Abstract
In addition to effects on appetite and metabolism, leptin influences many neuroendocrine and physiological systems, including the sympathetic nervous system. Building on my Carl Ludwig Lecture of the American Physiological Society, I review the sympathetic and cardiovascular actions of leptin. The review focuses on a critical analysis of the concept of selective leptin resistance (SLR) and the role of leptin in the pathogenesis of obesity-induced hypertension in both experimental animals and humans. We introduced the concept of SLR in 2002 to explain how leptin might increase blood pressure (BP) in obese states, such as diet-induced obesity (DIO), that are accompanied by partial leptin resistance. This concept, analogous to selective insulin resistance in the metabolic syndrome, holds that in several genetic and acquired models of obesity, there is preservation of the renal sympathetic and pressor actions of leptin despite attenuation of the appetite and weight-reducing actions. Two potential overlapping mechanisms of SLR are reviewed: 1) differential leptin molecular signaling pathways that mediate selective as opposed to universal leptin action and 2) brain site-specific leptin action and resistance. Although the phenomenon of SLR in DIO has so far focused on preservation of sympathetic and BP actions of leptin, consideration should be given to the possibility that this concept may extend to preservation of other actions of leptin. Finally, I review perplexing data on the effects of leptin on sympathetic activity and BP in humans and its role in human obesity-induced hypertension.
Keywords: sympathetic, blood pressure, renal, adipose tissue, renin-angiotensin system
the discovery of leptin by Jeffrey Friedman and colleagues in 1994 (161) revolutionized the understanding of the biology of appetite, metabolism, and obesity. Although the initial focus was on the role of leptin in the neurobiological regulation of appetite, metabolism and fat mass, it quickly became apparent that leptin is a highly pleiotropic hormone, influencing many neuroendocrine and physiological systems (38, 40).
Obesity-induced hypertension is multifactorial. For many years, the focus was on the role of renal mechanisms, hyperinsulinemia, the renin-angiotensin system, and the sympathetic nervous system (50, 71). In the past two decades, there has been increasing interest in the role of leptin and melanocortins in obesity-induced sympathoexcitation and hypertension (1, 22, 58, 119). More recently, brain oxidative stress and endoplasmic reticulum stress have been implicated as mediators of sympathetic activation and hypertension in obesity (104, 113, 114).
In this review, I address the sympathetic and cardiovascular actions of leptin in experimental animals and humans and the concept of selective leptin resistance (SLR) in obesity-induced hypertension. This review emanated from my Carl Ludwig Lecture of the Neural Control and Autonomic Regulation Section of the American Physiological Society.
Sympathetic and Blood Pressure Actions of Leptin
Shortly after the discovery of leptin, we (58) and others (29, 78, 88, 132) identified leptin-induced sympathetic nerve activation in experimental animals. Our studies were prompted by recognition that thermogenic metabolism in interscapular brown adipose tissue (BAT) is sympathetically mediated (19). It was not surprising, therefore, that leptin increased sympathetic nerve activity (SNA) and uncoupling protein 1 (UCP1) in BAT (58, 132) and white adipose tissue (WAT) (111, 123). The surprising finding was that leptin also increased SNA to the kidney, adrenal gland, and hindlimb, (29, 58, 78, 88), and it has subsequently been shown to regulate sympathetic activity to liver (153), bone (31), and the splanchnic circulation (75). The SNA responses to leptin were absent in obese Zucker rats and db/db mice with loss-of-function mutations in leptin receptors (LepR), indicating that SNA responses to leptin are receptor-mediated (58, 117). Thus, in experimental animals, leptin participates in the regulation of sympathetic activity to a range of tissues and organs.
Early studies indicated that leptin increased regional SNA by stimulating LepR in the brain. For example, cerebroventricular administration of leptin reproduced the regional SNA responses seen with systemic administration and electrolytic lesioning of the arcuate nucleus abolished BAT SNA responses to systemic injection of leptin (59). Many further studies have reinforced the view that the sympathoexcitatory actions of leptin result from stimulation of LepR in the central nervous system.
There is, however, growing evidence for an additional mechanism—the so-called adipose afferent reflex—that is garnering support as a contributor to renal sympathoexcitation and hypertension in rats with diet-induced obesity (DIO) (106, 107, 137, 155). In this reflex, leptin triggers afferent impulses originating in WAT that act via a central neural pathway involving the paraventricular nucleus to trigger increases in renal SNA and blood pressure (BP). Augmented activity of this reflex contributes to increases in renal SNA and BP in hypertensive diet-induced obese rats (155), as judged by the response to injection of a leptin antagonist into WAT or sensory denervation of WAT. These studies are likely to prompt a reassessment of the mechanisms of the sympathetic actions of leptin by suggesting that peripheral actions of leptin may be an additional trigger.
Leptin and blood pressure.
The leptin-induced increases in SNA to the kidney, adrenal gland, and hindlimb suggested that leptin might be involved in the regulation of BP. This soon received support from reports that administration of leptin increased BP (15, 16, 29, 78, 88, 136). For example, Shek et al. (136) reported that chronic leptin infusion (associated with plasma levels of the hormone within the pathophysiological range) increased BP in conscious rats. These increases were prevented with combined α- and β-adrenergic blockade (15).
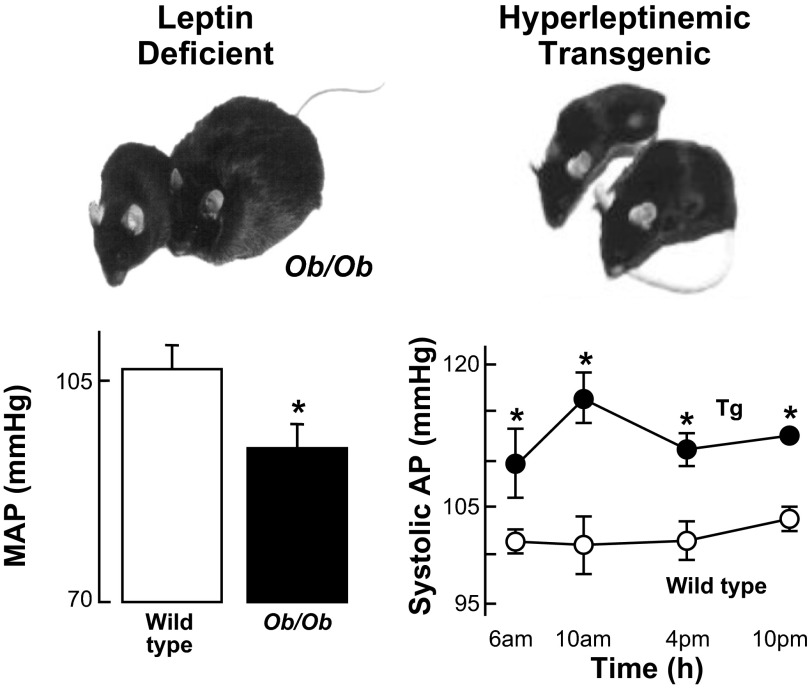
At this point, a key question was whether leptin was involved in the regulation of SNA and BP in physiological and pathophysiological states. In 1999, we reported that severely obese, leptin-deficient ob/ob mice had significantly lower BP than their lean littermate controls (81; Fig. 1, left). Concurrently, Aizawa-Abe et al. (1) described two key findings (Fig. 1, right). First, in leptin-deficient ob/ob mice, infusion of leptin for 3 days increased BP. Second, transgenic skinny mice overexpressing leptin from a liver promoter had increases in systolic BP and urinary catecholamines despite decreased adiposity. The elevated BP was abolished by adrenergic or ganglionic blockade at doses with no effect in nontransgenic littermates.
Fig. 1.
Left: data for mean arterial pressure (MAP) in wild-type vs. obese, leptin-deficient ob/ob mice (81). Right: systolic arterial pressure (AP) in wild-type vs. skinny, hyperleptinemic transgenic mice overexpressing leptin from a liver promoter (1). Obese leptin-deficient mice had lower AP than wild-type controls, whereas skinny hyperleptinemic mice had higher AP than wild-type controls. Tg, transgenic. *P < 0.05. [Adapted from Aizawa-Abe et al. (1) and Mark et al. (81).].
Studies of rabbits from Geoffrey Head's laboratory have provided interesting insights into the role of leptin in hypertension associated with DIO in rabbits (4, 76, 112). In rabbits fed a high-fat diet (HFD) for only 1 wk, there were increases in plasma insulin and leptin, renal SNA, BP, and body weight. With resumption of normal diet, plasma leptin and insulin returned to control, but body weight, BP, and renal SNA remained elevated (4). The investigators suggested that leptin and insulin contribute to increases in BP during a high-fat diet, in part, through “alterations in the response of neurons in the hypothalamus” (4). In support of this, cerebroventricular administration of leptin in rabbits fed HFD for 4 wk produced exaggerated increases in renal SNA response in the diet-induced obese vs. control rabbits, but surprisingly, leptin-induced c-Fos activation in virtually every region examined was lower in diet-induced obese vs. control rabbits (112). The explanation and significance of this intriguing finding—increased renal SNA responses to leptin despite decreased leptin-induced activation of c-Fos—are unclear.
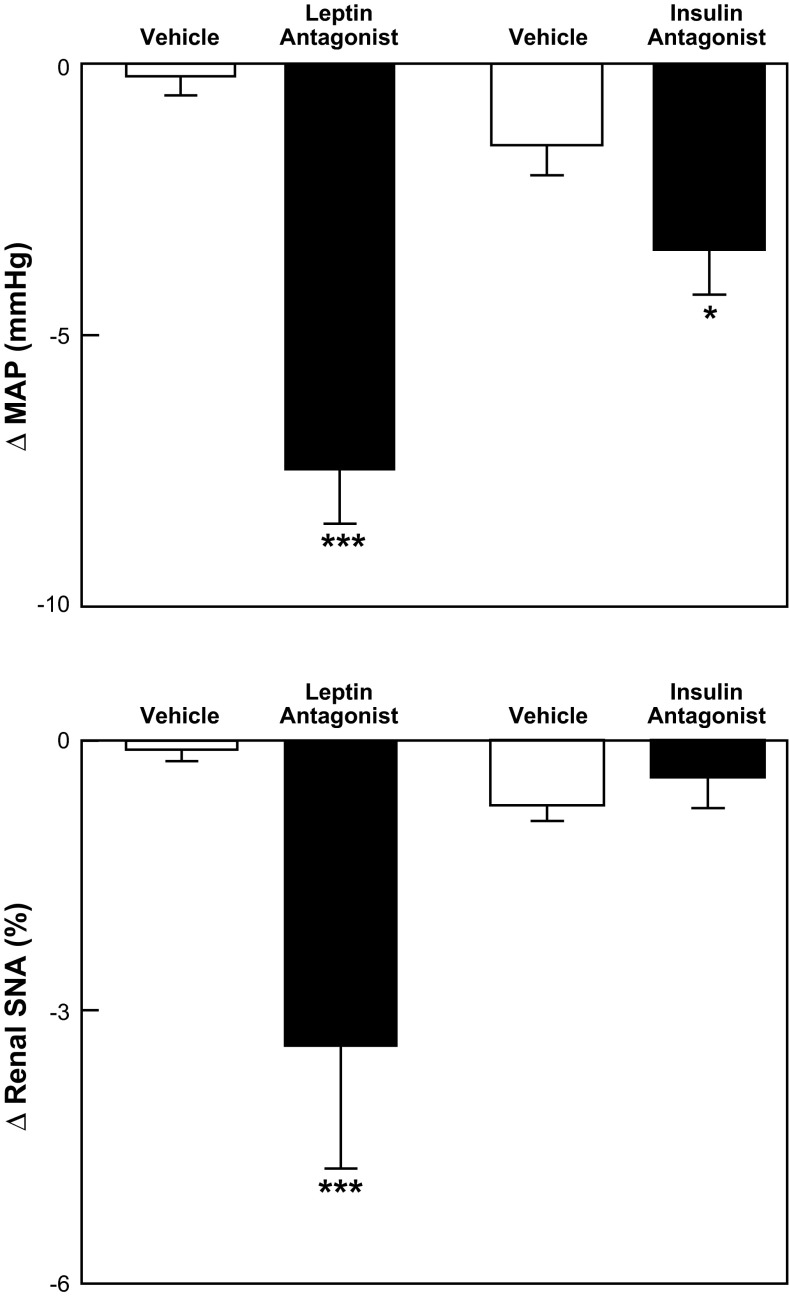
Using a leptin antagonist, these investigators recently reported that leptin plays a major role in DIO-induced renal sympathetic activation and hypertension (76). Specifically, after 3 wk of HFD, cerebroventricular administration of the leptin antagonist significantly decreased mean arterial pressure (MAP) by 9% and renal SNA by 17% (Fig. 2). Decreases in BP with an insulin antagonist were appreciably smaller than those with the leptin antagonist and were not accompanied by decreases in renal SNA (Fig. 2). The investigators concluded that “the elevation of blood pressure and renal SNA induced by HFD (in rabbits) is predominantly mediated by central actions of leptin” (69).
Fig. 2.
Changes in MAP (top) and renal sympathetic nerve activity (SNA; bottom) in diet-induced obese hypertensive rabbits after cerebroventricular administration of vehicle, a leptin antagonist, or an insulin antagonist (76). The leptin antagonist produced significant (***P < 0.001) decreases in MAP and renal SNA, whereas the insulin antagonist produced a smaller decrease in MAP (*P < 0.05) and did not decrease renal SNA.
