Abstract
The development of muscle fatigue is oxygen (O2)-delivery sensitive [arterial O2 content (CaO2) × limb blood flow (QL)]. Locomotor exercise in acute hypoxia (AH) is, compared with sea level (SL), associated with reduced CaO2 and exaggerated inspiratory muscle work (Winsp), which impairs QL, both of which exacerbate fatigue individually by compromising O2 delivery. Since chronic hypoxia (CH) normalizes CaO2 but exacerbates Winsp, we investigated the consequences of a 14-day exposure to high altitude on exercise-induced locomotor muscle fatigue. Eight subjects performed the identical constant-load cycling exercise (138 ± 14 W; 11 ± 1 min) at SL (partial pressure of inspired O2, 147.1 ± 0.5 Torr), in AH (73.8 ± 0.2 Torr), and in CH (75.7 ± 0.1 Torr). Peripheral fatigue was expressed as pre- to postexercise percent reduction in electrically evoked potentiated quadriceps twitch force (ΔQtw,pot). Central fatigue was expressed as the exercise-induced percent decrease in voluntary muscle activation (ΔVA). Resting CaO2 at SL and CH was similar, but CaO2 in AH was lower compared with SL and CH (17.3 ± 0.5, 19.3 ± 0.7, 20.3 ± 1.3 ml O2/dl, respectively). Winsp during exercise increased with acclimatization (SL: 387 ± 36, AH: 503 ± 53, CH: 608 ± 67 cmH2O·s−1·min−1; P < 0.01). Exercise at SL did not induce central or peripheral fatigue. ΔQtw,pot was significant but similar in AH and CH (21 ± 2% and 19 ± 3%; P = 0.24). ΔVA was significant in both hypoxic conditions but smaller in CH vs. AH (4 ± 1% vs. 8 ± 2%; P < 0.05). In conclusion, acclimatization to severe altitude does not attenuate the substantial impact of hypoxia on the development of peripheral fatigue. In contrast, acclimatization attenuates, but does not eliminate, the exacerbation of central fatigue associated with exercise in severe AH.
Keywords: altitude, respiratory muscle work, arterial O2 content, cerebral blood flow
the development of locomotor muscle fatigue during whole-body endurance exercise is highly sensitive to the delivery of oxygen [O2; arterial O2 content (CaO2) × leg blood flow (QL)]. Specifically, blunted O2 delivery exaggerates, and augmented O2 delivery attenuates the rate of development of locomotor muscle fatigue during exercise (1).
Acute exposure to hypoxia (AH) has a substantial impact on the two determinants of leg muscle O2 delivery during strenuous locomotor exercise. First, despite a marked hyperventilatory response, arterial partial pressure of O2 [PO2 (PaO2)] and arterial hemoglobin saturation (SaO2) fall below sea level (SL) values and cause a significant reduction in CaO2. In addition, inspiratory muscle work (Winsp) is increased substantially at any given workload in hypoxia (2, 58), and these high levels of Winsp compromise, in a dose-dependent manner, QL during exercise (34). Each of these two determinants of leg muscle O2 delivery, namely CaO2 and QL, accounts for, substantially and independently, the accelerated development of locomotor muscle fatigue in hypoxia (2).
During prolonged exposure to altitude, a progressive, time-dependent hyperventilation, which increases alveolar PO2, occurs over the initial hours and days and advances more gradually over the ensuing 1–2 wk of acclimatization (56). This ventilatory acclimatization adds to an accompanying reduction in the alveolar-arterial O2 gradient, which combined, substantially improves arterial oxygenation during exercise by increasing PaO2 and SaO2 (9, 13). Furthermore, chronic exposure to hypoxia (CH) is accompanied by erythropoiesis, and the combination of an increased hemoglobin concentration ([Hb]) plus improved oxygenation may serve to restore resting SL CaO2 (8, 13). In contrast to this beneficial effect on O2 delivery, QL, during intense leg exercise at a given submaximal absolute workload, has been suggested to decline from SL to CH (8, 49, 64). The net effect of these acclimatization-induced, opposing consequences on leg O2 delivery depends on the degree to which the increase in CaO2 can counterbalance potential reductions in QL. It has been documented previously that at a given absolute workload, locomotor muscle O2 delivery is reduced from SL to AH with no further changes following acclimatization (Pikes Peak, 4,300 m) (8, 64). Therefore, given the critical role of muscle O2 delivery in the development of fatigue, it could be argued that peripheral fatigue during constant-load endurance exercise is exacerbated in AH (vs. SL) and does not improve further during prolonged acclimatization. On the other hand, studies conducted at the same location as the present experiments [Mt. Chacaltaya (Bolivia), 5,260 m] document a reduction in locomotor muscle O2 delivery from SL to AH and a full recovery following prolonged exposure, with the net effect of similar values in SL and CH (13). Based on these findings, it could be argued that the development of peripheral fatigue during constant-load endurance exercise is fastened in AH but recovers to SL values in CH.
In this study, we sought to quantify exercise-induced locomotor muscle fatigue induced by the identical constant-load cycling trial performed at SL, in AH, and in CH (following 14 days at 5,260 m) to clarify the effects of acclimatization. We hypothesized that fatigue is, compared with SL, exacerbated significantly in AH and that altitude acclimatization would alleviate this impact.
METHODS
This study was conducted as part of the AltitudeOmics project, examining the integrative physiology of human responses to hypoxia. All procedures conformed to the Declaration of Helsinki and were approved by the Universities of Colorado, Oregon, and Utah Institutional Review Boards and the U.S. Department of Defense Human Research Protection Program Office. All subjects were born and raised below 1,500 m and had not traveled to elevations >1,000 m for 3 mo before the experiments. Eight subjects (age 21 ± 1 y, body weight 69 ± 11 kg, height 176 ± 10 cm) were studied at SL and following 14 days of altitude acclimatization at 5,260 m on Mt. Chacaltaya. At high altitude, subjects did not follow a systematic exercise-training program but were given the opportunity to participate, on a voluntary basis, in light hikes around the campsite (no significant change in altitude).
Experimental Protocol
All participants were familiarized thoroughly with various experimental procedures involved in this investigation. The SL experiments of the present study were conducted ∼130 m above SL [Eugene, OR; barometric pressure (BP) 750.0 ± 2.2 Torr]. The experiments in AH were conducted at the same altitude, while breathing a gas mixture containing 10.5% O2 balance nitrogen, and experiments in CH were conducted on the 14th day of acclimatization at 5,260 m (BP 408.9 ± 0.7 Torr). Two participants were tested every morning. To assure that all subjects were tested exactly on day 14 after arrival on the mountain, the groups' transport to the mountain was staged, i.e., two new participants arrived every day. SL peak power output (Wpeak) was obtained from a maximal incremental exercise test (70, 100, 130, and 160 W for 3 min, each followed by 15 W/min increases thereafter) on a computer-controlled bicycle ergometer (Velotron, Dynafit; RacerMate, Seattle, WA). The experimental trial consisted of the identical constant-load cycling exercise (same absolute workload and duration) in each condition. Preliminary experiments (using different subjects), conducted to identify a workload that causes voluntary exhaustion between 8 and 12 min when acutely exposed to 5,260 m, revealed that a constant workload equal to 50% of SL Wpeak was required to reach this goal. Based on this, the workload during the experimental trials was set to equal 50% (138 ± 14 W) of the subjects' SL Wpeak (275 ± 14 W). Since an individual's endurance/aerobic capacity is lowest in AH (vs. SL and CH) (13), the first trial was performed to voluntary exhaustion in AH, and the achieved time (10.6 ± 0.7 min) was then used for all subsequent trials. A 5-min warm-up at 10% Wpeak (27 ± 8 W) preceded each trial. Throughout exercise, subjects were instructed to maintain their preferred pedal frequency, as determined during the practice sessions (88 ± 3 rpm). Neuromuscular function was assessed before and within 2.5 min after exercise. During these procedures, subjects breathed ambient air at SL and in CH and a gas mixture (10.5% O2) in AH.
