Abstract
The purpose of this work was to explore the use of image registration-derived variables associated with computed tomographic (CT) imaging of the lung acquired at multiple volumes. As an evaluation of the utility of such an imaging approach, we explored two groups at the extremes of population ranging from normal subjects to severe asthmatics. A mass-preserving image registration technique was employed to match CT images at total lung capacity (TLC) and functional residual capacity (FRC) for assessment of regional air volume change and lung deformation between the two states. Fourteen normal subjects and thirty severe asthmatics were analyzed via image registration-derived metrics together with their pulmonary function test (PFT) and CT-based air-trapping. Relative to the normal group, the severely asthmatic group demonstrated reduced air volume change (consistent with air trapping) and more isotropic deformation in the basal lung regions while demonstrating increased air volume change associated with increased anisotropic deformation in the apical lung regions. These differences were found despite the fact that both PFT-derived TLC and FRC in the two groups were nearly 100% of predicted values. Data suggest that reduced basal-lung air volume change in severe asthmatics was compensated by increased apical-lung air volume change and that relative increase in apical-lung air volume change in severe asthmatics was accompanied by enhanced anisotropic deformation. These data suggest that CT-based deformation, assessed via inspiration vs. expiration scans, provides a tool for distinguishing differences in lung mechanics when applied to the extreme ends of a population range.
Keywords: lung mechanics, quantitative computed tomography, image registration, air trapping, asthma
asthma affects more than 25 million people in the US and can be characterized by different symptoms, such as airflow obstruction, bronchial hyperresponsiveness, and airway inflammation (8). Identification of phenotypes serving to separate nonsevere asthmatics from severe asthmatics has been the focus of the National Institutes of Health (NIH)-sponsored multicenter Severe Asthma Research Program (SARP) (12, 35, 40, 41, 54), and the search for phenotypes has included the acquisition of volumetric computed tomography (CT) scans of the lungs at total lung capacity (TLC) and functional residual capacity (FRC).
Imaging techniques such as hyperpolarized magnetic resonance imaging (MRI) (1, 9, 16, 17, 49, 61), positron emission tomography (PET), and single-photon emission CT (SPECT) (27, 29, 36, 43, 51) have been utilized for the exploration of functional defects and airway structural changes. Recently, hyperpolarized helium gas MRI has been compared with regional volume changes using paired lung volumes imaged via CT, and regional differences in lung function (expansion) were well matched (25). CT and MRI as tools for quantitative assessment of the lung have been reviewed recently (53). Although each method provides unique pieces of information regarding lung structure and function, CT has the ability to relate detailed structure together with regional lung function (33). Over the past 30 years now, CT has been validated with regard to its ability to reflect regional air content of the lung (31) and parenchymal destruction in chronic obstructive pulmonary disease (COPD) (15, 24).
Image-matching methods as used here have recently been demonstrated not only to reflect independent measures of regional volume changes (10, 22, 45, 58) but also to have shown the utility in differentiating airway vs. parenchymal phenotypes in a COPD population (23). In addition, the image-matching-derived variables via CT have been compared with different modalities such as MRI, SPECT, and Xenon-CT (11, 38, 45), and they have shown fairly significant correlations. With the demonstration that image registration between two lung volumes provides an accurate surrogate for regional lung function, we utilize CT image matching to assess regional differences in lung function of severe asthmatics relative to normal subjects.
In this study, we applied a mass-preserving nonrigid registration method (57, 58) with two breath-hold volumes (TLC and FRC) to study alteration of regional air volume change and lung deformation. In the previous studies (57, 58), the method demonstrated the relatively accurate results even in large deformation based on landmarks selected at vessel bifurcations. The registration-derived variables can measure the features of the lung when deforming from one static state to the other, unlike existing air trapping measurements (7, 44) that are based on density measurements using a single volumetric image at FRC [or in other cases, residual volume (RV)].
The purpose of this study was to apply a newly emerging tool allowing for the matching of lung volume pairs imaged via CT, and we selected normal and severely asthmatic groups to evaluate the utility of such image registration methods in mapping alterations in regional lung mechanics. In addition, we correlated the registration-derived variables with existing traditional measurements such as pulmonary function test (PFT) measurements and air trapping, which have been commonly used for the study of asthma, to illustrate the implications of registration-derived measurements.
METHODS
Human subject data sets.
Fourteen normal (10F) and 30 severe asthmatic (18F) subjects were chosen for this study. Demographic and pulmonary function data are provided in Table 1. Both CT images of normal subjects and severe asthmatics were acquired at the University of Pittsburgh as part of the SARP consortium (12, 20, 40, 54). The associated human studies, along with the imaging protocol, were approved by the Institutional Review Board at the University of Pittsburgh. CT images were gathered during coached breath-holds (TLC and FRC) in the supine position and then processed using the Pulmonary Workstation and Apollo software (VIDA Diagnostics, Coralville, IA). Scanning details are provided in Table 2. Major criteria used to define severe asthma are provided in Ref. 54 and include treatments with oral corticosteroids and high-dose inhaled corticosteroids as well as minor criteria such as requirement for daily treatment with a controller medication of long-acting β-agonist, theophylline, or leukotriene antagonist.
Table 1.
