Abstract
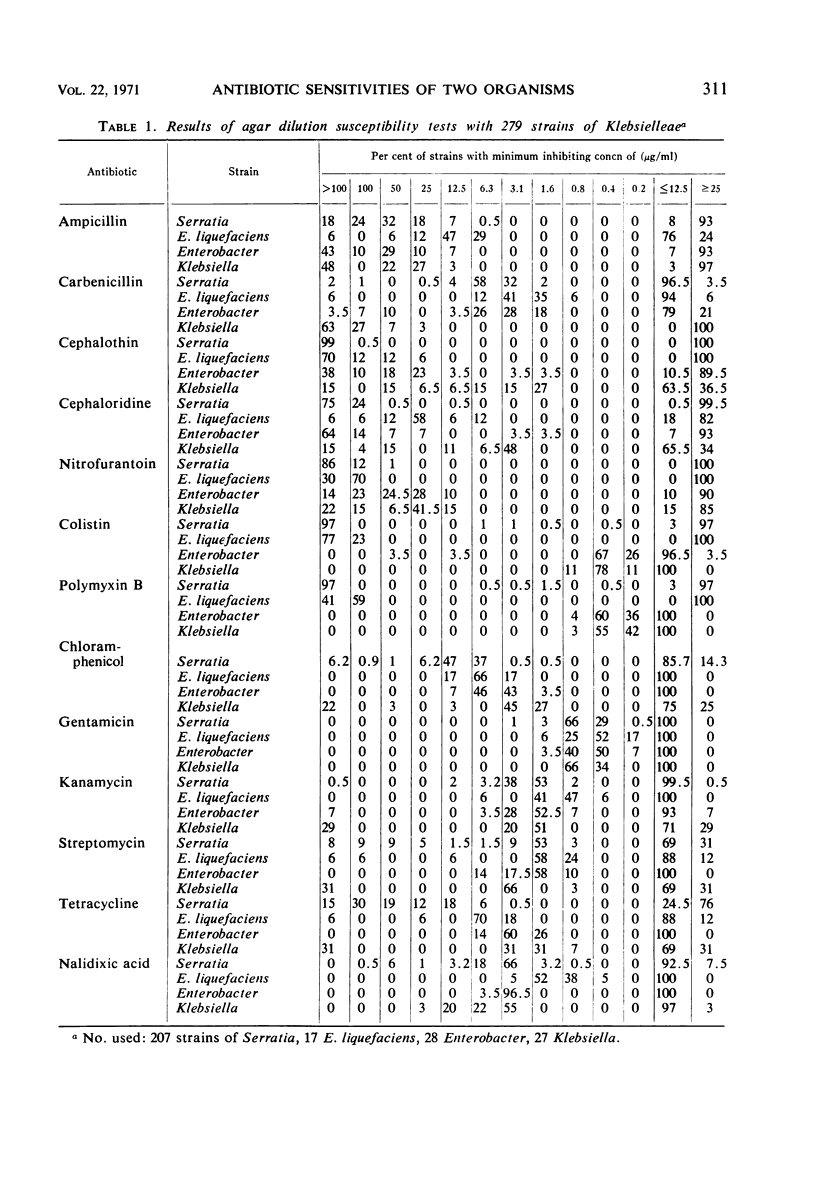
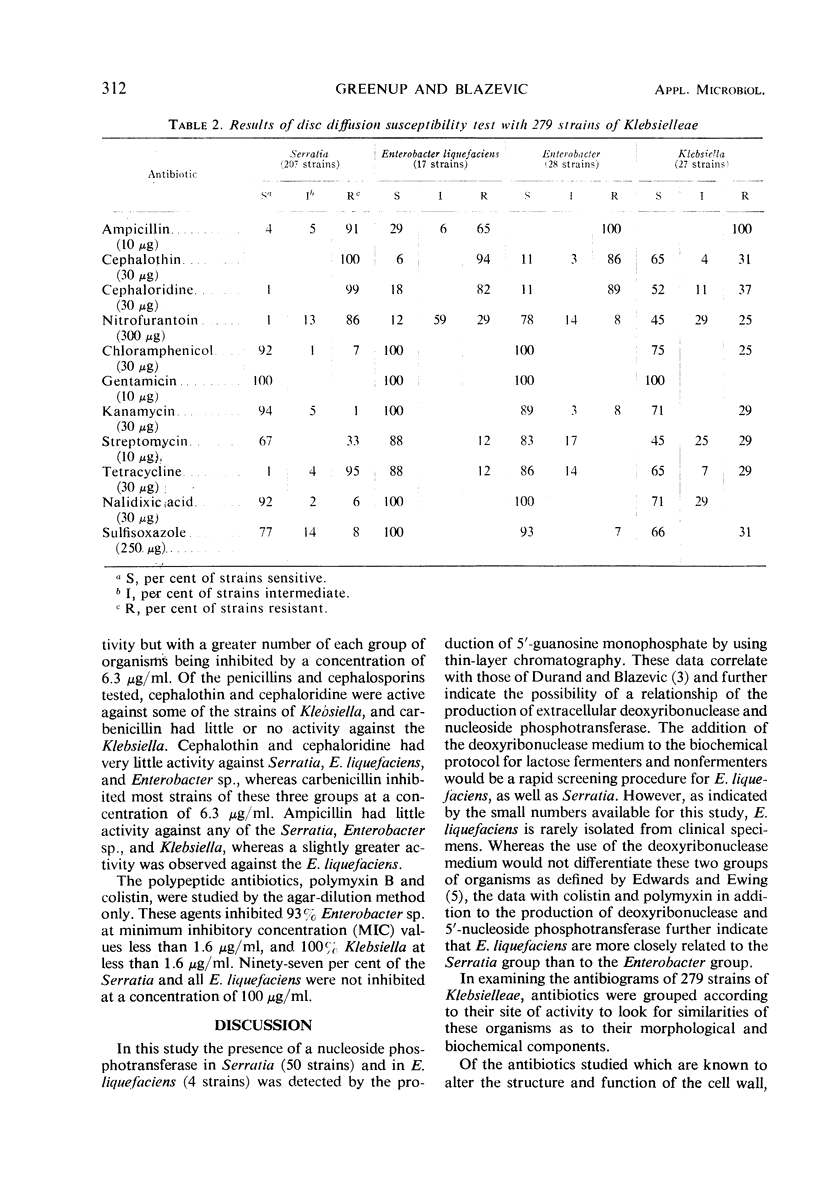
Production of 5′-nucleotides by Serratia marcescens and Enterobacter liquefaciens correlates with deoxyribonuclease production, indicating the close relationship between these two organisms. To determine further relationships, susceptibilities of 279 strains of the tribe Klebsielleae were determined by the high-potency disc method, agar-dilution method, or both, by using 14 antibiotics. Ninety-seven per cent of S. marcescens (201 of 207 strains) and 100% of E. liquefaciens (17 strains) had minimum inhibitory concentration (MIC) of 100 μg/ml or greater with colistin and polymyxin B. With these two antibiotics, 93% of other Enterobacter species (28 strains) had MIC values of less than 1.6 μg/ml, and 100% of Klebsiella (27 strains) had MIC values less than 1.6 μg/ml. Consistent patterns were not noted with the other antibiotics tested, but the results with colistin and polymyxin B provide additional evidence of the close relationship of S. marcescens and E. liquefaciens.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Durand A. M., Blazevic D. J. Differentiation of Serratia from Enterobacter on the basis of nucleoside phosphotransferase production. Appl Microbiol. 1970 Jan;19(1):134–137. doi: 10.1128/am.19.1.134-137.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson E. B., Sanford J. P. The Klebsiella-Enterobacter (Aerobacter)-Serratia group. A clinical and bacteriological evaluation. Medicine (Baltimore) 1967 Jul;46(4):323–340. doi: 10.1097/00005792-196707000-00002. [DOI] [PubMed] [Google Scholar]
- Greenfield S., Feingold D. S. The synergistic action of the sulfonamides and the polymyxins against Serratia marcescens. J Infect Dis. 1970 May;121(5):555–558. doi: 10.1093/infdis/121.5.555. [DOI] [PubMed] [Google Scholar]
- Martin W. J., Ewing W. H. The deoxyribonuclease test as applied to certain gram-negative bacteria. Can J Microbiol. 1967 May;13(5):616–618. doi: 10.1139/m67-080. [DOI] [PubMed] [Google Scholar]
- Oberhofer T. R., Hajkowski R. Evaluation of non-lactose-fermenting members of the Klebsiella-Enterobacter-Serratia division. II. Antibiotic susceptibility. Am J Clin Pathol. 1970 Nov;54(5):726–732. doi: 10.1093/ajcp/54.5.726. [DOI] [PubMed] [Google Scholar]