A study by Tümer et al. (148) employing a leptin antagonist has been cited as challenging the role of leptin in DIO-induced hypertension. In lean rats, hypothalamic overexpression of leptin produced an increase in BP that was reversed by a 14-day central neural infusion of a rat leptin antagonist. Rats with DIO produced by a high-fat diet for 5 mo had increased BP and tyrosine hydroxylase activity (an index of sympathetic neural drive). Central neural infusion of the leptin antagonist in the diet-induced obese rats reversed the increase in tyrosine hydroxylase, supporting the concept of augmented leptin-induced sympathoexcitation in DIO. In contrast, the leptin antagonist did not lower BP in diet-induced obese rats. At first glance, this challenges the view that leptin contributes to hypertension in DIO, but the leptin antagonist produced accelerated increases in food intake, adiposity, and body weight in the diet-induced obese rats. Accelerated hyperphagia, adiposity and weight gain in the diet-induced obese rats during the leptin antagonist would be expected to increase BP and may have masked a depressor response to loss of leptin action. In this regard, the study by Lim et al. (76), discussed above, focused on the acute responses to the leptin antagonist and avoided the potentially confounding effect of accelerated adiposity and weight gain on BP during administration of the leptin antagonist. Another factor that may have contributed to the difference in the response to the leptin antagonist in diet-induced obese animals in the studies by Tümer et al. (148) and Lim et al. (76) was the duration of the high-fat diet. Rats received the leptin antagonist after 5 mo on a high-fat diet in the study by Tümer et al. (148), whereas rabbits received the antagonist at 1 and 3 wk of high-fat diet in the study of Lim et al. (76). The contribution of leptin to DIO-induced hypertension might vary at different stages of hypertension.
I conclude that the body of evidence supports a role for leptin in the regulation of BP and in the pathogenesis of DIO-associated hypertension in experimental animals.
The Concept of Selective Leptin Resistance
In 1999, we reported that moderately obese, hyperleptinemic agouti yellow obese mice had higher BP than their lean controls in contrast to a lower BP in severely obese, leptin-deficient ob/ob mice (81). In related findings, Aizawa-Abe et al. (1) reported that in moderately obese, hyperleptinemic transgenic mice overexpressing agouti peptide, there was elevation in urinary catecholamine excretion and BP. Using an imaginative protocol, these investigators demonstrated that the increased BP in the agouti mice was dependent on elevated leptin. Specifically, with caloric restriction in the agouti mice, body weight, plasma leptin and BP decreased, but when plasma leptin was maintained at elevated levels during caloric restriction, BP remained elevated despite weight loss (1).
The finding of contrasting BP responses to obesity in leptin-deficient ob/ob vs. hyperleptinemic agouti obese mice prompted the concept that the BP response to obesity may be determined by the genetic and neurobiological mechanisms underlying the obesity (81, 82).
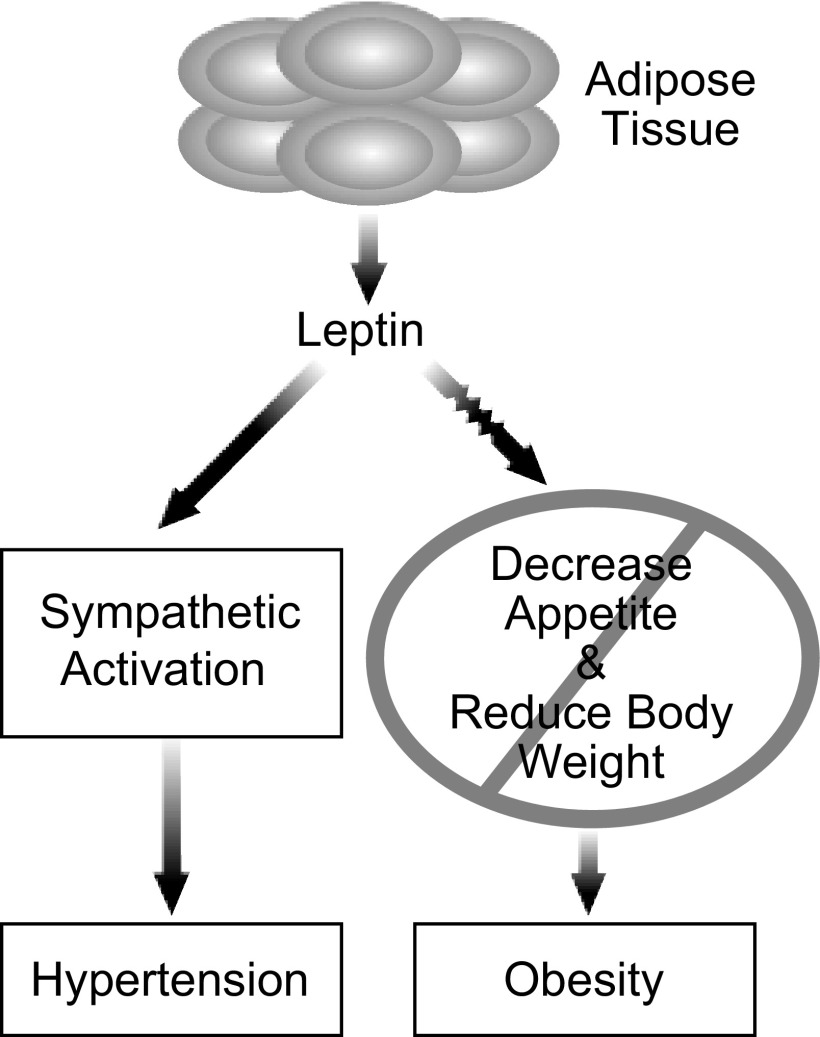
The studies of agouti mice by Aizawa-Abe et al. (1) also raised a paradox that led us to introduce the concept of SLR. Their studies demonstrated that hyperleptinemia was contributing to the elevated BP in agouti mice (1), but it was known that agouti obese mice are resistant to the food intake and body weight effects of leptin (48). To address this paradox, we asked how leptin could contribute to the elevated BP in agouti mice if the mice were resistant to leptin? We were drawn to an analogy with insulin resistance in the metabolic syndrome. In the metabolic syndrome, resistance to insulin is selective and not uniform (14, 70, 125, 126). There is resistance to several favorable actions of insulin but preservation of some detrimental actions of insulin, including sympathetic activation. On the basis of this analogy, we advanced the concept that in some forms of obesity, there may be resistance to the favorable satiety and weight-reducing actions of leptin but preservation of renal sympathetic and perhaps other actions of leptin that could contribute to obesity-induced hypertension (Ref. 83) (Fig. 3).
Fig. 3.
Schematic diagram depicting the concept of selective leptin resistance.
There is some evidence that physiological alterations in leptin sensitivity in Siberian hamsters may involve selective changes in leptin signaling pathways and action. This suggests that selectivity in leptin signaling and actions may have evolved to accommodate physiological needs (26, 149).
In our initial test of SLR, Correia et al. (20) observed that agouti obese mice were resistant to the food intake and body weight effects of systemic administration of leptin, but had preservation of leptin-induced renal sympathetic activation, supporting the concept of SLR (83). We subsequently obtained similar findings with cerebroventricular administration of leptin in agouti obese mice (115).
In subsequent studies, we also found evidence for SLR in diet-induced obese mice with preservation of leptin-induced increases in renal SNA and BP despite resistance to the anorexic and weight-reducing actions of leptin (119).
We considered the possibility that partial saturation of leptin transport across the blood-brain barrier (BBB) contributed to SLR in diet-induced obese mice. There is partial saturation of leptin transport across the BBB, as obesity progresses, but this does not explain the selectivity of leptin resistance in DIO. For example, Morgan et al. (95) reported that after 20 wk of high-fat diet in mice, there was partial saturation of BBB leptin transport. Nevertheless, cerebrospinal fluid (CSF) leptin levels were elevated. In addition, the renal SNA response to cerebroventricular administration of leptin was preserved, whereas the food intake, body weight, and BAT SNA responses were attenuated. Thus, despite partial saturation of leptin transport across the BBB, the elevated CSF levels and selectivity of central neural leptin actions provide the substrate for the phenomenon of SLR in DIO.
Additional findings in our studies of diet-induced obese mice and rats merit comment. Although there was preservation of the renal SNA response to leptin in the diet-induced obese mice, there was an attenuation of the BAT SNA response (119). The attenuation of leptin-induced increases in BAT SNA in diet-induced obese mice is consistent with an earlier study from our laboratory in rats (56). In lean Sprague-Dawley rats, leptin potentiated hypothermia-induced increases in thermogenic BAT SNA. This potentiation was absent in diet-induced obese rats. At first glance, these findings might seem to argue against the concept of SLR, since not all sympathetic responses to leptin were preserved. As a counter argument, we submit that attenuation of the BAT SNA response to leptin with preservation of the renal SNA response does not undermine the concept of SLR (119). BAT SNA is part of the “metabolic” response, not the cardiovascular response (renal SNA and BP), to leptin. The sympathetic nervous system is highly differentiated. SNA to BAT subserves metabolic function (98). Conversely, renal SNA subserves renal and cardiovascular function (24). Thus, in our study of diet-induced obese mice, there was metabolic leptin resistance with attenuation of the satiety, body weight, and BAT SNA responses to leptin, but preservation of the cardiovascular renal SNA and BP responses to leptin, i.e., the phenomenon of SLR.
In contrast to our findings, Enriori et al. (35), using interscapular BAT temperature as a marker of sympathetic activity, reported preservation of BAT sympathetic thermogenesis in diet-induced obese mice. Renal SNA and BP were not measured. The leptin-induced increase in BAT temperature was comparable in diet-induced obese and control mice, despite diet-induced obese mice being resistant to the anorectic action of leptin. The investigators concluded that the preservation of leptin-induced BAT sympathetic thermogenesis despite attenuation of the anorectic action represented selective leptin resistance and further concluded that “selective leptin resistance may be a crucial mechanism linking adiposity and metabolic syndrome.” The investigators demonstrated that the leptin-induced increases in BAT temperature emanate from LepRb neurons in the dorsomedial hypothalamus (DMH). As discussed later, the DMH appears to be importantly involved in the regulation of BAT sympathetic thermoregulatory circuits (162), but microinjection of leptin into the DMH fails to increase renal SNA (85). I cannot explain the differences in leptin-induced regulation of BAT sympathetic activity in the study by Enriori et al. (35) and those from our laboratories (56, 119), except that our two groups employed different methods for assessing sympathetic activity to BAT. Nevertheless, as discussed above, the studies from both laboratories support the concept of SLR.
Rahmouni et al. (121) performed studies of obesity-induced hypertension in mouse models of Bardet-Biedl syndrome (BBS) that provided further support for the role of SLR in obesity-induced hypertension. BBS is a rare form of syndromic, monogenic obesity that is often, but not always, accompanied by hypertension (94). Rahmouni reported studies on three different mouse models of BBS-mice with knockout of either Bbs 2, Bbs 4, or Bbs 6 genes. All three models were obese, hyperleptinemic, and resistant to the satiety and weight-reducing actions of leptin, i.e., metabolic leptin resistance.
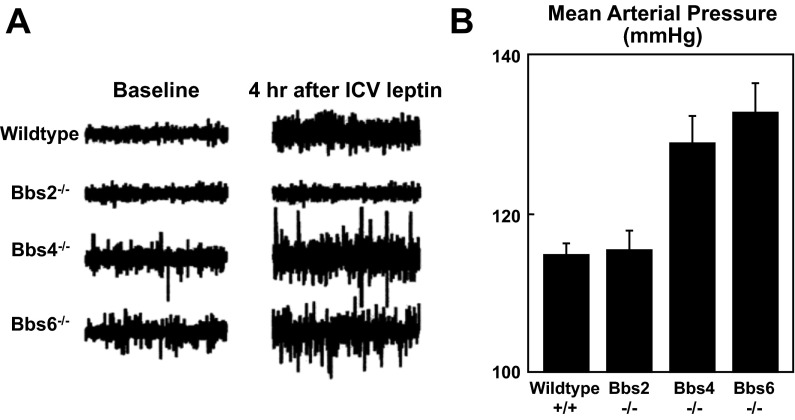
While all three models were obese and hyperleptinemic, the BP responses to obesity were different in the three models (121). Bbs 4 and Bbs 6 knockout mice had hypertension, but Bbs 2 knockout mice did not (Fig. 4). Of even greater interest, Bbs 4 and Bbs 6 knockout mice had preserved renal SNA responses to leptin, elevated baseline renal SNA, and exaggerated depressor responses to ganglionic blockade, indicating neurogenic hypertension (Fig. 4). In addition, the elevated baseline renal SNA and BP in the Bbs 4 and Bps 6 knockout mice were reversed when the endogenous hyperleptinemia was suppressed by fasting, whereas the elevated BP was maintained when hyperleptinemia was sustained by leptin treatment in the fasted mice. This strengthens the concept that endogenous hyperleptinemia is sufficient to increase renal SNA and BP in mice with SLR. In contrast to the Bbs 4 and Bbs 6 knockout mice, the Bbs 2 knockout mice had normal baseline renal SNA and loss of the renal SNA response to leptin and were normotensive (Fig. 4).
Fig. 4.
Evidence that selective leptin resistance (SLR) contributes to obesity-induced hypertension in mouse models of Bardet-Biedl syndrome (Bbs) (121). A: recordings of renal SNA at baseline and 4 h after cerebroventricular administration of leptin, 5 μg in Bbs 2, Bbs 4, and Bbs 6 knockout and in wild-type mice. Compared with wild-type mice, Bbs 4 and Bbs 6 knockout mice had elevated baseline renal SNA and preserved renal SNA responses to leptin. Bbs 2 knockout mice had normal baseline renal SNA and loss of renal SNA responses to leptin. B: baseline values for mean arterial pressure in the wild-type and Bbs 2, Bbs 4, and Bbs 6 knockout mice. Bbs 2 knockout mice were normotensive, whereas Bbs 4 and Bbs 6 knockouts were hypertensive.
