Abstract
Our aim was to establish in spontaneously breathing urethane-anesthetized rats, the relationship between the concentrations of H2S transported in the blood and the corresponding clinical manifestations, i.e., breathing stimulation and inhibition, during and following infusion of NaHS at increasing rates. The gaseous concentration of H2S (CgH2S, one-third of the total soluble form) was computed from the continuous determination of H2S partial pressure in the alveolar gas, while H2S, both dissolved and combined to hemoglobin, was measured at specific time points by sulfide complexation with monobromobimane (CMBBH2S). We found that using a potent reducing agent in vitro, H2S added to the whole blood had little interaction with the plasma proteins, as sulfide appeared to be primarily combined and then oxidized by hemoglobin. In vivo, H2S was undetectable in the blood in its soluble form in baseline conditions, while CMBBH2S averaged 0.7 ± 0.5 μM. During NaHS infusion, H2S was primarily present in nonsoluble form in the arterial blood: CMBBH2S was about 50 times higher than CgH2S at the lowest levels of exposure and 5 or 6 times at the levels wherein fatal apnea occurred. CgH2S averaged only 1.1 ± 0.7 μM when breathing increased, corresponding to a CMBBH2S of 11.1 ± 5.4 μM. Apnea occurred at CgH2S above 5.1 μM and CMBBH2S above 25.4 μM. At the cessation of exposure, CMBBH2S remained elevated, at about 3 times above baseline for at least 15 min. These data provide a frame of reference for studying the putative effects of endogenous H2S and for testing antidotes against its deadly effects.
Keywords: control of respiration, hydrogen sulfide, toxicity
during hydrogen sulfide (h2s) exposure, a large portion of H2S diffusing into the blood is oxidized into innocuous compounds (sulfite, sulfate, and thiosulfate) (8, 16, 22). This oxidation takes place both in the blood and in most tissues (cytoplasm and mitochondria) (for review, see Ref. 37), limiting, in turn, the dreadful effects of H2S toxicity. However, even at very low levels of exogenous H2S exposure, not all H2S is “oxidized”. H2S appears in the arterial blood (and thus by diffusion, increases in the tissue) during inhalation as low as 50 ppm or during venous infusion levels of H2S as low as 1 μmol/min in rats and humans (8, 29, 46, 54).
However, H2S can be transported in the blood in different forms, i.e., dissolved and combined. These forms are not equivalent in terms of their potential toxicity and physiological effects (8). The dissolved H2S consists of 1) H2S in gaseous form with a concentration proportional to its partial pressure (PH2S), according to Henry's law (3, 6, 10, 13, 17), and 2) the sulfhydryl anion, HS− (3, 34). The gaseous form of H2S is, at physiological pH, about 20–30% of the total dissolved H2S (27). H2S and HS− represent the only forms under which hydrogen sulfide can diffuse between blood and tissues or within cells. On the other hand, H2S can be combined with 1) metalloproteins (e.g., hemoglobin), sometimes referred to as acid-labile sulfides (7, 42, 43), and 2) cysteine residues leading to the formation of disulfide bonds (R-S-S-H), also referred to as persulfides or sulfhydrated proteins (35, 38, 51). From a toxicological standpoint, the combined forms play a dual role since they represent a mechanism of protection (41, 43, 47), trapping H2S in a nonsoluble state, but also account for some of the effects of H2S toxicity (e.g., interaction with cytochrome-c oxidase) (12, 16, 31, 33, 41). Similarly, the alteration of specific enzymatic activities by the process of sulfhydration offers novel pathways through which both exogenous and endogenous H2S could exert their toxic and physiological effects, respectively (38).
Despite decades of research on the fate of H2S in the blood (22), there is, as yet, no direct information on the relative contribution of the different forms of H2S transport and on the relationship between H2S concentrations and the acute clinical/toxic manifestations produced by sulfide during and following H2S exposure (8, 21, 41). As a consequence, any attempt to rationalize the use of potential antidotes, e.g., methemoglobin, hydroxocobalamin, bicarbonate, O2, or reducing agents (21, 41, 44, 51), remains speculative. In addition, H2S has long been shown to exert its main toxicity by inhibiting the activity of mitochondrial cytochrome-c oxidase (CCO) (12, 31) at concentrations between 10 and 50 μM in vitro. However, we do not know how much soluble/diffusible H2S must be present in vivo (16) to produce a reduction in CCO activity vs. other mechanisms that account for the main acute, clinical manifestations (41) of sulfide poisoning, such as hyperventilation (25, 28), apnea (1, 20), or coma (2).
In the current study, following experiments from Insko et al. (29) and Wintner et al. (54), we infused H2S intravenously in the form of NaHS to spontaneously breathing, urethane-anesthetized rats. We increased the infusion rate gradually until an apnea occurred (“lethal” exposure). At each step, the rate of H2S elimination and the level of dissolved H2S in the blood were measured along with breathing, used here as a clinical sign of toxicity. We determined the partial pressure of H2S in the arterial blood, and, thus, its concentration, from the measurement of the alveolar partial pressure of H2S. In addition, the concentration of H2S was measured in the arterial blood at specific time points, in steady-state conditions, using the HPLC-fluorescence technique based on complexation of reactive sulfide species with monobromobimane (MBB) (54). The chemical derivatization protocol that we used has been validated elegantly by Wintner et al. (54) as a method to measure sulfide in the blood, not only in its soluble form, but also combined with the red blood cells.
After characterizing the factors potentially affecting the measurement of H2S concentrations using the MBB technique, as well as alveolar H2S partial pressure, we present a quantitative description of the fate of the dissolved and combined forms of H2S in the blood and the limits of the approaches that we have used.
These results are discussed in the light of the potential benefits of putative antidotes against H2S toxicity and the debate over the levels of endogenous H2S required to affect structures involved in breathing control in vivo or in vitro.
METHODS
Animal Preparation
The experimental procedures were performed on 10 adult male, Sprague-Dawley rats (563 ± 136 g), as previously described (26). All procedures were approved by the Pennsylvania State University College of Medicine Institutional Animal Care and Use Committee. Anesthesia was induced with 3.5% isoflurane in O2 followed by an intraperitoneal injection of 1.2 g/kg of urethane. A tracheostomy was performed, and a catheter (14 gauge, 2.25 mm OD) was placed in the trachea. The catheter was attached to a Hans Rudolph low dead space two-way valve. The inspiratory port of the valve was connected to a pneumotachograph (1100 Series; Hans Rudolph, KS). Inspiratory flow was measured breath-by-breath. The expiratory port of the valve was connected to two 5-ml “mixing chambers” placed in series. The outlet of the second chamber was connected to a filter containing charcoal. Mixed expired CO2 and H2S fractions were measured continuously from the second mixing chamber, using a CO2 infrared (Vacumed 17630; Vacumed, Ventura, CA) and H2S (Interscan RM series; Interscan, Simi Valley, CA) analyzer, respectively. The range of the H2S analyzer is 0.001 to 1.00 ppm; at the level of infused H2S used in our study, the expired H2S fraction would be well above this range. In addition, to be able to use this analyzer in a large range of FH2S, an external source of air was introduced into the first mixing chamber in which flow (V̇add) was continuously measured via a second pneumotachograph (1100 series, Hans Rudolph, Shawnee, KS). V̇add was used to determine the actual fraction of H2S in the expired gas (see Measurements and data analysis).
Rats were placed on a heating pad, and body temperature was monitored with a rectal temperature probe (Thermalert TH-5; Physitemp, Clifton, NJ).