Demographic and PFT information of 14 normal and 30 severely asthmatic subjects
| Normal Subjects | Severe Asthmatics | P (From t-Test) | |
|---|---|---|---|
| Age, yr | 34.5 ± 3.9 | 47.2 ± 2.2 | <0.01 |
| BMI | 25.6 ± 1.5 | 31.1 ± 1.3 | <0.05 |
| Asthma duration | 28.2 ± 3.3 | ||
| Sex, No. (%Female) | 10 (71%) | 18 (60%) | |
| Race, No. (White nonhispanic/African American/Other) | 10/1/3 (71%/7%/21%) | 25/3/2 (83%/10%/7%) | |
| TLC, % predicted | 96 ± 3 | 97 ± 3 | 0.75 |
| FRC, % predicted | 90 ± 5 | 102 ± 5 | 0.12 |
| RV, % predicted | 90 ± 6 | 134 ± 9 | <0.0005 |
| FVC, % predicted | 98 ± 2 | 71 ± 3 | <1.0 × 10−7 |
| FEV1, % predicted | 96 ± 3 | 55 ± 4 | <1.0 × 10−10 |
| FEV1/FVC × 100 | 81 ± 1 | 60 ± 2 | <5.0 × 10−10 |
| RV/TLC × 100 | 27 ± 2 | 44 ± 2 | <5.0 × 10−6 |
Values are means ± SE. PFT, pulmonary function test; TLC, total lung capacity; FRC, functional residual capacity; RV, residual volume; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s. TLC, FRC, and RV of 1 normal subject and 1 severely asthmatic subject were not available.
Table 2.
Scanner and scanning protocol used for both normal subjects and severe asthmatics
| Scanner and Protocol | |
|---|---|
| Scanner model | GE VCT 64 slice |
| Scan type | Helical |
| Rotation time(s) | 0.5 |
| Detector configuration (channel no. × mm) | 64 × 0.625 mm |
| Pitch | 0.984 |
| Peak kilovoltage, kVp | 120 |
| Miliampere, mA | S-145 |
| M-180 | |
| L-270 | |
| Dose modulation | Auto mA OFF |
| Reconstruction algorithm | Standard or Detail |
| Lung algorithm | None |
| Additional image filters | No selection |
| Thickness, mm | 0.625 |
| Interval, mm | 0.5 |
| Iterative reconstruction (noise reduction algorithm) | No selection |
| Scan time(s), 30-cm length | <10 |
mA was varied for Severe Asthma Research Program protocol based on BMI size (small: BMI <20, medium: 20 ≤ BMI ≤30, large: BMI >30).
Image registration and regional air volume change.
The intensity-based mass-preserving image registration method (57, 58) was employed to match two CT lung images. Here, the CT images at TLC and FRC are used for the reference and floating images, respectively. The tissue and air fractions are estimated as follows.
| (1) |
where βtissue(x), βair(x), I(x), HUair, and HUtissue denote tissue fraction, air fraction, Hounsfield unit (HU) of a voxel, HU of air, and HU of tissue, respectively. HUair and HUtissue are set to −1,000 and 55, respectively (57, 58). The tissue volume Vtissue(x) and air volume Vair(x) are calculated by multiplying a local volume v(x) to the tissue and air fractions, respectively.
The image registration method is to determine a spatial transformation that matches the two images by minimizing a cost function C, which is called the sum of squared tissue volume difference (SSTVD), as shown below.
| (2) |
where Vtissueref(x) is the local tissue volume of the reference image, whereas Vtissuef[T(x)] is the local tissue volume of the floating image. T(x), known as the warping function, provides a transformation that maps a local volume at location x in the reference image to the corresponding location in the floating image. A multilevel B-spline transformation technique is adopted to describe the warping function T(x). The finest number of control grids in the entire image domain is selected as 32 × 32 × 32, which has been an optimal number when considering accuracy and computational cost (13, 58).
Once warping function T(x) is obtained, the corresponding local volume vf[T(x)] at floating image is calculated as vf[T(x)] = vref(x)/J, where J is the determinant of Jacobian matrix. At the floating image, the air fraction βairf[T(x)] is obtained by CT intensity value I[T(x)] (see Eq. 1) so that the air volume Vairf[T(x)] is calculated as vf[T(x)]βairf [T(x)]. As a result, the regional air volume change ΔVair is obtained by the air volume differences between the reference image and the floating image as follows (56):
| (3) |
Lung deformation.
The volume change (measured by J) and the anisotropic deformation index (ADI) are employed to quantify lung deformation (2). To obtain J and ADI, the deformation gradient tensor (F) is defined as follows (37):
| (4) |
where ∇ is the vector gradient operator. F could be decomposed into a rotation tensor (R) and a stretch tensor (U); R is orthogonal, whereas U is symmetric and positive definite:
| (5) |
| (6) |
| (7) |
Cauchy-Green deformation tensor (FTF) is symmetric and positive definite due to the orthogonality of R and the nature of U, s denotes the eigenvector, and λi* are the eigenvalues of the FTF of each local volume from TLC to FRC. In Eq. 7, both λi* and λi are positive, and λi represents the principal strains along the principal directions of a deformed lung tissue element from FRC to TLC, where λi = 1/ with λ1 > λ2 > λ3 > 0. With the eigenvalues (λ1, λ2, λ3), J and ADI are calculated as follows:
| (8) |
| (9) |
Physical interpretation of ΔVair, J, and ADI.