In other words, in the two models (Bbs 4 and Bbs 6) with SLR, there was leptin-induced neurogenic hypertension. In the model (Bbs 2) that had uniform leptin resistance with loss of the renal SNA, as well as metabolic effects of leptin, the mice were normotensive. The observation that Bbs 4 and Bbs 6 mice are hypertensive but Bbs 2 mice are normotensive parallels findings in humans in which patients with BBS 4 and BBS 6 mutations are hypertensive but those with BBS 2 mutations are not (94). Although physiological data relating to sympathetic activity are lacking in the patients, this parallel between hypertension in the mouse and human genotypes suggests that hyperleptinemia accompanied by SLR has a human counterpart.
Thus, in several monogenic mouse models of obesity and hypertension and in acquired DIO and hypertension in mice and rabbits, there is evidence of SLR. This concept has been met with support (68, 138), but also with skepticism (102). In a review that was broadly critical of the concept of leptin resistance, Myers et al. (102) argued against meaningful SLR in DIO. The authors suggested that the redundant and overlapping pathways and sites of leptin action make it unlikely that there could be a process that might interfere with some, but not other, actions of leptin in DIO. This argument may have been tenable in 2002 when the concept of SLR was introduced, but there is now abundant and mounting evidence for differential leptin molecular signaling pathways that mediate some, but not all, leptin actions and for brain site-specific leptin action. These advances, discussed below, provide potential mechanisms for SLR.
Potential Mechanisms of SLR
For several years after the discovery of leptin, the prevailing view was that the long signaling form of the leptin receptor (LepRb) occurred primarily in the hypothalamus, notably the arcuate nucleus (39, 49, 72) and that the JAK-STAT3 pathway mediated leptin actions (150). As this study focused on one brain region and one transduction pathway, this evidence did not provide likely neuroanatomical or molecular mechanisms for selective leptin action or resistance. In the past fifteen years, substantial evidence has accrued for 1) differential leptin molecular signaling pathways and 2) a distributed brain network of leptin receptors with site-specific leptin action. These advances provide potential molecular and neuroanatomical mechanisms for SLR.
In discussing these issues, it is important to understand that the sympathetic nervous system is not a uniform system. As mentioned previously, it is a highly differentiated system, with profound differences in both the control mechanisms and function of SNA to distinct regional tissues and organs. There may be qualitative, as well as quantitative differences, in SNA to different tissue and organs. For example, sophisticated methods have demonstrated that sympathetic activity to skeletal muscle and kidney is increased in obese humans, but surprisingly, sympathetic activity to the heart is decreased (43, 133, 151). A striking example of this exquisite selectivity in regulation of SNA to distinct tissues and organs is evidence of differential PI3K regulation of SNA to white vs. brown adipose tissue (111, 122). An understanding of the sympathetic actions of leptin and the concept of SLR necessitates recognition of this exquisite regional differentiation in the sympathetic nervous system.
Multiple, differential leptin molecular signaling pathways.
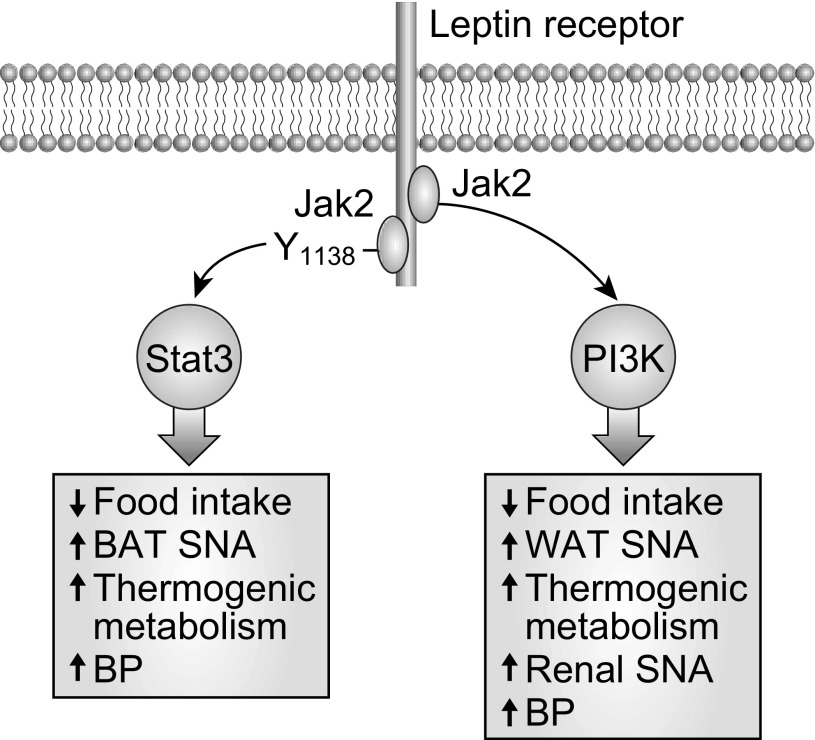
The identification of the leptin STAT3 pathway was followed by the discovery of other leptin signaling pathways, including those for phosphoinositol-3 kinase (PI3K) (108) and ERK/MAPK (6, 10, 11, 146). The delineation of STAT3, PI3K, and ERK/MAPK in leptin signaling has led to recognition of pathway-specific leptin action and has had major implications for understanding the sympathetic actions of leptin.
The most likely explanation for the renal sympathetic and BP actions of leptin and for selective leptin action and resistance has focused on arcuate proopiomelanocortin (POMC) neurons and PI3K signaling, but as discussed later, there is also evidence that leptin STAT3 signaling may contribute to the pressor actions of leptin independent of renal sympathetic activation. With a focus on the sympathetic and BP actions of leptin signaling pathways, I begin with comments on the role of arcuate POMC neurons. Before doing so, however, it should be mentioned that in addition to stimulating anorexogenic POMC neurons, leptin also acts to inhibit orexigenic neuropeptide Y-containing (NPY) neurons in arcuate nucleus. Cowley et al. (21) described a neuronal interaction between arcuate POMC and NPY neurons such that in addition to its direct effects on POMC neurons, leptin also stimulates POMC neurons indirectly by inhibiting NPY neurons. This interaction has potential but undefined significance in the sympathetic actions of leptin.
Proopiomelanocortin neurons in leptin signaling.
There are POMC neurons in both the arcuate nucleus and the nucleus of the solitary tract (NTS). There is convincing evidence for LepR neurons and leptin signaling in the NTS, but surprisingly, POMC neurons in the hindbrain do not appear to be involved in leptin action (42, 63). Thus, leptin signaling in POMC neurons appears restricted to the arcuate nucleus. There are distinct or separate arcuate POMC neurons for leptin receptors, insulin receptors, and serotonin receptors (61, 144, 154, 156). As stated by Williams et al. (154), the cross talk between leptin and insulin (and presumably serotonin) appears to occur between different neurons rather than within individual neurons.
After the discovery of leptin, it was thought that the arcuate nucleus was the major site for the effects of leptin on food intake and body weight, but in 2004 Balthasar et al. (5) reported that, surprisingly, POMC neurons had only modest effects on body weight and food intake. do Carmo et al. (25) reported that mice lacking LepR in POMC neurons (LepRflox/flox/POMC-Cre mice) were hyperglycemic, and hyperinsulinemic compared with control mice, but did not manifest the hypertension expected with the metabolic syndrome. Deletion of LepR in POMC neurons also prevented leptin-induced increases in BP and decreases in blood glucose. These investigators concluded that POMC neurons are critical for the BP and blood glucose responses to leptin, but do not play a major role in the appetite suppressant or body weight actions of leptin (25).
Leptin-PI3K signaling.
A landmark in understanding leptin signaling was the 2001 report by Niswender et al. (108) that PI3K is a crucial enzyme in the signal transduction pathway linking hypothalamic leptin to reduced food intake. Systemic administration of leptin in intact rats rapidly increased hypothalamic insulin receptor substrate-2 associated PI3K activity (Fig. 5). Wortmannin and LY294002, pharmacologic inhibitors of PI3K, inhibited leptin-induced decreases in food intake, but did not inhibit the anorexic responses to stimulation of melanocortin receptors (108). These findings led to an avalanche of studies on the role of PI3K in leptin actions using pharmacologic inhibitors and genetically modified mouse models.
Fig. 5.
Schematic diagram depicting several leptin-STAT3 and leptin-PI3K-mediated actions with emphasis on the differential regional SNA actions. STAT3 mediates increases in brown adipose tissue (BAT) SNA, whereas PI3K increases white adipose tissue (WAT) SNA but not BAT SNA. PI3K mediates leptin-induced increases in renal SNA, whereas STAT3 does not. Nevertheless, both STAT3 and PI3K mediate leptin-induced increases in blood pressure.
We presented the first evidence that PI3K was critically involved in the renal sympathetic responses to leptin (116). Cerebroventricular administration of pharmacologic inhibitors of PI3K attenuated the renal SNA responses to leptin but not to stimulation of melanocortin 3/4 receptors with MTII. In a subsequent study, we demonstrated that while PI3K antagonists prevented renal SNA responses to leptin, they did not attenuate leptin-induced increases in BAT SNA (122). This illustrates the concept that a leptin molecular signaling pathway can mediate selective leptin actions, including some (renal), but not all (BAT), sympathetic actions of leptin. There is even more to the story of PI3K and regulation of regional SNA. Although PI3K does not mediate leptin-induced increases in SNA to thermogenic BAT (122), it increases SNA to WAT and promotes “browning” of WAT (111, 123). This is intriguing because PI3K in LepR neurons appears to regulate adiposity primarily by modulating energy expenditure, not energy intake (111). The evidence that leptin-induced PI3K signaling increases SNA to WAT but not to BAT is a striking example of the extraordinary selectivity of leptin signaling pathways.
A recent study from Rahmouni's laboratory (54) strengthens the concept that PI3K signaling contributes importantly to the renal sympathetic and BP actions of leptin. The investigators employed two mouse models with bidirectional changes in PI3K function-one model with PI3K gain of function produced by ablation of PTEN in LepR neurons and another with PI3K loss of function caused by a heterozygous mutation in the p110α subunit of PI3K. The mice with PI3K gain of function in LepR neurons were hypertensive and had exaggerated leptin-induced increases in renal SNA and BP. Mice with PI3K loss of function had blunted increases in renal SNA and BP in response to leptin.
Leptin-STAT3 signaling.
Mice with genetically engineered disruption of leptin-STAT3 signaling (lepr s/s mice) are hyperphagic and profoundly obese, comparable to db/db mice that have a loss-of-function mutation in LepR (7). Like db/db mice, s/s mice with loss of leptin-STAT3 signaling have lower BAT UCP1 (8). This suggests that STAT3 mediates leptin-induced sympathetic thermogenic metabolism in BAT (Fig. 5). In contrast, s/s mice have preserved leptin-induced increases in renal SNA (52), whereas db/db mice have loss of leptin-induced renal sympathoactivation (116). This indicates that STAT3 does not contribute to leptin-induced increases in renal SNA. The contrasting role of STAT3 in the regulation of sympathetic thermogenic metabolism in BAT vs. renal SNA is an example of differential control of SNA to different tissues and organs.
Assuming that leptin-induced increases in renal SNA contribute importantly to hypertension in obesity, the finding that leptin-induced increases in renal SNA are preserved in s/s mice would predict that obese s/s mice have elevated BP and obesity-induced hypertension. In contrast to this prediction, Bodary et al. (12) observed that state-of-the-art radiotelemetric measurements of BP were lower in conscious obese s/s compared with wild-type mice. Furthermore, BP did not differ in s/s vs. db/db mice. Obese db/db mice with a loss-of-function mutation in the leptin receptor also had lower BP than wild-type mice (12). Bodary concluded that leptin-mediated effects on BP are mediated by the STAT3 pathway. This challenges the view that the leptin-STAT3 pathway is not important in regulation of BP. Loss of leptin-induced STAT3 activation appears to prevent or even reverse obesity-induced increases in BP.
As I was finalizing this review, a report appeared from Dubinion et al. (28) that reinforces the view that STAT3 signaling contributes to leptin-induced regulation of BP. These investigators studied mice with selective deletion of STAT3 in POMC neurons (STAT3flox/flox/POMC-Cre) with control mice (STAT3flox/flox). Mice with POMC neuronal deletion of STAT3 were heavier than control mice but there was no difference in baseline BP. This suggests that deletion of STAT3 signaling attenuated obesity-induced increases in BP. In addition, STAT3 deletion in POMC neurons prevented leptin-induced increases in BP.
How do we reconcile the findings that STAT3 contributes to leptin-induced increases in BP and to obesity-induced hypertension (12, 28) with a report that STAT3 does not mediate leptin-induced increases in renal SNA (52)? One possibility is that the pressor action of leptin-STAT3 signaling is mediated through mechanisms other than renal sympathetic activation. Another possibility is that the mechanisms mediating chronic and acute responses to leptin may differ (27). The studies supporting a role for STAT3 signaling in the BP effects of leptin and in obesity-induced hypertension assessed chronic effects of leptin (12, 28), whereas the study showing preservation of renal SNA responses to leptin evaluated acute responses (52).
Leptin-ERK signaling.