Catheters (PE-50 tubing) were introduced into the left external jugular or the left femoral vein and in the right femoral artery (the carotid artery was not used to prevent any unnecessary change in the CB or medullary blood flow). The arterial line allowed us to monitor arterial blood pressure (ABP) using a pressure transducer (TA-100; CWE, Ardmore, PA) and to sample blood for H2S measurement and blood gas analysis (see below). The vein was used for NaHS infusion. H2S infusion was stopped just when apnea occurred, and animals did resume their breathing activity either spontaneously or, more often, would require mechanical ventilation for a few minutes only.
Measurements and Data Analysis
The pneumotachograph and blood pressure transducers were calibrated prior to every experiment. The gas analyzers were calibrated using different gas mixtures containing 0 or 5% CO2 for the CO2 analyzer and 0 (air flowing through a charcoal filter) or 0.65 ppm of H2S.
Inspiratory flow, the additional flow of gas delivered (V̇add) to the first mixing chamber. ABP, CO2, and H2S fractions in the second mixing chamber, as well as the rectal temperature signals, were digitized using an analog-to-digital data acquisition system (PowerLab 16/35; AD Instruments; Colorado Springs, CO) at 200 Hz. Data were displayed online and stored for later analysis. Breathing frequency (f) and tidal volume (Vt) were determined using peak detection and integration of the inspiratory flow signal, respectively, and minute ventilation (V̇i) was computed as f×Vt.
Alveolar H2S Fraction, Partial Pressure, and Dissolved Concentrations of H2S in the Arterial Blood
The fraction of H2S was continuously measured from the second mixing chamber, defined as FchH2S. Assuming V̇e = V̇i, the mixed expired H2S fraction (FEH2S) was computed as FEH2S = FchH2S×(V̇e + V̇add/V̇e). The partial pressure of expired H2S (PEH2S) was then calculated as FEH2S×(PB mmHg).
As the diffusion of H2S is, by definition, only taking place in alveolar regions and not in the pulmonary dead space, the alveolar partial pressure of H2S (PAH2S) was computed as PAH2S = PEH2S× V̇e/V̇a, where V̇a is the alveolar ventilation.
V̇e/V̇a was determined from the PeCO2/PaCO2 ratio (PeCO2× V̇e = PaCO2× V̇a). PaCO2 was estimated from arterial Pco2 (PaCO2) sampled at various times during the experiment, while PeCO2 was continuously measured from the second mixing chamber.
PaH2S was equated to PAH2S (neglecting the possibility of pulmonary or extra-pulmonary shunts). The concentration of gaseous H2S in the blood (CgH2S) was calculated as CgH2S = 0.00012×PaH2S, with 0.00012 being the coefficient of solubility of H2S (0.09 mol·l−1·760 mmHg−1 at 37°C in saline) (17) (see discussion). Assuming that H2S is under the form of H2S gas and its sulfhydryl anion HS− at a ratio of 1/3 and 2/3 in the arterial blood, the concentration of dissolved H2S, CdH2S could be estimated as three times CgH2S. Finally, the rate of elimination of H2S by the lungs, V̇H2S, was determined as FchH2S× (V̇add+ V̇e).
H2S Determination in the Blood
Following a procedure validated by Wintner et al. (54), arterial blood (200 μl) was added with a syringe to a solution of MBB (20 mM in 200 μl of acetonitrile) and 200 μl HEPES (50 mM, pH 8.0) in a sealed vial. The resulting suspension was stirred for 10 min at room temperature, at which time 100 μl 0.1 N HCl was added to prevent any further reaction between MBB and H2S. The mixture was then extracted (3 × 1 ml) with ethyl acetate, dried over Na2SO4, filtered through glass wool, and the organic solvent was evaporated under vacuum. All of the extraction procedures were completed within 2 h after HCl was added. The residue was dissolved in 1 ml chloroform and purified by SFE. The columns were equilibrated with 20 ml chloroform, and the samples were applied and eluted with 30 ml chloroform, followed by 15 ml of 1% methanol/chloroform and 30 ml of 2% methanol/chloroform. Sulfide-dibimane was eluted in the 2% methanol/chloroform fraction, after being dried under vacuum and redissolved in acetonitrile prior to HPLC analysis.
Samples were analyzed using a Shimadzu HPLC system consisting of two 10AD VP pumps, a SCL-10AVP controller, and a Rheodyne injector, interfaced with a Hitachi L 7485 fluorescence detector. Data were recorded using a Hitachi D2500 integrator. A Phenomenex (Torrance, CA) C-18 Bondclone (4.6 × 300 mm, 10 μm) column was used. Solvent A was 2 mM ammonium acetate, pH 4.0, while solvent B was methanol. The flow rate was 1 ml/min. The following elution program was used: initial conditions 80% A and 20% B, followed by the addition of 2% B for 1 min to 50% B, held for 6 min, and then washed to 100% B for 2 min. The fluorescence excitation wavelength was 390 nm, and the emission wavelength was 470 nm. Under these chromatographic conditions, sulfide-bimane eluted at 19.4 min. The levels of sulfide-bimane in rat blood were determined on the basis of standard curves constructed for these analyses. The detection limit was 0.5 ng/injection. An example of a HPLC/fluorescence spectrum is shown in Fig. 1.
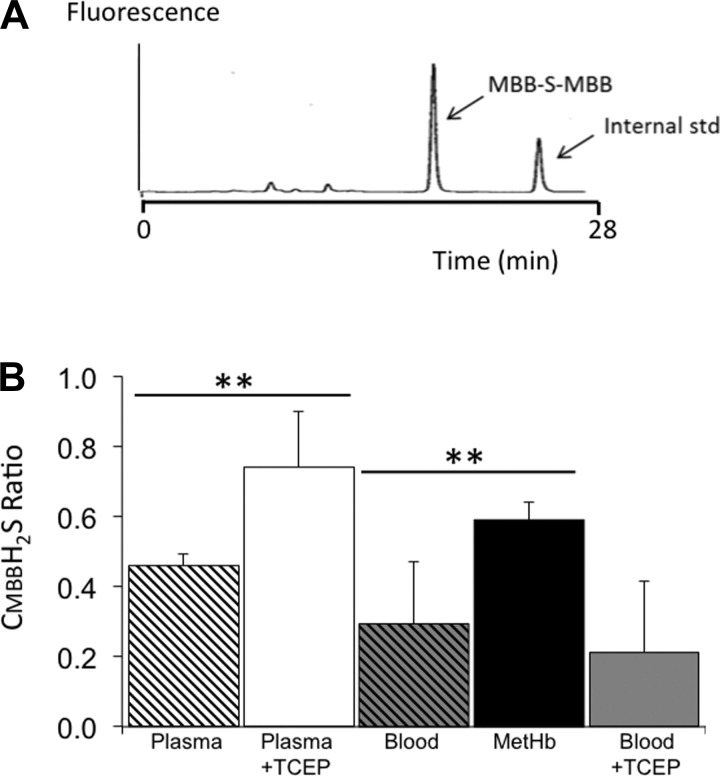
Fig. 1.
A: example of a HPLC/fluorescence spectrum of the monobromobimane (MBB)-S-MBB standard (sulfide dibimane), and MBB-derived internal standard. Note that the retention times of the MBB-S-MBB and the internal standard were 18.4 and 25.1 min, respectively. B: concentrations of H2S determined in vitro by the MBB technique after 10 min of incubating a solution of NaHS in saline, in whole blood in plasma, and plasma with methemoglobin. Data are expressed as a ratio from the saline solution containing 100 μM of H2S and analyzed at the same time. Although sulfide complexation with monobromobimane (CMBBH2S) remained unaffected in saline for up to 10 min, H2S decreased, reaching 29% of its initial concentration in the blood and 46% in the plasma. However, H2S concentrations were significantly higher in the presence of methemoglobin (59% of initial concentration) compared with whole blood, while the presence of reducing agent abolished the decrease in H2S in the plasma, but not in the whole blood. **P < 0.01.