The three registration-derived variables ΔVair, J, and ADI are used to evaluate air volume change and lung deformation. First of all, ΔVair reflects local air volume difference between the reference and floating images, measuring the amount of air entering (or leaving) a local region during inhalation (or exhalation). Second, J is defined as the ratio of νref(x) at TLC over νf[T(x)] at FRC. That is, if J = 1 at a local volume, the local volume remains unchanged between the two lung volumes. If J < 1, the volume decreases from FRC to TLC (i.e., contracts), whereas if J > 1, it increases (i.e., expands). Because the tissue volume inside a local volume can be assumed to be unchanged, the change in local volume is due primarily to the change of air volume. Basically, ΔVair and J are measurements for air volume change excluding tissue volume and lung volume change including tissue volume, respectively. In fact, both variables exhibit similar characteristics because tissue volumes during lung deformation remain unchanged. However, ΔVair is the volume difference, whereas J is the volume ratio, and thus both would not have linear correlations.
The third variable ADI provides information on the preferential deformation of local lung volume (2). For example, if a local volume is stretched isotropically in all directions, namely λ1 = λ2 = λ3, then Eq. 9 gives an ADI value of zero. With increasing anisotropy, ADI increases. An important feature of ADI is its independence from J. That is, even if two local volumes have the same J, their ADI values could be different (2). Note that ADI measures the degree of anisotropy rather than the direction of anisotropy. Intrinsically, ΔVair is derived from CT intensity I(x) at each local volume (Eq. 1), whereas J and ADI are derived from eigenvalues of deformation gradient tensor (Eq. 4) so that ΔVair could provide a discrete field sensitive to the local CT intensity, and J and ADI could generate more smoothed fields by the first-order derivative of warping function.
Air trapping.
A voxel is regarded as an air-trapped voxel if the Hounsfield unit of the voxel at FRC is below −856 (this number varies ±6 HU depending upon the studies) (7, 12). Air trapping percentage “AirT%” is defined as the ratio of the number of air-trapped voxels over the number of voxels in the respective lobes (lobar AirT%) or in the whole lung (total AirT%). Lobar contribution to total air-trapped voxels is denoted by “AirT*”, which is defined as the ratio of the number of air-trapped voxels in the lobe over the number of air-trapped voxels in the whole lung. Thus, the summation of AirT* values in the five lobes is equal to unity.
Data type and analysis.
The aforementioned air volume change, volume change, and anisotropic deformation index (ΔVair, J, and ADI) are calculated for each local volume. ΔVair, J, and ADI are then normalized by their respective medians of the same subject, denoted by ΔVair*, J*, and ADI*. Their spatial distributions are presented by lobe, lung height, and depth averaged over all subjects as well as for the whole lungs of selected individual subjects. Here, the normalized lung height Z* is measured from apical to basal (Z* = 0 − 1), i.e., along the cranio-caudal axis, which is perpendicular to the normalized lung depth Y* from the nondependent (ventral) region to the dependent (dorsal) region of the lung (Y* = 0 − 1). When being presented by lobe (or lung height), ΔVair*, J*, and ADI* are the medians over all values in lobe (or at a given lung height). In this study, the left upper lobe, left lower lobe, right upper lobe, right middle lobe, and right lower lobe are denoted by LUL, LLL, RUL, RML, and RLL, respectively. To distinguish the features of severe asthmatics from normal subjects a mixed analysis of variance (ANOVA) and independent t-tests are performed for significance check with software R (36a); statistical significance is taken at P < 0.05.
RESULTS
PFT.
Table 1 summarizes the PFT information for the 44 subjects (14 normal and 30 severe asthmatics) analyzed in this study. The predicted values of TLC, FRC, and RV are calculated with the equation of Stocks and Quanjer (48), and the predicted values of forced vital capacity (FVC) and FEV1 are obtained from the equation of Hankinson et al. (26). The measured values are then divided by the predicted values, yielding the “%predicted” values in the table. In severe asthmatics, the %predicted values of both TLC and FRC are within the normal range and close to 100%. On the other hand, the %predicted values of FVC, FEV1, and FEV1/FVC of severe asthma are significantly smaller than those of normal subjects, as would be expected in severe asthma (40, 41). In addition, consistent with severe asthma (47), the %predicted values of RV and RV/TLC in the severe asthmatics indicative of air-trapping are higher than those of normal subjects (P <0.0005 and P < 5.0 × 10−6, respectively).
Validation of CT-based lung volumes.
