Abstract
Objective
To measure the levels of early follicular phase Anti-Mullerian hormone (AMH) in Indian patients of IVF and to evaluate the AMH as a predictive marker of ovarian response in assisted reproductive technology outcome.
Methods
Sixty women (age 25–40 years) selected for in vitro fertilization treatment were included in this study. Analysis of day-2 serum samples was done for the AMH, FSH, Inhibin B, and LH by ELISA kit methods. USG was done for the antral follicle count (AFC) and oocytes’ retrieval. Hormone parameters were compared and correlated with the oocytes’ retrieval count and the AFC. The discriminant analysis was done to compare relevance of different parameters for predicting ovarian response.
Result(s)
The Anti-Mullerian hormone showed a significant correlation with the oocytes’ retrieval after ovulation induction for IVF (r = 0.648, p < 0.0001) and no correlation was seen with serum FSH, LH, and Inhibin. Serum AMH levels show 80 % sensitivity and 80 % specificity in predicting poor ovarian response.
Conclusion(s)
There is a significant correlation between day-2 serum AMH levels and the oocytes’ retrieval count in women undergoing ovulation induction for IVF, and the AMH is a good marker as the negative predictive values for the success of ART. There is no correlation found between other hormonal ovarian reserve markers and the oocytes’ retrieval count.
Keywords: Anti-Mullerian hormone, AMH/MIS, Antral follicle count, Oocytes retrieval count, Assisted reproductive technology (ART)
Introduction
The ovarian reserve, constituted by the size of the ovarian follicle pool and the quality of oocytes therein, declines with increasing age, resulting in the decrease of women’s reproductive function [1]. Diminished ovarian reserve has been recognized as an increasingly important cause of infertility. With age, ovarian reserve declines principally due to apoptotic loss of primordial follicles and not due to ovulation [2]. The only effective treatment for decreased ovarian reserve is early attempt at pregnancy; and therefore, identification of accurate predictors of ovarian reserve is a must.
The traditional assessment of ovarian reserve as serum levels of FSH, Inhibin B, and Estradiol has low sensitivity in the early stages of reduced ovarian reserve. Inhibin B and E2 produced by early antral follicles in response to FSH contribute to the classical feedback loop of the pituitary–gonadal axis to suppress FSH secretion. With the decline of the follicle pool, serum levels of Inhibin B and E2 decrease and subsequently serum FSH levels rise. These factors are part of a feedback system as their serum levels are not independent of each other. Furthermore, changes in serum levels of FSH, Inhibin B, and E2 occur relatively late in the reproductive aging process when reduction in ovarian reserve is critical and chances of pregnancy are significantly reduced [3]. So far, assessment of the number of antral follicle count (AFC) by ultrasonography best predicts the quantitative aspect of ovarian reserve.
Optimal evaluation of women and assessment of ovarian reserve are essential for the successful outcome of assisted reproductive technology (ART). The identification of both low and high responders before treatment may decrease the cycle cancelation rate and side effects such as ovarian hyper stimulation syndrome (OHSS) [4]. Age, day-3 FSH, Inhibin B, AFC, ovarian volume, and several dynamic tests have been correlated with ovarian response in ART. However, their predictive value remains controversial and disappointing [5].
Currently, the Anti-Mullerian hormone (AMH) is considered a promising and reliable marker, corresponding to the number of small antral follicles with constant levels across the cycle and superior intercycle reproducibility compared with FSH and early AFC [1, 6, 7]. Some studies reported a possible benefit of serum AMH measurement in IVF programs [8, 9]. However, urgently needed cut-off levels of AMH for supporting clinical decisions are still missing.
