Abstract
Objective
A prospective cohort study in a teaching hospital to assess the efficacy and safety of neoadjuvant chemotherapy in the treatment of locally advanced carcinoma cervix.
Method
Neoadjuvant chemotherapy in the form of cisplatin 75 mg/m2 and paclitaxel 135 mg/m2 on day 1 and repeated at 14 days’ interval for up to a maximum of three courses.
Results
Neoadjuvant chemotherapy in cervical cancer was effective in the downstaging of the disease. Downstaging was observed in 19.23 % of patients after two cycles and in 50 % of patients after three cycle of NACT. Operability increases to 33.3 and 38.4 % after two and three cycles of NACT, respectively. Complete pathological response was observed in 37.5 % of patients after NACT. No significant adverse effect in the feasibility of surgery was observed.
Conclusion
The present study showed that neoadjuvant chemotherapy was an effective and well-tolerated mode of therapy with significantly less morbidity and mortality.
Keywords: Cervical cancer, Neoadjuvant chemotherapy, Downstaging
Introduction
On a global scale, cervical cancer is the second most common cause of cancer mortality in women, and is the most prevalent female malignancy in many developing countries. It causes close to 234,000 deaths in developing countries, but only 40,000 in developed nations [1]. The incidence of cervical cancer has declined significantly in many developed countries due to effective screening. The lack of screening programs in developing countries is the main reason for the high number of cases diagnosed at advanced stages. The lifetime risk of developing cervical cancer is 3 % in developing countries and 1.1 % in developed countries.
In order to combat the above burden, we need to have some effective and affordable treatment modality to improve survival. For the early stage (FIGO stage IB–IIA), the standard treatment is radical radiotherapy or radical hysterectomy. Radical radiotherapy is the treatment of choice for locally advanced disease (FIGO stage IIB, III and IVA) and offers an alternative to radical surgery for stage IB bulky tumor (tumor size more than 4 cm confined to cervix). Survival varies according to the stage of the disease: 5-year survival for stage IA is above 95 %, while it is around 20 % for stage IV [1]. In developing countries, the radiotherapy facilities are often inadequate; hence, there is a need to explore neoadjuvant chemotherapy. Moreover, the patients are relatively young and the long-term side effects of radiotherapy are more. Neoadjuvant and concurrent chemotherapy have been the most common chemotherapy schedules studied in locally advanced cervical cancer [2]. A systematic review and meta-analysis suggest a large benefit of concomitant chemo-radiotherapy on the overall and progression-free survival, and it also reduces local and distant recurrence [3]. Thus, concomitant chemo-radiotherapy has become the standard of care for locally advanced disease, but the 5-year survival has yet not improved satisfactorily. Moreover, delayed toxicity of radiation is also observed. The neoadjuvant chemotherapy has given encouraging results by arresting growth of the tumor and control of micrometastasis and better tolerance due to non-overlapping of the of the two modalities of treatment. Chemotherapy delivered in a neoadjuvant fashion seems to be more effective (response rate 40–70 %) [2]. Neoadjuvant chemotherapy is predictive of the response to subsequent radiotherapy. The advantages of NACT are many, decrease in tumor bulk making surgery easier, better dose distribution of brachytherapy, less radiotherapy toxicity, and absolute increase of 15 % in 5-year survival [4]. The disadvantages of NACT are accelerated growth during ineffective chemotherapy and delay in radiotherapy. Cisplatin is considered to be the most effective drug for the treatment of cervical cancer and usually is an essential part of the NACT regimen. Timing and dose intensity of NACT have an important impact on the outcome.
Considering the inadequacy of the treatment modalities like surgery and radiotherapy separately or in combination, this study was planned to evaluate the impact of chemotherapy in neoadjuvant form in the management of advanced stage of cervical cancer.
