but not eplerenone, impairs glucose tolerance in a rat model of metabolic syndrome. J Vet Med Sci 74: 1015–1022, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Jean C, Rome S, Mathe V, Huneau JF, Aattouri N, Fromentin G, Achagiotis CL, Tome D. Metabolic evidence for adaptation to a high-protein diet in rats. J Nutr 131: 91–98, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Kim JH, Park Y, Kim D, Park Y. Dietary influences on nonexercise physical activity and energy expenditure in C57BL/6J mice. J Food Sci 77: H63–H68, 2012 [DOI] [PubMed] [Google Scholar]
- 30.L'Heureux-Bouron D, Tome D, Bensaid A, Morens C, Gaudichon C, Fromentin G. A very high 70%-protein diet does not induce conditioned taste aversion in rats. J Nutr 134: 1512–1515, 2004 [DOI] [PubMed] [Google Scholar]
- 31.L'Heureux-Bouron D, Tome D, Bensaid A, Morens C, Lacroix M, Huneau JF, Fromentin G. Preabsorptive factors are not the main determinants of intake depression induced by a high-protein diet in the rat. Physiol Behav 81: 499–504, 2004 [DOI] [PubMed] [Google Scholar]
- 32.L'Heureux-Bouron D, Tome D, Rampin O, Even PC, Larue-Achagiotis C, Fromentin G. Total subdiaphragmatic vagotomy does not suppress high protein diet-induced food intake depression in rats. J Nutr 133: 2639–2642, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Lacroix M, Gaudichon C, Martin A, Morens C, Mathe V, Tome D, Huneau JF. A long-term high-protein diet markedly reduces adipose tissue without major side effects in Wistar male rats. Am J Physiol Regul Integr Comp Physiol 287: R934–R942, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Latner JD, Schwartz M. The effects of a high-carbohydrate, high-protein or balanced lunch upon later food intake and hunger ratings. Appetite 33: 119–128, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Levin BE, Keesey RE. Defense of differing body weight set points in diet-induced obese and resistant rats. Am J Physiol Regul Integr Comp Physiol 274: R412–R419, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Liew M, Groll MC, Thompson JE, Call SL, Moser JE, Hoopes JD, Voelkerding K, Wittwer C, Spendlove RS. Validating a custom multiplex ELISA against individual commercial immunoassays using clinical samples. Biotechniques 42: 327–328, 330–323, 2007 [DOI] [PubMed] [Google Scholar]
- 37.McArthur LH, Kelly WF, Gietzen DW, Rogers QR. The role of palatability in the food intake response of rats fed high-protein diets. Appetite 20: 181–196, 1993 [DOI] [PubMed] [Google Scholar]
- 38.Mithieux G. [Glucose sensing: from gut to brain]. Bull Acad Natl Med 191: 911–920 discussion 920–911, 2007 [PubMed] [Google Scholar]
- 39.Morita S, Miyata S. Accessibility of low-molecular-mass molecules to the median eminence and arcuate hypothalamic nucleus of adult mouse. Cell Biochem Funct, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Olsson A, Vanderstichele H, Andreasen N, De Meyer G, Wallin A, Holmberg B, Rosengren L, Vanmechelen E, Blennow K. Simultaneous measurement of beta-amyloid(1–42), total tau, and phosphorylated tau (Thr181) in cerebrospinal fluid by the xMAP technology. Clin Chem 51: 336–345, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Pittner RA, Albrandt K, Beaumont K, Gaeta LS, Koda JE, Moore CX, Rittenhouse J, Rink TJ. Molecular physiology of amylin. J Cell Biochem 55 Suppl: 19–28, 1994 [DOI] [PubMed] [Google Scholar]
- 42.Potteiger JA, Jacobsen DJ, Donnelly JE. A comparison of methods for analyzing glucose and insulin areas under the curve following nine months of exercise in overweight adults. Int J Obes Relat Metab Disord 26: 87–89, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Powley TL, Chi MM, Schier LA, Phillips RJ. Obesity: should treatments target visceral afferents? Physiol Behav 86: 698–708, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Rubin NH, Alinder G, Rietveld WJ, Rayford PL, Thompson JC. Restricted feeding schedules alter the circadian rhythms of serum insulin and gastric inhibitory polypeptide. Regul Pept 23: 279–288, 1988 [DOI] [PubMed] [Google Scholar]