Anti-Mullerian hormone, a member of the transforming growth factor-b family, is essentially involved in the regression of Mullerian ducts in the male fetus, the initial step of organogenesis of the male genital tract. In females, it is a product of the granulosa cells from pre-antral and small antral follicles. It has direct or indirect roles in various phases of folliculogenesis from the primordial to the FSH-sensitive follicular stages, probably via AMH II receptors, expressed in granulosa theca cells. Therefore, AMH secretion might reflect the activity of pre-antral and early antral follicles, making it a promising parameter in the evaluation of ovarian follicular reserve [10].
The present study was planned to evaluate whether serum AMH levels could predict the ovarian response in women undergoing ART in comparison to conventional markers FSH, Inhibin, Estradiol, and chronological age in the Indian population.
Methods
This study included sixty women (age 25–40 years) attending the IVF program of the department of Obstetrics and Gynecology. The informed consent was taken from each patient and the ethical clearance was obtained from the institute’s ethical board. The inclusion criteria were as follows: (1) Regular menstrual cycle; (2) Presence of both ovaries; (3) No evidence of endocrine disorders (normal thyroid hormone, Prolactin, Testosterone); and (4) Age < 42 years. Women with genital tuberculosis, endometriosis, and autoimmune disorders were excluded from the study.
Hormone Measurement
The venous blood sample for hormonal estimation was taken on the 2nd–5th day of the menstrual cycle under strict aseptic conditions, centrifuged at 3,500 rpm for 15 min, and the serum was stored in 1.5 ml Eppendorf tubes at −20 °C. The IVF procedures were performed in the month after the blood sampling.
Serum AMH was measured by EIA AMH/MIH kit (A Beckman Coulter Company) following the manufacturer’s protocol. For the AMH, the analytical sensitivity was 0.14 ng/ml and intra-assay and inter-assay CVs were <12.3 and <14.2 %, respectively.
Serum Inhibin levels were determined by the sandwich ELISA technique using the INHIBIN B DSL-10-84100i kit following the manufacture’s protocol. The analytical sensitivity was 7 pg/ml and intra-assay and inter-assay CVs were <3.5 and <6.2 %, respectively.
Serum FSH levels were determined by the immune enzymometric assay ELISA technique using the EIAGEN FSH kit following the manufacture’s protocol. The analytical sensitivity was 0.6 mIU/ml and intra-assay and inter-assay CVs were <8.5 and <9.4 %, respectively.
The patients were stimulated using standard long protocol using GnRH analog for pituitary suppression followed by stimulation using recombinant FSH and HMG, and in some short protocol, an antagonist (Cetrotide) was used.
Long GnRH Agonist Protocol
GnRH agonists are started in the mid-luteal phase of the cycle preceding the planned IVF, leading to both pituitary and ovarian desensitization. Following this, ovarian stimulation with gonadotropins is started and GnRH agonist injection is continued until hCG is administered. This is the most widely used method.
Antagonist Protocol
GnRH antagonists like Cetrorelix or Ganirelix are given either as a single bolus dose of Cetrorelix 3 mg or in multiple doses of 0.25 mg daily. Next, HCG is given to trigger ovulation. Ovum pick-up is done after 34–36 h and inseminated with washed and processed sperms.
Fertilized ovum is cultured till either the 6–8 cell. Two–three morphologically normal embryos are transferred back in the uterus.
The main outcome measures were the number of retrieved oocytes. The poor ovarian response was defined as <4 oocytes or cancelation due to impaired or absent follicular growth and response to ovarian stimulation, the normal ovarian response as a collection of 4–8 oocytes, and the good ovarian response as a collection of 9–16 oocytes. Patients were considered as high responders when more than 16 oocytes were retrieved or when the cycle was canceled because of exaggerated response.
The pregnancy rates were not measured as the outcome measure because of the absence of homogeneity in the couples as couples with tubal factor and idiopathic infertility were also included in the study and the sample size was small. The group has planned another study to look for correlation of AMH values and the pregnancy rates.