In vitro studies implicated ERK as a signaling pathway of the leptin receptor (6, 10, 11, 146). Leptin-induced activation of ERK 1/2 is mediated through Shp 2 emanating from the Tyr-985 residue of LepRb and through direct interaction with JAK2 (6, 11). We demonstrated that ERK plays an important role in leptin-mediated regulation of food intake, body weight, and thermogenic SNA (122). Leptin produced dose-dependent, receptor-mediated activation of ERK 1/2. This was restricted to the hypothalamic arcuate nucleus and dependent on JAK2. Cerebroventricular administration of the ERK antagonists (PD98059 and U0165) blocked decreases in food intake and body weight with systemic administration of leptin but did not alter responses to the melanocortin receptor agonist, MTII. The ERK antagonists prevented leptin-induced increases in BAT thermogenic SNA but did not attenuate renal SNA responses to leptin. Thus, while ERK contributes to the thermogenic BAT sympathetic responses to leptin, it does not mediate the renal SNA responses to leptin. This resembles the role of STAT3 in the regional sympathetic responses to leptin, but as noted above, it contrasts with the role of PI3K, which mediates the renal but not thermogenic BAT SNA responses to leptin. Again assuming that increased renal SNA is a critical contributor to obesity-induced hypertension, the finding that ERK does not mediate the renal SNA responses to leptin would suggest that leptin-ERK signaling would not contribute to increases in BP in obesity or during leptin. To put it another way, one would predict that disrupting leptin-ERK signaling would not prevent obesity-induced hypertension. However, to my knowledge, the role of leptin-ERK signaling in the regulation of BP in obesity or during administration of leptin has not been evaluated in mice with selective disruption of leptin-ERK signaling.
In summary, distinct leptin transduction pathways produce differential regulation of sympathetic metabolic vs. sympathetic cardiovascular function (Fig. 5). This provides a potential molecular mechanism for SLR. The evidence indicates that POMC neurons and leptin-PI3K signaling contribute importantly to the renal sympathetic and BP actions of leptin. In contrast, STAT3 and ERK/MAPK contribute to sympathetic metabolic control, but reportedly, do not contribute to regulation of renal sympathetic activity. Although STAT3 does not contribute to leptin-induced increases in renal SNA, it is involved in leptin-induced increases in BP and obesity-induced increases in BP. The role of ERK/MAPK in leptin-induced regulation of BP is not known. In other words, although studies of leptin-induced regulation of BP have focused on the PI3K pathway (55), there is evidence that the STAT3 pathway contributes to leptin-induced increases in BP and the role of the ERK/MAPK pathway has not been reported.
Is there pathway-specific leptin resistance in DIO?
We cannot leave a discussion of the role of selective leptin signaling pathways in SLR without asking a critical question. Is there pathway-specific leptin resistance in DIO? If there is resistance in all leptin signaling pathways in DIO, then it would be difficult to explain selective leptin resistance (with preservation of leptin-induced renal SNA and BP responses) and a hypertensive role of leptin on the basis of differential, selective leptin signaling pathways. In contrast, preservation of leptin-induced PI3K signaling in DIO could lead to preservation of the renal SNA and BP responses to leptin and obesity-induced hypertension. So is there pathway-specific leptin resistance in DIO and, more specifically, is there preservation of leptin-PI3K signaling in the face of resistance to leptin-STAT3 signaling in DIO? Morgan et al. (95) demonstrated that the preserved renal SNA response to leptin in both DIO and agouti obese mice is blocked by a PI3K inhibitor. This supports preservation of leptin-induced PI3K signaling in DIO, which is characterized by resistance to leptin STAT3 signaling. In contrast, Metlakunta et al. (89, 90) and Sahu and Metlakunta (130) demonstrated that the leptin inhibitor, SOCS3, modulates leptin-induced PI3K, as well as leptin STAT3 signaling. These investigators reported that hypothalamic PI3K signaling is impaired in DIO and following chronic central neural infusion of leptin (89, 90, 130). However, the impairment in leptin signaling was partial, so that with high leptin levels in DIO, there was still leptin signaling through these pathways. Further research is needed to evaluate the existence and mechanisms of pathway-specific leptin resistance in DIO.
Brain site-specific leptin actions.
Given the initial evidence for the hypothalamic arcuate nucleus as the linchpin of leptin action, the rapid emergence of evidence for a distributed brain network of leptin action has been staggering (44, 57, 103, 134). A notable feature of many of these studies has been evidence for brain site-specific leptin action (103). I focus here on brain site-specific leptin action, as it relates to sympathetic cardiovascular actions of leptin (53) and then discuss how this may relate to selective leptin resistance.
Hypothalamic arcuate nucleus.
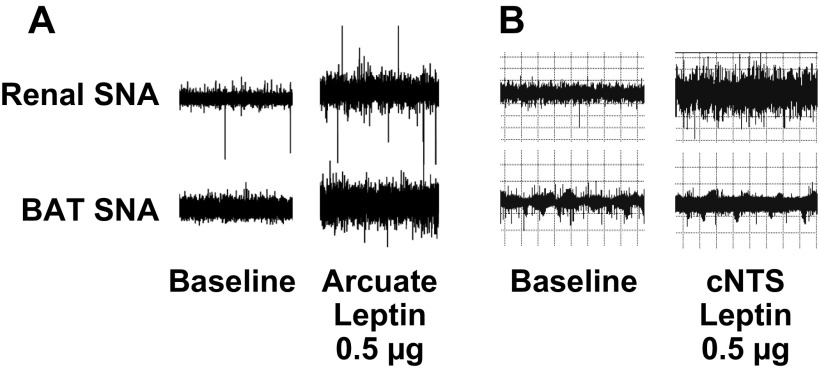
In addition to its role in the metabolic actions of leptin, the hypothalamus is a key site of the sympathetic actions. Microinjection of leptin into the hypothalamic arcuate nucleus of rats increases SNA to both kidney and BAT (120) (Fig. 6). BAT SNA responses to intravenous leptin are abolished by electrolytic lesioning of the arcuate nucleus in rats (59). In addition, we demonstrated that site-specific deletion of leptin receptors in the arcuate nucleus abrogates leptin-induced increases in both BAT and renal SNA (51). Thus, the arcuate nucleus plays a critical role in mediating the full spectrum of the sympathetic actions of leptin. These observations are consistent with studies of the neuroanatomic pathways underlying sympathetic actions of leptin (33, 34). In these studies, neurons in the lateral arcuate nucleus that innervate sympathetic preganglionic neurons in the thoracic spinal cord were activated by leptin (33, 34).
Fig. 6.
Recordings of renal and BAT SNA at baseline and 5 to 6 h after microinjection of leptin, 0.5 μg, into the arcuate nucleus or caudal nucleus tractus solitarii (cNTS) in Sprague-Dawley rats. A: arcuate injection of leptin increased SNA to both kidney and BAT (120) [Adapted from Rahmouni and Morgan (120)]. B: in contrast, cNTS injection of leptin increased SNA to kidney but not to BAT (84).
Ventromedial and dorsomedial hypothalamus.
The ventromedial hypothalamus (VMH) contributes substantially to the control of food intake, energy expenditure, and body weight (65, 157). The VMH is also involved in the circulating catecholamine and renal, lumbar, and BAT SNA responses to leptin (85, 91, 93, 131, 147). As discussed earlier, the dorsomedial hypothalamus (DMH) appears to be importantly involved in the regulation of BAT thermoregulatory circuits that are presumably sympathetic. Using retrograde trans-synaptic tracer pseudorabies virus (PRV) injected into BAT of mice, Zhang et al. (162) identified PRV-labeled LepR neurons in the DMH and median preoptic nucleus, and cold exposure induced neuronal activity in LepR neurons. In contrast, microinjection of leptin into the DMH increased heart rate and BP but failed to increase renal SNA (85). This suggests that the DMH contributes to leptin-induced regulation of thermogenic BAT SNA but not to regulation of renal SNA—an example of brain site-specific leptin sympathetic action.
Nucleus tractus solitarii.
In 2002, Grill and colleagues (44, 45) introduced the concept of the hindbrain, notably the caudal nucleus tractus solitarius (NTS), as a target for the inhibitory effects of leptin on food intake and body weight. This concept derived from the observation that injection of leptin into the fourth cerebroventricle (in the vicinity of the NTS) or the dorsal vagal complex of the rat hindbrain decreased food intake and body weight and increased core body temperature (44). The responses to stimulation of hindbrain leptin receptors were abolished by injection of a melanocortin antagonist, SHU 9119, into the fourth cerebroventricle (139).
Two studies employing different strategies support a physiological role of hindbrain leptin receptors in energy regulation. Hayes et al. (57) demonstrated that knockdown of NTS and area postrema leptin receptors using adeno-associated viral short hairpin RNAi in rats resulted in hyperphagia and increased adiposity and body weight. Scott et al. (135) reported that mice with selective ablation of LepR from glucagon-like peptide-1-expressing neurons in the NTS were hyperphagic and gained weight at a faster rate than wild-type controls. These two studies provide evidence for a physiologically important role of LepR and endogenous leptin signaling in the hindbrain.
Studies in our laboratories (84) and others (17) demonstrated that microinjection of leptin into the caudal NTS of rats increased renal SNA and BP. Although Skibicka and Grill (139) found that injection of leptin into the fourth cerebroventricle increased core body temperature, we found that selective NTS microinjection of leptin did not increase thermogenic BAT SNA (84). Thus, while arcuate injection of leptin increases both BAT and renal SNA (120), NTS injection of leptin increases renal but not BAT SNA (84 (Fig. 6). In other words, whereas the arcuate nucleus mediates the full spectrum of the sympathetic actions of leptin, the NTS triggers selective sympathetic actions of leptin—another example of brain site-specific leptin sympathetic action.
Subfornical organ.
The subfornical organ (SFO) is a forebrain circumventricular organ that lacks a blood-brain barrier and is, thus, bathed in circulating humoral factors and nutrients. With projections to hypothalamic and hindbrain structures, it plays a pivotal role in integrating circulating humoral factors and neural control of the circulation. Smith et al. (140) reported in vitro studies in rats, indicating the presence of functionally significant leptin receptors in SFO neurons. Neurochemical studies demonstrated mRNA for LepR in SFO preparations. In addition, intracerebroventricular administration of leptin activated STAT3 phosphorylation in SFO neurons. In whole cell current-clamp recordings from dissociated SFO neurons, leptin altered the excitability of 64% of SFO neurons with depolarization in 39% and hyperpolarization in 25%.
In a subsequent study, Smith and Ferguson (142) reported that microinjection of leptin into the SFO of rats produced dose-related rapid, small, transient decreases in BP. In diet-induced obese rats, SFO injection of leptin was without effect on BP. The investigators concluded that the SFO normally acts as a relay center that buffers pressor actions of leptin to maintain BP within physiological limits. They further suggested that loss of this transient depressor action of leptin in DIO likely contributes to the development of obesity-induced hypertension. This study did not, however, provide evidence that the early, small depressor response to SFO leptin was receptor-mediated.
A recent study by Young et al. (159) suggests a different role for SFO leptin receptors. Responses to systemic and cerebroventricular administration of leptin were studied in mice with intact LepR compared with LepRflox/flox mice with selective deletion of SFO LepR produced using Cre-LoxP technology. The mice lacking SFO LepR had normal food intake, body weight, and BAT SNA responses to systemic or cerebroventricular leptin. In contrast, deletion of SFO LepR abrogated renal SNA responses to leptin. This suggests that the SFO, like the NTS (84), contributes to the renal but not BAT SNA responses to leptin and that SFO LepR appear to be sympathoexcitatory (159). This study provides intriguing evidence for site-specific leptin action with increases in renal SNA in the absence of changes in food intake, body weight, or BAT SNA emanating from the SFO LepR. To our knowledge, this is the first evidence for site-specific functionally significant LepR regulating renal SNA without effect on food intake, thermogenic BAT SNA, or body weight.
Implications of site-specific leptin resistance to SLR.
How might brain site-specific leptin action relate to SLR? The answer is brain site-specific leptin resistance. Munzberg et al. (99) systematically examined leptin-activation of cells throughout the brain of mice during development of DIO using STAT3 phosphorylation as a marker of leptin sensitivity. The arcuate nucleus was a major site of DIO-induced leptin resistance, beginning as early as 6 days of a high-fat diet. Surprisingly, other hypothalamic and extra-hypothalamic sites, including the VMH, DMH, and NTS, remained leptin-sensitive. These investigators further demonstrated that DIO increased the leptin inhibitor SOCS3 in the arcuate nucleus but not in the DMH or VMH.
Matheny et al. (87) have contributed importantly to this topic. These investigators studied effects of either DIO or chronic adeno-associated viral mediated central neural leptin overexpression on leptin-induced STAT3 phosphorylation in multiple brain regions, including the ventral tegmental area (VTA), a midbrain region involved in ingestive reward behavior that is regulated by leptin. There were two key observations. First, a high-fat diet impaired leptin-STAT3 phosphorylation in the VTA, in addition to the arcuate nucleus. Leptin signaling in the lateral and dorsomedial hypothalamus was preserved. This confirmed the presence of site-specific leptin resistance in DIO and demonstrated that the site-specific leptin resistance extends to some extrahypothalamic regions and is not confined to the hypothalamus. Second, whereas DIO produced site-specific leptin resistance, chronic central neural overexpression of leptin produced leptin resistance in every brain region examined. This indicates that site-specific leptin resistance is distinctive to DIO and is not a nonspecific central neural response produced by exposure to high leptin.
Munzberg et al. (99, 100, 101) speculated that site-specific leptin resistance might contribute to SLR, and subsequent studies lend credence to this speculation. Given that leptin appears to act in the NTS (84) and SFO (159) to increase renal but not BAT SNA, one could speculate that maintenance of NTS and/or SFO actions of leptin in DIO could lead to preservation of renal SNA and BP actions of leptin, despite loss of metabolic actions of leptin in the arcuate nucleus.
A selective interaction of the brain renin-angiotensin system and leptin.