Materials and Methods
This prospective cohort study was carried out in the department of obstetrics and gynecology with the collaboration of the department of radiotherapy, Chhatrapati Shahuji Maharaj Medical University, Lucknow, after taking institutional ethical clearance, from August 2010 to August 2011. Twenty six patients admitted in the department of Obstetrics and Gynecology with histologically proven locally advanced carcinoma cervix were studied after obtaining informed consent. Women with deranged prechemotherapy blood tests (hemogram, liver function, kidney function) and systemic illness (cardiac, respiratory, and hepatorenal) were excluded from the study. Women with preexisting neuropathy, mental illness, pregnancy, or lactation and those who had prior chemotherapy or radiotherapy or distant metastasis were also excluded from the study. Women with histopathologically proven carcinoma cervix in stage IIA, IIB, IIIA, IIIB with performance status based on the Karnofsky performance status (KPS > 70) were included in our study.
Complete gynecological examination including abdominal, vaginal, and rectal examinations was done. Blood investigation, intravenous pyelography (IVP), cystoscopy, X-ray chest, and ultrasound of abdomen/pelvis and whenever possible CT scan and MRI were done. Clinical staging was done according to the current International Federation of Gynecology and Obstetrics (FIGO) classification in all cases.
In all cases, chemotherapy regimen used was cisplatin 75 mg/m2 and paclitaxel 135 mg/m2 on day 1 with the supportive therapy including methylprednisolone, chlorpheniramine, ranitidine, and ondansetron, 30 min before the treatment delivery along with IV hydration. Chemotherapy was given at 14 days’ interval with routine monitoring. A maximum of three courses was given. Operability was evaluated 2 weeks after the second course of chemotherapy. Operable patients were taken up for radical hysterectomy and the rest were given the 3rd course of chemotherapy. After 2 weeks of the 3rd course, operability was assessed again and the patient was taken up either for surgery or for radiotherapy. Assessment of operability was done with per speculum, per vaginal, and per rectal examinations. Patients with growth confined to the cervix without parametrial or vaginal extension were considered operable. The downstaging of the disease and an objective response regarding tumor size were also taken into account. The WHO criteria for tumor response were used. Safety (toxicity profile) of NACT was assessed by common toxicity criteria version-2 with proper grading of each and every side effect of the drug used in the study.
Statistical Methods
The statistical analysis was done using Statistical Package for Social Sciences (SPSS) version 15.0 statistical analysis software. Chi square test/Fisher exact test has been used to see the significant association between two co-variates. A p value of <0.05 is significant. The association between stage of disease and operability after NACT was analyzed.
Results
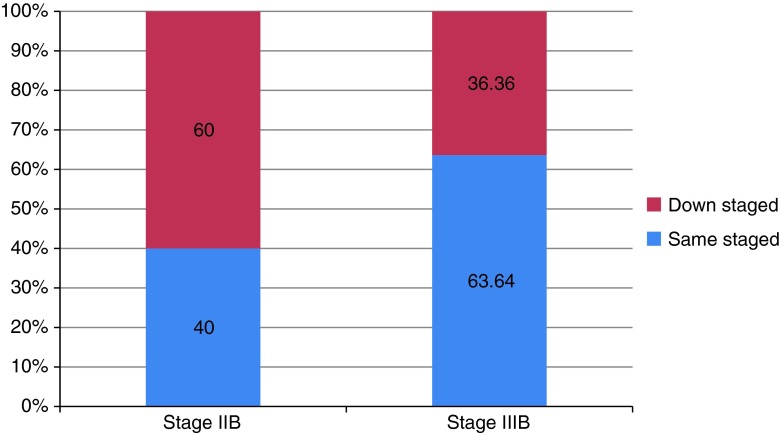