Statistical Analysis
Data were analyzed with SPSS 13 and presented as mean if normally distributed or median (10th–90th percentiles) if not normally distributed. Correlations between different parameters were determined by means of bivariate correlation statistics and are expressed as Spearman correlation coefficients. Student’s t test was used to compare the endocrine profile and basic characteristics of the patients. The relevance of different parameters for predicting ovarian reserve was performed by discriminant analysis to calculate the sensitivities and specificities. For the AMH alone, cut-off values and Receiver operating characteristic (ROC) curves were calculated to minimize the false positive and negative rates and to find an optimal threshold for discrimination between women with poor and normal responses.
Results
Fifty-five patients were included with the aim of having their first IVF attempt. Among them, 80 % of patients were suffering from primary infertility. Five patients’ cycles were canceled due to OHSS. The baseline characteristics of poor and good responder groups are shown in Table 1.
Table 1.
Baseline characteristics and IVF cycle outcome
| Good responders (≥8 oocytes) |
Poor Responders (≤4 oocytes) |
||
|---|---|---|---|
| 1. | Number of patients | 45 | 10 |
| 2. | Age (in years) | 32.6 ± 5.2 | 36 ± 4.2 |
| 3. | Infertility duration (years) | <10 | >15 |
| 4. | FSH (IU/l) | 4.94 ± 1.07 | 10.52 ± 1.82 |
| 5. | AMH (ng/ml) | 2.918 ± 1.96 | 0.689 ± 0.21 |
| 6. | Inhibin B (pg/ml) | 66.11 ± 8.0 | 50.8 ± 4.3 |
| 7. | LH (IU/l) | 4.5 ± 0.41 | 4.3 ± 2.5 |
| 8. | Oocytes retrieved | 10.8 ± 6.5 | 2.1 ± 1.44 |
| 9. | AFC | 13.37 ± 5.7 | 10.8 ± 6.2 |
Values are represented as median range. Student’s t test is performed to compare good and poor responders. Number of patients is 45 in the good responder group (five cancellations due to high response)
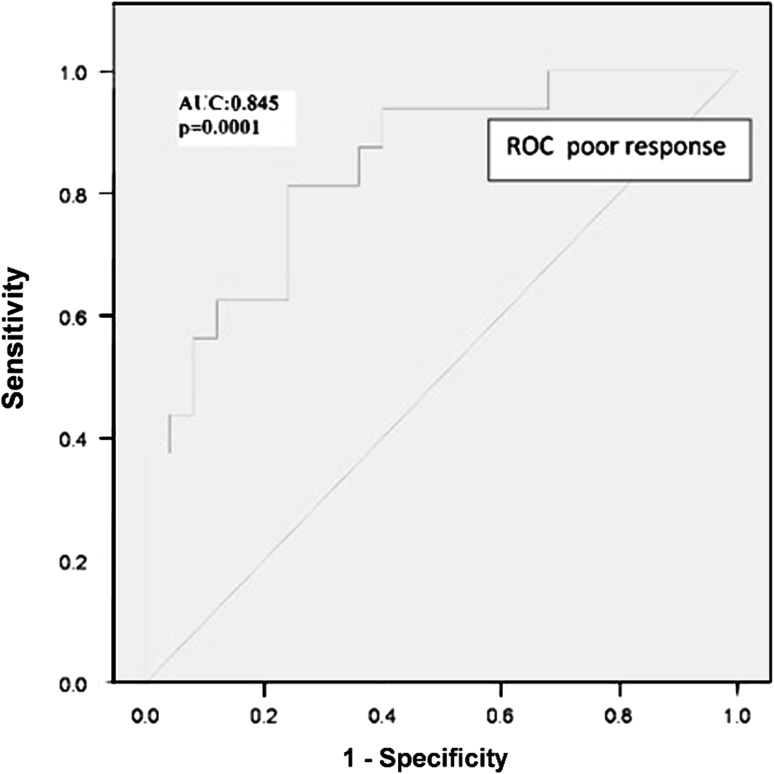
The mean AMH levels of all treated patients were 2.358 ± 0.596. Linear discriminant analysis was done to know the correlation of the AMH with poor ovarian response, and the AMH cut-off level for poor ovarian response was 1.4 ng/ml with the least false positive and false negative results. Figure 1 shows the typical ROC for the AMH indicating poor ovarian response with sensitivity of 80 % and specificity of 80 %. ROC curve analysis for poor response showed that the AMH had the largest area under the curve (AUC; 0.845; p = 0.0001) as compared to the FSH (AUC; 0.601 p = 0.04), age (AUC; 0.455; p = 0.05). The patients who responded poorly were older and had less oocytes retrieved with lower AMH than normal responders. FSH was elevated in the poor responder group, though it was in the normal range and it could not discriminate between low and normal responders (p = 0.041).