See appendix for information regarding reagents and preparation of internal standards.
In Vitro Experiments
In addition to the validation of the MBB technique by Wintner et al. (54), who showed that the CMBBH2S reflects the concentration of soluble H2S and H2S combined to hemoglobin in the blood (see discussion), we sought to determine whether H2S present in the blood also interacts with the plasma proteins, or is primarily interacting with red cells. We measured CMBBH2S in a NaHS solution (final concentration 100 μM) mixed in 0.9% saline (n = 9), fresh rat blood collected from naïve, untreated rats (n = 8), fresh plasma (n = 10), or a solution of MetHb ([Hb]=10.0 g/100 ml, >90% MetHb) (n = 4). CMBBH2S was also measured in the plasma with and without the reducing agent Tris(2-carboxyethyl)phosphine hydrochloride (TCEP; Soltec Bio Science, Beverly, MA). The rationale was that in the presence of the reducing agent TCEP, which should limit the sulfhydration of proteins, one would expect CMBBH2S to be higher with TCEP than in control plasma. When added to the blood containing TCEP, H2S concentration should also be higher than in control blood, if the plasma proteins are interacting with H2S in the whole blood. Finally, in the presence of methemoglobin, we anticipate H2S to remain higher than in the blood if CMBBH2S is primarily influenced by the combination of H2S with hemoglobin. Samples were incubated in hermetically sealed, glass vials and processed by the MBB method, as described in the previous paragraphs. The samples were analyzed for CMBBH2S after 10 min of contact between H2S and the various solutions.
Protocol
NaHS infusion.
NaHS (sodium hydrosulfide hydrate; Sigma Aldrich, St. Louis, MO) was dissolved in sterile saline at a concentration corresponding to 10.5 μmol/ml (10.5 mM). H2S was prepared minutes before the infusion and kept in airtight syringes. Infusion rates were determined on the basis of a series of pilot experiments not included in the present data. The solution was infused intravenously starting at a rate of 2.09 μmol/min using an infusion pump (Fusion 100; Chemyx, Stafford, TX). The flow rate was increased by about 2 μmol/min every 4 min until an apnea occurred. H2S administration was stopped as soon as breathing ceased. Apneic periods were defined as breaths with an increase in TTOT by more than 5 times (see results). While FAH2S was measured continuously, blood was sampled at the third min into each infusion rate. After the animal recovered from the apneic period and when minute ventilation was back to baseline, a new infusion of a sublethal level of H2S (4.42 μmol/min) was used for 5 min, and blood was sampled again during the last minute of the infusion period, and then at 5, 10, and 15 min into recovery.
Validation of FAH2S measurement.
First, the effects of the contact time between H2S and the gas coming from the alveolar region to the analyzer and the composition of the expired gas were characterized (Fig. 2, A and B). This was achieved by interposing tubing of two different lengths, i.e., 2 m (100 ml, short circuit) or 6.5 m (325 ml, long circuit) corresponding to 0.5 s and 1.4 s, respectively, between the first and second mixing chamber and mixing a gas containing H2S with an original concentration of 25 ppm diluted with N2, 100% O2, or air (see results). Second, the influence of the additional flow of a gas (V̇add) on the measurement of FH2S was determined using a gas mixture containing H2S (0.65 ppm) in N2. This gas was delivered to the first mixing chamber at a flow ranging from 0 to 22 l/min, while FH2S was determined from the second mixing chamber.
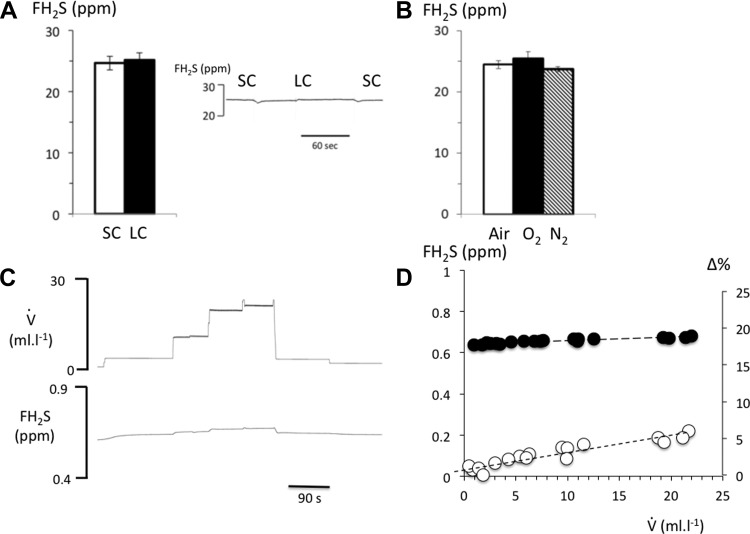
Fig. 2.
A: fraction of H2S was recomputed from the data obtained with our H2S analyzer using a calibration gas containing H2S in nitrogen (25 ppm) diluted in air to remain within the range of the analyzer (see methods). H2S was delivered and mixed with the flow of air in the first chamber, while tubing was interposed between the first and second chamber, allowing a time of contact between H2S and the air of 600 ms (short circuit, SC) or 1.5 s (long circuit, LC). The time of contact had no effect on the concentration of gaseous H2S. B: changing the composition of gas introduced into the respiratory circuit, (N2, 100% O2, or air), which mixed with the H2S calibration gas, had no effect on H2S concentration. C: effects of increasing the flow of a gas with an original FH2S of 0.65 ppm on the measured H2S fraction. D: relationship between FH2S and additional air flow, V̇add, in absolute levels (closed circles) and % of change. Note that there was a small, but systematic, increase in H2S determination by 6% when flow was increased from 0 up to 25 l/min.
Statistics
All data are expressed as means ± SD. The relationship between alveolar H2S and H2S in the blood was established in keeping with the dose infused and the level of minute ventilation. Exponential regression analysis was used to describe the relationship between CH2S and the rate of H2S infusion. In addition, a Mann-Whitney rank sum test compared baseline values to measurements taken during the different infusion rates of NaHS. Post hoc comparisons were performed using an unpaired t-test (SigmaStat 2.0; USA). The concentration of H2S at which breathing was increased by 30% was chosen as the threshold for CB stimulation (H2S-induced respiratory symptoms).
FH2S at two different contact times was compared using a paired t-test, and the values of CgH2S and CMBBH2S at the different rates of infusion were compared using one-way ANOVA. For the in vitro experiments, data were compared using one-way ANOVA. In all instances, P < 0.05 was considered as significant.
RESULTS
In vitro determination of CMBBH2S in plasma and whole blood.
In contrast to saline, in which CMBBH2S remained unchanged over time, CMBBH2S decreased dramatically in the plasma reaching 46 ± 3% of CMBBH2S in saline at 10 min. CMBBH2S also decreased over time in the whole blood, reaching 29 ± 18% of CMBBH2S in saline. As shown in Fig. 1, adding 1 mM of the reducing agent TCEP prevented the decrease in CMBBH2S in plasma (74 ± 16%; P < 0.01) but not in whole blood (21 ± 20%). Finally, CMBBH2S was found to be significantly higher after MetHb than in the blood (59 ± 0.1%; P < 0.01).
Determination of H2S in the Expired Gas
Effect of the time of contact between H2S and the expiratory gas and effect of gas composition.