Exercise Responses
Pulmonary ventilation (VE) and gas exchange were measured at rest and throughout exercise using an open circuit system (Ultima PFX; Medical Graphics, St. Paul, MN, and O2cap; Oxigraf, Mountain View, CA). Arterial O2 saturation (SpO2) was estimated continuously at rest and during exercise using a pulse oximeter (Nellcor N-200; Pleasanton, CA) with adhesive forehead sensors. A correction factor based on arterial blood gases was used to adjust for the nonlinearity associated with the obtained pulse oximeter values (error between 60% and 80% saturation: 6%; error between >90% saturation: 3%). Heart rate was measured from the R–R interval of an ECG, using a three-lead arrangement. Ratings of perceived exertion were obtained using Borg's modified CR10 scale (10). [Hb] was measured (Radiometer OSM-3) in resting arterial blood samples collected at SL and on the 16th day at 5,260 m. CaO2 was estimated as 1.39 [Hb] × (SpO2/100). During all constant workload trials, esophageal pressure (Pes) was measured via a nasopharyngeal balloon (Cooper Surgical, Trumbull, CT), using standard procedures (7). To estimate Winsp, Pes was integrated over the period of inspiratory flow, and the results were multiplied by respiratory frequency (fR) and labeled the inspiratory muscle pressure-time product. Vastus lateralis oxygenation was assessed using a multichannel near-infrared spectroscopy (NIRS) instrument (Oxymon Mk III; Artinis, Zetten, The Netherlands). As described previously (5), a NIR emitter and detector pair was affixed over the belly of the left vastus lateralis muscle (∼15 cm proximal and 5 cm lateral to the midline of the superior border of the patella), using a spacer with an optode distance of 5.0 cm. Probes were secured to the skin using double-sided tape and shielded from light using elastic bandages. The Beer-Lambert Law was used to calculate micrometer changes in tissue oxygenation [oxyhemoglobin (O2Hb) and deoxyhemoglobin (HHb)] across time. using received optical densities from two continuous wavelengths of NIR light (780 and 850 nm) and a fixed differential path-length factor of 4.95 (26). Total hemoglobin (THb) was calculated as the sum of [O2Hb] and [HHb] changes to give an index of change in regional blood volume (59). Data were recorded continuously at 10 Hz and expressed relative to the resting baseline recorded in each experimental condition. Mean cerebral blood flow (CBF) was estimated from blood velocity (CBFv) in the left middle cerebral artery (MCA; 50 ± 4 mm deep), determined using a 2-MHz transcranial Doppler (Spencer Technologies, Seattle, WA). An index of cerebral O2 delivery was calculated as the product of CBFv and CaO2. Changes in CBFv were assumed to reflect changes in CBF, based on evidence that the MCA changes minimally in response to hypoxia and hypocapnia (47, 54). The validity of this assumption at altitude has been challenged recently (62). Evidence of MCA dilation was demonstrated in subjects at altitudes above 6,400 m, but no changes in MCA diameter were observed at altitudes comparable with the present study (<5,300 m) (63). We acknowledge that these measurements must be interpreted with caution until definitive studies of MCA diameter at altitude are conducted.
Expiratory Flow Limitations and Lung Volume Responses
Expiratory flow limitations.
Subjects performed three maximal volitional flow-volume (FV) maneuvers before and after exercise (after assessment of neuromuscular function). Exercise tidal FV loops (FVLs) were plotted within the best of the six maximal loops (MFVLs), based on measured inspiratory capacity (IC) maneuvers (rest, 3 min of exercise, and immediately before the termination of exercise). Acceptable IC maneuvers during exercise required that peak inspiratory Pes match that obtained at rest. The amount of expiratory flow limitation was defined as the percentage of the tidal volume (VT) that met the boundary of the expiratory portion of the MFVL (38).
Lung volumes.
Functional residual capacity (FRC) was measured in a body plethysmograph (Platinum Elite Series; Medical Graphics), and total lung capacity (TLC) was calculated as the sum of FRC and IC. End-expiratory lung volume (EELV) was determined by subtracting the maximal IC, as measured during exercise from TLC, as measured at rest. End-inspiratory lung volume (EILV) was calculated as the sum of EELV and VT. Inspiratory reserve volume, during exercise, was calculated by subtracting EILV from TLC, and expiratory reserve volume, during exercise, was determined by subtracting the residual volume from EELV.
Force and Compound Muscle Action Potentials
Knee-extensor force during voluntary and evoked contractions was measured using a calibrated load cell (Tedea, Basingstoke, UK). The load cell was fixed to a custom-built chair and connected to a noncompliant cuff, attached around the participant's right leg, just superior to the ankle malleoli. Participants sat upright in the chair with the hips and knees at 90° of flexion. Compound muscle action potentials (M-waves) were recorded from surface electrodes placed 2 cm apart over the vastus lateralis muscle belly. A reference electrode was placed over the patella. Evoked signals were amplified [gain: 1,000; force: custom-built bridge amplifier; electromyographic (EMG): PowerLab 26T; ADInstruments (Oxfordshire, UK)], band-pass filtered (EMG only: 20–2,000 Hz), digitized (4 kHz; PowerLab 26T, ADInstruments), acquired, and later analyzed (LabChart v7.0; ADInstruments) for peak-to-peak amplitude.
Neuromuscular Function
Force and EMG variables were assessed before and immediately (<2.5 min) after each trial. Before each trial, maximum voluntary contraction (MVC) force was determined from three control contractions. Femoral nerve stimulation was delivered during each 5-s MVC, and an additional stimulus was delivered after the MVC to determine the potentiated quadriceps twitch force (Qtw,pot) and voluntary muscle activation (VA) (42). Briefly, the force produced during the superimposed twitch (SIT), delivered within 0.5 s of attaining peak force during the MVC, was to be compared with the force produced by the single twitch, delivered during relaxation, ∼2 s after the MVC: VA (%) = [1 − (SIT/Qtw,pot)] × 100. The contraction sets were repeated three times, with 30 s between each set. Visual feedback of the target force was provided via a computer monitor.
Femoral nerve stimulation.
Single electrical stimuli (200 μs pulse width) were delivered to the right femoral nerve via surface electrodes (32 mm diameter; CF3200; Nidd Valley Medical, North Yorkshire, UK) and a constant-current stimulator (DS7AH; Digitimer, Welwyn Garden City, Hertfordshire, UK). The cathode was positioned over the nerve, high in the femoral triangle; the anode was placed midway between the greater trochanter and the iliac crest (32). The site of stimulation that produced the largest resting twitch amplitude and M-wave was located. Single stimuli were delivered, beginning at 100 mA and increasing by 20 mA, until plateaus occurred in twitch amplitude and M-wave. Supramaximal stimulation was ensured by increasing the final intensity by 30% (mean current, 250 ± 55 mA). Muscle contractility was assessed for each potentiated twitch as twitch amplitude (Qtw,pot: peak force − onset force), maximum rate of force development (MRFD), contraction time, maximum relaxation rate (MRR), and one-half relaxation time (RT0.5). Sarcolemmal membrane excitability was inferred from the peak-to-peak amplitude of the electrically evoked M-wave (27).
Reliability Measures
On a separate day, measures of neuromuscular function were repeated twice in all subjects at SL. The two assessment procedures were separated by a 2-min walk around the laboratory, followed by a 5-min rest period. Coefficient of variation (CV) and Pearson product-moment correlation coefficients (r) were calculated to evaluate test-retest error (precision) and test-retest reliability of the neuromuscular function-assessment procedure. All correlations were significant and indicated; in combination with the CVs, acceptable degrees of reproducibility include: MVC, CV = 3.1%, r = 0.97; Qtw,pot, CV = 4.1%, r = 0.98; M-wave peak, CV = 4.8%, r = 0.98; VA, CV = 3.3%, r = 0.77.
Statistical Analysis
A one-way repeated-measures ANOVA was performed to evaluate differences among trials. A least-significance difference test identified the means that were significantly different with P < 0.05. Results are expressed as mean ± SE.