In this prospective clinical study, a majority (57.69 %) of the patients were of stage IIB and the remaining 42.31 % were in stage IIIB. Mean age of patients with stage IIB was 47.06 ± 9.26 years, whereas it was 45.60 ± 12.67 years in stage IIIB. High parity was found to be strongly associated with cervical cancer in our study. Forty percent of patients of stage IIB had parity ≥5, followed by 26.67 % with parity 4 and 2. In stage IIIB, each of para 2 and para ≥5 constituted 36.36 %, followed by para 4 (18.18 %) and para 1 (9.09 %). After 2 cycles of chemotherapy, 13.33 % of patients of stage IIB and 27.27 % patients of stage IIIB were downstaged. Change in stage after three cycles of chemotherapy was more significant. Fifty percent of stage IIB and 12.5 % of stage IIIB patients were downstaged (n = 24). The overall observation for downstaging was that a majority (60 %) of stage IIB patients were downstaged as compared to 36.6 % patients of stage IIIB (Fig. 1). Out of 26 enrolled patients, 24 patients received the 3rd cycle of chemotherapy and 10 patients became operable after NACT. Nine patients belonged to stage IIB and one belonged to stage IIIB before therapy (Table 1). Statistically, the change in the status of patients was significant (p = 0.008). The difference in treatment response between the two age group, ≤40 and ≥40 years, was not statistically significant (p = 0.106). Overall, tumor response to NACT in the present study was 88.4 %. Complete response was observed in 13 (50 %) patients, followed by 10 (38.4 %) patients who showed partial response. Disease remained stable in 3 (11.5 %) patients; none of the patient showed progression of disease (Table 2). Prechemotherapy tumor size was an important factor for the improvement of the patient. Downstaging, operability, and tumor response were significantly better in patients with tumor size ≤4 cm as compared to patients with tumor size ≥4 cm (Table 3). Histopathologically, 50 % of tumors were keratinizing and the other 50 % were non-keratinizing squamous cells in nature. Patients with keratinizing tumors were more inclined toward downstaging (61.5 %) and operability (46.1 %) as compared to non-keratinizing tumors with downstaging in 38.4 % and operability in 30.7 % (χ2 = 1.384; p = 0.239).
Fig. 1.
Overall downstaging observed after NACT
Table 1.
Post-neoadjuvant chemotherapy operability in cases
| Stage | Pre-neoadjuvant chemotherapy (n) | Post-neoadjuvant chemotherapy | |
|---|---|---|---|
| Operable | Inoperable | ||
| IIB | 15 | 9 (60 %) | 6 (40 %) |
| IIIB | 11 | 1 (9.09 %) | 10 (90.91 %) |
| Total | 26 | 10 (38.4 %) | 16 (61.5 %) |
χ2 = 6.949, p = 0.008
Table 2.
Overall tumor response after neoadjuvant chemotherapy
| S.No. | Finding | No. of patients | Percentage |
|---|---|---|---|
| 1 | Complete response | 13 | 50 |
| 2 | Partial response | 10 | 38.4 |
| 3 | Stable disease | 3 | 11.5 |
| 4 | Progressive disease | 0 | 0 |
Table 3.
Neoadjuvant chemotherapy response in relation to prechemotherapy sizes of tumor
| Mean size | Downstaging | Operability | Tumor response | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Observed | Not observed | Operable | Inoperable | CR | PR | SD | PD | |||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| ≤4 cm (n = 14) | 9 | 64.2 | 5 | 35.7 | 7 | 50 | 7 | 50 | 9 | 64.2 | 2 | 14.2 | 3 | 21.4 | 0 | 0 |
| >4 cm (n = 12) | 4 | 33 | 8 | 66.6 | 3 | 25 | 9 | 75 | 4 | 33.3 | 8 | 66.6 | 0 | 0 | 0 | 0 |
Out of 10 patients who regressed to IB/operable after three cycle of NACT, two patients refused surgery and opted for radiotherapy; hence, only 8 (30.76 %) patients underwent surgery and 18 (69.23 %) patients were given radiotherapy as treatment. The post-surgical histopathologic findings of the eight operated patients are demonstrated in Table 4. Tumor response to neoadjuvant chemotherapy observed by various trails is shown in Table 5.