- 45.Salvante KG, Brindle E, McConnell D, O'Connor K, Nepomnaschy PA. Validation of a new multiplex assay against individual immunoassays for the quantification of reproductive, stress, and energetic metabolism biomarkers in urine specimens. Am J Hum Biol 24: 81–86, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Semon BA, Leung PM, Rogers QR, Gietzen DW. Effect of type of protein on food intake of rats fed high protein diets. Physiol Behav 41: 451–458, 1987 [DOI] [PubMed] [Google Scholar]
- 47.Soenen S, Westerterp-Plantenga MS. Proteins and satiety: implications for weight management. Curr Opin Clin Nutr Metab Care 11: 747–751, 2008 [DOI] [PubMed] [Google Scholar]
- 48.St Jeor ST, Howard BV, Prewitt TE, Bovee V, Bazzarre T, Eckel RH. Dietary protein and weight reduction: a statement for healthcare professionals from the Nutrition Committee of the Council on Nutrition, Physical Activity, and Metabolism of the American Heart Association. Circulation 104: 1869–1874, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Stengel A, Coskun T, Goebel-Stengel M, Craft LS, Alsina-Fernandez J, Wang L, Rivier J, Taché Y. Chronic injection of pansomatostatin agonist ODT8-SST differentially modulates food intake and decreases body weight gain in lean and diet-induced obese rats. Regul Pept 167: 201–208, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stengel A, Goebel-Stengel M, Wang L, Shaikh A, Lambrecht NW, Rivier JE, Taché YF. Abdominal surgery inhibits circulating acyl ghrelin and ghrelin-O-acyltransferase levels in rats: role of the somatostatin receptor subtype 2. Am J Physiol Gastrointest Liver Physiol 301: G239–G248, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thorens B. Glucagon-like peptide-1 and control of insulin secretion. Diabetes Metab 21: 311–318, 1995 [PubMed] [Google Scholar]
- 52.Thorpe KE, Florence CS, Howard DH, Joski P. The impact of obesity on rising medical spending. Health Aff (Millwood) Suppl Web Exclusives: W4-480–486, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Vidal J. Updated review on the benefits of weight loss. Int J Obes Relat Metab Disord 26 Suppl 4: S25–S28, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 378: 815–825, 2011 [DOI] [PubMed] [Google Scholar]
- 55.Weigle DS, Breen PA, Matthys CC, Callahan HS, Meeuws KE, Burden VR, Purnell JQ. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am J Clin Nutr 82: 41–48, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Weisburger JH. Dietary fat and risk of chronic disease: mechanistic insights from experimental studies. J Am Diet Assoc 97: S16–S23, 1997 [DOI] [PubMed] [Google Scholar]
- 57.Westerterp-Plantenga MS, Rolland V, Wilson SA, Westerterp KR. Satiety related to 24 h diet-induced thermogenesis during high protein/carbohydrate vs high fat diets measured in a respiration chamber. Eur J Clin Nutr 53: 495–502, 1999 [DOI] [PubMed] [Google Scholar]
- 58.Westerterp KR, Wilson SA, Rolland V. Diet induced thermogenesis measured over 24 h in a respiration chamber: effect of diet composition. Int J Obes Relat Metab Disord 23: 287–292, 1999 [DOI] [PubMed] [Google Scholar]
- 59.Whitehead JM, McNeill G, Smith JS. The effect of protein intake on 24-h energy expenditure during energy restriction. Int J Obes Relat Metab Disord 20: 727–732, 1996 [PubMed] [Google Scholar]
- 60.Woods SC. The control of food intake: behavioral versus molecular perspectives. Cell Metab 9: 489–498, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woods SC. Gastrointestinal satiety signals. I. An overview of gastrointestinal signals that influence food intake. Am J Physiol Gastrointest Liver Physiol 286: G7–G13, 2004 [DOI] [PubMed] [Google Scholar]
- 62.Zhao K, Ao Y, Harper RM, Go VL, Yang H. Food-intake dysregulation in type 2 diabetic Goto-Kakizaki rats: Hypothesized role of dysfunctional brainstem thyrotropin-releasing hormone and impaired vagal output. Neuroscience 247C: 43–54, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]