RESULTS
CaO2 and Cerebral O2 Delivery
CaO2 at rest was significantly lower in AH compared with SL and CH (17.3 ± 0.5, 19.3 ± 0.7, 20.3 ± 1.3 ml O2/dl, respectively). Acclimatization to altitude significantly increased [Hb] and SpO2, resulting in similar CaO2 at SL and in CH (P = 0.16). Resting CBFv was similar among SL, AH, and CH (50.5 ± 3.7, 52.7 ± 2.3, and 55.7 ± 3.0 cm/s, respectively; P = 0.45). In all three conditions, CBFv increased significantly from rest to the final minute of exercise (22 ± 3%, 39 ± 6%, and 28 ± 5% for SL, AH, and CH, respectively; Table 1). The percent increase was significantly greater in AH compared with that observed at SL and in CH. The cerebral O2 delivery index during the last minute of exercise was 18 ± 5% lower in AH vs. SL (Table 1) and 17 ± 8% greater in CH vs. SL (Table 1).
Table 1.
Mean responses to the final minute of exercise (138 ± 14 W, 10.6 ± 0.7 min)
| Sea Level | Acute Hypoxia | Chronic Hypoxia | |
|---|---|---|---|
| HR, beats/min | 152 ± 5 | 174 ± 4* | 166 ± 4*† |
| VE, l min−1 | 64 ± 4 | 113 ± 8* | 133 ± 10*† |
| fR, breaths min−1 | 32 ± 2 | 50 ± 3* | 54 ± 3* |
| VT, liter | 2.0 ± 0.1 | 2.2 ± 0.2 | 2.6 ± 0.2*† |
| V̇o2, l min−1 | 2.58 ± 0.19 | 2.44 ± 0.19* | 2.39 ± 0.16*† |
| V̇co2, l min−1 | 2.51 ± 0.22 | 2.81 ± 0.21* | 2.40 ± 0.15*† |
| VE/V̇o2 | 25 ± 1 | 50 ± 4* | 56 ± 3*† |
| VE/V̇co2 | 26 ± 1 | 41 ± 2* | 58 ± 3*† |
| SpO2, % | 94.1 ± 1.0 | 62.2 ± 1.8* | 75.6 ± 1.2*† |
| CBFv, cm/s | 59.1 ± 4.8 | 74.2 ± 3.8* | 73.2 ± 3.4* |
| Cerebral O2 delivery, a.u. | 1,105 ± 62 | 895 ± 40* | 1,289 ± 42*† |
| Ti/Ttot | 0.35 ± 0.01 | 0.39 ± 0.01* | 0.39 ± 0.01* |
| Te, s | 1.30 ± 0.08 | 0.74 ± 0.05* | 0.70 ± 0.04* |
| Winsp, cmH2O · s−1 · min−1 | 387 ± 36 | 503 ± 53* | 608 ± 67*† |
| IC, liter | 3.29 ± 0.22 | 3.13 ± 0.23 | 3.60 ± 0.23* |
| VT/IC | 0.60 ± 0.03 | 0.68 ± 0.02* | 0.72 ± 0.02*† |
| IRV, liter | 1.30 ± 0.14 | 0.99 ± 0.13* | 0.96 ± 0.05* |
| ERV, liter | 1.98 ± 0.25 | 2.14 ± 0.29 | 1.67 ± 0.25*† |
| EILV, %TLC | 80.5 ± 1.6 | 85.4 ± 1.7* | 85.2 ± 0.9* |
| EELV, %TLC | 51.5 ± 1.8 | 53.8 ± 2.5 | 46.9 ± 2.1*† |
| Expiratory flow limitation, n out of 8 subjects | 0/8 | 2/8 | 4/8 |
| RPE | 12.3 ± 1.0 | 19.8 ± 0.1* | 17.9 ± 0.6*† |
| Dyspnea | 11.5 ± 0.7 | 19.5 ± 0.2* | 19.3 ± 0.2* |
HR, heart rate; VE, minute ventilation; fR, breathing frequency; VT, tidal volume; V̇o2, maximum oxygen (O2) uptake; V̇co2, carbon dioxide production; SpO2, arterial O2 saturation; CBFv, cerebral blood flow velocity; Ti, duration of inspiration; Ttot, duration of entire breath; Te, duration of expiration; Winsp, inspiratory muscle work; IC, inspiratory capacity; IRV, inspiratory reserve volume; ERV, expiratory reserve volume; EILV, end-inspiratory lung volume; TLC, total lung capacity; EELV, end-expiratory lung volume; RPE, rating of perceived exertion.
P < 0.05 vs. sea level;
P < 0.05 vs. acute hypoxia, n = 8.
Ventilatory Effects
Ventilatory response.
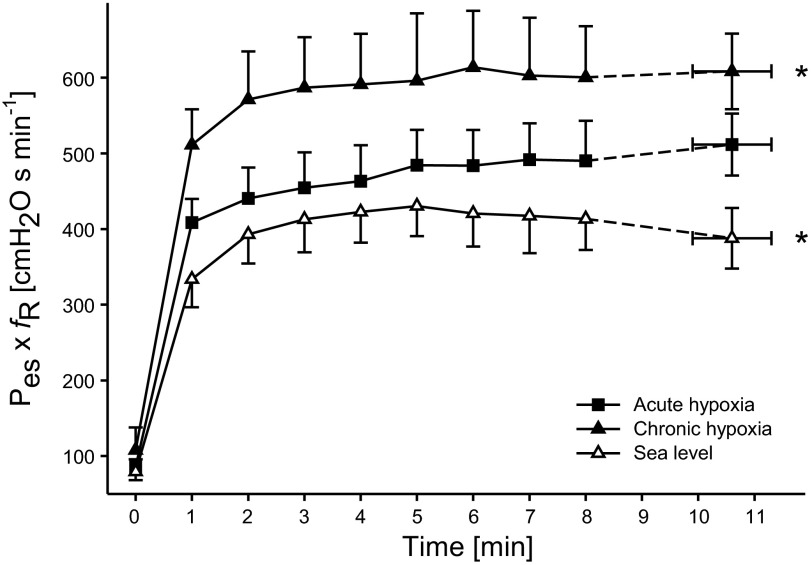
AH increased Winsp work by 34 ± 8% above that at SL (P < 0.01) and dropped SpO2 by 36 ± 3% during the final minute of exercise. Following 14 days of acclimatization, Winsp was increased further by 23 ± 8% from AH, and SpO2, during the final minute of exercise, was 36 ± 5% higher in CH vs. AH. Breathing frequency and VE rose substantially over the time of exercise in AH and CH, and VE was, during the final minute, 79 ± 13% and 110 ± 12%, respectively, higher compared with SL (P < 0.01). Pulmonary VE during the final minute of exercise was 19 ± 4% higher in CH vs. AH (P < 0.01). Compared with SL, O2 uptake, during the final minute of exercise, was 5 ± 2% and 7 ± 2% lower in AH and CH, respectively (both P < 0.05; Fig. 1).
Fig. 1.
Inspiratory muscle pressure-time product [esophageal pressure (Pes) × respiratory frequency (fR)] during the identical constant-load cycling exercise performed in all 3 conditions. *P < 0.05 vs. acute hypoxia (AH), n = 8.
Expiratory flow limitation.
At SL, exercise flow rates during tidal breathing were well within the MFVL in all eight subjects. At end-exercise in AH, 6–51% of the VT in two of the eight subjects reached flow limitation, as lung volume approached end-expiration. As VE increased further in CH, expiratory flow rate became more limited, and 10–64% of the VT in four of the eight subjects met the limit imposed by the MFVL.
Membrane Excitability and Contractile Function
M-waves.
As a measure of membrane excitability we examined pre- vs. postexercise vastus lateralis M-wave amplitudes in conjunction with the quadriceps muscle mechanical properties. Pre-exercise M-wave amplitudes were similar in all three conditions (10.2 ± 1.0 mV, 9.4 ± 0.7 mV, and 12.9 ± 1.8 mV for SL, AH, and CH, respectively; P = 0.15). Postexercise M-wave amplitudes were unchanged from pre-exercise baseline values at SL and in AH (10.2 ± 1.0 mV and 9.6 ± 0.9 mV, respectively; P > 0.3). However, following exercise in CH, M-wave amplitudes (7.8 ± 2.1 mV) were reduced significantly from pre-exercise baseline levels (range: 1–18%; P < 0.01).
Quadriceps twitch force.