We found no difference between the concentrations of the H2S in air, N2, or 100% O2 or between the short (2 m) or long (6.5 m) circuit (see methods section) as shown in Fig. 2. In other words, the time of contact, as well as the composition of the expired gas, had no effect on the fraction of H2S coming from the alveolar region up to 1.5 s. The latter is longer than the time required for a given aliquot of expired gas to reach the analyzer using a sampling flow of about 500 ml/min.
Effect of Gas Flow
To evaluate the influence of V̇add on the determination of FH2S in the mixing chamber where the expired gas was sampled by our H2S analyzer, the effects of the flow of a gas with a known concentration of H2S in N2 was evaluated in keeping with the levels of V̇add that we used in vivo (Fig. 2). FH2S increased by less than 6% between 0 and 22 l/min, likely due to the change in pressure in the mixing chamber at a high flow.
CgH2S and CMBBH2S During H2S Infusion: Effects on Breathing
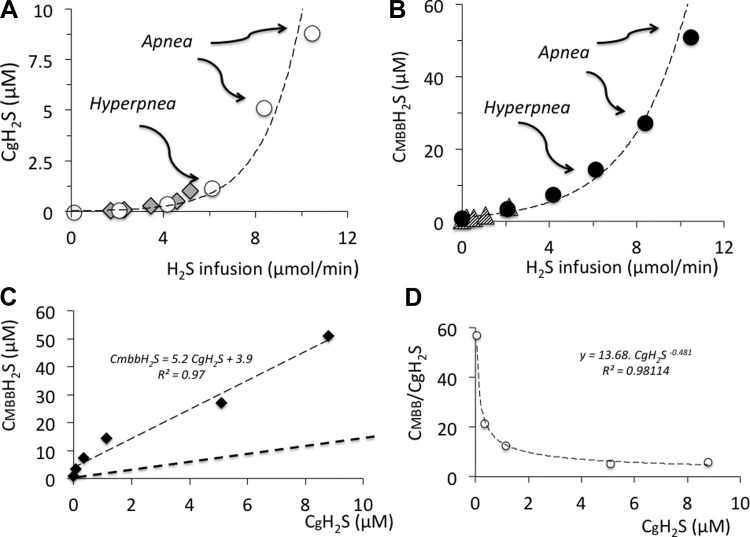
Figure 3 is an example of the change in the alveolar fraction of H2S at various rates of NaHS infusion along with ventilation. H2S was undetectable in its soluble form in baseline conditions, while CMBBH2S averaged 0.7 ± 0.5 μM. Clearly, CgH2S increased according to the level of H2S infused, and CMBBH2S followed a very similar pattern, but at a level that was about 50 times that of CgH2S at low concentrations and 5 or 6 times that of the lethal level. The average values of CgH2S and CMBBH2S, as a function of the rate of H2S infusion, are displayed in Figs. 4 and 5.
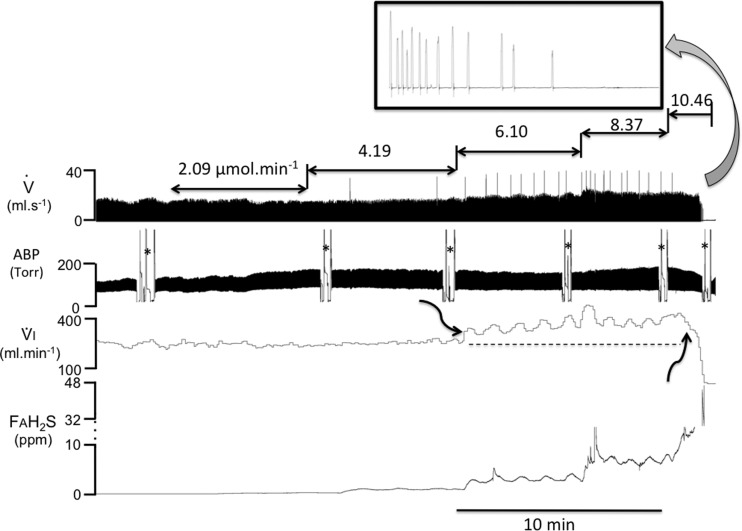
Fig. 3.
Recording from a rat receiving an incremental rate of H2S infusion from 2.09 to 10.46 μmol/min. Inspiratory flow (V̇), minute ventilation (V̇I), additional airflow (V̇add), and the alveolar fraction of H2S (FAH2S) are displayed. The symbol * indicates when arterial blood was sampled for CMBBH2S determination. Minute ventilation was stimulated (first arrow) only when the rate of H2S infusion was 6.10 μmol/min. Note that this increase in breathing was associated with a production of augmented breaths (sighs) along with periodic breathing. FAH2S increased along with the rate of NaHS infused. At the highest rate, breathing decreased dramatically (second arrow), and then an apnea occurred. In this example, infusion was stopped, allowing breathing to slowly recover following 30 s of mechanical ventilation (not shown).
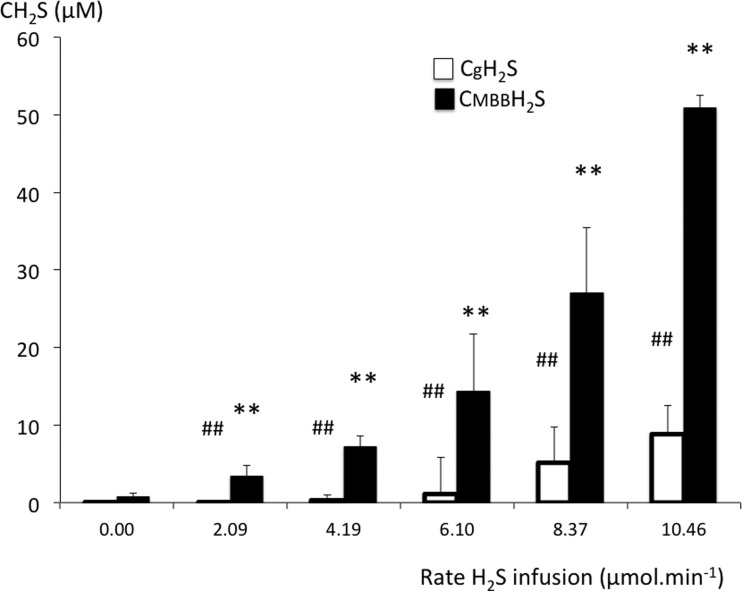
Fig. 4.
Concentration (mean ± SD) of the gaseous form of H2S in the arterial blood (CgH2S; open bars) and of H2S measured in the blood using MBB (CMBBH2S; solid bars). Increasing the rate of NaHS infusion caused an increase in CgH2S and CMBBH2S. The majority of H2S found in the blood was not in the dissolved form. CgH2S increased from zero to a maximum of 8.8 μM. Baseline CMBBH2S averaged 0.74 μM and increased up to 50.88 μM, which represents the lethal level of H2S. ##CgH2S significantly different from baseline at P < 0.001. **Significantly different from CgH2S at P < 0.001.
Fig. 5.
A: CgH2S as a function of the rate of H2S infused (only mean values are shown). Breathing was stimulated at a concentration of CgH2S around 1.1 μM, while the highest values corresponding to the lethal level ranged from 5.1 to 8.8 μM. The data recomputed from the study of Insko et al. (diamonds) fits with the relationship established in the present study (29). B: CMBBH2S as a function of the rate of H2S infused compared with data from Wintner et al. (triangles) (54). C: relationship between mean CgH2S and mean CMBBH2S. D: relationship between the CMBBH2S/CgH2S ratio and CgH2S. Note that this ratio is very high and decreases in keeping with the rate of H2S infused.