- Poupard J. A., Dewees L. B., Morton H. E. Antibiotic susceptibility of Klebsiella-Enterobacter as determined by a single high-concentration disc method. Antimicrob Agents Chemother (Bethesda) 1969;9:489–494. [PubMed] [Google Scholar]
- Ramirez M. J. Differentiation of Klebsiella-Enterobacter (Aerobacter)-Serratia by biochemical tests and antibiotic susceptibility. Appl Microbiol. 1968 Oct;16(10):1548–1550. doi: 10.1128/am.16.10.1548-1550.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford J. P. Sensitivity tests of klebsiella, enterobacter, and serratia. J Infect Dis. 1969 Apr-May;119(4):388–390. doi: 10.1093/infdis/119.4-5.388. [DOI] [PubMed] [Google Scholar]
- Schreier J. B. Modification of deoxyribonuclease test medium for rapid identification of Serratia marcescens. Am J Clin Pathol. 1969 Jun;51(6):711–716. doi: 10.1093/ajcp/51.6.711. [DOI] [PubMed] [Google Scholar]
- Stemper J. E., Matsen J. M. Device for turbidity standardizing of cultures for antibiotic sensitivity testing. Appl Microbiol. 1970 Jun;19(6):1015–1016. doi: 10.1128/am.19.6.1015-1016.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub W. H., Raymond E. A., Linehan J. Identification of Enterobacteriaceae in the clinical microbiology laboratory. Appl Microbiol. 1970 Sep;20(3):303–308. doi: 10.1128/am.20.3.303-308.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilfert J. N., Barrett F. F., Ewing W. H., Finland M., Kass E. H. Serratia marcescens: biochemical, serological, and epidemiological characteristics and antibiotic susceptibility of strains isolated at Boston City Hospital. Appl Microbiol. 1970 Feb;19(2):345–352. doi: 10.1128/am.19.2.345-352.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabransky R. J., Hall J. W., Day F. E., Needham G. M. Klebsiella, Enterobacter, and Serratia: biochemical differentiation and susceptibility to ampicillin and three cephalosporin derivatives. Appl Microbiol. 1969 Aug;18(2):198–203. doi: 10.1128/am.18.2.198-203.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Graevenitz A. Sensitivity studies on Serratia strains from clinical specimens. Chemotherapy. 1967;12(4):215–225. doi: 10.1159/000220503. [DOI] [PubMed] [Google Scholar]