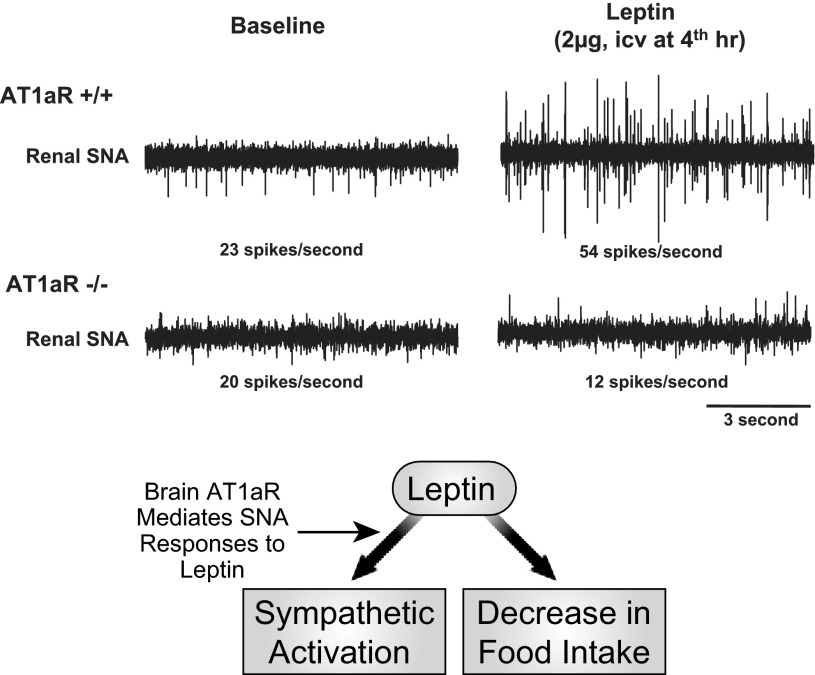
We have recently identified another contributor to selective leptin action, namely a novel interaction, whereby the brain renin-angiotensin system (RAS) mediates the renal (cardiovascular) and BAT (thermogenic) SNA responses to leptin but does not alter leptin-induced decreases in food intake (62) (Fig. 7). These studies were prompted by evidence for both leptin and angiotensin receptors in several brain regions involved in the regulation of SNA (141) and by mounting evidence that the brain RAS participates in the sympathetic regulation of energy expenditure and adiposity (46, 47), as well as BP (23, 46). Prompted by this background, we demonstrated that deletion of ANG II type 1a receptors (AT1aR) in mice and pharmacological blockade of brain AT1R in rats attenuated leptin-induced increases in renal and BAT SNA but did not alter effects of leptin on food intake. The selectivity of the brain AT1R-leptin interaction was indicated by the finding that blockade of brain AT1R did not impair SNA responses to melanocortin receptor stimulation. These findings suggest surprisingly that the brain RAS mediates the actions of leptin on BAT (thermogenic) and renal (cardiovascular) SNA without affecting leptin actions on food intake. These observations suggest that increases or decreases in activity of the brain RAS could modulate the effects of leptin on energy expenditure and BP without influencing leptin-induced decreases in food intake.
Fig. 7.
Top: changes in renal SNA in Sprague-Dawley rats after cerebroventricular administration of leptin (vehicle-leptin), losartan (losartan-vehicle), or leptin following losartan (losartan-leptin). Leptin alone increased renal SNA. Losartan blocked leptin-induced increases in renal SNA. Losartan alone had no significant effect on renal SNA. As depicted in the schematic (lower panel), these studies suggest that brain AT1R mediate the renal SNA responses to leptin (62).
Leptin and arginine vasopressin.
Another potential contributor to the cardiovascular actions of leptin is AVP. In rats, cerebroventricular administration of leptin increases AVP mRNA in the paraventricular nucleus and supraoptic nucleus and increases plasma AVP (158) and, thereby, stimulates the hypothalamic-pituitary-adrenal axis (96). In addition, cerebroventricular administration of leptin in conscious rabbits increases plasma AVP in addition to renal SNA and BP (88). Increases in AVP have been implicated in the hypertension caused by increased activity of the brain RAS (23, 77, 97). Given that leptin (like the brain RAS) increases AVP secretion and plasma levels, AVP merits further consideration as a contributor to SNA and/or BP actions of leptin.
Other Cardiovascular Actions of Leptin
In addition to sympathetic stimulation, leptin increases in endothelial nitric oxide (NO) and promotes peripheral adrenergic desensitization, which are depressor actions and may modulate the pressor response to sympathetic stimulation. For example, leptin increases NO and vasorelaxation in vascular rings (64, 73, 152). Leptin also produced brachial artery flow-mediated dilation, presumably endothelial dependent, in vivo in humans (13). These studies are juxtaposed with reports from our group that leptin lacks an endothelium-dependent dilator action in conscious rats (92, 118). Indeed, studies from Tune's laboratory (66, 67, 110) suggest that increases in either exogenous or perivascular adipose tissue leptin promote coronary endothelial dysfunction. Despite the challenges to endothelial NO-dependent dilation with leptin, Frühbeck (41) reported that inhibition of NO synthase enhanced the pressor response to leptin in acute experiments in anesthetized rats. In addition, ganglionic blockade unmasked a depressor response to leptin that was blocked by NO synthesis inhibition. Furthermore, in chronic experiments Kuo et al. (69) observed that inhibition of NO synthesis produced a modest enhancement of the chronic renal hemodynamic and pressor responses to increases in plasma leptin comparable to those found in obesity-induced hypertension. I conclude that the weight of evidence, particularly the studies by Frühbeck (41) and Kuo et al. (69), suggests that endothelial NO depressor mechanisms oppose—albeit modestly—the sympathetic pressor action of leptin.
Belin de Chantemèle et al. (9) demonstrated that leptin-induced sympathetic overdrive triggers a compensatory reduction in vascular adrenergic reactivity and α-1 adrenergic receptor expression. This compensatory adrenergic desensitization may protect against the development of leptin-induced hypertension.
These observations suggest that the BP response to leptin may be influenced by the balance between sympathetic pressor actions of leptin and opposing depressor actions. Once again, there is an analogy with the BP actions of insulin in the metabolic syndrome where the BP response is determined by the balance between the sympathetic pressor and vascular depressor actions.
Sympathetic and Blood Pressure Actions of Leptin in Humans
Compared with the large and persuasive body of work on the sympathetic and BP effects of leptin in experimental animals, there has been relatively little attention to the sympathetic and BP effects of leptin in humans. Why is this? I suggest two reasons. First, in many studies involving administration of leptin in humans, data on BP have either not been reported or, when reported, have not been discussed. Second, the availability of recombinant human leptin for mechanistic experimental studies in humans has been limited. Despite this, there are substantial data on the effects of leptin on BP in humans. The results of the various studies are perplexing and in some instances challenge the role of leptin in the regulation of BP in humans. As these studies in humans have not been a prominent feature of reviews on the sympathetic and cardiovascular effects of leptin, I will expand on this topic.
Leptin and sympathetic activity in humans.
In a placebo-controlled study of lean human subjects, Mackintosh and Hirsch (80) found that treatment with leptin for 6 days did not increase urinary catecholamines. Rosenbaum et al. (127) reported that diet-induced weight loss in humans produced small decreases in urinary epinephrine and norepinephrine (NE) and also decreased sympathetic tone assessed from analysis of heart rate and responses to adrenergic blockade. Low-dose leptin replacement restored sympathetic tone and reversed the decrease in urinary epinephrine but did not reverse the small decrease in urinary NE.
There are several studies correlating plasma leptin levels in humans with indices of sympathetic activity. Eikelis et al. (32) observed that plasma leptin levels correlated with renal NE spillover but not with other indices of sympathoadrenal activity. Snitker et al. (143) reported a weak correlation between muscle SNA and plasma leptin concentration in healthy men fed a controlled diet, but other investigators have not (2, 105). For example, Alvarez et al. (2) demonstrated that muscle SNA did not differ in men with subcutaneous obesity compared with nonobese men despite 2.6-fold higher plasma leptin in the men with subcutaneous obesity. In contrast, muscle SNA was correlated with visceral fat without a significant relationship to plasma leptin (2, 3). These investigators questioned the role of leptin in the sympathetic activation of human obesity, but did not evaluate indices of renal sympathetic activity.
Masuo et al. (86) reported that normotensive Caucasian males with two different putative loss-of-function alleles in the leptin receptor gene (homozygous for the Arg223 allele of Gln223Arg and carriers of the Asn656 allele of Lys656Asn) had slightly higher values for body mass index (BMI), but lower levels of whole body NE spillover. This contrasts with increased levels of whole body NE spillover seen in most obese humans. The decreases in NE spillover in the Arg223 homozygotes and Asn656 carriers were not associated with lower BP, but hypertension was an exclusion criterion in the study. The finding that the subjects with the polymorphisms in the leptin receptor gene had decreased NE spillover despite high BMI suggests that even subtle loss of function in the leptin receptor decreases sympathetic activity in humans. This finding is consistent with studies in our laboratory showing that obese db/db mice with loss-of-function mutations in the leptin receptor lack SNA responses to leptin (117).
Ozata et al. (109) studied three adults and one child with morbid obesity and complete leptin deficiency secondary to loss-of-function mutations in the leptin gene. The morbid obesity with leptin deficiency was associated with sympathetic hypofunction, manifest by orthostatic hypotension and an attenuated cold pressor response, whereas common human obesity is usually associated with sympathetic hyperactivity. There were no data on plasma or urinary catecholamines, but the orthostatic hypotension and attenuated cold pressor test suggest that loss of leptin action in humans decreases sympathetic activity.
Thus, the study of whole body NE turnover in subjects with variants in the leptin receptor gene and the clinical observations in patients with complete leptin deficiency suggest that leptin regulates sympathetic nerve activity in humans, but there are no reports of state-of-the-art measurements of sympathetic activity (total body or renal NE spillover or direct microneurographic intraneural recordings of SNA) during administration of leptin in humans.
Leptin and blood pressure in humans.
Two reports of monogenic human obesity secondary to loss-of-function mutations in the leptin receptor gene failed to mention BP or hypertension (18, 37). Saeed et al. (129) reported 10 children with morbid obesity secondary to complete leptin deficiency but made no mention of BP or hypertension. Farooqi et al. (36) described a 9-yr-old girl with severe, early-onset obesity, secondary to complete leptin deficiency, who had a BP of 118/70. This value is in the high normal range for BP in a 9-yr-old girl and neither supports nor refutes a role for leptin in regulation of BP.
In the three adults with morbid obesity and complete leptin deficiency described by Strobel et al. (145) and Ozata et al. (109), all three individuals had cited values of elevated baseline BP, despite leptin deficiency, and sympathetic hypofunction, but there was no mention of hypertension in these individuals and no comparison of BP with weight- and age-matched subjects.
Thus, the evidence that loss of leptin action modulates BP and attenuates obesity-induced hypertension in humans is inconclusive.
Rosmond et al. (128) reported that several polymorphisms in the leptin receptor gene were associated with significant differences in BP in middle-aged men. The results relating to Lys109 and Arg109 alleles in exon 4 and Gln223 and Arg223 alleles in exon 6 were the most intriguing. Systolic and diastolic BP was lower among Arg109 and Arg223 homozygotes than in subjects with Lys109 or Gln223 alleles, respectively, even after adjusting for adiposity, body fat distribution, and leptin levels. The differences in BP were said to be “considerable”. The men who were homozygous for both Arg109 and Arg223 alleles had systolic and diastolic BP 13 and 10 mmHg lower, respectively, than men homozygous for both Lys109 and Gln223 alleles. The investigators concluded that leptin is associated with BP regulation through the leptin receptor and further stated that when BMI and leptin are elevated, increased BP is found only with the most prevalent leptin receptor genotypes at codons 109 and 223, whereas variants of the leptin receptor gene seem to protect from hypertension.
In a family-based association study within the National Heart, Lung, and Blood Institute Family Heart Study (79), there was an association between variants in the leptin gene, BP, and hypertension but only in women, particularly postmenopausal women. There was also a correlation between plasma leptin levels and BP in women, but this correlation weakened or disappeared after adjustment for BMI.
Thus, two studies (79, 128) suggest that variants in leptin receptor or leptin gene are associated with variation in BP, but the association was weak in one of the studies (79). Furthermore, in the study by Masuo (86), which described an association between variants in the leptin receptor gene and whole body NE turnover, there was not a corresponding association with BP.
This brings us to a discussion of the BP response to administration of leptin in humans. Licinio et al. (74) described a favorable body weight and metabolic response to 18 mo of leptin replacement in three obese adults with complete leptin deficiency, but there was no comment on the BP response. Farooqi et al. (36) reported that there were “no changes in blood pressure” during treatment with leptin over 12 mo in the 9-yr-old girl, who had severe obesity, secondary to complete leptin deficiency.
Heymsfield et al. (60) reported a placebo-controlled, randomized trial of graded doses of recombinant human leptin (rL) in 73 obese and 54 lean humans. There were no data on BP, but the investigators stated that “none of the subjects taking rL experienced clinically significant adverse effects on major organ systems … (cardiovascular …) as evidenced by … physical examinations and vital signs.” Zelissen et al. (160) reported values for BP in a randomized, double-blind, placebo controlled multicenter study of 284 overweight/obese subjects assigned to one of three doses of leptin or placebo for 12 wk. Leptin did not increase BP.
Brook et al. (13) analyzed the BP effects of recombinant leptin in two randomized, placebo-controlled studies—a study of the acute effects of leptin on endothelial function in lean adults and a study of the chronic effects of leptin on weight loss. Leptin did not increase BP in either the acute or chronic study.