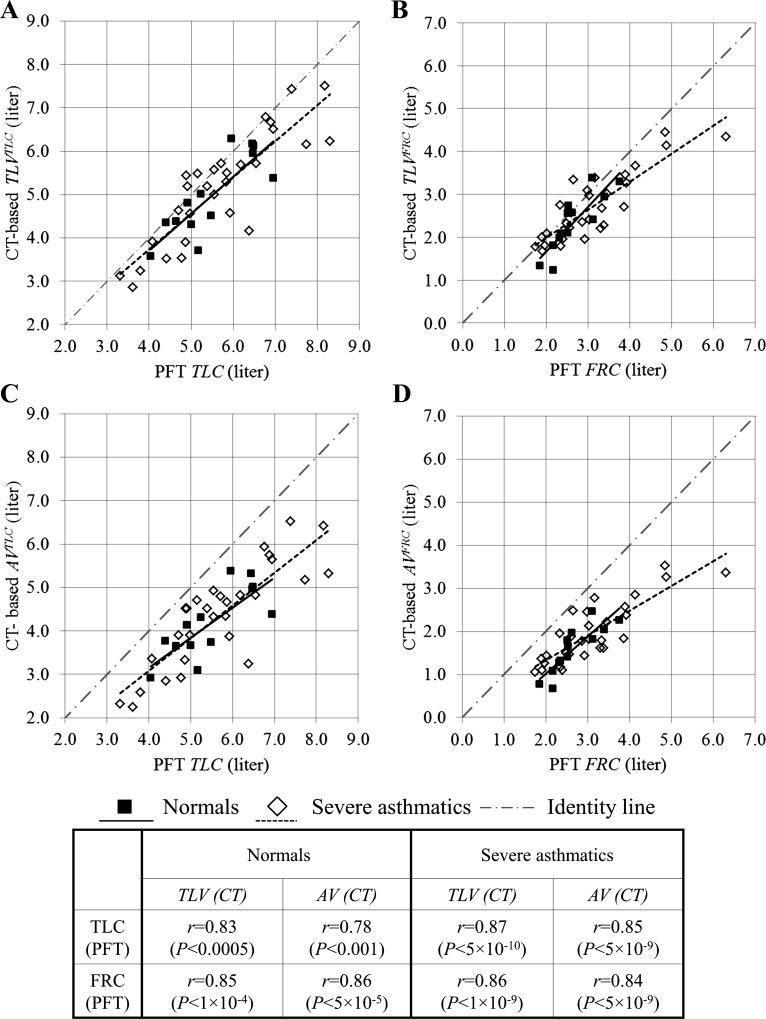
Figure 1 shows the linear correlations of lung volumes between upright PFT and supine CT. CT-based total lung volumes (TLV), including both air volume (AV) and tissue volume (TV), are significantly correlated with PFT-based measurements of TLC and FRC (see Fig. 1, A and B). The upright PFT volumes and supine CT-based TLV are in similar ranges, being adjacent to the identity line. On the other hand, as shown in Fig. 1, C and D, CT-based AV tends to be consistently less than PFT volumes. Table 3 compares the ratios of CT-based TLV, AV, and inspiratory capacity (IC) over PFT measurements between normal subjects and severe asthmatics. In normal subjects, the CT-based air volumes at TLC (AVTLC) and FRC (AVFRC) decrease ∼24 and 42%, respectively, compared with their corresponding PFT volumes. Similarly, AVTLC and AVFRC in severe asthmatics decrease ∼24 and 36%, respectively, relative to their corresponding PFT volumes. The CT-based IC (CT) is reduced only 6 and 8% for normal subjects and severe asthmatics, respectively, compared with PFT measurements. Hence, the ratios of CT supine volumes to PFT upright volumes in severe asthmatics are not statistically different from those of normal subjects, as shown in Table 3.
Fig. 1.
Comparisons of total lung volume at total lung capacity (TLVTLC; A), total lung volume at functional residual capacity (TLVFRC; B), air volume at TLC (AVTLC; C), and air volume at FRC (AVFRC; D) from computed tomography (CT) scans with the corresponding pulmonary function test (PFT) volumes in normal (■) and severely asthmatic subjects (◇). Solid (normal subjects) and dashed lines (severe asthmatics) indicate linear fitted regression lines.
Table 3.
Comparison of the ratio of upright PFT volumes to supine CT volumes and AV, TV, and TV difference from supine CT between normal subjects and severe asthmatics
| Normal Subjects | Severe Asthmatics | P (From t-Test) | |
|---|---|---|---|
| TLVTLC (CT)/TLC (PFT) × 100 | 91 ± 2 | 91 ± 2 | 0.98 |
| TLVFRC (CT)/FRC (PFT) × 100 | 88 ± 3 | 89 ± 3 | 0.76 |
| supAVTLC (CT)/TLC (PFT) × 100 | 76 ± 2 | 76 ± 2 | 0.98 |
| AVFRC (CT)/FRC (PFT) × 100 | 58 ± 4 | 64 ± 2 | 0.23 |
| IC (CT)/IC (PFT) × 100 | 94 ± 7 | 92 ± 4 | 0.85 |
| AVTLC, liters | 4.14 ± 0.2 | 4.27 ± 0.2 | 0.66 |
| AVFRC, liters | 1.57 ± 0.1 | 1.95 ± 0.1 | 0.06 |
| TVTLC, liters | 0.79 ± 0.04 | 0.79 ± 0.03 | 0.96 |
| TVFRC, liters | 0.76 ± 0.04 | 0.75 ± 0.02 | 0.70 |
| (∣TVTLC − TVFRC∣/TVTLC) × 100 | 6 ± 1 | 6 ± 1 | 0.72 |
Values are means ± SE. CT, computed tomography; TLV, total lung volume (air + tissue); AV, air volume; TV tissue volume; IC, inspiratory capacity. PFT volumes (TLC, FRC, and IC) of 1 normal subject and 1 severely asthmatic subject were not available.
Table 3 also shows that TV differences between TLC and FRC are ∼0.03 and 0.04 liters, respectively, whereas AV differences between TLC and FRC are ∼2.57 and 2.32 liters for normal subjects and severe asthmatics, respectively. The means of TV differences over TLC tissue volume are ∼6% in both normal subjects and severe asthmatics. As a result, the difference of TV between TLC and FRC is much smaller than that of AV, supporting the assumption of SSTVD that TV change is negligible (see Table 3).
Mixed ANOVA.
