Abstract
Aim/Objective
Peripartum cardiomyopathy (PPCM) is a disorder of unknown etiology in which symptoms of heart failure occur between the last month of pregnancy and 5 months post-partum. These findings prompted us to carry out a more detailed study aimed at correlating plasma levels of C-reactive protein TNF-α and IL-6 as prognostic value for major clinical in-hospital events and 6-month follow-up in patients with PPCM.
Materials and Methods
After ethical clearance, in the present prospective case–control study, a total of 86 subjects were enrolled [patients (n = 46) and controls (n = 40)]. After checking for the inclusion and exclusion criteria, informed consent was obtained and patients were enrolled. The details of history of pre-eclampsia and mode of delivery were obtained from the patients. The history of onset of symptoms and signs was recorded at the first presentation and at 6 months. Clinical assessment, echocardiography, and blood analysis were done at baseline and after 6 months of standard therapy. All patients received treatment with diuretics and the ACE inhibitor (ramipril), Carvedilol if not contraindicated, and inotropic support inj-Dobutamine. Inflammatory markers (C-reactive protein, TNF-α, and IL-6) were measured at baseline and at 6 months. Data were analyzed using the SAS version 9.1 statistical program.
Results
The characteristics of the study population at first presentation to the cardiac clinic are similar (compared with controls): 0.91 % of the study patients were diagnosed as PPCM patients for the first time and 49 % patients presented within one month after delivery. C-reactive protein (22 vs 08 mg/dl, p < 0.05), TNF-α (9.6 vs 3.2 pg/dl, p < 001), and IL-6 (73.19 ± 34.4 vs 31.52 ± 8.83 pg/dl, p < 0.005) were significantly abnormal, and these patients showed significantly higher LV dimensions, LV EDD (61.6 ± 7.1 vs 46 ± 9 mm p < 0.004) LV ESD (53.1 ± 7 vs 32 ± 8, p < 0.005), and significantly lower echocardiographic left ventricular ejection fraction (LVEF) (25.9 ± 8.2 vs 55 ± 12 p < 0.001) and correlate well with NYHA FC and death. LVEF improved from 25.9 ± 8.2 to 42.9 + 13.6 % at 6 months (p < 0.0001). Patients who completed 6 months of standard care showed a significant reduction of heart rate, LV dimensions, and NYHA FC (p < 0.001). However, normalization of LVEF (>50 %) was only observed in 11 (35 %) patients. Seven patients died within 6 months of diagnoses and eight patients were lost to follow-up.
Conclusions
Plasma markers of inflammation were significantly elevated in PPCM patients and correlated with increased LV dimensions and lower EF at presentation. Baseline CRP, IL-6, TNF-α, and higher NYHA FC were the only predictors of mortality. These results contribute to inflammation which may contribute to the pathogenesis of PPCM and its complications and predictors of mortality.
Keywords: Peripartum cardiomyopathy , IL-6, Tumor Necrotic Factor-α, Left ventricular failure, LV ejection fraction
Introduction
Peripartum cardiomyopathy (PPCM) is a disorder of unknown etiology in which symptoms of heart failure occur between the last month of pregnancy and 5 months post-partum. Adherence to this time interval was emphasized to exclude preexisting causes of heart failure that may be exacerbated by pregnancy rather than arising as a result of pregnancy [1–4].
We studied of the mechanism of pathogenesis, clinical features, and prognostic markers in this disease. Heart failure is characterized by activation of pro-inflammatory cytokines TNF-α and IL-6 and elevation of C-reactive protein [5, 6]. C-reactive protein is an acute-phase protein which, due to its binding specificity for phosphocholine present in a large variety of molecules, recognizes a range of pathogenic targets including membranes of apoptotic and reactive cells [7]. Cardiac biomarkers, including B-type natriuretic peptide, are elevated in patients presenting with PPCM, although these markers are not unique to PPCM. Elevations of troponin T (TnT) appear to have prognostic significance in this group. The TnT level ≥0.04 ng/ml at presentation predicts persistence of systolic dysfunction with a sensitivity of 55 % and specificity of 91 % [8]. Inflammatory cytokines (TNF-α and IL-6) are elevated in women with PPCM compared to pregnancy controls [9, 10]. However, these cytokines are elevated in patients with other cardiomyopathies. As this inflammatory marker is associated with adverse prognosis in patients with idiopathic dilated cardiomyopathy [5, 6, 11], we investigated whether levels of plasma C-reactive protein and IL-6 at baseline could predict clinical outcome in patients with PPCM. In chronic heart failure, low levels of total cholesterol have been found to be a predictor of impaired survival. The lipid profile in patients with PPCM has not been studied to date. In addition, cardiac myocytes have been noted to undergo apoptosis in animal models of PPCM [12, 14] as well in the failing human heart [14]. These findings prompted us to carry out a more detailed study aimed at correlating clinical evaluation with plasma levels of C-reactive protein and IL-6.