CgH2S was found to average 1.1 ± 0.7 μM when breathing was stimulated (H2S partial pressure of 9.5 10−3 ± 5.5 10−3 mmHg), corresponding to a CMBBH2S of 11.1 ± 5.4 μM. At lower concentrations, no visible effect on breathing was observed, as illustrated in Fig. 3. The change in breathing occurred as soon as the rate of infusion was increased to 6.10 μmol/min. Minute ventilation increased significantly from 284 ± 6 ml/min to 383 ± 8 ml/min (P < 0.01). This phase of hyperventilation was associated with the production of numerous augmented breaths, along with phases of periodic breathing typical of carotid body stimulation. Depression of breathing, leading to apnea, occurred between 5.1 and 8.8 μM for CgH2S and 25.4 and 50.9 μM for CMBBH2S, as illustrated in Figs. 3 and 5.
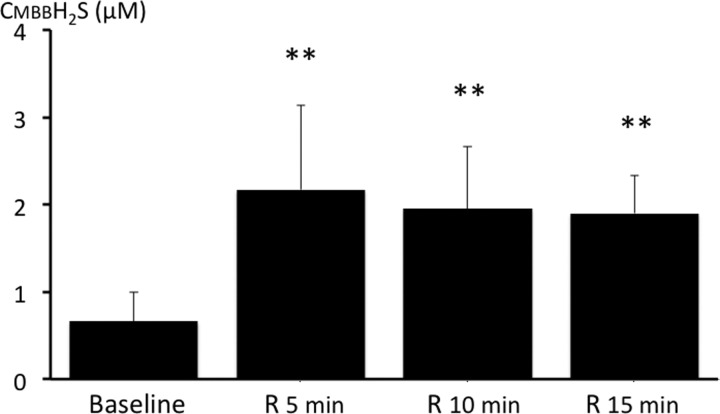
The rate of infusion, which produced a terminal apnea (8.37–10.46 μmol/min), was only 37% higher than the rate at which NaHS increased breathing. This small rise in the NaHS infusion rate increased the concentration of gaseous H2S by about 8 times, while CMBBH2S rose by 4.6 times. Typically, the apneic response triggered by NaHS consisted of a rapid depression in breathing frequency for about 1 min followed by a complete cessation of breathing. In a few tests, the period of depression was limited to about 10 s, leading to an apnea. Apneic rats were mechanically ventilated, and all rats but two resumed a spontaneous breathing pattern within 30–60 s after the cessation of NaHS infusion. In five of these animals, CH2S was determined during recovery following a new infusion of a sublethal level of NaHS at an average rate of 4.42 μmol/min for 4 min (See methods section). We had previously measured the time constant of our H2S analyzer and the respiratory circuit and found it to be between 10 and 12 s at a sampling rate of 500 ml/min. As soon as NaHS infusion was interrupted, CgH2S dropped dramatically. The kinetics of this response was indiscernible from the off-transient of the analyzer. CMBBH2S subsided progressively over time and remained above baseline, even after 15 min (Fig. 6).
Fig. 6.
CMBBH2S during recovery from NaHS infusion. CMBBH2S was still significantly higher than baseline 15 min into recovery from H2S exposure. **P < 0.01.
Balance of H2S
The rate of H2S eliminated per minute by the lungs averaged 0.0058 ± 0.0023 μmol/min for the lowest rate of infusion (2.09 μmol/min), corresponding, therefore, to 0.28% of the rate of H2S infused. The rate of elimination by the lungs reached 0.57 ± 0.36 μmol/min during infusion of lethal levels of H2S (about 5.4% of 10.46 μmol/min). For all of the rates of infusion, except the one leading to apnea, FH2S remained stable during infusion, suggesting, therefore, that the rate of elimination of H2S (e.g., via oxidation, or combination to proteins) was about the same as the rate of infusion.
DISCUSSION
We found that in the rat, the concentrations of both dissolved and combined H2S displayed an exponential relationship with the rate/concentration of H2S infused in the venous blood, until breathing was abolished (lethal dose). CgH2S represents a small portion of the total concentration of H2S present in the arterial blood. CMBBH2S/CgH2S ratio ranged from 50 for the lowest concentrations to 6 when lethal levels were reached. When the first clinical signs of H2S exposure occurred, i.e., breathing stimulation, H2S averaged 1.14 μM for CgH2S and 11.1 μM for CMBBH2S. H2S produced a terminal apnea, and was thus lethal, at concentrations of gaseous and “combined” H2S, which ranged from 5.1 to 9.8 μM and from 27.1 to 50.8 μM, respectively. These results complement the data previously published on the concentrations of H2S in the blood using the MBB technique in the rat (54) and also offer a simple way to estimate the concentrations of dissolved H2S in the blood based on the continuous determination of alveolar H2S partial pressure. Collectively, these data allow us to propose a frame of reference that can be used to study and clarify the clinical relevance of studies using exogenous sulfide to mimic the effects of endogenous H2S, as well as the potential impacts of antidotes against H2S intoxication.
What Was Measured in the Blood?
Wintner et al. (54) have already used and validated the approach based on the formation of sulfide-dibimane resulting from the reaction of H2S and MBB to determine the change in H2S concentrations in the blood during H2S inhalation or intravenous infusion. Sulfide-dibimane was identified, and its concentration was determined by reverse-phase HPLC separation coupled to fluorescence detection. Because we used a different and larger range of H2S concentrations, not all of our data could be compared with those reported by Wintner et al. (54), who studied low levels of exposure. We found, however, values very similar to those reported in that low range (See Fig. 5). We also found that CMBBH2S increased sharply, with a very steep slope, when the rate of H2S administration was close to the lethal levels of exposure, corresponding to a CMBBH2S around 30–40 μM. Wintner et al. (54) also showed, using a series of in vitro studies, that the MBB technique can identify H2S present in various forms in the blood. First, H2S concentrations in the plasma were similar to the values obtained using amperometry, which only measures soluble H2S (15, 30, 37). We found that in saline, CMBBH2S did not change within 10 min, if evaporation of H2S was prevented, in keeping with our previous results (49, 50). In addition, since H2S is present in the form of HS− (70–80%) and H2S (30–20%) at physiological pH in PBS, CMBBH2S includes all forms of dissolved H2S (gaseous and sulfhydryl anion HS−).
Perhaps more importantly, these authors reported that when exogenous H2S was added to whole blood, it disappeared virtually within seconds in its soluble form, as determined by amperometry, while CMBBH2S remained elevated for more than 20 min, subsiding progressively with time. The dissociation between the concentrations obtained from amperometry and from the MBB method in the whole blood, was not observed in the plasma (54). Although the exact nature of the H2S sink measured by MBB was not described in these studies, we found that CMBBH2S was higher when Hb was replaced by MetHb. The latter has a much higher affinity for H2S than its ferrous counterpart. Finally, adding the reducing agent TCEP (1 mM concentration) prevented the decrease in H2S concentrations in the plasma but not in the blood. Taken together, the data from Wintner al. (54) using amperometry vs. the MBB technique in the blood and plasma combined with our present results, suggest that 1) the MBB method does not only allow for the determination of H2S in its dissolved form but also measures sulfide combined, in part, with Hb, 2) the MBB technique does not measure H2S sulfhydrated on the cysteine residues of protein in the plasma, 3) H2S is primarily interacting with hemoglobin when entering the blood, preventing further reaction with the proteins in the plasma, and 4) the progressive decrease in H2S in the whole blood or in the MetHb solution in plasma reflects its oxidation by the ferrous/ferric iron.