Pre-exercise Qtw,pot was similar in all three conditions (106 ± 4 N, 109 ± 4 N, and 110 ± 5 N for SL, AH, and CH, respectively; P = 0.18). Exercise in both hypoxic conditions caused a substantial (P < 0.01) but similar (P = 0.14) reduction in Qtw,pot in all eight subjects. In contrast, exercise at SL did not induce measurable locomotor muscle fatigue; the postexercise Qtw,pot was similar to pre-exercise baseline.
MVC force.
Pre-exercise MVC was similar in all three conditions (391 ± 30 N, 394 ± 25 N, and 372 ± 30 N for SL, AH, and CH, respectively; P = 0.21). At SL, postexercise MVC was similar to pre-exercise baseline (P = 0.42). In contrast, exercise in AH and CH caused a substantial reduction in MVC in all eight subjects. However, the exercise-induced reduction in MVC was 30 ± 9% less in CH vs. AH (P < 0.05).
Muscle activation.
Pre-exercise baseline values were similar in all three conditions (94 ± 1%, 94 ± 1%, and 93 ± 1% for SL, AH, and CH, respectively; P = 0.19). Following the exercise at SL, muscle activation was unchanged from pre-exercise baseline (P = 0.88). In both AH and CH, postexercise muscle activation was significantly lower compared with pre-exercise baseline values. However, the pre- to postexercise decrease in muscle activation was 52 ± 12% less in CH vs. AH (P < 0.01).
Within-twitch measurements.
MRFD, MRR, and RT0.5 complement the findings reported for Qtw,pot. The pre- to postexercise changes in within-twitch measurements of MRFD, MRR, and RT0.5 were similar in CH vs. AH.
Vastus Lateralis Tissue Oxygenation
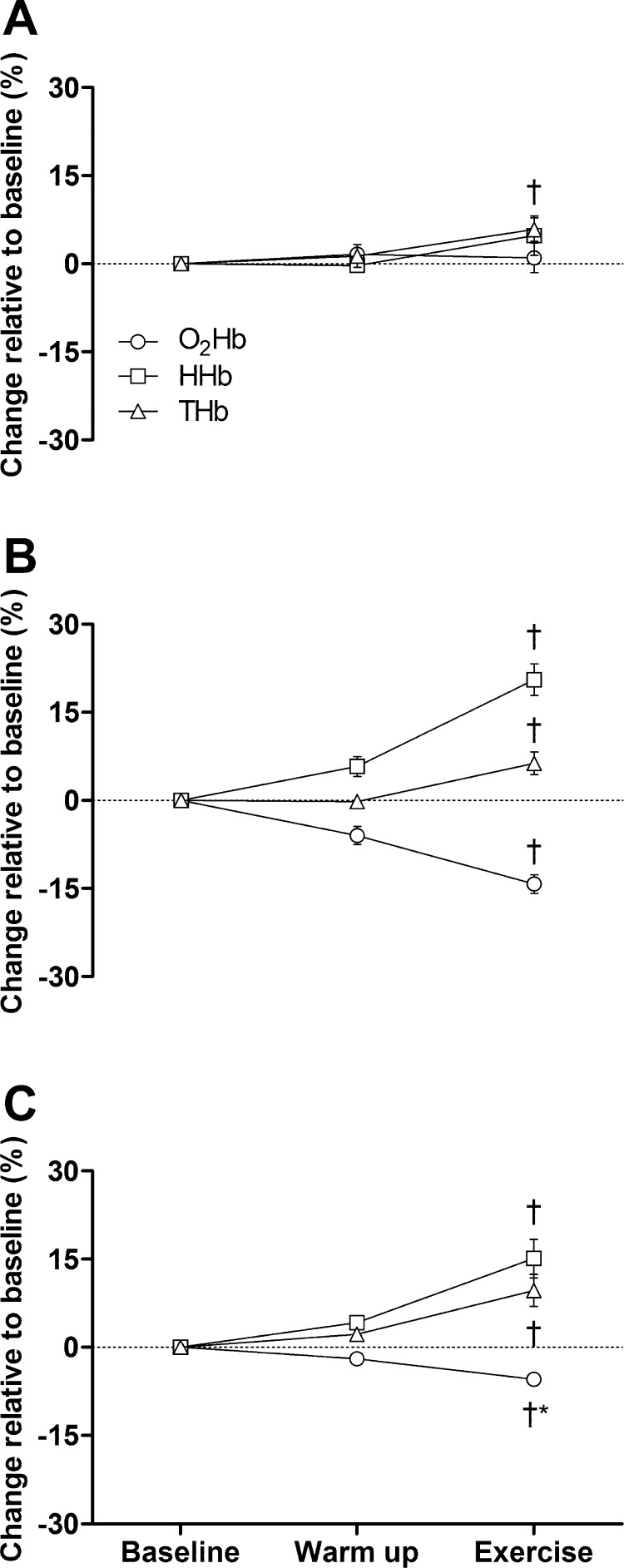
O2Hb was unchanged from baseline to warm-up at SL (P = 0.40) but decreased in AH (P < 0.05) and CH (P = 0.05). Compared with baseline, O2Hb was unchanged during the final minute of exercise at SL (P = 0.73) but was significantly lower in AH and CH (both P < 0.01). This decrease was significantly greater in AH vs. CH. HHb was unchanged from baseline to warm-up at SL (P = 0.80) but decreased significantly in AH and CH. Compared with baseline, HHb was unchanged during the final minute of exercise at SL (P = 0.24) but similarly increased in AH and CH (both P < 0.01). THb was unchanged from baseline to warm-up in all three conditions. In contrast, compared with baseline, THb was increased significantly and similarly (P = 0.37) during the final minute of exercise in all three conditions.
DISCUSSION
The purpose of this investigation was to evaluate the effect of altitude acclimatization on the development of fatigue during whole-body endurance exercise. Subjects repeated the identical constant-load cycling exercise at SL, in AH, and in CH. No measurable degree of fatigue was found following the exercise at SL. However, the identical exercise in AH, characterized by a reduced CaO2 and increased Winsp, resulted in a substantial degree of both peripheral and central fatigue. Two weeks of exposure to 5,260 m restored CaO2 to SL values but increased Winsp further over that observed in AH. The critical finding was that the rate of development of peripheral locomotor muscle fatigue failed to recover from AH to CH and was similar in both conditions. In contrast, the development of central fatigue was attenuated significantly in CH (vs. AH) but still greater compared with SL. Taken together, our findings suggest that acclimatization to high altitude attenuates the impact of AH on the development of central fatigue but fails to improve the exacerbated development of peripheral fatigue present during exercise in AH.
Peripheral Fatigue
Acute hypoxia.
The cycling bout in AH was, compared with SL, characterized by a substantially exaggerated rate of peripheral fatigue (Table 2 and Fig. 2). These observations confirm numerous earlier findings using whole-body (4, 31, 57) and single-muscle exercise (28, 39).
Table 2.
Effects of constant-load cycling exercise on quadriceps muscle function
| Percent Change from Pre- to Immediately Postexercise | |||
|---|---|---|---|
| Sea Level | Acute Hypoxia | Chronic Hypoxia | |
| Qtw,pot | −3.1 ± 1.8* | −20.9 ± 2.4 | −18.8 ± 3.4 |
| MRFD | −4.1 ± 2.5* | −21.2 ± 4.2 | −17.9 ± 3.5 |
| MRR | 2.7 ± 2.8* | −13.2 ± 3.1 | −9.0 ± 2.2 |
| RT0.5 | 1.0 ± 2.2* | 9.2 ± 1.3 | 8.2 ± 1.4 |
| MVC | −1.3 ± 1.2* | −12.3 ± 1.2 | −8.9 ± 1.3† |
| Voluntary muscle activation | −0.1 ± 1.0* | −6.9 ± 1.1 | −3.7 ± 1.2† |
| M-wave amplitude | 0.7 ± 2.7* | 2.5 ± 2.0* | −7.8 ± 2.1 |
Changes in muscle function are expressed as a percent change from pre-exercise baseline. All exercise trials were performed for the same duration (10.6 ± 0.7 min) and at the same absolute workload (138 ± 14 W).Values are expressed as means ± SE. Qtw,pot, potentiated single twitch; MRFD, maximal rate of force development; MRR, maximal rate of relaxation; RT0.5, 1/2 relaxation time; MVC, maximal voluntary contraction force; M-wave, compound muscle action potential. Percent muscle activation is based on superimposed twitch technique. Various variables in acute and chronic hypoxia were, compared with baseline, altered significantly, 2.5 min after exercise (P < 0.01).