A mixed ANOVA test consisting of one between-subject variable and one within-subject variable was performed for two independent groups (normal subjects and severe asthmatics) and five lung regions (LUL, LLL, RUL, RML, and RLL), as shown in Table 4. The ANOVA test evaluated six dependent variables, including lobar fraction of air volume change, ΔVair*, J*, ADI*, AirT%, and AirT*. For the between-subject variable “groups,” AirT% of severe asthmatics is different from normal subjects (P < 0.05) in entire lungs. As a result, a followup t-test was conducted to compare total AirT% between normal subjects and severe asthmatics (see Table 6). On the other hand, the differences in lobar fraction of air volume change, ΔVair*, J*, ADI* and AirT* between normal subjects and severe asthmatics are not significant. This is because the dependent variables ΔVair*, J*, and ADI* were normalized by the respective medians, and the summations of lobar fractions of air volume change and AirT* values in the five lobes are equal to unity, as described in methods. For within-subject variable “lung regions”, six dependent variables indicate that each lobe has the different characteristics of air volume change, volume change, anisotropic deformation, and air trapping. The significant interactions between groups and lung regions of the lobar fraction of air volume change, J*, ADI*, and AirT* imply that the effect of the severely asthmatic group can affect regional difference of air volume change, deformation, and air trappings. Accordingly, the t-tests of these variables in five lobes of both normal subjects and severe asthmatics were performed.
Table 4.
Mixed (between-group and within-group) ANOVA test performed with normal subjects vs. severe asthmatics (between) and 5 lobes (repeated measures) as a grouping and a within variable, respectively
| ANOVA (F-Test, P Value) | Groups (Normal Subjects vs. Asthmatics) | Lung regions (LUL, LLL, RUL, RML and RLL) | Interactions (Groups × Lung Regions) |
|---|---|---|---|
| Ventilation fraction | 0.69 | <5 × 10−22 | <0.005 |
| ΔVair* | 0.94 | <5.0 × 10−7 | 0.13 |
| J* | 0.90 | <5.0 × 10−15 | <0.005 |
| ADI* | 0.07 | <1.0 × 10−5 | <0.05 |
| AirT% | <0.05 | <0.05 | 0.07 |
| AirT* | 0.49 | <5 × 10−8 | <0.05 |
LUL, left upper lobe; LLL, left lower lobe; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe. Type III error is employed for F-test, and sphericity is corrected by Greenhouse-Geiser epsilon.
Table 6.
AirT% and AirT* of air-trapped voxels
| Normal Subjects | Severe Asthmatics | P (t-Test) | |
|---|---|---|---|
| Total AirT% | 3.4 ± 1.2 | 10.9 ± 1.9 | <0.005 |
| AirT*, % | |||
| LUL | 33.7 ± 2.9 | 24.8 ± 1.8 | <0.05 |
| LLL | 9.3 ± 1.6 | 15.7 ± 1.6 | <0.01 |
| RUL | 16.2 ± 2.0 | 19.2 ± 1.6 | 0.258 |
| RML | 30.2 ± 3.8 | 25.9 ± 2.2 | 0.349 |
| RLL | 10.6 ± 1.6 | 14.4 ± 1.3 | 0.075 |
Values are means ± SE. AirT%, air trapping percentage; AirT*, lobar contribution. A voxel at FRC image is treated as an air-trapped region if its CT intensity of less than −856 Hounsfield units. The difference between AirT% and AirT* is described in Air Trapping.