The concentrations of dissolved H2S in the arterial blood in vivo can be measured by the amperometry technique (15, 30), which requires careful and repetitive calibrations of the electrode. As pointed out in the previous paragraph, the concentrations of H2S reported by amperometry greatly differ in vitro from CMBBH2S in the blood (54), but not in the plasma or in saline. This is also true in vivo: for instance, in Fig. 11 of their paper, Wintner et al. (54) showed that the concentrations of dissolved H2S in the blood barely reach 1 μM, while CMBBH2S averages 30 μM during sodium sulfide infusion at a rate of 1 mg·kg−1·min−1. This ratio between dissolved H2S and CMBBH2S is of the same magnitude as the ratio between dissolved and combined H2S we found in our study, while using the partial pressure of H2S in the alveolar gas. This ratio decreased as the concentration or rate of H2S infusion reached lethal levels. We determined the arterial partial pressure of H2S in the blood from the measurement of mixed expired H2S and the ratio between mixed expired and arterial Pco2 (or V̇e/V̇a), allowing the estimation of the concentration of dissolved H2S in the arterial blood in gaseous form. Indeed, when the H2S rate of disappearance equals its rate of infusion, H2S should be in equilibrium in all body compartments. A plateau of expired H2S values was reached for all the sublethal levels of exposure of H2S, so PH2S is expected to be similar in the alveolar gas and in the blood leaving the lungs. When reaching values close to the levels producing apnea, no steady state could be reached (Fig. 5) and PH2S was probably no longer in equilibrium in the lungs and the blood. This approach, which differs from traditional amperometry, relies on a certain number of prerequisites and limits. We found that the RM17-1000b hydrogen sulfide detector, which was previously used to estimate the rate of elimination of H2S by the lungs (29), is sensitive to change in humidity, which would require it to re-zero when connected to expired gas unless dry gas is used. Second, the sampling flow of these analyzers is high (around 0.5 l/min, which is about 2 times the minute ventilation of a rat) but does fluctuate around its set value. As developed in the methods, we used an additional flow of dry gas, which overcame the possible variable dilution related to this nonstable flow and, more importantly, the additional flow allowed us to use the same analyzer in a much higher range and avoided the effect of humidity. Yet, the FAH2S values that we are reporting are very close to those recomputed from the study of Insko et al. (29)—with the same analyzer—using the values of mixed, expired H2S, and assuming a V̇a/V̇e ratio of 0.7.
We selected a Henry's (H0) coefficient of 0.084 M·l−1·atm−1 for a temperature of 36°C. The solubility (H) of H2S was computed using a value of H0 determined from the studies of Douabul and Riley. (17) and Barrett et al. (6) after correction with a Setchenow coefficient of 0.064 (13) [H = H0e(−0.064·M)]. This yielded values similar to the actual data reported in a saline solution by Douabul et al. (17). Also, at a pKa of 6.9, at least 70 to 80% or more of H2S is present under the HS− form (3, 34), so that the total concentration of dissolved hydrogen sulfide can be assumed to be about 3 times CgH2S.
Even if we assume that H2S was in equilibrium in all body “water compartments” and taking into account the rate of pulmonary elimination (see results), from only 1 to 5% of the anticipated concentrations of H2S was found in the blood in the form of CgH2S. Five to 20% of the expected H2S concentrations were identified by MBB. Clearly, the majority of H2S may have been rapidly oxidized in the blood and in the tissues (32). The possibility that some of the H2S infused could have been combined with compounds “invisible” to the MBB technique should be also tested.
Baseline H2S and Recovery From H2S Exposure
Haggard (22), in his seminal paper on “the fate of H2S in the blood,” was among the first to report that as soon as H2S diffuses in the blood, it virtually disappears. Furne et al. (19) convincingly showed that in baseline conditions H2S could only be present in the blood at best in the picomolar range. Whitfield et al. (52) also challenged the numerous studies wherein the presence of endogenous H2S was found to be in the micromolar range in the blood in many species, including in humans, as well as the use of a micromolar concentration of H2S to mimic physiological changes produced by endogenous H2S (for review, see Ref. 36).
We found no measurable level of expired H2S in baseline conditions, while baseline CMBBH2S ranged between 0.3 and 0.8 μM; similar results have been reported by Wintner et al. (54) in rats and by Tokuda et al. (45) in mice. Baseline CMBBH2S origin and the exact nature remain to be determined: whether CMBBH2S is a marker or can be used as a surrogate of the presence of endogenous H2S, or other thiol compounds, is unknown.
Finally, it is quite interesting that CMBBH2S remains elevated in the blood for at least 15 min after the cessation of H2S exposure. This suggests that H2S may remain combined with metalloprotein compounds in the tissue (such as cytochrome-c oxidase) well after the end of exposure and could account for the beneficial effects of antidotes administered following H2S intoxication in humans (24).
Concentrations of H2S and Breathing
The levels of H2S partial pressure, and, therefore, dissolved H2S concentrations, present in the blood were determined in keeping with the clinical respiratory symptoms of H2S toxicity (41). Since the very first description of the effects of exogenous H2S on breathing (23, 28), it has been well established that 1) the ventilatory stimulation produced by H2S is exclusively mediated by the arterial chemoreceptors (28, 53), and 2) H2S-induced apnea is the result of the direct effects of H2S on medullary respiratory neurons (20) and to some extent the stimulation of pulmonary vagal afferents (1), although this latter notion has been challenged. Indeed, in the sheep, the apneic response to H2S is unaltered after bilateral vagotomy (25). Typically, hyperventilation occurs with H2S around 500 ppm in humans, while death occurs by apnea (inaccurately termed “respiratory paralysis”) around 1,000 ppm in the course of an extremely short period of exposure (4, 8, 9, 18, 21, 41). Although these figures should be regarded more as a general frame of reference rather than absolute thresholds for toxicity, we found that the concentrations of dissolved H2S required to produce these effects were very reproducible between animals. They range within less than 1 μM for breathing stimulation and less than 8 μM for the cessation of breathing, i.e., 3 and 24 μM if total dissolved H2S and HS− are considered. These concentrations should be compared with the much higher levels of H2S used to stimulate CB in vitro, i.e., between 30 and 100 μM (39) or the 300 μM of H2S required to affect breathing control when applied to a brain stem preparation (11). Our present results suggest, that H2S partial pressures and, thus, H2S concentrations required to stimulate breathing in vivo can be quite low, i.e., low micromolar range, in the blood and are, thus, probably even lower in the tissue. This offers the possibility, at least for the stimulatory response, that H2S can act through nonmitochondrial mechanisms. The other possibility is that, in vivo, much lower concentrations of H2S than those reported from in vitro experiments, i.e., using cells, tissue, or mitochondria, are already impeding cytochrome-c oxidase activity in the CB (12, 31).
Clinical Relevance
H2S is traditionally regarded as a chemical hazard in oil and gas production (5, 18) and in well drilling and gas refining (9, 18). Because H2S can be easily manufactured, using material readily available to everyone, it has sadly become an appalling method of suicide, which incidence has increased over the last few years, first in Japan and now in the United States (40, 48). For the same reasons, H2S is also regarded as a possible threat by the Department of Homeland Security (14).
Using a model based on the separation of diffusible H2S determined from alveolar partial pressure and its combined form, a rational frame of reference can be proposed to study how the various antidotes could affect H2S during, and more importantly, following H2S intoxication. Even if 1) the concentrations and kinetics of H2S will remain unknown in humans, and 2) the absolute levels of sulfide may well differ among species, the model developed in this study offers a clinically relevant approach to study the possible benefit of antidotes, in keeping with the acute clinical/respiratory manifestations both during and following H2S exposure.