Not significantly different from pre-exercise baseline.
P < 0.05 vs. acute hypoxia, n = 8.
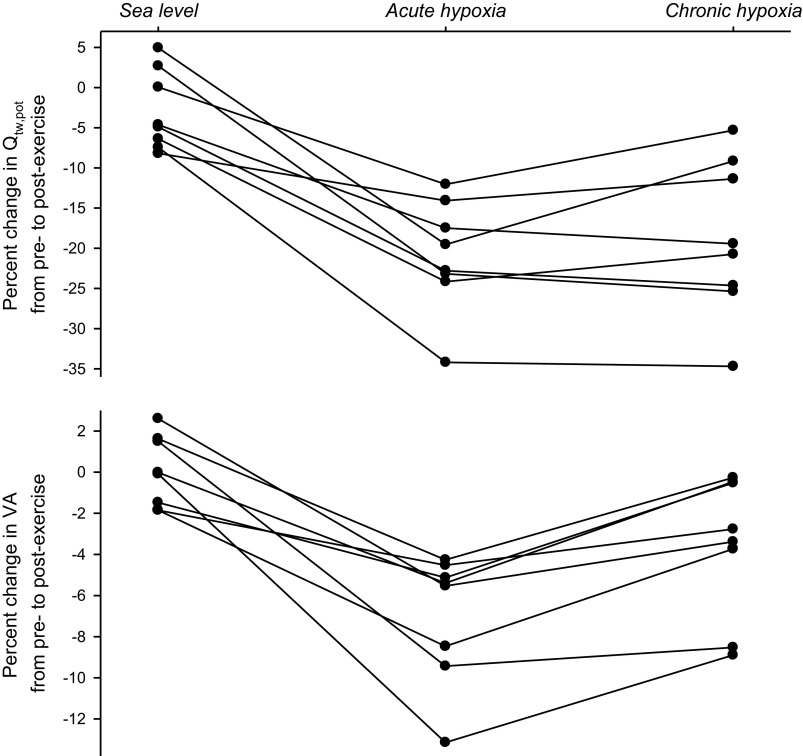
Fig. 2.
Individual data illustrating the effects of constant-load bike exercise (138 ± 14 W; 10.6 ± 0.7 min) on potentiated quadriceps twitch force (Qtw,pot; top) and voluntary muscle activation (VA; bottom) at sea level [SL; resting arterial oxygen (O2) content: 19.3 ± 0.7 ml O2/dl] and in AH (17.3 ± 0.5 ml O2/dl) and chronic hypoxia (CH; 20.3 ± 1.3 ml O2/dl).
Compared with SL, CaO2 was approximately one-third lower and Winsp, ∼34% higher during exercise in AH. These substantial alterations are known to contribute about equally to the exacerbated development of peripheral fatigue in AH (2). The impact of an acutely lowered CaO2 on muscle fatigability is mediated via the facilitating effects of the associated reduction in muscle O2 delivery on the intramuscular accumulation of metabolites known to cause peripheral fatigue, i.e., hydrogen ion and inorganic phosphate (37, 61). The Winsp-induced exacerbation of peripheral fatigue results from the same intramuscular metabolic consequences associated with reductions in locomotor muscle O2 delivery. However, in the case of the Winsp-related impairment in peripheral fatigue, the compromised O2 delivery is the consequence of a sympathetically mediated impact on QL, secondary to the activation of the respiratory muscle metaboreflex (34). Taken together, the combined effects of a significantly reduced CaO2 and a higher Winsp has a profound impact on leg O2 delivery and thus peripheral locomotor muscle fatigue (1).
Chronic hypoxia.
Despite 2 wk of acclimatization to altitude, the rate of development of peripheral locomotor muscle fatigue was similar in AH and CH (Table 2 and Fig. 2). Somewhat conflicting data from earlier investigations suggest different mechanisms as a potential explanation of this finding. On the one hand, studies conducted by Reeves and colleagues (8, 64), following 2–3 wk at 4,300 m, report similar locomotor muscle O2 delivery during submaximal endurance exercise in AH and CH. Given the critical dependency of the development of peripheral fatigue on muscle O2 delivery, this similarity might explain the nearly identical levels of end-exercise locomotor muscle fatigue in AH and CH. On the other hand, experiments conducted at the same location as the present study (Mt. Chacaltaya, 5,260 m) have documented a significant improvement in leg muscle O2 delivery from AH to CH, with the net effect of similar values during submaximal bike exercise at SL and in CH (13). It might be important to emphasize that these latter experiments involved a greater altitude (5,260 m vs. 4,300 m) and a 9–10 wk acclimatization period vs. only a 2–3 wk period, as in the experiments by Reeves and colleagues (8, 64), as well as the present study. Regardless, based on the findings from the earlier Chacaltaya experiments, it appears that the similar degrees of end-exercise fatigue in AH and CH in the present study (Fig. 2) might have occurred in the face of a significant difference in bulk muscle O2 delivery, i.e., higher in CH vs. AH.
QL was not measured directly in the present study. However, changes in THb, a NIRS-derived variable, are thought to reflect changes in regional blood volume and potentially QL (24, 59). The previously documented similarity in resting QL at SL, in AH, and in CH (11, 12, 36, 49, 50) is a critical prerequisite when using THb as an estimate of potential differences in QL and O2 delivery during exercise. Since CaO2 was comparable at SL and CH (see results), the same exercise-induced increase in THb (Fig. 3) suggests a similar degree of O2 delivery in these conditions. Furthermore, the combination of a lower CaO2 in AH vs. CH (and SL; see results) plus the similar increase in THb during exercise (Fig. 3) insinuates a lower locomotor muscle O2 delivery in AH vs. CH (and by extension, SL). Both of these observations might support earlier blood flow studies conducted at the same location as the present experiments (13) but might contradict others performed at a lower altitude (8, 64). However, NIRS findings obtained from skeletal muscle need to be interpreted with caution. A significant limitation associated with NIRS is that this measurement is confined to a finite location, and changes in THb might not be representative of the whole muscle. Indeed, significant blood flow heterogeneity has been documented previously in skeletal muscle (35). Whereas heterogeneity diminishes with higher exercise intensities and is not affected by hypoxia (36), the exact location of NIRS probe placement from day to day is a potential source of error. To minimize this risk, we had strict criteria regarding probe placement (see methods), and at least two investigators independently assured correct probe positioning before each experiment.
Fig. 3.
Vastus lateralis oxygenation at resting baseline, during the final 30 s of a 3-min warm-up (28 W), and during the final 30 s of constant-load exercise (131 W) at SL (A), in AH (B), and in CH (C). †P < 0.05 vs. respective baseline; *P < 0.05 vs. AH, n = 8. O2Hb, oxyhemoglobin; HHb, deoxyhemoglobin; THb, total hemoglobin.
Assuming that the similar degrees of peripheral fatigue in AH vs. CH occurred in the face of a greater O2 delivery in CH, other, rather disadvantageous adaptations associated with acclimatization must have outweighed this benefit. A potential candidate is the documented impairment in the capacity of skeletal muscle to extract O2 in CH, i.e., a decreased capillary muscle O2 conductance (41). This impact might, despite a similar O2 delivery at SL and in CH, potentially lower extracellular PO2 to or beyond a previously suggested critical value (∼30 Torr) associated with exacerbated development of peripheral fatigue (55). Alternatively, the higher O2 delivery in CH vs. AH (13), combined with the same degree of peripheral fatigue, might suggest that CaO2 and bulk O2 delivery, per se, might not depict key determinants of the exaggerated fatigability in hypoxia. Important here is the fact that despite the normalized CaO2 and bulk O2 delivery in CH, PaO2 only partially recovers with acclimatization and remains fairly low in CH. This could hint toward a key role of PaO2 in exacerbating the development of peripheral fatigue at altitude.