Lobar fraction of air volume change.
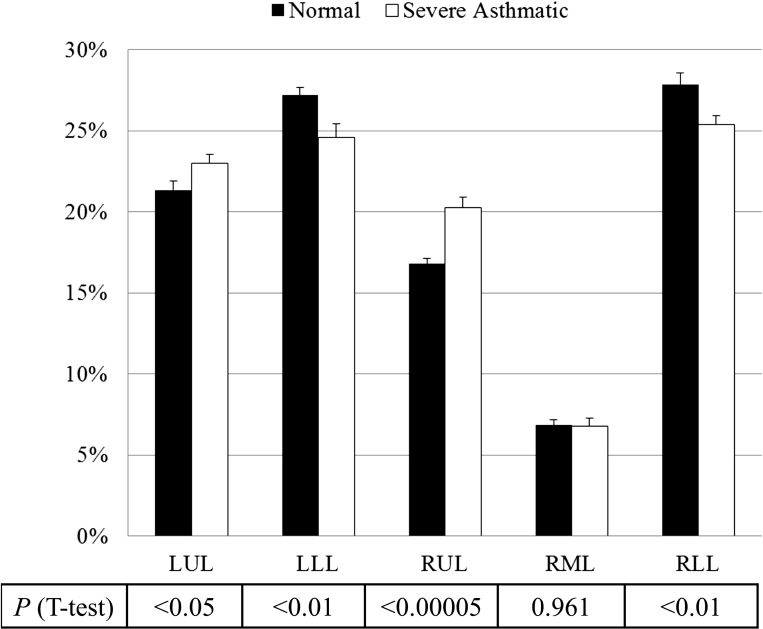
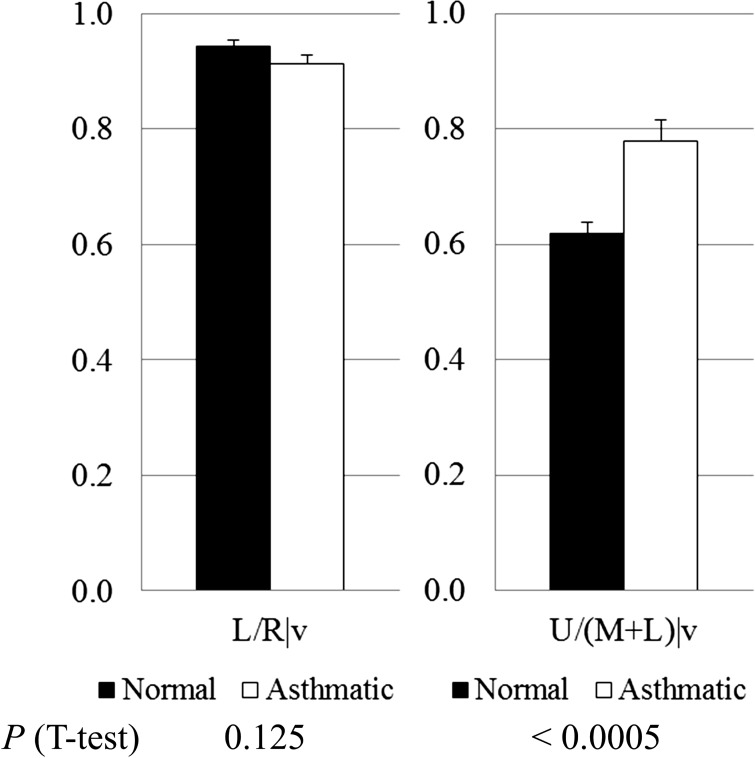
Based upon lobar segmentation data, Fig. 2 shows the mean ± SE of lobar fractions of air volume change (lobar air volume change/whole lung air volume change) for normal subjects (black bars) and severe asthmatics (open bars). The t-test indicates that the difference in air volume change fraction in the upper and lower lobes between normal subjects and severe asthmatics is significant (P < 0.05), especially in RUL (P < 0.00005). Figure 3 also displays the ratios of air volume change to the left lung over the right lung L/R ∣ v for normal subjects (black bars) and severe asthmatics (open bars) and the ratios of air volume to the upper lobes over the middle and lower lobes U/(M + L) ∣ v [P = 0.125 for L/R ∣ v and P < 0.0005 for U/(M + L) ∣ v]. The difference in U/(M + L) ∣ v is still observed in age-controlled (P < 0.01) and BMI-controlled (P < 0.01) subgroups by controlling the sample number of severe asthmatics with age <50 yr (P = 0.33 for age difference between 14 normal subjects and 17 severe asthmatics) and BMI <35 (P = 0.25 for BMI difference between 14 normal subjects and 22 severe asthmatics), respectively. Thus, the difference between normal subjects (black bar) and severe asthmatics (open bar) is significant in upper and lower lungs rather than left and right lungs.
Fig. 2.
Means ± SE of the fraction of air volume changes in normal subjects (black bars) and severe asthmatics (open bars) by lobe. LUL, left upper lobe; LLL, left lower lobe; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe.
Fig. 3.
Means ± SE of L/R ∣ v (left) and U/(M + L) ∣ v ratio (right) of air volume change in normal subjects (black bars) and severe asthmatics (open bars).
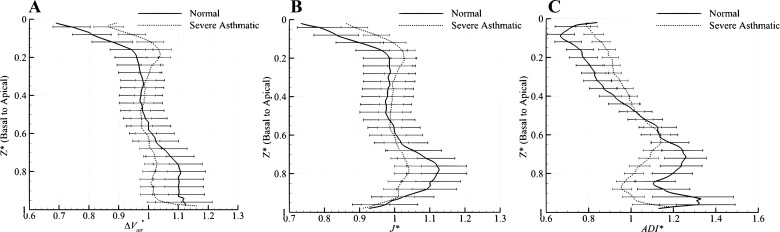
Spatial characteristics of averaged ΔVair*, J*, and ADI*.
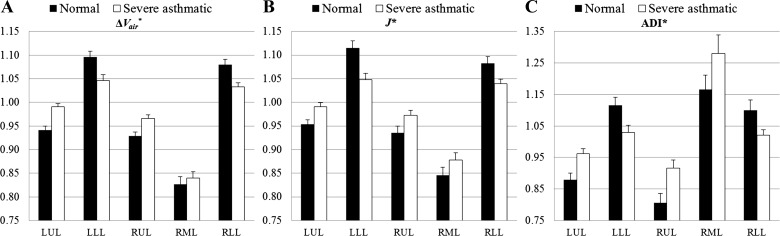
Figure 4 shows that lobar air volume changes (ΔVair*), volume changes (J*), and anisotropic deformations (ADI*) in normal subjects (black bars) and severe asthmatics (open bars) are significantly different, except for the RML. In normal subjects, ΔVair*, J*, and ADI* of the lower lobes are higher than those of the upper lobes, as shown in Fig. 4, but the difference diminishes in severe asthmatics. The redistributions of these quantities between upper and lower lobes in asthmatics are particularly evident in Fig. 5, where the data are presented by lung height along the basal-apical axis. Figure 4 also shows that RML has the smallest air volume change (Fig. 4A) and volume change (Fig. 4B) but the highest anisotropic deformation (Fig. 4C) among five lobes in both normal subjects and severe asthmatics.
Fig. 4.
Means ± SE of air volume change (ΔVair*; P < 0.05 in LUL and RLL, P = 0.06 at LLL, P = 0.07 at RUL, P = 0.717 at RML; A), volume change (J*; P < 0.05 in upper and lower lobes, and P = 0.183 in RML; B), and anisotropic deformation (ADI*; P < 0.05 in upper and lower lobes, and P = 0.13 in RML; C) in normal subjects (black bars) and severe asthmatics (open bars).