Fig. 1.
Receiver operating characteristic curve for AMH as an indicator of poor ovarian reserve and oocytes’ retrieval
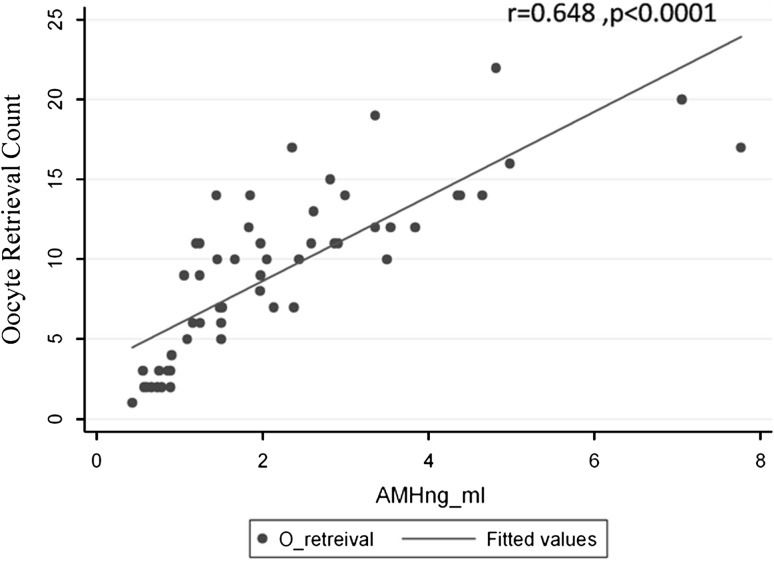
A statistically significant positive correlation was observed between the number of oocytes retrieved and the AMH (r = 0.648, p = 0.0001) (Fig. 2). Significant correlation was also seen between the number of oocytes retrieved and AFC (r = 0.495, p = 0.0001). The AFC and AMH also showed a significant correlation (r = 0.321, p = 0.022). The negative correlation was seen between the Oocyte retrieval count and FSH, though less significant (r = −0.395, p = 0.01). No correlation was identified between number of retrieved oocytes and Inhibin B (r = 0.125, p > 0.1) or LH (r = 0.012, p > 0.1).
Fig. 2.
Correlation of number of oocytes after ovum retrieval with AMH in IVF patients. r is Spearman’s correlation coefficient followed by the p value
Discussion
In the present study, authors investigated the value of serum AMH levels for predicting ovarian response in patients undergoing IVF treatment in the Indian population. The study evaluates the relationship between serum AMH levels, measured by an ultrasensitive ELISA technique, and the oocytes retrieved after gonadotropins’ stimulation in IVF patients and compares the strength of correlations between the various hormonal parameters in predicting the positive outcome of IVF.
The ovarian response during exposure to high levels of gonadotropins is considered to be a measure of the selectable cohort of antral follicles. As the number of antral follicles is related to the size of the primordial follicle pool, ovarian response can be regarded as a reflection of ovarian reserve. Recent studies have shown that low response to exogenous gonadotropins’ stimulation is associated with early menopause, supporting the idea that ovarian response indeed reflects the ovarian aging process [11]. The excellent correlation between initial AMH levels and subsequent ovarian response in IVF implies that the AMH is a promising marker for ovarian reserve.