In CH, VE was ∼20% higher compared with AH. Given the substantially lower air density at 5,260 m (0.64 kg/m3 vs. 1.18 kg/m3 at 130 m, where AH experiments occurred), it could be argued that in terms of respiratory muscle work, the reduced density might balance the acclimatization-induced increase in VE, with the net effect of a similar Winsp in CH and AH. However, Winsp was, similar to VE, ∼20% higher in CH vs. AH. This observation, per se, might suggest that the lower air density at altitude had no effect on the relationship between minute VE and respiratory muscle work. However, it has been shown that bronchoconstriction, associated with severe hypoxia, increases the resistive component of respiratory work and offsets the theoretical benefit of a reduced air density (22). This results in a similar respiratory muscle work for a given VE at altitude and at SL (18). Therefore, any increase in Winsp observed in hypobaric CH is attributable to the exaggerated ventilatory response associated with altitude acclimatization.
The increase in minute VE in the present study was mainly due to the increase in VT; fR was similar in both conditions. The higher VT was achieved via reductions in EELV (Table 1), which is compared with increasing EILV to raise VT, more economical, since higher lung volumes are associated with a reduced compliance (38). We therefore conclude that the 23% higher Winsp at the same workload in CH vs. AH resulted from the substantially higher VE following acclimatization. Finally, this exaggerated Winsp likely aggravated the respiratory muscle metaboreflex and associated impact on leg vascular conductance (25) and presumably blunted exercise QL more in CH compared with AH.
In contrast to our findings, it was suggested previously that acclimatization to high altitude might eliminate the impact of AH on the rate of development of fatigue during single muscle exercise (adductor pollicis) and restore it to that observed at SL (28). However, submaximal, intermittent exercise, including a small muscle mass, does not maximally challenge O2 delivery and use. Therefore, the observed positive effect could, at least in part, be explained by the use of the available reserve capacity. Specifically, various compensatory mechanisms, including increases in cardiac output and muscle O2 delivery and extraction, could have reduced the hypoxia-induced impact on the development of fatigue. Such an effective compensation might not—or only to a much smaller degree–be possible during intense, whole-body exercise, performed close to a human's maximal circulatory and ventilatory capacity (14, 15).
CH had a significant impact on the effect of exercise on M-wave amplitude. Reductions in M-wave amplitude have been associated with decreases in sarcolemma excitability (19). The attenuated excitability results from reduced sarcolemma sodium (Na+)-potassium (K+)-ATPase activity (46) and can contribute to compromised muscle force output (21). Pre-exercise M-wave amplitudes (and Qtw,pot) in our experiments were similar in all three conditions. This suggests that neither severe AH nor CH impairs sarcolemma Na+-K+-ATPase activity and membrane excitability of resting locomotor muscle. This confirms earlier findings (40); however, it contrasts with others (16) who report decreased resting M-wave amplitudes following 10 days of exposure to severe hypoxia (>4,300 m). Regardless, although M-wave amplitudes did not change from pre- to postexercise at SL and in AH, we observed, in contrast to Garner et al. (30), a significant exercise-induced decrease in CH (Table 2). AH has recently been shown to have no effect on exercise-induced changes in Na+-K+-ATPase activity, which explains the similar M-wave behavior in SL and AH (51). However, altitude acclimatization causes a downregulation of Na+-K+-ATPase pump concentration, and although this does not alter resting M-wave characteristics, it likely explains the exercise-induced decrease in M-wave amplitude observed in CH (20, 33).
The lower postexercise M-wave amplitude in CH indicates a failure of the motor nerve/sarcolemma to propagate evoked stimuli to the contractile apparatus and might have masked potential benefits of acclimatization on fatigue resistance. Put simply, postexercise twitch forces might have been larger in CH if M-waves had remained unchanged from pre-exercise. If so, this would have resulted in a smaller exercise-induced reduction in Qtw,pot in CH. Regardless, failure of neuromuscular transmission/sarcolemmal excitability contributes to reduced force output in response to a given central nervous activation and can therefore be considered a key determinant of the impaired fatigue resistance in CH.
Central Fatigue
Exercise in AH induced a substantial degree of central fatigue, which was attenuated by ∼50% when the same trial was repeated in CH (Table 2). This significant improvement, associated with acclimatization, clearly contrasts with the absence of a beneficial effect of CH on peripheral fatigue, as described above. Since the development of central fatigue is highly sensitive to O2 (1), we attribute this improvement to the effects of high-altitude acclimatization on O2 availability within the brain. Specifically, the cerebral O2 delivery index at the end of exercise in CH was improved from AH (Table 1) (65) and may explain the lower degree of central fatigue in CH vs. AH.
Despite the similar CBFv and a slightly higher brain O2 delivery in CH vs. SL (Table 1), which agrees with earlier Chacaltaya studies using the Kety-Schmidt technique to measure CBF/O2 delivery (44), exercise-induced central fatigue was greater in CH. Two considerations discussed previously might account for this observation. First, the significant degree of peripheral fatigue in CH (vs. no fatigue at SL) presumably facilitated central fatigue via increases in inhibitory neural feedback from locomotor muscle (mediated by group III/IV muscle afferents), which limit central motor drive (3, 6). Second, although CaO2 and brain O2 delivery were similar/higher in CH vs. SL, the still substantially lower PaO2 might have contributed to the greater degree of central fatigue during exercise in this condition. Indeed, a low PaO2 was recently suggested to impair cerebral metabolism (48) and alterations in neurotransmitter turnover (23), and both of these factors have been linked to the development of central fatigue (17, 53).
Taken together, the current findings provide a global indication of the positive effects of altitude acclimatization on the development of central fatigue during exercise. However, we cannot comment on the specific sites of the central motor pathway involved or the relative contribution of CaO2 and PaO2 in mediating these beneficial adaptations.
Implications of Findings for Performance-Related Questions in CH
AH generally impairs endurance exercise performance (60). Prolonged exposure to hypoxia is known to recover some of this impairment (29, 52); however, SL performance is never matched at altitude. Our current findings indicate that the acclimatization-induced partial recovery of endurance performance occurs independent of any improvement of peripheral locomotor muscle fatigue from AH to CH. This insinuates that peripheral locomotor muscle fatigability, per se, does not contribute to the improvement of endurance performance observed from AH to CH. We therefore propose that the significantly attenuated central fatigue during exercise in severe CH likely accounts, at least in part, for the improvement of endurance performance associated with altitude acclimatization.
Mechanisms underlying the hypoxia-induced curtailment of central motor drive (i.e., increase in central fatigue) and endurance exercise performance have been documented previously to differ depending on the severity of arterial hypoxemia. Specifically, peripheral fatigue might depict the dominant determinant of central motor drive and thus the limiting factor above 70–75% SpO2. At more severe degrees of hypoxemia (<70% SpO2), central motor drive and endurance performance might primarily—but not exclusively—be determined/limited by central nervous system (CNS) hypoxia (5). Since peripheral fatigue did not change with acclimatization in the present study, but SpO2 increased from below to above the “threshold” described previously (5), reductions in central fatigue might be mediated mainly by improved arterial oxygenation and associated smaller influence of CNS hypoxia on central motor drive.
A recent Point:Counterpoint debate in this journal has focused on the potential existence/relevance of differences in physiological responses to exercise performed in normobaric vs. hypobaric hypoxia (43, 45). Since the present AH and CH experiments were performed in normobaric and hypobaric hypoxia, respectively, these potential differences, if indeed existent, might have influenced our findings.
Conclusion
AH exacerbates central and peripheral fatigue during endurance exercise. Our experiments indicate that acclimatization to high altitude significantly attenuates the development of central fatigue but does not improve the development of peripheral fatigue observed during whole-body endurance exercise in AH.
GRANTS
Funding for AltitudeOmics was provided, in part, by grants from the Department of Defense (W81XWH-11-2-0040 Telemedicine and Advanced Technology Research Center to R. C. Roach and W81XWH-10-2-0114 to A. T. Lovering); the Cardiopulmonary & Respiratory Physiology Laboratory, University of Oregon; and the Altitude Research Center and the Charles S. Houston Endowed Professorship, Department of Emergency Medicine, School of Medicine, University of Colorado Denver. Additional funding for this study of fatigue in hypoxia was provided by the National Heart, Lung, and Blood Institute (HL-103786 and HL-116579 to M. Amann).