Methods
Study Design and Patient Enrollment
All patients (n = 46) and controls (n = 40) gave written informed consent before study entry. The study was conducted at Narayana medical college, Nellore, AP. History of pre-eclampsia and mode of delivery were obtained from the patient and confirmed by examining the obstetric card carried by each patient. The history of onset of symptoms and signs was recorded at the first presentation of the patients (baseline) and after a follow-up period of 6 months (6 months visit). These were the two time points of the study.
Inclusion criteria were as follows: (i) age ≥ 16 and ≤40, (ii) New York Heart Association functional classes (NYHA FCs) II–IV, (iii) symptoms of congestive heart failure that developed in the last month of pregnancy or during the first 5 months post-partum, (iv) no other identifiable cause for heart failure, (v) left ventricular ejection fraction (LVEF) ≤40 % by transthoracic echocardiography, and (vi) sinus rhythm.
Exclusion criteria were as follows: (i) significant organic valvular heart disease, (ii) systolic blood pressure (SBP) ≥160 mmHg and/or diastolic blood pressure ≥100 mmHg, (iii) clinical conditions other than cardiomyopathy that could increase inflammatory markers by screening serum for rheumatoid arthritis and HIV and evidence of sepsis, (iv) treatment with anti-inflammatory drugs, (v) severe anemia (hemoglobin concentration <9 g/dl), (vi) serum creatinine level more than 2 mg/dl, and (vii) metabolic disorders affecting lipoprotein metabolism, i.e., thyroid disease.
Clinical assessment, echocardiography, and blood analysis were done at baseline and after 6 months of standard therapy. Inflammatory markers were measured at baseline only. All patients received treatment with diuretics and the angiotensin-converting enzyme inhibitor (ramipril) and inotropic support inj-Dobutamine. Patients with an EF ≤ 25 % or LV thrombus received anti-coagulation therapy. Carvedilol was added after resolution of overt heart failure, and the dose was slowly titrated up to a target of 25 mg twice daily as long as SBP was ≥100 mmHg or symptoms such as dizziness did not occur. Patients attended the cardiac clinic at least once a month for routine follow-up.
Plasma levels of C-reactive protein were measured as part of the routine investigation by the hospital laboratory using a commercially available enzyme-linked immunosorbent assay (Roche Diagnostics). The assay had a sensitivity of 0.1–10 mg/L and included standards that were run in parallel. All patients had serum IL-6 levels measured by the ELISA method, M/s Immunetech France (IL-6 ELISA KIT-Beckmen). Obtained values were used to calculate plasma levels in patient and control samples.
The manufacturer supplied a reference range for normal values. However, as those values were obtained in samples from western population groups, we collected blood from 20 otherwise healthy age-, race-, sex-, body mass index-, and parity-comparable controls recruited from the local population.
For ROC curve, the best cutoff point of IL-6 (40 pg/ml) was identified to assess the increased risk of cardiovascular events (sensitivity 83.7 %, specificity 63 %, AUC 0.671, p = 0.007). The normal range for IL-6 by this method was 0–39.9 pg/ml in the local population. In the group with <40 pg/ml (Group I), there were 40 patients and the average IL-6 was 31.52 ± 8.83 pg/ml (range: 24–39.8 pg/ml), whereas in the group with >40 pg/ml (group II), there were 46 patients with an average IL-6 of 73.19 ± 34.4 pg/ml (range: 40.1–210 pg/ml) (p < 0.0001).