Table 4.
Histopathologic finding in operated patients (n = 8)
| S. no. | Specimen | No. of patients | Percentage |
|---|---|---|---|
| 1. | Cervix | ||
| Squamous cell carcinoma | 5 | 62.5 | |
| Chronic cervicitis | 2 | 25 | |
| High-grade CIN | 1 | 12.5 | |
| 2 | Parametrium | ||
| Normal histology | 8 | 100 | |
| Tumor invasion | 0 | 0 | |
| 3 | Lymph node | ||
| Normal histology | 7 | 87.5 | |
| Tumor invasion | 1 | 12.5 | |
| 4 | Uterus | ||
| Secretory endometrium | 5 | 62.5 | |
| Proliferative endometrium | 1 | 12.5 | |
| Simple cystic hyperplasia | 1 | 12.5 | |
| Adenomyosis | 1 | 12.5 | |
Table 5.
Observations in different series
| Study | Overall response (%) | CR (%) | PR (%) |
|---|---|---|---|
| Taneja [8] | 47.8 | 8.7 | 39.1 |
| Kangnam-KU | 83 | 24 | 59 |
| Present study | 88.4 | 50 | 38.4 |
Hair loss (alopecia) was the most common toxicity observed and was present in 100 % of the patients who had undergone chemotherapy. Gastrointestinal side effects, anorexia and nausea, were observed in 60.7 %. Other side effects were rare (Table 6).
Table 6.
Toxicity profile observed with neoadjuvant chemotherapy (n = 26)
| S. no. | Adverse effect | Grade 1 | Grade 2 | Grade 3 | Grade 4 | No. of Pt | % |
|---|---|---|---|---|---|---|---|
| 1 | Allergic reaction | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | Ototoxicity | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | Myelosuppresion | ||||||
| Leucopenia | 0 | 1 | 0 | 0 | 1 | 3.8 | |
| Lymphopenia | 0 | 0 | 0 | 0 | 0 | 0 | |
| Neutropenia | 0 | 0 | 0 | 0 | 0 | 0 | |
| Anemia | 3 | 2 | 1 | 6 | 23 | ||
| Transfusion of PRBC | Yes | Yes | Yes | 6 | 23 | ||
| 4 | Alopecia | 7 | 8 | 6 | 5 | 26 | 100 |
| 5 | Injection site reaction | 0 | 0 | 0 | 0 | 1 | 3.8 |
| 6 | Gastrointestinal | ||||||
| Anorexia | 6 | 4 | 5 | 1 | 16 | 60.7 | |
| Diarrhea | 2 | 3 | 1 | 0 | 6 | 22.6 | |
| Vomiting | 2 | 3 | 2 | 0 | 7 | 27.6 | |
| Nausea | 6 | 5 | 5 | 0 | 16 | 60.7 | |
| 7 | Renal toxicity | ||||||
| Creatinine | 0 | 0 | 0 | 0 | 0 | 0 | |
| Renal failure | 0 | 0 | 0 | 0 | 0 | 0 | |
| 8 | Electrolyte | ||||||
| Hypokalemia | 2 | 0 | 0 | 0 | 2 | 7.6 | |
| Hypocalcemia | 0 | 0 | 0 | 0 | 0 | 0 | |
| 9 | Fever | 2 | 3 | 3 | 1 | 9 | 34.5 |
Discussion
Pelvic failures with or without a systemic component are frequent problems for patients with locally advanced cervical carcinoma, despite the recent encouraging results of concomitant chemo-radiation based on cisplatin or other radiosensitizing drugs. These results emphasize the need to test new treatment alternatives in order to improve survival.