DISCLOSURES
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Author contributions: M.A., S.G., and A.W.S. conception and design of research; M.A., S.G., R.T., and A.W.S. performed experiments; M.A., S.G., and R.T. analyzed data; M.A., S.G., R.T., and A.W.S. interpreted results of experiments; M.A. and S.G. prepared figures; M.A. and A.W.S. drafted manuscript; M.A., S.G., R.T., A.W.S., A.T.L., and R.C.R. edited and revised manuscript; M.A., S.G., R.T., A.W.S., A.T.L., and R.C.R. approved final version of manuscript.
ACKNOWLEDGMENTS
This paper is part of a series, titled “AltitudeOmics,” which together, represents a group of studies that explored the basic mechanisms controlling human acclimatization to hypoxia and its subsequent retention. Many people and organizations invested enormous time and resources to make AltitudeOmics a success. Foremost, the study was made possible by the tireless support, generosity, and tenacity of our research subjects. AltitudeOmics principal investigators were Colleen G. Julian, Andrew T. Lovering, Andrew W. Subudhi, and Robert C. Roach. We also thank Mr. Jui-lin Fan and Drs. Bengt Kayser and Nicolas Bourdillon (University of Geneva, Switzerland); Mr. Jim Davis, Mr. Jonathan Elliot, Dr. Steve Laurie, Ms. Julia Kern, Ms. Kara Beasley, and Mr. Henry Norris (University of Oregon); and Mr. Oghenero Evero (University of Colorado) for valuable technical assistance during data collection. In addition, we thank Drs. Lee Romer and Emma Ross for allocating nerve stimulation equipment from Brunel University and the University of Brighton (UK). Finally, we thank Dr. Jerry Dempsey for valuable advice and feedback on the manuscript.
REFERENCES
- 1.Amann M, Calbet JA. Convective oxygen transport and fatigue. J Appl Physiol 104: 861–870, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Amann M, Pegelow DF, Jacques AJ, Dempsey JA. Inspiratory muscle work in acute hypoxia influences locomotor muscle fatigue and exercise performance of healthy humans. Am J Physiol Regul Integr Comp Physiol 293: R2036–R2045, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol 587: 271–283, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amann M, Romer LM, Pegelow DF, Jacques AJ, Hess CJ, Dempsey JA. Effects of arterial oxygen content on peripheral locomotor muscle fatigue. J Appl Physiol 101: 119–127, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Amann M, Romer LM, Subudhi AW, Pegelow DF, Dempsey JA. Severity of arterial hypoxaemia affects the relative contributions of peripheral muscle fatigue to exercise performance in healthy humans. J Physiol 581: 389–403, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amann M, Venturelli M, Ives SJ, McDaniel J, Layec G, Rossman MJ, Richardson RS. Peripheral fatigue limits endurance exercise via a sensory feedback-mediated reduction in spinal motoneuronal output. J Appl Physiol; 10.1152/japplphysiol.00049.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baydur A, Behrakis PK, Zin WA, Jaeger M, Milic-Emili J. A simple method for assessing the validity of the esophageal balloon technique. Am Rev Respir Dis 126: 788–791, 1982 [DOI] [PubMed] [Google Scholar]
- 8.Bender PR, Groves BM, McCullough RE, McCullough RG, Huang SY, Hamilton AJ, Wagner PD, Cymerman A, Reeves JT. Oxygen transport to exercising leg in chronic hypoxia. J Appl Physiol 65: 2592–2597, 1988 [DOI] [PubMed] [Google Scholar]
- 9.Bisgard GE, Forster HV. Ventilatory response to acute and chronic hypoxia. In: Handbook of Physiology, Section 4: Environmental Physiology, edited by Fregly MJ, Blatteis CM. Bethesda, MD: Oxford University Press, 1996, p. 1207–1239 [Google Scholar]
- 10.Borg G. Borg's Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics, 1998 [Google Scholar]
- 11.Brooks GA, Wolfel EE, Butterfield GE, Cymerman A, Roberts AC, Mazzeo RS, Reeves JT. Poor relationship between arterial [lactate] and leg net release during exercise at 4,300 m altitude. Am J Physiol Regul Integr Comp Physiol 275: R1192–R1201, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Calbet JA. Chronic hypoxia increases blood pressure and noradrenaline spillover in healthy humans. J Physiol 551: 379–386, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calbet JA, Boushel R, Radegran G, Sondergaard H, Wagner PD, Saltin B. Why is VO2 max after altitude acclimatization still reduced despite normalization of arterial O2 content? Am J Physiol Regul Integr Comp Physiol 284: R304–R316, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Calbet JA, Lundby C. Air to muscle O2 delivery during exercise at altitude. High Alt Med Biol 10: 123–134, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Calbet JA, Radegran G, Boushel R, Saltin B. On the mechanisms that limit oxygen uptake during exercise in acute and chronic hypoxia: role of muscle mass. J Physiol 587: 477–490, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caquelard F, Burnet H, Tagliarini F, Cauchy E, Richalet JP, Jammes Y. Effects of prolonged hypobaric hypoxia on human skeletal muscle function and electromyographic events. Clin Sci (Lond) 98: 329–337, 2000 [PubMed] [Google Scholar]
- 17.Chaudhuri A, Behan PO. Fatigue and basal ganglia. J Neurol Sci 179: 34–42, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Cibella F, Cuttitta G, Romano S, Grassi B, Bonsignore G, Milic-Emili J. Respiratory energetics during exercise at high altitude. J Appl Physiol 86: 1785–1792, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Clausen T. Na+-K+ pump regulation and skeletal muscle contractility. Physiol Rev 83: 1269–1324, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Clausen T, Nielsen OB, Harrison AP, Flatman JA, Overgaard K. The Na+,K+ pump and muscle excitability. Acta Physiol Scand 162: 183–190, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Clausen T, Overgaard K, Nielsen OB. Evidence that the Na+-K+ leak/pump ratio contributes to the difference in endurance between fast- and slow-twitch muscles. Acta Physiol Scand 180: 209–216, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Cruz JC. Mechanics of breathing in high altitude and sea level subjects. Respir Physiol 17: 146–161, 1973 [DOI] [PubMed] [Google Scholar]
- 23.Davis JN, Carlsson A, MacMillan V, Siesjo BK. Brain tryptophan hydroxylation: dependence on arterial oxygen tension. Science 182: 72–74, 1973 [DOI] [PubMed] [Google Scholar]
- 24.De Blasi RA, Ferrari M, Natali A, Conti G, Mega A, Gasparetto A. Noninvasive measurement of forearm blood flow and oxygen consumption by near-infrared spectroscopy. J Appl Physiol 76: 1388–1393, 1994 [DOI] [PubMed] [Google Scholar]
- 25.Dempsey JA, Amann M, Romer LM, Miller JD. Respiratory system determinants of peripheral fatigue and endurance performance. Med Sci Sports Exerc 40: 457–461, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Duncan A, Meek JH, Clemence M, Elwell CE, Tyszczuk L, Cope M, Delpy DT. Optical pathlength measurements on adult head, calf and forearm and the head of the newborn infant using phase resolved optical spectroscopy. Phys Med Biol 40: 295–304, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Fowles JR, Green HJ, Tupling R, O'Brien S, Roy BD. Human neuromuscular fatigue is associated with altered Na+-K+-ATPase activity following isometric exercise. J Appl Physiol 92: 1585–1593, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Fulco CS, Cymerman A, Muza SR, Rock PB, Pandolf KB, Lewis SF. Adductor pollicis muscle fatigue during acute and chronic altitude exposure and return to sea level. J Appl Physiol 77: 179–183, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Fulco CS, Kambis KW, Friedlander AL, Rock PB, Muza SR, Cymerman A. Carbohydrate supplementation improves time-trial cycle performance during energy deficit at 4,300-m altitude. J Appl Physiol 99: 867–876, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Garner SH, Sutton JR, Burse RL, McComas AJ, Cymerman A, Houston CS. Operation Everest II: neuromuscular performance under conditions of extreme simulated altitude. J Appl Physiol 68: 1167–1172, 1990 [DOI] [PubMed] [Google Scholar]