Functional Class, Echocardiography, and Cardiac
Scintigraphy
A physician who was provided the clinical data, but was blinded to the protocol and unaware of the results of the laboratory tests, performed the assignments of each patient to the NYHA FC during baseline and follow-up visits.
The same physician evaluated all patients. Two-dimensional and M-mode echocardiography with Doppler color flow mapping was performed using an echocardiograph (Philips i 33, WA, USA machine) attached to a 2.5 or 3.5 MHz transducer. All studies were performed and interpreted by the same operator who was unaware of the other parameters investigated. LV dimensions were measured according to the American Society of Echocardiography Guidelines [15]. Measurements of LV dimensions and function were determined on an average of ≥3 beats. LVEF and calculations of LV performance were made.
Statistical Analysis
Data were analyzed using the SAS version 9.1 statistical program. Results are expressed as mean ± SD or median (range). The paired t test was used for the comparison of baseline data with the 6-month data. The McNemar test was used for calculating the differences on the basis of the NYHA FC by grouping the patients into two classes (I, II and III, IV). The multiple logistic regression was performed including predictor variables [NYHA FC, end-diastolic diameter (EDD), end-systolic diameter (ESD), EF, CRP, serum IL-6, and SBP] that had a p value less than 0.15 from the univariate analysis. The resulting model for mortality selected IL-6 and TNF-α and NYHA FC as explanatory variables with a p value less than 0.05 being considered significant.
Results
Study Characteristics
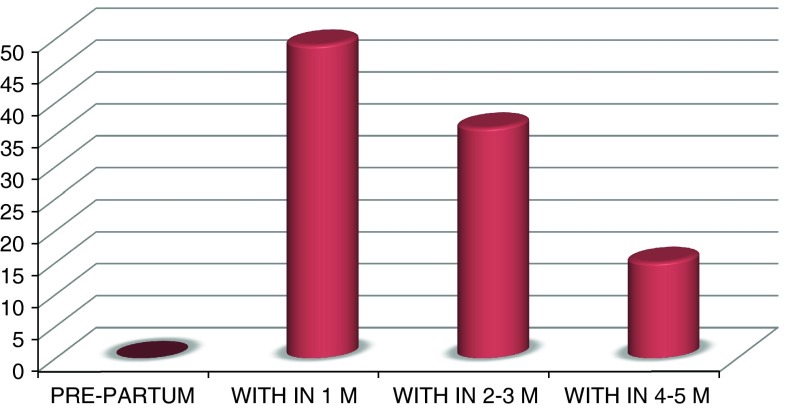
The characteristics of the study population at first presentation to the cardiac clinic are similar (compared with controls) as shown in Table 1. Although 91 % of the study patients were diagnosed as PPCM patients for the first time, the remaining 9 (9 %) PPCM patients had been diagnosed at a previous pregnancy. Clinical presentation of PPCM patients within 1 month post-partum was 49 %, within 2–3 month post-partum was 36 %, and within 4–5 month post-partum was 15 % (Graph 1).
Table 1.
Baseline characteristics of study population
| Patients | Control | |
|---|---|---|
| Age (years) | 21 ± 14.6 | 23 ± 15 |
| Gravidity (median) | 3 (1–7) | 2(1–5) |
| Type of delivery (%) | 82 % vaginal | 84 % vaginal |
| 18 % cesarean | 16 % cesarean | |
| Body mass index (kg/cm2) | 25.6 ± 5.1 | 24.2 ± 6.2 |
| Blood pressure (mmHg) systolic | 108.1 ± 19 | 112 ± 18 |
| Blood pressure (mmHg) diastolic | 70.4 ± 13 | 68 ± 15 |
| Heart rate (b.p.m.) | 93.5 ± 18 | 89 ± 14 |
Graph 1.
Patients presented with symptoms
Mean plasma levels of hemoglobin and glucose of the PPCM patients were low compared to the control (176 ± 34 vs 189 ± 42 mg/dl). There was an inverse correlation between baseline total cholesterol level and LV EDD and ESD, and therefore a positive correlation to EF (rs = 0.36, p = 0.006) was observed.