After a large number of randomized trials, neoadjuvant chemotherapy has been explored as an adjunct to either radiotherapy or surgery, in comparison with radiotherapy alone. Cisplatin-based regimens have been favored because of impressive response rate. Currently, cisplatin-based chemo-radiation is the standard of care for locally advanced carcinoma cervix [2]. Malignancies of the genital tract are common in the elderly age group. The mean ages of cervical cancer patients in three different arms of the trials of Gonzalez et al. [5] were 48, 44, and 49 years. In comparison to western countries, developing countries present a different picture with most cases diagnosed with locally advanced disease [4]. In our study, the mean age of cervical cancer patients was 47.06 ± years for stage IIB and 45.6 ± years for stage IIIB, and 57.69 % of patients were of stage IIB, while 42.31 % patients were in stage IIIB.
Cervical cancer is considered as a disease of multiparous women. In this study, the maximum number of patients had parity ≥5. Sexually active women are two to four times more likely to develop cervical cancer than sexually inactive women. Early coital activity, frequency of coitus, number of coital partners, and non-circumcised husbands (Hindus) are associated with a high incidence of cervical cancer. A similar association was observed in this study also. Histopathologically, Squamous cell carcinoma was found in 92, 90, and 87 % in three different arms of the trial by Gonzalez et al. [5]. In the present study, all the patients had squamous cell carcinoma prechemotherapy. Histopathologic changes like fibrosis, hyalinization, necrosis, myxoid changes, scattered inflammatory cells, etc., are seen in the tumor following chemotherapy, especially in high-grade and vascular tumor. Similar findings were observed in the present study.
For more than two decades, neoadjuvant chemotherapy has been introduced in clinical practice, but no standard drug regimen is unanimously accepted. There are many regimens for neoadjuvant chemotherapy in carcinoma cervix. PVB (cisplatin, vincristine, bleomycin), BOMP (bleomycin, ifosamide, cisplatin), BIP (bleomycine, ifosamide, cisplatin), and a quick VBP regimen were used by different investigators [6, 7], and modified VBP regimen consists of (vincristine 1 mg/m2 on day 1 and cisplatin 50 mg/m2 on day 1, bleomycin 25 mg/m2 on day 1 and 2) on weekly basis for three courses [8]. TIP (ifosamide 5 gm/m2 on day 1, cisplatin 75 mg/m2 and paclitaxel 175 mg/m2 on day 3) was given every 3 weeks for a total of three courses [9]. Most of the regimens are repeated for two or three courses. In the present study, cisplatin 75 mg/m2 and paclitaxel 135 mg/m2 on day 1 at 14-day intervals for up to three courses were given.
Most of the trials have used intravenous administration of the chemotherapeutic drugs. Intra-arterial administration seems to have a theoretic advantage for the local control, and usually causes less toxicity than intravenous route. Disadvantages include troublesome management, risk of arterial catheterization, and increased morbidity [10]. In the present study, chemotherapeutic drugs were administered intravenously. Traditionally, the chemotherapeutic regimen had been cycled on a three-weekly basis and required at least 9–12 weeks before definitive therapy. A “quick-cycle” regime which is cycled weekly not only decreases time to definitive therapy but also prevents the rapid regrowth of the tumor cells without any increase in side effects having been reported. Trials using chemotherapy cycle length shorter than 14 days (HR = 0.83, 95 % CI 0.69–1.00, p = 0.046) or cisplatin dose intensities greater than 25 mg/m2 have tended to show an increase in survival (HR = 0.91, 95 % CI 0.78–1.05, p = 0.20). In contrast, trials using a cycle length longer than 14 days (HR = 1.25, 95 % CI 1.07–1.46, p = 0.005) or cisplatin dose intensities lower than 25 mg/m2 per week (HR = 1.35, 95 % CI 1.11–1.14, p = 0.002) tended to show a detrimental effect of neoadjuvant therapy on survival [11]. In the present study, cisplatin dose intensity was 75 mg/m2 and the cycle length was no longer than 14 days. After two cycles of chemotherapy, more patients (27.7 %) of stage IIIB shifted toward downstaging as compared to that of stage IIB (13.3 %). After three cycles of chemotherapy, downstaging was observed in 50 % of patients. A prospective randomized trial was carried out in patients with squamous cell carcinoma stage IIIB in three different arms—radiotherapy, neoadjuvant chemotherapy followed by radiotherapy, neoadjuvant chemotherapy followed by surgery and whole pelvic irradiation [6]. The overall survival after 4-year follow-up was 37, 53, and 63 % indicating that surgery is the best treatment after NACT. This was mainly due to a decrease in pelvic recurrences. A systematic review and meta-analysis of individual patient data (IPD) also demonstrated that NACT followed by surgery is superior to radiotherapy alone in terms of overall survival [12]. In the present study also, NACT followed by surgery was used. Usually, there are apprehensions regarding increased vascularity, difficulty in dissection during surgery following chemotherapy. In our study, post-chemotherapy operability was better, no increase in vascularity observed, and the dissection was not difficult.