Ebihara et al. (30) evaluated the efficacy and safety of leptin-replacement therapy from 4 mo to 3 yr in seven Japanese patients with leptin deficiency caused by generalized lipodystrophy. There was improvement in diabetes, dyslipidemia, fatty liver, and albuminuria. No patient showed hypertension at baseline and, as shown, there was “no distinct elevation of blood pressure … at any time throughout the therapy period.”
Summary of human studies.
There is some evidence supporting a role for leptin in the regulation of sympathetic activity in humans, but the current evidence for a role of leptin in regulation of BP in humans is not convincing. In reports of three adults with severe obesity secondary to complete leptin deficiency, there were cited values of elevated baseline BP, suggesting that these individuals had some obesity-induced increase in BP. In addition, data from studies in relatively large numbers of lean and obese subjects have failed to show an increase in BP with either chronic or acute administration of leptin. These data challenge a role for leptin in regulation of BP and in the pathogenesis of obesity-induced hypertension in humans.
Further research employing other methods and strategies is needed to determine the role of leptin in the regulation of sympathetic activity and BP in humans and in the pathogenesis of human obesity-induced hypertension.
State-of-the-art studies of sympathetic activity and BP in patients with complete leptin deficiency before and during treatment would be valuable, but these studies are unlikely, among other reasons, because patients with complete leptin deficiency are rare and are often children.
There is, however, a group of subjects that might provide further insight into the role of leptin in the regulation of BP in obese humans. These are obese individuals who have relatively low levels of circulating leptin. Although serum leptin concentrations are elevated in most individuals with common human obesity, there is a minority of obese individuals who have relatively low levels of circulating leptin (124). A comparison of sympathetic activity and BP in obese subjects with relatively low levels of leptin vs. age, weight- and body fat-matched subjects with high leptin levels might provide useful insight into the role of leptin in obesity-induced regulation of BP in humans.
Evaluating the contribution of leptin to obesity-induced human hypertension is impeded by the lack of safe, effective, reversible leptin antagonists for studies in humans. Understanding the role of the sympathetic nervous system, the renin-angiotensin system, and calcium channels in the pathogenesis and treatment of hypertension has depended substantially on the availability of safe, effective antagonists. Since a leptin antagonist would not have obvious widespread or compelling therapeutic potential, it seems unlikely that such an antagonist for use in humans will emerge soon.
Final comment on SLR.
The concept of SLR arose from our interest in obesity-induced hypertension and an effort to explain how leptin could contribute to obesity-induced hypertension in diet-induced obesity despite partial leptin resistance. The focus of most of the work on SLR has, therefore, been on preservation of the renal sympathetic and blood pressure actions of leptin in DIO despite metabolic leptin resistance. I speculate that further study may yield other physiologically and clinically significant examples of preserved leptin actions in DIO despite resistance to other actions of leptin.
Conclusions.
In addition to its classic effects on appetite, metabolism and adiposity, leptin increases regional sympathetic nerve activity to a spectrum of tissues and organs, including brown and white adipose tissue and cardiovascular control regions, notably the kidney. In several mouse models of obesity-induced hypertension (diet-induced obese mice; agouti obese mice; and Bardet-Biedl syndrome mice), there is selective leptin resistance with preservation of the renal sympathetic and blood pressure responses to leptin despite resistance to the appetite, thermogenic sympathetic, and body weight responses. Two possible mechanisms for SLR have emerged in the past decade: 1) differential leptin molecular signaling pathways that mediate selective as opposed to uniform or universal leptin actions and 2) brain site-specific leptin actions and resistance. In addition, recent studies suggest surprisingly that the brain renin-angiotensin system mediates the actions of leptin on renal and brown adipose tissue thermogenic SNA without affecting leptin actions on food intake. These observations suggest that increases or decreases in activity of the brain RAS could modulate the effects of leptin on energy expenditure and blood pressure without influencing leptin-induced decreases in food intake. There is evidence that genetic variants in the leptin receptor influence sympathetic activity and blood pressure in humans, but studies in humans have failed to show an increase in BP with administration of leptin. Consequently, the current evidence supporting a role for leptin in regulation of sympathetic activity and BP in humans is perplexing and unconvincing and compels further studies in humans. In closing, although the concept of SLR in DIO has so far focused on preservation of sympathetic actions of leptin in the face of resistance to the food intake and body weight responses, further consideration should be given to the possibility that this concept may extend to preservation of other physiologically and clinically significant actions of leptin.
GRANTS
Research discussed in this review from the laboratories of the author and his colleagues was supported by the following grants from the NHLBI HL-84207, HL-44546, HL-55006, HL-63887, HL-96571, and HL-14388 and by research funds from the Roy J. and Lucille A. Carver Trust.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
I acknowledge the contributions of Kamal Rahmouni, William Haynes, Marcelo Correia, Aline Hilzendeger, Justin Grobe, and Curt Sigmund to studies from the University of Iowa discussed in the review and of Colin Young and Robin Davisson to our studies on this topic at Weill Cornell Medical College, Cornell University. This review was written, in part, during a Visiting Fellowship in the laboratory of Professor Davisson at Weill Cornell Medical College. I thank Mark Chapleau and the Neural Control and Autonomic Regulation Section of the American Physiological Society for the privilege of presenting the Carl Ludwig Lecture. Finally, I appreciate the review of this manuscript by Robin Davisson, Justin Grobe, William Haynes, Ted Kurtz, Anne Kwitek, Kamal Rahmouni, and Colin Young.
REFERENCES
- 1.Aizawa-Abe M, Ogawa Y, Masuzaki H, Ebihara K, Satoh N, Iwai H, Matsuoka N, Hayashi T, Hosoda K, Inoue G, Yoshimasa Y, Nakao K. Pathophysiological role of leptin in obesity-related hypertension. J Clin Invest 105: 1243–1252, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez GE, Ballard TP, Beske SD, Davy KP. Subcutaneous obesity is not associated with sympathetic neural activation. Am J Physiol Heart Circ Physiol 287: H414–H418, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Alvarez GE, Beske SD, Ballard TP, Davy KP. Sympathetic neural activation in visceral obesity. Circulation 106: 2533–2536, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Armitage JA, Burke SL, Prior LJ, Barzel B, Eikelis N, Lim K, Head GA. Rapid onset of renal sympathetic nerve activation in rabbits fed a high-fat diet. Hypertension 60: 163–171, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, Lowell BB. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 42: 983–991, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Banks AS, Davis SM, Bates SH, Myers MG., Jr Activation of downstream signals by the long form of the leptin receptor. J Biol Chem 75: 14563–14572, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery JH, Hag AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG., Jr STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 421: 856–859, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Bates SH, Dundon TA, Seifert M, Carlson M, Maratos-Flier E, Myers MG., Jr LRb-STAT3 signaling is required for the neuroendocrine regulation of energy expenditure by leptin. Diabetes 53: 3067–3073, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Belin de Chantemèle EJ, Muta K, Mintz J, Tremblay ML, Marrero MB, Fulton DJ, Stepp DW. Protein tyrosine phosphatase 1B, a major regulator of leptin-mediated control of cardiovascular function. Circulation 120: 753–763, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjorbaek C, Uotani S, da Silva B, Flier JS. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem 272: 32686–32695, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Bjorbaek C, Buchholz RM, Davis SM, Bates SH, Pierroz DD, Gu H, Neel BG, Myers MG, Jr, Flier JS. Divergent roles of SHP-2 in ERK activation by leptin receptors. J Biol Chem 276: 4747–4755, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Bodary PF, Shen Y, Ohman M, Bahrou KL, Vargas FB, Cudney SS, Wickenheiser KJ, Myers MG, Jr, Eitzman DT. Leptin regulates neointima formation after arterial injury through mechanisms independent of blood pressure and the leptin receptor/STAT3 signaling pathways involved in energy balance. Arterioscler Thromb Vasc Biol 27: 70–76, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Brook RD, Bard RL, Bodary PF, Eitzman DT, Rajagopalan S, Sun Y, Depaoli AM. Blood pressure and vascular effects of leptin in humans. Metab Syndr Relat Disord 5: 270–274, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab 7: 95–96, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Carlyle M, Jones OB, Kuo JJ, Hall JE. Chronic cardiovascular and renal actions of leptin: role of adrenergic activity. Hypertension 39: 496–501, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Casto RM, VanNess JM, Overton JM. Effects of central leptin administration on blood pressure in normotensive rats. Neurosci Lett 246: 29–32, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Ciriello J, Moreau JM. Leptin signaling in the nucleus of the solitary tract alters the cardiovascular responses to activation of the chemoreceptor reflex. Am J Physiol Regul Integr Comp Physiol 303: R727–R736, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Clément K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte JM, Basdevant A, Bougnères P, Lebouc Y, Froquel P, Guy-Grand B. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 392: 398–401, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Collins S, Kuhn CM, Petro AE, Swick AG, Chrunyk BA, Surwit RS. Role of leptin in fat regulation. Nature 380: 677, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Correia ML, Haynes WG, Rahmouni K, Morgan DA, Sivitz WI, Mark AL. The concept of selective leptin resistance: evidence from agouti yellow obese mice. Diabetes 51: 439–442, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411: 480–484, 2001 [DOI] [PubMed] [Google Scholar]
- 22.da Silva AA, do Carmo JM, Hall JE. Role of leptin and central nervous system melanocortins in obesity hypertension. Curr Opin Nephrol Hypertens 22: 135–140, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davisson RL, Yang G, Beltz TG, Cassell MD, Johnson AK, Sigmund CD. The brain renin-angiotensin system contributes to the hypertension in mice containing both the human renin and human angiotensinogen transgenes. Circ Res 83: 1047–1058, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Di Bona GF, Kopp UC. Neural control of renal function. Physiol Rev 77: 75–197, 1997 [DOI] [PubMed] [Google Scholar]
- 25.do Carmo J, da Silva A, Cai Z, Lin S, Dubinion J, Hall J. Control of blood pressure, appetite, and glucose by leptin in mice lacking leptin receptors in proopiomelanocortin neurons. Hypertension 57: 918–926, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drazen DL, Demas GE, Nelson RJ. Leptin effects on immune function and energy balance are photoperiod dependent in Siberian hamsters (Phodopus sungorus). Endocrinology 142: 2768–2775, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Dubinion JH, da Silva AA, Hall JE. Chronic blood pressure and appetite responses to central leptin infusion in rats fed a high fat diet. J Hypertens 29: 758–762, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Dubinion JH, do Carmo JM, Adi A, Hama S, da Silva AA, Hall JE. Role of proopiomelanocortin neuron Stat3 in regulating arterial pressure and mediating the chronic effects of leptin. Hypertension 61: 1066–1074, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunbar JC, Hu Y, Lu H. Intracerebroventricular leptin increases lumbar and renal sympathetic nerve activity and blood pressure in normal rats. Diabetes 46: 2040–2043, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Ebihara K, Kusakabe T, Hirata M, Masuzaki H, Miyanaga F, Kobayashi N, Tanaka T, Chusho H, Miyazawa T, Hayashi T, Hosoda K, Ogawa Y, DePaoli AM, Fukushima M, Nakao K. Efficacy and safety of leptin-replacement therapy and possible mechanisms of leptin actions in patients with generalized lipodystrophy. J Clin Endocrinol Metab 92: 532–541, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434: 514–520, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Eikelis N, Schlaich M, Aggarwal A, Kaye D, Esler M. Interactions between leptin and the human sympathetic nervous system. Hypertension 41: 1072–1079, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, Kuhar MJ, Saper CB, Elmquist JK. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron 21: 1375–1385, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Elmquist JK. Hypothalamic pathways underlying the endocrine, autonomic, and behavioral effects of leptin. Physiol Behav 74: 703–708, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Enriori PJ, Sinnayah P, Simonds SE, Garcia Rudaz C, Cowley MA. Leptin action in the dorso-medial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J Neurosci 31: 12189–12197, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O'Rahilly S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med 341: 879–884, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Farooqi IS, Wangensteen T, Collins S, Kimber W, Matarese G, Keogh JM, Lank E, Bottomley B, Lopez-Fernandez J, Ferraz-Amaro I, Dattani MT, Ercan O, Myhre AG, Retterstol L, Stanhope R, Edge JA, McKenzie S, Lessan N, Ghodsi M, De Rosa V, Perna F, Fontana S, Barroso I, Undlien DE, O'Rahilly S. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med 356: 237–247, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farooqi IS, O'Rahilly S. Leptin: a pivotal regulator of human energy homeostasis. Am J Clin Nutr 89: 980S–984S, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Fei H, Okano HJ, Li C, Lee GH, Zhao C, Darnell R, Friedman JM. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc Natl Acad Sci USA 94: 7001–7005, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friedman JM. Leptin at 14 y of age: an ongoing story. Am J Clin Nutr 89: 973S–979S, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frühbeck G. Pivotal role of nitric oxide in the control of blood pressure after leptin administration. Diabetes 48: 903–908, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Garfield AS, Patterson C, Skora S, Gribble FM, Reimann F, Evans ML, Myers MG, Jr, Heisler LK. Neurochemical characterization of body weight-regulating leptin receptor neurons in the nucleus of the solitary tract. Endocrinology 153: 4600–4607, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grassi G, Seravalle G, Cattaneo BM, Bolla GB, Lanfranchi A, Colombo M, Giannattasio C, Brunani A, Cavagnini F, Mancia G. Sympathetic activation in obese normotensive subjects. Hypertension 25: 560–563, 1995 [DOI] [PubMed] [Google Scholar]