C-reactive protein (22 vs 08 mg/dl, p < 0.05), TNF-α (9.6 vs 3.2 pg/dl, p < 001), and IL-6 (73.19 ± 34.4 vs 31.52 ± 8.83 pg/dl, p < 0.005) were significantly abnormal (Table 2).
Table 2.
Blood parameters
| Control | Patients | p | |
|---|---|---|---|
| Hemoglobin (g/dL) | 11.5 ± 2.1 | 10.5 ± 1.7 | |
| Glucose (mg/dL) | 96 ± 18 | 89 ± 12 | |
| Total cholesterol (mg/dL) | 189 ± 42 | 176 ± 34 | |
| C-reactive protein (mg/L), | 08 | 22 (1–90) | <0.05 |
| TNF α (pg/mL), | 3.2 | 9.6 (0.2–20.0) | <0.001 |
| IL6 (pg/mL) | 31.52 ± 8.83 | 73.19 ± 34.4 | <0.005 |
Patients showed significantly higher LV dimensions, LV EDD (61.6 ± 7.1 mm vs 46 ± 9 mm p < 0.004) and LV ESD (53.1 ± 7 vs 32 ± 8, p < 0.005), and significantly lower echocardiographic LVEF (25.9 ± 8.2 vs 55 ± 12 p < 0.001). LV thrombi were detected on echocardiography in 12 % of the patients (Table 3).
Table 3.
Echocardiographic findings
| Control | Patient | p | |
|---|---|---|---|
| Left ventricular EDD (mm) | 46 ± 9 | 61.6 ± 7.1 | <0.004 |
| Left ventricular ESD (mm) | 32 ± 8 | 53.1 ± 7 | <0.005 |
| Ejection fraction (%) | 55 ± 12 | 25.9 ± 8.2 | <0.001 |
| LV thrombus is present | 0 % | 12 % | <0.005 |
Follow-Up
At 6 months, only 32 patients’ data were available. Seven patients died within the follow-up period of 6 months and eight patients moved to remote areas and were not available for full follow-up assessments. Four patients died despite optimal medical therapy because of progression of heart failure in the hospital and the other three patients experienced sudden death; all the patients died during the first 3 months after enrollment in the longitudinal study.
Women who had a prior history of PPCM had no difference in mortality when compared with the women who had no previous history of PPCM. However, they had intensive monitoring by the cardiologist and obstetrician throughout their pregnancy and post-partum period. Cardiac transplantation or the LV assist device for the population studied was unavailable for the duration of the trial because of economic reasons. During the first months after enrollment, patients received standard therapy for heart failure, which included furosemide [median daily dose 160 mg (80–240)], Ramipril [median daily dose 5 mg (5–20)], and carvedilol [median daily dose 25 mg (6.25–50)]. Carvedilol was titrated up as long as SBP was >100 mmHg or symptoms such as dizziness occurred. Clinical data and NYHA FC were compared at first presentation at the cardiac clinic at baseline (n = 46) and after 6 months of standard care (n = 32) as detailed in Table 4.
Table 4.
Clinical variables and left ventricular function at baseline and after 6-month follow-up of survivors (n = 36)
| Baseline | 6 months | p value | |
|---|---|---|---|
| Systolic BP (mmHg) | 111.1 ± 17.4 | 116.1 ± 17.6 | 0.016 |
| HR (b.p.m.) | 93.5 ± 18.5 | 72.6 ± 11.3 | 0.087 |
| NYHA FC (n) | 13 | 30 | <0.001 |
| FCs I & II | 23 | 6 | |
| FCs III &IV | |||
| EDD (mm) | 61.6 ± 7.1 | 55.6 ± 8.9 | <0.0001 |
| ESD (mm) | 53 ± 7 | 43 ± 10.3 | <0.0001 |
| Echo EF (%) | 24.9 ± 8.2 | 42 + 13.6 | <0.0001 |
LV Function, Dimension, and Heart Rate
Patients who completed 6 months of standard care showed a significant reduction of heart rate, LV dimensions, and significant improvement in echocardiographic LVEF (p < 0.0001, Table 4) and NYHA FC (p < 0.001). However, normalization of LVEF (>50 %) was only observed in 11 (35 %) patients.