Sardi et al. [6] commented that response to NACT was strongly associated with the initial volume of the tumor. Our patients with a tumor size <4 cm showed a complete response in 64.2 %, while in patients with a tumor size >4 cm, complete response was seen in 33.3 %, reflecting similar results. Patients having similar tumor sizes but belonging to a higher stage were associated with decreased response to NACT.
After neoadjuvant chemotherapy, up to 25 % of patients achieved complete responses. Our patients showed better response as complete response was observed in 50 %. Complete pathological response is associated with longer survival (98.3 %) as compared to partial response which has a survival of 83 % after NACT or chemo-radiotherapy (p = .009) [13]. In the present study, complete pathological response was seen in three (37.5 %) and positive pelvic lymph node was seen in only one (12.5 %) of the operated patients.
The current intense dose and short cycle NACT were efficient, safe, and well tolerated. The most common side effects were alopecia, anorexia, and nausea. Similar results were observed by Gonzalez et al. [14]. In the present study, 100 % of the patients were having grade II alopecia. With regard to late toxicity, no late surgical complications were observed in the eight operated patients. Similar results were observed by Gonzalez et al. [14].
All the patients are being followed up and long-term survival would be commented on at the end of 5 years.
Conclusion
From the above study, we conclude that neoadjuvant chemotherapy is an effective and well-tolerated mode of therapy with acceptable side effects and toxicity. These preliminary results, while encouraging, nevertheless merit a long period of follow-up in a larger group of patients in order to establish neoadjuvant chemotherapy as a standard therapy in locally advanced carcinoma cervix. The study outcome may be useful in formulating future guidelines for chemotherapy in locally advanced cervical cancer.
Acknowledgments
Conflict of interest
None.
References
- 1.Poveda A, Martin AG. Multimodality treatment in locoregional gynecological cancer: cervical cancer treatment update. Ann Oncol. 2008;19:70–76. doi: 10.1093/annonc/mdn465. [DOI] [PubMed] [Google Scholar]
- 2.Rose PG. Combined-modality therapy of locally advanced cervical cancer. J Clin Oncol. 2003;21:211s–217s. doi: 10.1200/JCO.2003.01.222. [DOI] [PubMed] [Google Scholar]
- 3.Green JA, Klirwan JM, Tierney JF, Symnds P, Fresco L, Collingwood M, et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis. Lancet. 2001;358:781–786. doi: 10.1016/S0140-6736(01)05965-7. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez AD, Cetina L, Mariscal I, Garza J. Modern management of locally advanced cervical carcinoma. Cancer Treat Rev. 2003;29:389–399. doi: 10.1016/S0305-7372(03)00068-9. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez AD, Lopea-Graniell CM, Mota A, Mohar A. Neoadjuvant chemotherapy followed by surgery in locally advanced cervical carcinoma. J Clin Oncol. 2002;20:2908–2910. doi: 10.1200/JCO.2002.20.12.2908. [DOI] [PubMed] [Google Scholar]