- 44.Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology 143: 239–246, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab 16: 296–309, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grobe JL, Grobe CL, Beltz TG, Westphal SG, Morgan DA, Xu D, deLange WJ, Li H, Sakai K, Thedens DR, Cassis LA, Rahmouni K, Mark AL, Johnson AK, Sigmund CD. The brain renin-angiotensin system controls divergent efferent mechanisms to regulate fluid and energy balance. Cell Metab 12: 431–442, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grobe JL, Rahmouni K, Liu X, Sigmund CD. Metabolic rate regulation by the renin-angiotensin system: brain vs. body. Pflügers Arch 465: 167–175, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci USA 94: 8878–8883, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Halaas JL, Friedman JM. Leptin and its receptor. J Endocrinol 155: 215–216, 1997 [DOI] [PubMed] [Google Scholar]
- 50.Hall JE. The kidney, hypertension, and obesity. Hypertension 41: 625–633, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Harlan SM, Morgan DA, Agassandian K, Guo DF, Cassell MD, Sigmund CD, Mark AL, Rahmouni K. Ablation of the leptin receptor in the hypothalamic arcuate nucleus abrogates leptin-induced sympathetic activation. Circ Res 108: 808–812, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harlan SM, Morgan DA, Dellsperger DJ, Myers MG, Jr, Mark AL, Rahmouni K. Cardiovascular and sympathetic effects of disrupting tyrosine 985 of the leptin receptor. Hypertension 57: 627–632, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harlan SM, Rahmouni K. Neuroanatomical determinants of the sympathetic nerve responses evoked by leptin. Clin Auton Res 23: 1–7, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harlan S, Guo DF, Morgan DA, Fernandes-Santos C, Rahmouni K. Hypothalamic mTORC1 signaling controls sympathetic nerve activity and arterial pressure and mediates leptin effects. Cell Metab 17: 599–606, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harlan SM, Rahmouni K. PI3K signaling: A key pathway in the control of sympathetic traffic and arterial pressure by leptin. Mol Metab 2: 69–73, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hausberg M, Morgan DA, Mitchell JL, Sivitz WI, Mark AL, Haynes WG. Leptin potentiates thermogenic sympathetic response to hypothermia: A receptor-mediated effect. Diabetes 51: 2434–2440, 2002 [DOI] [PubMed] [Google Scholar]
- 57.Hayes MR, Skibicka KP, Leichner TM, Guarnieri DJ, DiLeone RJ, Bence KK, Grill HJ. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab 11: 77–83, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest 100: 270–278, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haynes WG. Interaction between leptin and sympathetic nervous system in hypertension. Curr Hypertens Rep 2: 311–318, 2000 [DOI] [PubMed] [Google Scholar]
- 60.Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, McCamish M. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA 282: 1568–1575, 1999 [DOI] [PubMed] [Google Scholar]
- 61.Hill JW, Elias CF, Fukuda M, Williams KW, Berglund ED, Holland WL, Cho YR, Chuang JC, Xu Y, Choi M, Lauzon D, Lee CE, Coppari R, Richardson JA, Zigman JM, Chua S, Scherer PE, Lowell BB, Brüning JC, Elmquist JK. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab 11: 286–297, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hilzendeger AM, Morgan DA, Brooks L, Dellsperger D, Liu X, Grobe JL, Rahmouni K, Sigmund CD, Mark AL. A brain leptin-renin angiotensin system interaction in the regulation of sympathetic nerve activity. Am J Physiol Heart Circ Physiol 303: H197–H206, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huo L, Grill HJ, Bjorbaek C. Divergent regulation of proopiomelanocortin neurons by leptin in the nucleus of the solitary tract and in the arcuate hypothalamic nucleus. Diabetes 55: 567–573, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Kimura K, Tsuda K, Baba A, Kawabe T, Boh-oka S, Ibata M, Moriwaki C, Hano T, Nishio I. Involvement of nitric oxide in endothelium-dependent arterial relaxation by leptin. Biochem Biophys Res Commun 273: 745–749, 2000 [DOI] [PubMed] [Google Scholar]
- 65.King BM. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol Behav 87: 221–244, 2006 [DOI] [PubMed] [Google Scholar]
- 66.Knudson JD, Dincer UD, Zhang C, Swafford AN, Jr, Koshida R, Picchi A, Focardi M, Dick GM, Tune JD. Leptin receptors are expressed in coronary arteries, and hyperleptinemia causes significant coronary endothelial dysfunction. Am J Physiol Heart Circ Physiol 289: H48–H56, 2005 [DOI] [PubMed] [Google Scholar]
- 67.Knudson JD, Dincer UD, Dick GM, Shibata H, Akahane R, Saito M, Tune JD. Leptin resistance extends to the coronary vasculature in prediabetic dogs and provides a protective adaptation against endothelial dysfunction. Am J Physiol Heart Circ Physiol 289: H1038–H1046, 2005 [DOI] [PubMed] [Google Scholar]
- 68.Konner AC, Bruning JC. Selective insulin and leptin resistance in metabolic disorders. Cell Metab 16: 144–152, 2012 [DOI] [PubMed] [Google Scholar]
- 69.Kuo JJ, Jones OB, Hall JE. Inhibition of NO synthesis enhances chronic cardiovascular and renal actions of leptin. Hypertension 37: 670–676, 2001 [DOI] [PubMed] [Google Scholar]
- 70.Landsberg L. Diet, obesity and hypertension: an hypothesis involving insulin, the sympathetic nervous system, and adaptive thermogenesis. Q J Med 61: 1081–1090, 1986 [PubMed] [Google Scholar]
- 71.Landsberg L, Aronne LJ, Beilin LJ, Burke V, Igel LI, Lloyd-Jones D, Sowers J. Obesity-related hypertension: Pathogenesis, cardiovascular risk, and treatment. A position paper of The Obesity Society and the American Society of Hypertension. J Clin Hypertens (Greenwich) 15: 14–33, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature 379: 632–635, 1996 [DOI] [PubMed] [Google Scholar]
- 73.Lembo G, Vecchione C, Fratta L, Marino G, Trimarco V, d'Amati G, Trimarco B. Leptin induces direct vasodilation through distinct endothelial mechanisms. Diabetes 49: 293–297, 2000 [DOI] [PubMed] [Google Scholar]
- 74.Licinio J, Caglayan S, Ozata M, Yildiz BO, de Miranda PB, O'Kirwan F, Whitby R, Liang L, Cohen P, Bhasin S, Krauss RM, Veldhuis JD, Wagner AJ, DePaoli AM, McCann SM, Wong ML. Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc Natl Acad Sci USA 101: 4531–4536, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li B, Shi Z, Cassaglia PA, Brooks VL. Leptin acts in the forebrain to differentially influence baroreflex control of lumbar, renal, and splanchnic sympathetic nerve activity and heart rate. Hypertension 61: 812–819, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lim K, Burke SL, Head GA. Obesity-related hypertension and the role of insulin and leptin in high-fat-fed rabbits. Hypertension 61: 628–634, 2013 [DOI] [PubMed] [Google Scholar]
- 77.Littlejohn NK, Siel RB, Jr, Ketsawatsomkron P, Pelham CJ, Pearson NA, Hilzendeger AM, Buehrer BA, Weidemann BJ, Li H, Davis DR, Thompson AP, Liu X, Cassell MD, Sigmund CD, Grobe JL. Hypertension in mice with transgenic activation of the brain renin-angiotensin system is vasopressin-dependent. Am J Physiol Regul Integr Comp Physiol 304: R818–R828, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lu H, Duanmu Z, Houck C, Jen KL, Buison A, Dunbar JC. Obesity due to high fat diet decreases the sympathetic nervous and cardiovascular responses to intracerebroventricular leptin in rats. Brain Res Bull 47: 331–335, 1998 [DOI] [PubMed] [Google Scholar]
- 79.Ma D, Feitosa MF, Wilk JB, Laramie JM, Leiendecker-Foster C, Myers RH, Province MA, Borecki IB. Leptin is associated with blood pressure and hypertension in women from the National Heart, Lung, and Blood Institute Family Heart Study. Hypertension 53: 473–479, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mackintosh RM, Hirsch J. The effects of leptin administration in non-obese human subjects. Obes Res 9: 462–469, 2001 [DOI] [PubMed] [Google Scholar]
- 81.Mark AL, Shaffer RA, Correia ML, Morgan DA, Sigmund CD, Haynes WG. Contrasting blood pressure effects of obesity in leptin-deficient ob/ob mice and agouti yellow obese mice. J Hypertens 17: 1949–1953, 1999 [DOI] [PubMed] [Google Scholar]
- 82.Mark AL, Correia M, Morgan DA, Shaffer RA, Haynes WG. State-of-the-art-lecture: Obesity-induced hypertension: new concepts from the emerging biology of obesity. Hypertension 33: 537–541, 1999 [DOI] [PubMed] [Google Scholar]
- 83.Mark AL, Correia ML, Rahmouni K, Haynes WG. Selective leptin resistance: a new concept in leptin physiology with cardiovascular implications. J Hypertens 20: 1245–1250, 2002 [DOI] [PubMed] [Google Scholar]
- 84.Mark AL, Agassandian K, Morgan DA, Liu X, Cassell MD, Rahmouni K. Leptin signaling in the nucleus tractus solitarii increases sympathetic nerve activity to the kidney. Hypertension 53: 375–380, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marsh AJ, Fontes MA, Killinger S, Pawlak DB, Polson JW, Dampney RA. Cardiovascular responses evoked by leptin acting on neurons in the ventromedial and dorsomedial hypothalamus. Hypertension 42: 488–493, 2003 [DOI] [PubMed] [Google Scholar]
- 86.Masuo K, Straznicky NE, Lambert GW, Katsuya T, Sugimoto K, Rakugi H, Socratous F, Hastings J, Lambert EA, Ogihara T, Esler MD. Leptin-receptor polymorphisms relate to obesity through blunted leptin-mediated sympathetic nerve activation in a Caucasian male population. Hypertens Res 31: 1093–1100, 2008 [DOI] [PubMed] [Google Scholar]
- 87.Matheny M, Shapiro A, Tümer N, Scarpace PJ. Region-specific diet-induced and leptin-induced cellular leptin resistance includes the ventral tegmental area in rats. Neuropharmacology 60: 480–487, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Matsumura K, Abe I, Tsuchihashi T, Fujishima M. Central effects of leptin on cardiovascular and neurohormonal responses in conscious rabbits. Am J Physiol Regul Integr Comp Physiol 278: R1314–R1320, 2000 [DOI] [PubMed] [Google Scholar]
- 89.Metlakunta AS, Sahu M, Sahu A. Hypothalamic phosphatidylinositol 3-kinase pathway of leptin signaling is impaired during the development of diet-induced obesity in FVB/N mice. Endocrinology 149: 1121–1128, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Metlakunta AS, Sahu M, Yasukawa H, Dhillon SS, Belsham DD, Yoshimura A, Sahu A. Neuronal suppressor of cytokine signaling-3 deficiency enhances hypothalamic leptin-dependent phosphatidylinositol 3-kinase signaling. Am J Physiol Regul Integr Comp Physiol 300: R1185–R1193, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Minokoshi Y, Haque MS, Shimzau T. Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats. Diabetes 48: 287–291, 1999 [DOI] [PubMed] [Google Scholar]
- 92.Mitchell JL, Morgan DA, Correia ML, Mark AL, Sivitz WI, Haynes WG. Does leptin stimulate nitric oxide to oppose the effects of sympathetic activation? Hypertension 38: 1081–1086, 2001 [DOI] [PubMed] [Google Scholar]
- 93.Montanaro MS, Allen AM, Oldfield BJ. Structural and functional evidence supporting a role for leptin in central neural pathways influencing blood pressure in rats. Exp Physiol 90: 689–696, 2005 [DOI] [PubMed] [Google Scholar]
- 94.Moore SJ, Green JS, Fan Y, Bhogal AK, Dicks E, Fernandez BA, Stefanelli M, Murphy C, Cramer BC, Dean JC, Beales PL, Katsanis N, Bassett AS, Davidson WS, Parfrey PS. Clinical and genetic epidemiology of Bardet-Biedl syndrome in Newfoundland: A 22-year prospective, population-based, cohort study. Am J Med Genet Part A 132A: 352–360, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Morgan DA, Thedens DR, Weiss R, Rahmouni K. Mechanisms mediating renal sympathetic activation to leptin in obesity. Am J Physiol Regul Integr Comp Physiol 295: R1730–R1736, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Morimoto I, Yamamoto S, Kai K, Fujihira T, Morita E, Eto S. Centrally administered murine-leptin stimulates the hypothalamus-pituitary-adrenal axis through arginine-vasopressin. Neuroendocrinology 71: 366–374, 2000 [DOI] [PubMed] [Google Scholar]