We observed a significant correlation of the AMH with ovarian response as expressed by the number of oocytes retrieved as compared to serum levels of Inhibin B, Estradiol, FSH, and LH. These results expand the clinical data reported previously by other investigators [12].
The AFC is regarded as a good marker to predict poor ovarian response in ART programs, providing better information than the patient’s age alone or several endocrine markers. However, it is affected by high inter-observer variability due to duration of the vaginal ultrasound examination, observers’ experience, and expected ovarian reserve due to the patient’s age. Therefore, we have focused on assessing endocrine markers to avoid observer-related variability.
Our results show that the AMH levels are superior parameters predicting ovarian response as compared to other hormones conventionally used. However, it has been observed in various studies that even in women of comparable age, there was a wide variation in the individual ovarian reserve. For them, the AMH is a very promising and possibly the best actual candidate to evaluate their individual ovarian response to gonadotrophins’ stimulation and to detect poor responders with levels of AMH 1.4 ng/ml (80 % sensitivity and 80 % specificity). With an AMH of 1.4 ng/ml, correct prediction of poor response (<4 oocytes) to gonadotrophins’ stimulation of 80 % can be achieved.
Although we found a strong correlation of AMH levels and ovarian response in ART cycles by using the cut-off level of AMH 1.4 ng/ml, we can only advocate caution as to use of this cut-off predictive value as the size of our cohort was small. The study of a large cohort will need to be set up to determine not only the cut-off values and cycle outcome (pregnancy or not), but also the ovarian response. With a large sample size, the specificity and “correct prediction rate of poor response” will increase significantly, although sensitivity and “correct prediction rate of normal response” are much more robust and will remain unchanged whether or not a high age-related heterogeneity is given. Therefore, the AMH measurement and subsequent AFC should be combined in unselected groups of patients to minimize false positive results [8, 13].
Advantages of the use of the AMH over AFC for the prediction of ovarian response are that all predictive information is obtained from blood and additional ultrasound is not needed. Furthermore, since there is no change in AMH levels in response to gonadotrophins and the magnitude of fluctuation at three different time points is very less during the menstrual cycle [14], the AMH can be measured throughout the cycle in contrast to the other parameters, an advantage for both patients and clinicians. Therefore, the AMH can be a reliable screening marker for reduced ovarian response in IVF patients.
The Anti-Mullerian hormone can also be a promising marker for the detection of OHSS. Our study shows an elevation of the AMH in the hyper-responders as compared to good responders, although due to the small size, it did not meet statistical significance. The women with PCOS were excluded from the study because they had high levels of AMH and the oocytes’ retrieval count was very high after the gonadotrophins’ stimulation. So, our results also confirm the findings in previous studies showing the link between the AMH and OHSS risk in the general infertility population.
Our data strongly support the previous published studies dealing with AMH measurement and prediction of ovarian response in ART and this is the first study being conducted in the Indian population, predicting the cut-off values in poor responders. The AMH is significantly correlated with the number of eggs collected and is a good negative predictive marker for the success of ART, although further studies are needed to determine the cut-off values with pregnancy as the outcome instead of ovarian response.