- 6.Sardi J, Giaroli A, Sananes C, Rueda NG, Vighi S, Ferreira M, Bastardas M, Paniceres G, Di Paola G. Randomized trial with neoadjuvant chemotherapy in stage IIIB squamous carcinoma cervix uteri: an unexpected therapeutic management. Int J Gynecol Cancer. 2002;6:85–93. doi: 10.1046/j.1525-1438.1996.06020085.x. [DOI] [Google Scholar]
- 7.Singh KC, Agarwal A, Agarwal S, Rajaram S, Goel N, Agarwal N. ‘Quick course’ neoadjuvant chemotherapy with cisplatin, bleomycin and vincristine in advanced cervical cancer. Gynecol Obstet Invest. 2004;58:109–113. doi: 10.1159/000078863. [DOI] [PubMed] [Google Scholar]
- 8.Taneja A, Rajaram S, Agarwal S, Singh KC, Sahni S, Goel N. “Quick cycle” neoadjuvant chemotherapy in squamous cell carcinoma of cervix. Indian J Pharmacol. 2005;37(5):320–324. doi: 10.4103/0253-7613.16857. [DOI] [Google Scholar]
- 9.Lissoni AA, Colombo N, Pellegrino A, Parma G, Zola P, Katsaros D, Chiari S, Buda A, Landoni F, Peiretti M, Dell’Anna T, Fruscio R, Signorelli M, Grassi R, Floriani I, Fosati R, V Torri, Rulli E. A phase II, randomized trial of neo-adjuvant chemotherapy comparing a three-drug combination of paclitaxel, ifosfamide, and cisplatin (TIP) versus paclitaxel and cisplatin (TP) followed by radical surgery in patients with locally advanced squamous cell cervical carcinoma: the Snap-02 Italian Collaborative Study. Ann Oncol. 2008;20:660–665. doi: 10.1093/annonc/mdn690. [DOI] [PubMed] [Google Scholar]
- 10.Toita T, Sakumoto K, Hiqashi M, Kakinohana OK, Shinzato S, Moromizato H, Kanazawa K, Sawada S. Therapeutic value of neoadjuvant intra-arterial chemotherapy (cisplatin) and irradiation for locally advanced uterine cervical cancer. Gynecol Oncol. 1997;64:421–424. doi: 10.1006/gyno.1997.4702. [DOI] [PubMed] [Google Scholar]
- 11.Neoadjuvant Chemotherapy for Cervical Cancer Meta-Analysis Collaboration (NACCCMA) Collaboration. Neoadjuvant chemotherapy for locally advanced cervix cancer. Cochrane Database Syst Rev. 2004;(2):CD001774 Review. PMID: 15106161. [DOI] [PMC free article] [PubMed]
- 12.Martin AG, Cortijo LG, Carballo N, Garcia JF, Lapueente F, Rojo A, Chiva LM. The current role of neoadjuvant chemotherapy in the management of cervical carcinoma. Gynecol Oncol. 2008;110:S36–S40. doi: 10.1016/j.ygyno.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Candelaria M, Vilchis JC, Cetina L, Estrada DF, Graniel CL, Enciso AG, Cantú D, Poitevin A, Rivera L, Hinojosa J, Garza J, Gonzalez AD. Prognostic significance of pathological response after neoadjuvant chemotherapy or chemoradiation for locally advanced cervical carcinoma. Int Semin Surg Oncol. 2006;3:3. doi: 10.1186/1477-7800-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez AD, Graniell CL, Enciso AG, Mohar A, Rivera L, Mota A, Guadarrama R, Chanona G, Garza J. Concomitant chemoradiation versus neoadjuvant chemotherapy in locally advanced cervical carcinoma: result from two consecutive phase II studies. Ann Oncol. 2002;13:1212–1219. doi: 10.1093/annonc/mdf196. [DOI] [PubMed] [Google Scholar]