Conclusions
We have established that 1) H2S is predominantly present in the blood in nondissolved forms, and 2) relatively low concentrations of gaseous H2S were found in the blood when signs of toxicity occurred: around 1 μM when breathing was stimulated and between 5 and 8 μM when breathing stopped. These data challenge the use of high micromolar concentrations of exogenous sulfide to study the effects of endogenous sulfide. The combined measurement of CgH2S and CMBBH2S represents an interesting tool and frame of reference that could be used to study the potential effects and benefits of H2S antidotes during and following sulfide exposure.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: C.M.K., N.T., B.P., and P.H. performed experiments; C.M.K., N.T., B.P., and P.H. analyzed data; C.M.K. and P.H. interpreted results of experiments; C.M.K., N.T., B.P., and P.H. prepared figures; C.M.K. and P.H. drafted manuscript; C.M.K. and P.H. edited and revised manuscript; C.M.K. and P.H. approved final version of manuscript; P.H. conception and design of research.
ACKNOWLEDGMENTS
This work was supported by the CounterACT Program, National Institutes of Health Office of the Director, and the National Institute of Neurological Disorders and Stroke, Grant 1R21NS-080788-01.
Appendix
H2S Determination in the Blood
Reagents.
Monobromobimane (MBB), 1,2-ethanedithiol, HEPES, solid-phase extraction (SFE) columns (silica, 1 g/6 ml), and silica gel (200–400 mesh, 60 Å) were purchased from Sigma-Aldrich (St. Louis, MO). Sodium sulfide was obtained from Alfa Aesar (Ward Hill, MA). Ethyl acetate and hydrochloric acid (0.1 N) were obtained from Fisher Scientific (Pittsburg, PA).
Preparation of standards.
Sulfide-dibimane was prepared according to a previously published method (54). Briefly, MBB (0.06 mmol) was dissolved in 250 ml of acetonitrile and added to 750 μl HEPES (100 mM, pH 8.0) followed by the addition of sodium sulfide (0.03 mmol in water). The reaction mixture was stirred under N2 at room temperature for 50 min followed by extraction with ethyl acetate. The organic extracts were evaporated under vacuum, and the resulting residue was dissolved in chloroform and purified by silica gel column chromatography. The column was eluted progressively with chloroform, 1% methanol, 2% methanol, and 5% methanol in chloroform. The final product eluted in 5% methanol/chloroform. The organic solvent containing the sulfide-dibimane was evaporated under vacuum. The identity of the product was confirmed by proton NMR and mass spectrometry. NMR (CDCl3)d 3.8 (s, 4H, CH2), 2.3 (s, 6H, CH3), 1.9 (s, 6H, CH3), 1.85 (s,6H, CH3); m/z (M + H) 415.
1,2-Ethanedithiol-dibimane was synthesized as follows: 1,2-ethanedithiol (0.6 mmol) was dissolved in 500 μl of acetonitrile. 500 μl of HEPES (5 mM, pH 8.0) was added to this solution followed by 0.25 mmol MBB in 1.25 ml of acetonitrile. The solution was stirred under N2 at room temperature for 2 h and extracted with ethyl acetate. The organic extracts were dried over Na2SO4, and the organic solvent was evaporated under vacuum. The resulting product was purified by silica gel column chromatography in a similar manner as described above for purification of sulfide-dibimane. The identity of the product was confirmed by proton NMR and mass spectrometry.
REFERENCES
- 1.Almeida AF, Guidotti TL. Differential sensitivity of lung and brain to sulfide exposure: a peripheral mechanism for apnea. Toxicol Sci 50: 287–293, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Almeida AF, Nation PN, Guidotti TL. Mechanism and treatment of sulfide-induced coma: a rat model. Int J Toxicol 27: 287–293, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Almgren T, Dyrssen D, Elgquist B, Johannsson O. Dissociation of hydrogen sulfide in seawater and comparison of pH scales. Mar Chem 4: 289–297, 1976 [Google Scholar]
- 4.Ammann HM. A new look at physiological respiratory response to H2S poisoning. J Hazard Mat 13: 369–374, 1986 [Google Scholar]
- 5.Arnold IM, Dufresne RM, Alleyne BC, Stuart PJ. Health implication of occupational exposures to hydrogen sulfide. J Occup Med 27: 373–376, 1985 [DOI] [PubMed] [Google Scholar]
- 6.Barrett TJ, Anderson GM, Lugowski J. The solubility of hydrogen sulphide in 0–5 m NaCl solutions at 25–95°C and one atmosphere. Geochim Cosmochim Acta 52: 807–811, 1988 [Google Scholar]
- 7.Baxter CF, Van Reen R. The oxidation of sulfide to thiosulfate by metalloprotein complexes and by ferritin. Biochim Biophys Acta 28: 573–578, 1958 [DOI] [PubMed] [Google Scholar]
- 8.Beauchamp RO, Jr, Bus JS, Popp JA, Boreiko CJ, Andjelkovich DA. A critical review of the literature on hydrogen sulfide toxicity. Crit Rev Toxicol 13: 25–97, 1984 [DOI] [PubMed] [Google Scholar]
- 9.Bronstein AC, Spyker DA, Cantilena LR, Jr, Green J, Rumack BH, Heard SE. 2006 Annual Report of the American Association of Poison Control Centers' National Poison Data System (NPDS). Clin Toxicol (Phila) 45: 815–917, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Carroll JJaM AE. The solubility of hydrogen sulphide in water from 0 to 90°C and pressures to 1 MPa. Geochim Cosmochim Acta 53: 1163–1170, 1989 [Google Scholar]
- 11.Chen L, Zhang J, Ding Y, Li H, Nie L, Zhou H, Tang Y, Zheng Y. Site-specific hydrogen sulfide-mediated central regulation of respiratory rhythm in medullary slices of neonatal rats. Neuroscience 233: 118–126, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Cooper CE, Brown GC. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J Bioenerg Biomembr 40: 533–539, 2008 [DOI] [PubMed] [Google Scholar]
- 13.De Bruyn WJ, Swartz E, Hu JH, Shorter JA, Davidovits P, Worsnop DR, Zahniser S, Kolb CE. Henry's law solubilities and Setchenow coefficients for biogenic reduced sulfur species obtained from gas-liquid uptake measurements. J Geophys Res 100: 7245–7251, 1995 [Google Scholar]
- 14.Department of Homeland Security Appendix to Chemical Facility Anti-Terrorism Standards; Final rule (FR No 07-5585) Washington, DC: Department of Homeland Security, 2007 [Google Scholar]
- 15.Doeller JE, Isbell TS, Benavides G, Koenitzer J, Patel H, Patel RP, Lancaster JR, Jr, Darley-Usmar VM, Kraus DW. Polarographic measurement of hydrogen sulfide production and consumption by mammalian tissues. Anal Biochem 341: 40–51, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Dorman DC, Dautrebande L, Moulin FJ, McManus BE, Mahle KC, James RA, Struve MF. Cytochrome oxidase inhibition induced by acute hydrogen sulfide inhalation: correlation with tissue sulfide concentrations in the rat brain, liver, lung, and nasal epithelium. Toxicol Sci 65: 18–25, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Douabul AA, Riley JP. The solubility of gases in distilled water and seawater-V. Hydrogen sulphide. Deep-Sea Res 26A: 259–268, 1979 [Google Scholar]
- 18.Environmental Protection Agency Toxicological Review of Hydrogen Sulfide (CAC No 7783-06-04) Washington, DC: United States Environmental Protection Agency, 2003 [Google Scholar]