- 97.Morimoto S, Cassell MD, Sigmund CD. The brain renin-angiotensin system in transgenic mice carrying a highly regulated human renin transgene. Circ Res 90: 80–86, 2002 [DOI] [PubMed] [Google Scholar]
- 98.Morrison SF, Madden CJ, Tupone D. Central control of brown adipose tissue thermogenesis. Front Endocrinol (Lausanne) 3: 1–19, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 145: 4880–4889, 2004 [DOI] [PubMed] [Google Scholar]
- 100.Munzberg H. Differential leptin access into the brain—a hierarchical organization of hypothalamic leptin target sites? Physiol Behav 94: 664–669, 2008 [DOI] [PubMed] [Google Scholar]
- 101.Munzberg H. Leptin-signaling pathways and leptin resistance. Forum Nutr 63: 123–132, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Myers MG, Jr, Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab 21: 643–651, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Myers MG, Jr, Munzberg H, Leinninger GM, Leshan RL. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab 9: 117–123, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nagae A, Fujita M, Kawarazaki H, Matsui H, Ando K, Fujita T. Sympathoexcitation by oxidative stress in the brain mediates arterial pressure elevation in obesity-induced hypertension. Circulation 119: 978–986, 2009 [DOI] [PubMed] [Google Scholar]
- 105.Narkiewicz K, Kato M, Phillips BG, Pesek CA, Choe I, Winnicki M, Palatini P, Sivitiz WI, Somers VK. Leptin interacts with heart rate but not sympathetic nerve traffic in healthy male subjects. J Hypertens 19: 1089–1094, 2001 [DOI] [PubMed] [Google Scholar]
- 106.Niijima A. Afferent signals from leptin sensors in the white adipose tissue of the epididymis, and their reflex effect in the rat. J Auton Nerv Syst 73: 19–25, 1998 [DOI] [PubMed] [Google Scholar]
- 107.Niijima A. Reflex effects from leptin sensors in the white adipose tissue of the epididymis to the efferent activity of the sympathetic and vagus nerve in the rat. Neurosci Lett 262: 125–128, 1999 [DOI] [PubMed] [Google Scholar]
- 108.Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Jr, Schwartz MW. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature 413: 794–795, 2001 [DOI] [PubMed] [Google Scholar]
- 109.Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: Multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab 84: 3686–3695, 1999 [DOI] [PubMed] [Google Scholar]
- 110.Payne GA, Borbouse L, Kumar S, Neeb Z, Alloosh M, Sturek M, Tune JD. Epicardial perivascular adipose-derived leptin exacerbates coronary endothelial dysfunction in metabolic syndrome via a protein kinase C-beta pathway. Arterioscler Thromb Vasc Biol 30: 1711–1717, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Plum L, Rother E, Münzberg H, Wunderlich FT, Morgan DA, Hampel B, Shanabrough M, Janoschek R, Könner AC, Alber J, Suzuki A, Krone W, Horvath TL, Rahmouni K, Brüning JC. Enhanced leptin-stimulated Pi3k activation in the CNS promotes white adipose tissue transdifferentiation. Cell Metab 6: 431–445, 2007 [DOI] [PubMed] [Google Scholar]
- 112.Prior LJ, Eikelis N, Armitage JA, Davern PJ, Burke SL, Montani JP, Barzel B, Head GA. Exposure to a high-fat diet alters leptin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension 55: 862–868, 2010 [DOI] [PubMed] [Google Scholar]
- 113.Purkayastha S, Zhang G, Cai D. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-β and NF-κB. Nat Med 17: 883–887, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Purkayastha S, Zhang H, Zhang G, Ahmed Z, Wang Y, Cai D. Neural dysregulation of peripheral insulin action and blood pressure by brain endoplasmic reticulum stress. Proc Natl Acad Sci USA 108: 2939–2944, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Selective resistance to central neural administration of leptin in agouti obese mice. Hypertension 39: 486–490, 2002 [DOI] [PubMed] [Google Scholar]
- 116.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Intracellular mechanisms involved in leptin regulation of sympathetic outflow. Hypertension 41: 763–767, 2003 [DOI] [PubMed] [Google Scholar]
- 117.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosci 23: 5998–6004, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rahmouni K, Jalali A, Morgan DA, Haynes WG. Lack of dilator effect of leptin in the hindlimb vascular bed of conscious rats. Eur J Pharmacol 518: 175–181, 2005 [DOI] [PubMed] [Google Scholar]
- 119.Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes 54: 2012–2018, 2005 [DOI] [PubMed] [Google Scholar]
- 120.Rahmouni K, Morgan DA. Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Hypertension 49: 647–652, 2007 [DOI] [PubMed] [Google Scholar]
- 121.Rahmouni K, Fath MA, Seo S, Thedens DR, Berry CJ, Weiss R, Nishimura DY, Sheffield VC. Leptin resistance contributes to obesity and hypertension in mouse models of Bardet-Biedl syndrome. J Clin Invest 118: 1458–1467, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rahmouni K, Sigmund CD, Haynes WG, Mark AL. Hypothalamic ERK mediates the anorectic and thermogenic sympathetic effects of leptin. Diabetes 58: 536–542, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ramadori G, Fujikawa T, Fukuda M, Anderson J, Morgan DA, Mostoslavsky R, Stuart RC, Perello M, Vianna CR, Nillni EA, Rahmouni K, Coppari R. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab 12: 78–87, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ravussin E, Pratley RE, Maffei M, Wang H, Friedman JM, Bennett PH, Bogardus C. Relatively low plasma leptin concentrations precede weight gain in Pima Indians. Nat Med 3: 238–240, 1997 [DOI] [PubMed] [Google Scholar]
- 125.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 37: 1595–1607, 1988 [DOI] [PubMed] [Google Scholar]
- 126.Reaven GM. Why Syndrome X? From Harold Himsworth to the insulin resistance syndrome. Cell Metab 1: 9–14, 2005 [DOI] [PubMed] [Google Scholar]
- 127.Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, Gallagher D, Mayer L, Murphy E, Leibel RL. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest 115: 3579–3586, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rosmond R, Chagnon YC, Holm G, Chagnon M, Pérusse L, Lindell K, Carlsson B, Couchard C, Björntorp P. Hypertension in obesity and the leptin receptor gene locus. J Clin Endocrinol Metab 85: 3126–3131, 2000 [DOI] [PubMed] [Google Scholar]
- 129.Saeed S, Butt TA, Anwer M, Arslan M, Froguel P. High prevalence of leptin and melanocortin-4 receptor gene mutations in children with severe obesity from Pakistani consanguineous families. Mol Genet Metab 106: 121–126, 2012 [DOI] [PubMed] [Google Scholar]
- 130.Sahu A, Metlakunta AS. Hypothalamic phosphatidylinositol 3-kinase-phosphodiesterase 3B-cyclic AMP pathway of leptin signalling is impaired following chronic central leptin infusion. J Neuroendocrinol 17: 720–726, 2005 [DOI] [PubMed] [Google Scholar]
- 131.Satoh N, Ogawa Y, Katsuura G, Numata Y, Tsuji T, Hayase M, Ebihara K, Masuzaki H, Hosoda K, Yoshimasa Y, Nakao K. Sympathetic activation of leptin via the ventromedial hypothalamus: leptin-induced increase in catecholamine secretion. Diabetes 48: 1787–1793, 1999 [DOI] [PubMed] [Google Scholar]
- 132.Scarpace PJ, Matheny M. Leptin induction of UCP1 gene expression is dependent on sympathetic innervation. Am J Physiol Endocrinol Metab 275: E259–E264, 1998 [DOI] [PubMed] [Google Scholar]
- 133.Scherrer U, Randin D, Tappy L, Vollenweider P, Jequier E, Nicod P. Body fat and sympathetic nerve activity in healthy subjects. Circulation 89: 2634–2640, 1994 [DOI] [PubMed] [Google Scholar]
- 134.Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, Elmquist JK. Leptin targets in the mouse brain. J Comp Neurol 514: 518–532, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Scott MM, Williams KW, Rossi J, Lee CE, Elmquist JK. Leptin receptor expression in hindbrain Glp-1 neurons regulates food intake and energy balance in mice. J Clin Invest 121: 2413–2421, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertension 31: 409–414, 1998 [DOI] [PubMed] [Google Scholar]
- 137.Shi Z, Chen WW, Xiong XQ, Han Y, Zhou YB, Zhang F, Gap XY, Zhu GQ. Sympathetic activation by chemical stimulation of white adipose tissues in rats. J Appl Physiol 112: 1008–1014, 2012 [DOI] [PubMed] [Google Scholar]
- 138.Simonds SE, Cowley MA. Hypertension in obesity: is leptin the culprit? Trends Neurosci 36: 121–132, 2013 [DOI] [PubMed] [Google Scholar]
- 139.Skibicka KP, Grill HJ. Hindbrain leptin stimulation induces anorexia and hyperthermia mediated by hindbrain melanocortin receptors. Endocrinology 150: 1705–1711, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Smith PM, Chambers AP, Price CJ, Ho W, Hopf C, Sharkey KA, Ferguson AV. The subfornical organ: a central nervous system site for actions of circulating leptin. Am J Physiol Regul Integr Comp Physiol 296: R512–R520, 2009 [DOI] [PubMed] [Google Scholar]
- 141.Smith PM, Ferguson AV. Circulating signals as critical regulators of autonomic state—central roles for the subfornical organ. Am J Physiol Regul Integr Comp Physiol 299: R405–R415, 2010 [DOI] [PubMed] [Google Scholar]
- 142.Smith PM, Ferguson AV. Cardiovascular actions of leptin in the subfornical organ are abolished by diet-induced obesity. J Neuroendocrinol 24: 504–510, 2012 [DOI] [PubMed] [Google Scholar]
- 143.Snitker S, Pratley RE, Nicolson M, Tataranni PA, Ravussin E. Relationship between muscle sympathetic nerve activity and plasma leptin concentration. Obes Res 5: 338–340, 1997 [DOI] [PubMed] [Google Scholar]
- 144.Sohn JW, Xu Y, Jones JE, Wickman K, Williams KW, Elmquist JK. Serotonin 2C receptor activates a distinct population of arcuate pro-opiomelanocortin neurons via TRPC channels. Neuron 71: 488–497, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Strobel A, Issad T, Camoin L, Ozata M, Strosberg AD. A leptin missense mutation associated with hypogonadism and morbid obesity. Nat Genet 18: 213–215, 1998 [DOI] [PubMed] [Google Scholar]
- 146.Takahashi Y, Okimura Y, Mizuno I, Iida K, Takahashi T, Kaji H, Abe H, Chihara K. Leptin induces mitogen-activated protein kinase-dependent proliferation of C3H10T1/2 cells. J Biol Chem 272: 12897–12900, 1997 [DOI] [PubMed] [Google Scholar]
- 147.Tanida M, Nagai K, Kaneko H. Activation of the renal sympathetic nerve by leptin microinjection into the ventromedial hypothalamus in rats. In Vivo 17: 213–217, 2003 [PubMed] [Google Scholar]
- 148.Tümer N, Erdos B, Matheny M, Cudykier I, Scarpace PJ. Leptin antagonist reverses hypertension caused by leptin overexpression, but fails to normalize obesity-related hypertension. J Hypertens 25: 2471–2478, 2007 [DOI] [PubMed] [Google Scholar]
- 149.Tups A. Physiological models of leptin resistance. J Neuroendocrinol 21: 961–971, 2009 [DOI] [PubMed] [Google Scholar]
- 150.Vaisse C, Halaas JL, Horvath CM, Darnell JE, Jr, Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet 14: 95–97, 1996 [DOI] [PubMed] [Google Scholar]
- 151.Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation 96: 3423–3429, 1997 [DOI] [PubMed] [Google Scholar]
- 152.Vecchione C, Maffei A, Colella S, Aretini A, Poulet R, Frati G, Gentile MT, Fratta L, Trimarco V, Trimarco B, Lembo G. Leptin effect on endothelial nitric oxide is mediated through Akt-endothelial nitric oxide synthase phosphorylation pathway. Diabetes 51: 168–173, 2002 [DOI] [PubMed] [Google Scholar]
- 153.Warne JP, Alemi F, Reed AS, Varonin JM, Chan H, Piper ML, Mullin ME, Myers MG, Jr, Corvera CU, Xu AW. Impairment of central leptin-mediated PI3K signaling manifested as hepatic steatosis independent of hyperphagia and obesity. Cell Metab 14: 791–803, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 154.Williams KW, Margatho LO, Lee CE, Choi M, Lee S, Scott MM, Elias CF, Elmquist K. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci 30: 2472–2479, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Xiong XQ, Chen WW, Han Y, Zhou YB, Zhang F, Gao XY, Zhu GQ. Enhanced adipose afferent reflex contributes to sympathetic activation in diet-induced obesity hypertension. Hypertension 60: 1280–1286, 2012 [DOI] [PubMed] [Google Scholar]
- 156.Xu Y, Jones JE, Kohno D, Williams KW, Lee CE, Choi MJ, Anderson JG, Heisler LK, Zigman JM, Lowell BB, Elmquist JK. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron 60: 582–589, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xu Y, Hill JW, Fukuda M, Gautron L, Sohn JW, Kim KW, Lee CE, Choi MJ, Lauzon DA, Dhillon H, Lowell BB, Zigman JM, Zhao JJ, Elmquist JK. PI3K signaling in the ventromedial hypothalamic nucleus is required for normal energy homeostasis. Cell Metab 12: 88–95, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yamamoto S, Morimoto I, Kai K, Arao T, Fujihira T, Morita E, Kannan H, Eto S. Centrally administered murine leptin stimulates plasma arginine-vasopressin secretion and increases the level of mRNA expression in the supraoptic nucleus of conscious rats. Neuroendocrinology 70: 207–212, 1999 [DOI] [PubMed] [Google Scholar]
- 159.Young CN, Morgan DA, Butler SD, Mark AL, Davisson RL. The brain subfornical organ mediates leptin-induced increases in renal sympathetic activity but not its metabolic effects. Hypertension 61: 737–744, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zelissen PM, Stenlof K, Lean ME, Fogteloo J, Keulen ET, Wilding J, Finer N, Rössner S, Lawrence E, Fletcher C, McCamish M. Effect of three treatment schedules of recombinant methionyl human leptin on body weight in obese adults: a randomized, placebo-controlled trial. Diabetes Obes Metab 7: 755–761, 2005 [DOI] [PubMed] [Google Scholar]
- 161.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432, 1994 [DOI] [PubMed] [Google Scholar]
- 162.Zhang Y, Kerman IA, Laque A, Nguyen P, Faouzi M, Louis GW, Jones JC, Rhodes C, Münzberg H. Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. J Neurosci 31: 1873–1884, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]