Levels of C-Reactive Protein and Pro-inflammatory Cytokines
The median plasma level of C-reactive protein for the 46 PPCM patients was 10.0 mg/L (range 1–90) with 45 % patients having values of >10 mg/L. Only ten patients had a C-reactive protein level of 3 mg/L. Baseline plasma levels of C-reactive protein correlated positively with LV end-diastolic (rs = 0.33, p = 0.0026) and end-systolic dimensions (rs = 0.35, p = 0.0012), whereas the correlation with LVEF (rs = 20.27, p = 0.015) was inverse (Graph 2).
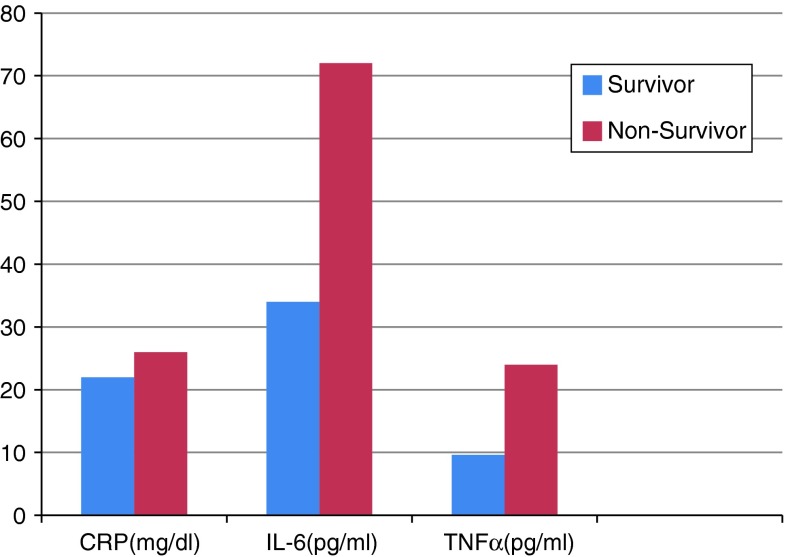
Graph 2.
Baseline plasma inflammatory markers of deceased patients versus survivors. Only differences in baseline plasma levels of IL-6 and TNF-α were significant, p < 0.002
Baseline plasma levels of C-reactive protein, TNF-α, and IL-6 were elevated in patients with PPCM when compared with age-, sex-, body mass index-, and parity-comparable controls (TNF-α 9.6 vs 3.2 pg/ml, IL-6 73.19 ± 34.4 vs 31.52 ± 8.83 pg/ml, C-reactive protein 20.8 ± 13.2 vs 3.1 ± 0.9 mg/L, p, 0.01). Plasma C-reactive protein, TNF-α, and IL-6 levels also correlated directly with NYHA FC-IV, complications, and deaths.
Predictors of Mortality
In the population studied, mortality remained high (15 %). Significant differences in the baseline data between the deceased patients and survivors were seen in NYHA FC and values of SBP, end-diastolic and end-systolic dimensions, LVEF, plasma levels of CRP, IL-6, and TNF-α (Table 5). Logistic regression analysis of NYHA FC, SBP, EDD, ESD, EF, CRP, IL6, and TNF-α revealed that only the baseline plasma levels of IL-6, TNF-α, and NYHA FC (OR = 2.67, CI 95 %, 1.04–6.83) were independent predictors of death (Table 5).
Table 5.
Baseline characteristics of deceased versus surviving patients
| Deceased | Survivors | p value | |
|---|---|---|---|
| Age (years) | 22 ± 1.2 | 28 ± 6 | 0.24 |
| No. of children (n), median (range) | 2(1–4) | 3(1–7) | 0.14 |
| Onset of symptoms after delivery (months), median (range) | 1(1–5) | 2(1–5) | 0.6 |
| NYHA FC | 3.2 ± 0.8 | 2.0 ± 0.6 | 0.04 |
| EDD (mm) | 64 ± 5 | 60 ± 6 | 0.03 |
| ESD (mm) | 57.6 ± 7 | 53.2 ± 8 | 0.03 |
| Echo EF (%) | 20 ± 4 | 26 ± 8 | 0.04 |
| C-reactive protein (mg/L), median (range) | 26 | 22 | 0.10 |
| TNF (pg/mL) | 9.6 | 24 | <0.001 |
| IL-6(pg/mL) | 34 | 72 | <0.001 |
Discussion
This study documented the clinical profile of 46 PPCM patients recruited prospectively and examined the role of inflammatory markers at time of diagnosis, in-hospital events, and clinical outcome after 6 months of standard clinical care.