- 31.Goodall S, Gonzalez-Alonso J, Ali L, Ross EZ, Romer LM. Supraspinal fatigue after normoxic and hypoxic exercise in humans. J Physiol 590: 2767–2782, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodall S, Ross EZ, Romer LM. Effect of graded hypoxia on supraspinal contributions to fatigue with unilateral knee-extensor contractions. J Appl Physiol 109: 1842–1851, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Green H, Roy B, Grant S, Burnett M, Tupling R, Otto C, Pipe A, McKenzie D. Downregulation in muscle Na(+)-K(+)-ATPase following a 21-day expedition to 6,194 m. J Appl Physiol 88: 634–640, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Dempsey JA. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol 82: 1573–1583, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Heinonen I, Nesterov SV, Kemppainen J, Nuutila P, Knuuti J, Laitio R, Kjaer M, Boushel R, Kalliokoski KK. Role of adenosine in regulating the heterogeneity of skeletal muscle blood flow during exercise in humans. J Appl Physiol 103: 2042–2048, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Heinonen IH, Kemppainen J, Kaskinoro K, Peltonen JE, Borra R, Lindroos M, Oikonen V, Nuutila P, Knuuti J, Boushel R, Kalliokoski KK. Regulation of human skeletal muscle perfusion and its heterogeneity during exercise in moderate hypoxia. Am J Physiol Regul Integr Comp Physiol 299: R72–R79, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Hogan MC, Richardson RS, Haseler LJ. Human muscle performance and PCr hydrolysis with varied inspired oxygen fractions: a 31P-MRS study. J Appl Physiol 86: 1367–1373, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Johnson BD, Saupe KW, Dempsey JA. Mechanical constraints on exercise hyperpnea in endurance athletes. J Appl Physiol 73: 874–886, 1992 [DOI] [PubMed] [Google Scholar]
- 39.Katayama K, Amann M, Pegelow DF, Jacques AJ, Dempsey JA. Effect of arterial oxygenation on quadriceps fatigability during isolated muscle exercise. Am J Physiol Regul Integr Comp Physiol 292: R1279–R1286, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Kayser B, Bokenkamp R, Binzoni T. Alpha-motoneuron excitability at high altitude. Eur J Appl Physiol 66: 1–4, 1993 [DOI] [PubMed] [Google Scholar]
- 41.Lundby C, Sander M, van Hall G, Saltin B, Calbet JA. Maximal exercise and muscle oxygen extraction in acclimatizing lowlanders and high altitude natives. J Physiol 573: 535–547, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Merton PA. Voluntary strength and fatigue. J Physiol 123: 553–564, 1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Millet GP, Faiss R, Pialoux V. Point: hypobaric hypoxia induces different physiological responses from normobaric hypoxia. J Appl Physiol 112: 1783–1784, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Moller K, Paulson OB, Hornbein TF, Colier WN, Paulson AS, Roach RC, Holm S, Knudsen GM. Unchanged cerebral blood flow and oxidative metabolism after acclimatization to high altitude. J Cereb Blood Flow Metab 22: 118–126, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Mounier R, Brugniaux JV. Counterpoint: hypobaric hypoxia does not induce different responses from normobaric hypoxia. J Appl Physiol 112: 1784–1786, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Nielsen OB, Clausen T. The Na+/K(+)-pump protects muscle excitability and contractility during exercise. Exerc Sport Sci Rev 28: 159–164, 2000 [PubMed] [Google Scholar]
- 47.Poulin MJ, Fatemian M, Tansley JG, O'Connor DF, Robbins PA. Changes in cerebral blood flow during and after 48 h of both isocapnic and poikilocapnic hypoxia in humans. Exp Physiol 87: 633–642, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Rasmussen P, Nielsen J, Overgaard M, Krogh-Madsen R, Gjedde A, Secher NH, Petersen NC. Reduced muscle activation during exercise related to brain oxygenation and metabolism in humans. J Physiol 588: 1985–1995, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roberts AC, Butterfield GE, Cymerman A, Reeves JT, Wolfel EE, Brooks GA. Acclimatization to 4,300-m altitude decreases reliance on fat as a substrate. J Appl Physiol 81: 1762–1771, 1996 [DOI] [PubMed] [Google Scholar]
- 50.Rowell LB, Saltin B, Kiens B, Christensen NJ. Is peak quadriceps blood flow in humans even higher during exercise with hypoxemia? Am J Physiol Heart Circ Physiol 251: H1038–H1044, 1986 [DOI] [PubMed] [Google Scholar]
- 51.Sandiford SD, Green HJ, Duhamel TA, Schertzer JD, Perco JD, Ouyang J. Muscle Na-K-pump and fatigue responses to progressive exercise in normoxia and hypoxia. Am J Physiol Regul Integr Comp Physiol 289: R441–R449, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Schuler B, Thomsen JJ, Gassmann M, Lundby C. Timing the arrival at 2340 m altitude for aerobic performance. Scand J Med Sci Sports 17: 588–594, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Secher NH, Seifert T, Van Lieshout JJ. Cerebral blood flow and metabolism during exercise: implications for fatigue. J Appl Physiol 104: 306–314, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke 31: 1672–1678, 2000 [DOI] [PubMed] [Google Scholar]
- 55.Stary CM, Hogan MC. Effect of varied extracellular PO2 on muscle performance in Xenopus single skeletal muscle fibers. J Appl Physiol 86: 1812–1816, 1999 [DOI] [PubMed] [Google Scholar]
- 56.Sutton JR, Reeves JT, Wagner PD, Groves BM, Cymerman A, Malconian MK, Rock PB, Young PM, Walter SD, Houston CS. Operation Everest II: oxygen transport during exercise at extreme simulated altitude. J Appl Physiol 64: 1309–1321, 1988 [DOI] [PubMed] [Google Scholar]
- 57.Taylor AD, Bronks R, Smith P, Humphries B. Myoelectric evidence of peripheral muscle fatigue during exercise in severe hypoxia: some references to m. vastus lateralis myosin heavy chain composition. Eur J Appl Physiol Occup Physiol 75: 151–159, 1997 [DOI] [PubMed] [Google Scholar]
- 58.Thoden JS, Dempsey JA, Reddan WG, Birnbaum ML, Forster HV, Grover RF, Rankin J. Ventilatory work during steady-state response to exercise. Fed Proc 28: 1316–1321, 1969 [PubMed] [Google Scholar]
- 59.Van Beekvelt MC, Colier WN, Wevers RA, Van Engelen BG. Performance of near-infrared spectroscopy in measuring local O(2) consumption and blood flow in skeletal muscle. J Appl Physiol 90: 511–519, 2001 [DOI] [PubMed] [Google Scholar]
- 60.Wehrlin JP, Hallen J. Linear decrease in VO2max and performance with increasing altitude in endurance athletes. Eur J Appl Physiol 96: 404–412, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Westerblad H, Allen DG, Lannergren J. Muscle fatigue: lactic acid or inorganic phosphate the major cause? News Physiol Sci 17: 17–21, 2002 [DOI] [PubMed] [Google Scholar]
- 62.Willie CK, Macleod DB, Shaw AD, Smith KJ, Tzeng YC, Eves ND, Ikeda K, Graham J, Lewis NC, Day TA, Ainslie PN. Regional brain blood flow in man during acute changes in arterial blood gases. J Physiol 590: 3261–3275, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilson MH, Edsell ME, Davagnanam I, Hirani SP, Martin DS, Levett DZ, Thornton JS, Golay X, Strycharczuk L, Newman SP, Montgomery HE, Grocott MP, Imray CHCaudwell Xtreme Everest Research Group Cerebral artery dilatation maintains cerebral oxygenation at extreme altitude and in acute hypoxia—an ultrasound and MRI study. J Cereb Blood Flow Metab 31: 2019–2029, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wolfel EE, Groves BM, Brooks GA, Butterfield GE, Mazzeo RS, Moore LG, Sutton JR, Bender PR, Dahms TE, McCullough RE, Huang SY, Sun SF, Grover RF, Hultgren HN, Reeves JT. Oxygen transport during steady-state submaximal exercise in chronic hypoxia. J Appl Physiol 70: 1129–1136, 1991 [DOI] [PubMed] [Google Scholar]
- 65.Xu K, Lamanna JC. Chronic hypoxia and the cerebral circulation. J Appl Physiol 100: 725–730, 2006 [DOI] [PubMed] [Google Scholar]