Acknowledgments
The authors sincerely thank the Institute Research grant received from the All India Institute of Medical Sciences, New Delhi, for the study.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Visser JA, de Jong FH, Laven JSE, et al. Anti-Müllerian hormone: a new marker for ovarian function. Reproduction. 2006;131(1):1–9. doi: 10.1530/rep.1.00529. [DOI] [PubMed] [Google Scholar]
- 2.Tremellen KP, Kolo M, Gilmore A, et al. Anti-mullerian hormone as a marker of ovarian reserve. Aust N Z J Obstet Gynaecol. 2005;45(1):20–24. doi: 10.1111/j.1479-828X.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- 3.Burger HG, Dudley EC, Hopper JL, et al. Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J Clin Endocrinol Metab. 1999;84(11):4025–4030. doi: 10.1210/jc.84.11.4025. [DOI] [PubMed] [Google Scholar]
- 4.La Marca A, Giulini S, Tirelli A, et al. Anti-Müllerian hormone measurement on any day of the menstrual cycle strongly predicts ovarian response in assisted reproductive technology. Hum Reprod. 2007;22(3):766–771. doi: 10.1093/humrep/del421. [DOI] [PubMed] [Google Scholar]
- 5.Bancsi LF, Broekmans FJ, Eijkemans MJ, et al. Predictors of poor ovarian response in in vitro fertilization: a prospective study comparing basal markers of ovarian reserve. Fertil Steril. 2002;77(2):328–336. doi: 10.1016/S0015-0282(01)02983-1. [DOI] [PubMed] [Google Scholar]
- 6.Gnoth C, Schuring AN, Friol K, et al. Relevance of anti-Mullerian hormone measurement in a routine IVF program. Hum Reprod. 2008;23(6):1359–1365. doi: 10.1093/humrep/den108. [DOI] [PubMed] [Google Scholar]
- 7.Feyereisen E, Méndez Lozano DH, Taieb J, et al. Anti-Müllerian hormone: clinical insights into a promising biomarker of ovarian follicular status. Reprod Biomed Online. 2006;12(6):695–703. doi: 10.1016/S1472-6483(10)61081-4. [DOI] [PubMed] [Google Scholar]
- 8.Ebner T, Sommergruber M, Moser M, et al. Basal level of anti-Müllerian hormone is associated with oocyte quality in stimulated cycles. Hum Reprod. 2006;21(8):2022–2026. doi: 10.1093/humrep/del127. [DOI] [PubMed] [Google Scholar]
- 9.Fréour T, Mirallié S, Colombel A, et al. Anti-mullerian hormone: clinical relevance in assisted reproductive therapy. Ann Endocrinol (Paris) 2006;67(6):567–574. doi: 10.1016/S0003-4266(06)73008-6. [DOI] [PubMed] [Google Scholar]
- 10.Baarends WM, Hoogerbrugge JW, Post M, et al. Anti-müllerian hormone and anti-müllerian hormone type II receptor messenger ribonucleic acid expression during postnatal testis development and in the adult testis of the rat. Endocrinology. 1995;136(12):5614–5622. doi: 10.1210/en.136.12.5614. [DOI] [PubMed] [Google Scholar]
- 11.Nikolaou D, Lavery S, Turner C, et al. Is there a link between an extremely poor response to ovarian hyperstimulation and early ovarian failure? Hum Reprod. 2002;17(4):1106–1111. doi: 10.1093/humrep/17.4.1106. [DOI] [PubMed] [Google Scholar]
- 12.Hazout A, Bouchard P, Seifer DB, et al. Serum antimüllerian hormone/müllerian-inhibiting substance appears to be a more discriminatory marker of assisted reproductive technology outcome than follicle-stimulating hormone, inhibin B, or estradiol. Fertil Steril. 2004;82(5):1323–1329. doi: 10.1016/j.fertnstert.2004.03.061. [DOI] [PubMed] [Google Scholar]
- 13.Muttukrishna S, McGarrigle H, Wakim R, et al. Antral follicle count, anti-mullerian hormone and inhibin B: predictors of ovarian response in assisted reproductive technology? BJOG. 2005;112(10):1384–1390. doi: 10.1111/j.1471-0528.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- 14.Cook CL, Siow Y, Taylor S, et al. Serum Müllerian-inhibiting substance levels during normal menstrual cycles. Fertil Steril. 2000;73(4):859–861. doi: 10.1016/S0015-0282(99)00639-1. [DOI] [PubMed] [Google Scholar]