- 19.Furne J, Saeed A, Levitt MD. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am J Physiol Regul Integr Comp Physiol 295: R1479–R1485, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Greer JJ, Reiffenstein RJ, Almeida AF, Carter JE. Sulfide-induced perturbations of the neuronal mechanisms controlling breathing in rats. J Appl Physiol 78: 433–440, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Guidotti TL. Hydrogen sulfide: advances in understanding human toxicity. Int J Toxicol 29: 569–581, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Haggard HW. The fate of sulfides in the blood. J Biol Chem 49: 519–529, 1921 [Google Scholar]
- 23.Haggard HW, Henderson Y. The influence of hydrogen sulphide upon respiration. Am J Physiol 41: 289–297, 1922 [Google Scholar]
- 24.Hall AH, Rumack BH. Hydrogen sulfide poisoning: an antidotal role for sodium nitrite? Vet Hum Toxicol 39: 152–154, 1997 [PubMed] [Google Scholar]
- 25.Haouzi P. Ventilatory and metabolic effects of exogenous hydrogen sulfide. Respir Physiol Neurobiol 184: 170–177, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Haouzi P, Van de Louw A. Uncoupling mitochondrial activity maintains body V̇o2 during hemorrhage-induced O2 deficit in the anesthetized rat. Respir Physiol Neurobiol 186: 87–94, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Hershey JP, Plese T, Millero FJ. The Pk1-Star for the dissociation of H2S in various ionic media. Geochim Cosmochim Acta 52: 2047–2051, 1988 [Google Scholar]
- 28.Heymans C, Bouckaert JJ, Regneirs P. Le Sinus Carotidien et la Zone Homologue Cardio-Aortique. Paris: G. Doin & Co, 1933, p. 334 [Google Scholar]
- 29.Insko MA, Deckwerth TL, Hill P, Toombs CF, Szabo C. Detection of exhaled hydrogen sulphide gas in rats exposed to intravenous sodium sulphide. Br J Pharmacol 157: 944–951, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeroschewski P, Haase K, Trommer A, Grundler P. Galvanic Sensor for the Determination of Hydrogen-Sulfide in Aqueous-Media. Fresenius J Anal Chem 346: 930–933, 1993 [Google Scholar]
- 31.Khan AA, Schuler MM, Prior MG, Yong S, Coppock RW, Florence LZ, Lillie LE. Effects of hydrogen sulfide exposure on lung mitochondrial respiratory chain enzymes in rats. Toxicol Appl Pharmacol 103: 482–490, 1990 [DOI] [PubMed] [Google Scholar]
- 32.Lagoutte E, Mimoun S, Andriamihaja M, Chaumontet C, Blachier F, Bouillaud F. Oxidation of hydrogen sulfide remains a priority in mammalian cells and causes reverse electron transfer in colonocytes. Biochim Biophys Acta 1797: 1500–1511, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Leschelle X, Goubern M, Andriamihaja M, Blottiere HM, Couplan E, Gonzalez-Barroso MD, Petit C, Pagniez A, Chaumontet C, Mignotte B, Bouillaud F, Blachier F. Adaptative metabolic response of human colonic epithelial cells to the adverse effects of the luminal compound sulfide. Biochim Biophys Acta 1725: 201–212, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Millero FJ. The thermodynamics and kinetics of hydrogen sulfide system in natural waters. Mar Chem 18: 121–147, 1986 [Google Scholar]
- 35.Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang G, Wang R, Snyder SH. H2S signals through protein S-sulfhydration. Sci Signal 2: ra72, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olson KR. Is hydrogen sulfide a circulating “gasotransmitter” in vertebrate blood? Biochim Biophys Acta 1787: 856–863, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Olson KR. A practical look at the chemistry and biology of hydrogen sulfide. Antioxid Redox Signal 17: 32–44, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paul BD, Snyder SH. H2S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol 13: 499–507, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Peng YJ, Nanduri J, Raghuraman G, Souvannakitti D, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR. H2S mediates O2 sensing in the carotid body. Proc Natl Acad Sci USA 107: 10719–10724, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reedy SJ, Schwartz MD, Morgan BW. Suicide fads: frequency and characteristics of hydrogen sulfide suicides in the United States. West J Emerg Med 12: 300–304, 2011 [PMC free article] [PubMed] [Google Scholar]
- 41.Reiffenstein RJ, Hulbert WC, Roth SH. Toxicology of hydrogen sulfide. Annu Rev Pharmacol Toxicol 32: 109–134, 1992 [DOI] [PubMed] [Google Scholar]
- 42.Smith RP. The oxygen and sulfide binding characteristics of hemoglobins generated from methemoglobin by two erythrocytic systems. Mol Pharmacol 3: 378–385, 1967 [PubMed] [Google Scholar]
- 43.Smith RP, Gosselin RE. On the mechanism of sulfide inactivation by methemoglobin. Toxicol Appl Pharmacol 8: 159–172, 1966 [DOI] [PubMed] [Google Scholar]
- 44.Smith RP, Kruszyna R, Kruszyna H. Management of acute sulfide poisoning. Effects of oxygen, thiosulfate, and nitrite. Arch Environ Health 31: 166–169, 1976 [DOI] [PubMed] [Google Scholar]
- 45.Tokuda K, Kida K, Marutani E, Crimi E, Bougaki M, Khatri A, Kimura H, Ichinose F. Inhaled hydrogen sulfide prevents endotoxin-induced systemic inflammation and improves survival by altering sulfide metabolism in mice. Antioxid Redox Signal 17: 11–21, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toombs CF, Insko MA, Wintner EA, Deckwerth TL, Usansky H, Jamil K, Goldstein B, Cooreman M, Szabo C. Detection of exhaled hydrogen sulphide gas in healthy human volunteers during intravenous administration of sodium sulphide. Br J Clin Pharmacol 69: 626–636, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Truong DH, Mihajlovic A, Gunness P, Hindmarsh W, O'Brien PJ. Prevention of hydrogen sulfide (H2S)-induced mouse lethality and cytotoxicity by hydroxocobalamin (vitamin B12a). Toxicology 242: 16–22, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Truscott A. Suicide fad threatens neighbours, rescuers. CMAJ 179: 312–313, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van de Louw A, Haouzi P. Ferric iron and cobalt (III) compounds to safely decrease hydrogen sulfide in the body? Antioxid Redox Signal 19: 510–516, 2013 [DOI] [PubMed] [Google Scholar]
- 50.Van de Louw A, Haouzi P. Oxygen deficit and H2S in hemorrhagic shock in rats. Crit Care 16: R178, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Warenycia MW, Goodwin LR, Francom DM, Dieken FP, Kombian SB, Reiffenstein RJ. Dithiothreitol liberates non-acid labile sulfide from brain tissue of H2S-poisoned animals. Arch Toxicol 64: 650–655, 1990 [DOI] [PubMed] [Google Scholar]
- 52.Whitfield NL, Kreimier EL, Verdial FC, Skovgaard N, Olson KR. Reappraisal of H2S/sulfide concentration in vertebrate blood and its potential significance in ischemic preconditioning and vascular signaling. Am J Physiol Regul Integr Comp Physiol 294: R1930–R1937, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Winder CV, Winder HO. The seat of action of sulfide on pulmonary ventilation. Am J Physiol 105: 337–352, 1933 [Google Scholar]
- 54.Wintner EA, Deckwerth TL, Langston W, Bengtsson A, Leviten D, Hill P, Insko MA, Dumpit R, VandenEkart E, Toombs CF, Szabo C. A monobromobimane-based assay to measure the pharmacokinetic profile of reactive sulphide species in blood. Br J Pharmacol 160: 941–957, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]