Fig. 5.
ΔVair* (A), J* (B), and ADI* (C) between normal subjects (solid lines) and severe asthmatics (dashed lines) along lung height (basal-apical axis). Values are normalized by the respective median of the entire lung and presented as means ± SE.
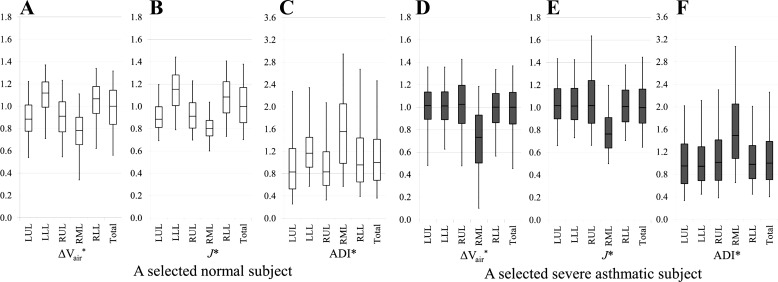
Subject-specific distributions of ΔVair*, J*, and ADI*.
To illustrate and inspect the spatial distributions of ΔVair*, J*, and ADI* in individuals, a normal subject with a lobar air volume change ratio of U/(M + L) ∣ v = 0.55 and a severely asthmatic subject with U/(M + L) ∣ v = 0.81 were chosen because these U/(M + L) ∣ v values are close to the respective group (normal subjects and severe asthmatics) mean values. Both selected normal and severely asthmatic subjects have normal BMI, same sex, and race, but the selected severely asthmatic subject has the characteristics of airflow obstruction (low FEV1) and air trapping (high RV) (see Table 5). In addition, air volumes of two selected subjects, AVTLC and AVFRC, are in the similar range. Figure 6 shows the lobar distributions of normalized air volume change (Fig. 6, A and D), volume change (Fig. 6, B and E), and anisotropic deformation (Fig. 6, C and F) for the selected two subjects. The selected normal subject exhibits the overall characteristics found in the normal group, and the selected asthmatic subject shows an obvious shift in the upper- and lower-lobe functions. As further shown in Fig. 6, the medians of ΔVair*, J*, and ADI* by lobe are fairly uniform in the severely asthmatic subjects, except for the RML.
Table 5.
Demographic, PFT, and CT volume information of the selected normal and severely asthmatic subjects
| Selected Normal Subject | Selected Severely Asthmatic Subject | |
|---|---|---|
| Age, yr | 57.3 | 47.7 |
| BMI | 19.7 | 23.9 |
| Asthma duration | 19.7 | |
| Sex | Female | Female |
| Race | White nonhispanic | White nonhispanic |
| TLC, %predicted | 93 | 127 |
| FRC, %predicted | 92 | 115 |
| RV, %predicted | 88 | 151 |
| FVC, %predicted | 92 | 80 |
| FEV1, %predicted | 95 | 40 |
| FEV1/FVC × 100 | 80 | 40 |
| RV/TLC × 100 | 36 | 42 |
| AVTLC (CT)/TLC (PFT) × 100 | 78 | 78 |
| AVFRC (CT)/FRC (PFT) × 100 | 56 | 62 |
| IC (CT)/IC (PFT) × 100 | 105 | 95 |
RV, residual volume; FEV1, forced expiratory volume in 1 s.
Fig. 6.
Lobar distributions of ΔVair* (A and D), J* (B and E), and ADI* (C and F) for a selected normal subject (left) and a selected severely asthmatic subject (right). Normalized values are presented as boxes (bottom line: 25th percentile; middle line: median; top line: 75th percentile) and whisker plots (bottom line: 5th percentile; top line: 95th percentiles).
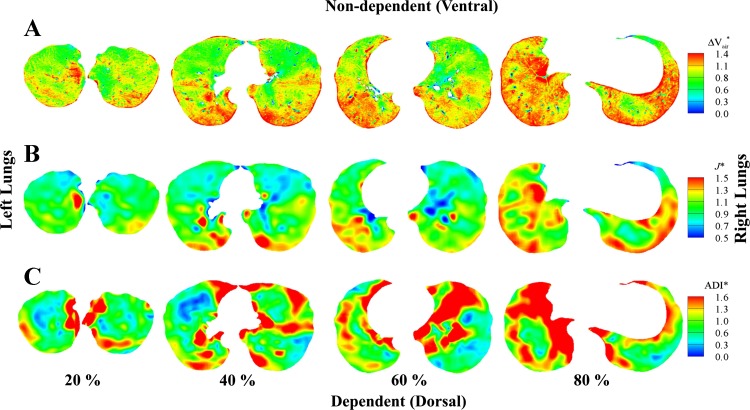
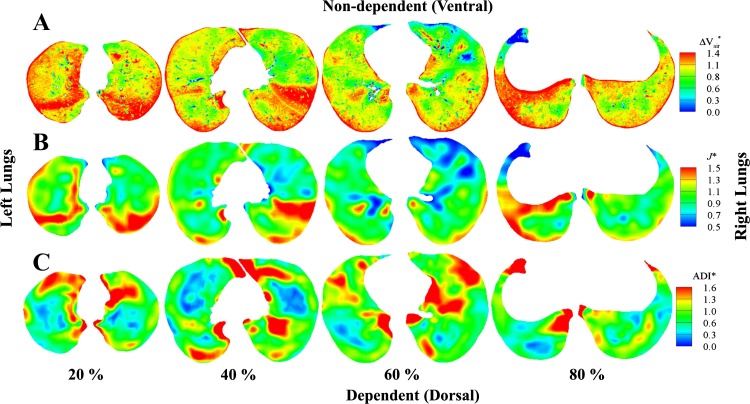
For the normal subject, Fig. 7, A and B, demonstrates that the apical-basal and ventral-dorsal gradients exist with larger ΔVair* and J* in the lower (80%, near base) and dependent (dorsal) regions and smaller ΔVair* and J* in the upper (20%, near apex) and nondependent (ventral) regions. Figure 7C shows anisotropic deformation in the lower regions of the normal subject and relatively isotropic deformation in the upper regions. In contrast, for the severely asthmatic subject, Fig. 8, A–C, shows increased heterogeneity of air volume change, volume change, and anisotropic deformation with the lack of regional characteristics. For example, higher air volume change, larger volume change, and increased anisotropic deformation are found near the apex.