Recovery of systolic function occurs in roughly half of affected women and usually occurs within 6 months of symptom onset [16]. A rapid recovery of EF is often seen in patients after initial diagnosis and dieresis [17]. EF > 45 % at 2 months after diagnosis predicts full functional recovery in 75 % of women with this result [18].
Of these, 15 % of patients died and only 23 % of the studied population normalized their LVEF after 6 months of therapy. Patients who died had lower NYHA FC, LVEF, larger LV dimensions, and higher CRP, IL-6, and TNF-α at diagnosis compared with those who survived, whereas age, parity, or onset of symptoms did not appear to play a role. None of the patients with PPCM presented with symptoms during the pre-partum period. This is in contrast with studies performed by others [3, 19] and more in keeping with a study from Haiti[4] documenting that 96 % of patients with PPCM developed heart failure in the post-partum period. There was no evidence of chronic disease or cardiac cachexia, which could account for a low lipid profile being a marker of severe chronic disease. We found an association of low total cholesterol levels with larger LV dimensions and lower EF.
Levels of low plasma cholesterol correlated positively with the levels of the inflammatory marker C-reactive protein, IL-6, and TNF-α. Plasma sampled from almost half of the population studied had significantly raised levels of C-reactive protein, IL-6, and TNF-α reflecting possibly the presence of a low-grade chronic inflammatory process due to the release of endotoxin or endotoxin-like substances and subsequent release of pro-inflammatory cytokines [20]. As C-reactive protein, IL-6, and TNF-α measurements are relatively inexpensive, they could provide us with a readily available tool to identify patients with an ongoing inflammatory process and high-risk complications.
This is supported by other previous research in PPCM patients presenting with subsequent pregnancy where we observed an exaggerated post-partum pro-inflammatory cytokine surge, possibly playing a role in the development of PPCM [21]. Plasma levels of CRP, IL-6, and TNF α as markers of potential cardiac inflammation were significantly higher in PPCM patients when compared with healthy controls and are predictors of mortality.
Conclusions
Plasma markers of inflammation were significantly elevated in PPCM patients and correlated with increased LV dimensions and lower EF at presentation. Baseline CRP, IL-6, TNF-α, and higher NYHA FC were the only predictors of mortality. These results contribute to inflammation which may contribute to the pathogenesis of PPCM and its complications [7, 8, 22].
References
- 1.Pearson GD, Veille JC, Rahimtoola S. Peripartum cardiomyopathy: National Institutes of Health workshop recommendations and review. JAMA. 2000;283:262–267. doi: 10.1001/jama.283.9.1183. [DOI] [PubMed] [Google Scholar]
- 2.Reimold S, Rutherford J. Peripartum cardiomyopathy. N Engl J Med. 2000;344:1629–1630. doi: 10.1056/NEJM200105243442110. [DOI] [PubMed] [Google Scholar]
- 3.Lampert M, Lang RM. Peripartum cardiomyopathy. Am Heart J. 1995;130:860–870. doi: 10.1016/0002-8703(95)90089-6. [DOI] [PubMed] [Google Scholar]
- 4.Demakis JG, Rahimtoola SH, Sutton GC. Natural course of peripartum cardiomyopathy. Circulation. 1971;44:1053–1061. doi: 10.1161/01.CIR.44.6.1053. [DOI] [PubMed] [Google Scholar]
- 5.Sato Y, Takatsu Y, Kataoka K. Serial circulating concentrations of C-reactive protein, interleukin-4, and IL-6 in patients with acute left heart decompensation. Clin Cardiol. 1999;22:811–813. doi: 10.1002/clc.4960221211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanebo K, Kanada T, Yamauchi Y. C-reactive protein in dilated cardiomyopathy. Cardiology. 1999;91:215–219. doi: 10.1159/000006913. [DOI] [PubMed] [Google Scholar]
- 7.Hayakawa Y, Chandra M, Wenfeng M, et al. Inhibition of cardiac myocyte apoptosis improves cardiac function and abolishes mortality in peripartum cardiomyopathy of Galpha(q) transgenic mice. Circulation. 2003;108:1037–1042. doi: 10.1161/01.CIR.0000101920.72665.58. [DOI] [PubMed] [Google Scholar]
- 8.Sliwa K, Skudicky D, Bergemann A. The addition of pentoxifylline to conventional therapy improves outcome in patients with peripartum cardiomyopathy. Eur J Heart Fail. 2002;4:305–309. doi: 10.1016/S1388-9842(02)00008-9. [DOI] [PubMed] [Google Scholar]
- 9.Sliwa K, Skudicky D, Bergemann A. Peripartum cardiomyopathy: analysis of clinical outcome, left ventricular function, plasma levels of cytokines and Fas/APO-1. J Am Coll Cardiol. 2000;35(3):701–705. doi: 10.1016/S0735-1097(99)00624-5. [DOI] [PubMed] [Google Scholar]
- 10.Ansari A, Fett JD, Carraway RE. Autoimmune mechanisms as the basis for human peripartum cardiomyopathy. Clin Rev Allergy Immunol. 2002;23(3):301–324. doi: 10.1385/CRIAI:23:3:301. [DOI] [PubMed] [Google Scholar]
- 11.Felker GM, Thompson R, Joshua MH. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–1084. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 12.Rauchhaus M, Clark AL, Doehner W. The relationship between cholesterol and survival in patients with chronic heart failure. J Am Coll Cardiol. 2003;42:1933–1940. doi: 10.1016/j.jacc.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 13.Horwich TB, Hamilton MA, Fonarow GC. Low serum cholesterol is associated with marked increase in mortality in advanced heart failure. J Card Fail. 2002;8:216–224. doi: 10.1054/jcaf.2002.0804216. [DOI] [PubMed] [Google Scholar]
- 14.Narula J, Haider N, Virmani R. Apoptosis in myocytes in end-stage heart failure. N Engl J Med. 1996;335:1182–1189. doi: 10.1056/NEJM199610173351603. [DOI] [PubMed] [Google Scholar]
- 15.Sahn DJ, DeMaria A, Kisslo J. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. doi: 10.1161/01.CIR.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 16.Hu CL, Li YB, Zou YG, et al. Troponin T measurement can predict persistent left ventricular dysfunction in peripartum cardiomyopathy. Heart. 2007;93(4):488–490. doi: 10.1136/hrt.2006.087387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amos AM, Jaber WA, Russell SD. Improved outcomes in peripartum cardiomyopathy with contemporary. Am Heart J. 2006;152(3):509–513. doi: 10.1016/j.ahj.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Cole P, Cook F, Plappert T. Longitudinal changes in left ventricular architecture and function in peripartum cardiomyopathy. Am J Cardiol. 1987;60(10):871–876. doi: 10.1016/0002-9149(87)91039-3. [DOI] [PubMed] [Google Scholar]
- 19.Hameed A, Elkayam U. Peripartum cardiomyopathy. In: Crawford M, DiMarco J, editors. Cardiology. 1. London: Mosby; 2001. p. 513.1. [Google Scholar]
- 20.Rauchhaus M, Coats AJS, Anker SD. The endotoxin-lipoprotein hypothesis. Lancet. 2000;346:930–933. doi: 10.1016/S0140-6736(00)02690-8. [DOI] [PubMed] [Google Scholar]
- 21.Sliwa K, Forster O, Zhanje F. Outcome of subsequent pregnancy in patients with documented peripartum cardiomyopathy. Am J Cardiol. 2004;93:1441–1443. doi: 10.1016/j.amjcard.2004.02.053. [DOI] [PubMed] [Google Scholar]
- 22.Ansari AA, Fett JD, Carraway RE. Autoimmune mechanism as the basis for human peripartum cardiomyopathy. Clin Rev Allergy Immunol. 2002;23:301–324. doi: 10.1385/CRIAI:23:3:301. [DOI] [PubMed] [Google Scholar]