Fig. 7.
Distributions of ΔVair* (A), J* (B), and ADI* (C) of a normal subject at 20 (near apex), 40, 60, and 80% (near base) from apical to basal. In each slice, the left lungs are on the left and the right lungs are on the right.
Fig. 8.
Distributions of ΔVair* (A), J* (B), and ADI* (C) of a severe asthma subject at 20 (near apex), 40, 60, and 80% (near base) from apical to basal. In each slice, the left lungs are on the left and the right lungs are on the right.
Air trapping.
Table 6 shows the mean and ± SE of total lung air trapping percentage (AirT%) and lobar contribution of the air-trapped voxels (AirT*) for normal subjects and severe asthmatics. It is noted that the total AirT% of severe asthmatics is much higher than that of normal subjects (P < 0.005), as observed in the ANOVA test. In addition, lobar contribution of air trapping (AirT*) is observed mainly in the upper lobes of both normal subjects and severe asthmatics, and AirT* is the highest in RML irrespective of asthma. Based on the t-test, the most statistically significant differences are found mainly in left lungs: LUL (P < 0.05) and LLL (P < 0.01). Specifically, AirT* increases in lower lobes of severe asthmatics relative to normal subjects.
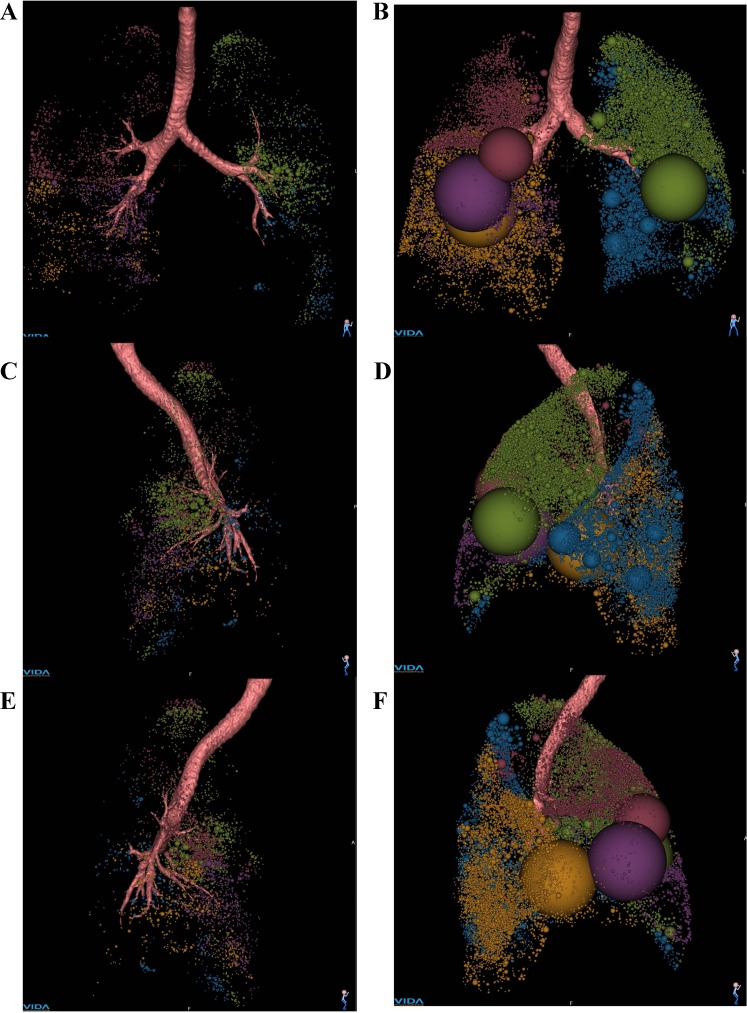
Figure 9 shows the spatial distributions of air-trapped clusters (CT intensity less than −856) in a normal subject and a severely asthmatic subject. Note that the two subjects are those discussed before in Figs. 6–8. Figure 9 shows frontal (Fig. 9 A and B), left lateral (Fig. 9, C and D), and right lateral views (Fig. 9, E and F) of the two subjects with a series of spheres embedded, representing the volume and location of contiguous air-trapped voxels. Air-trapped clusters are color coded by lobes. More air-trapped regions are found in the entire lung of asthmatic subject, as shown in Fig. 9, B, D, and F. The percentages of air-trapped voxels (AirT%) in the entire lungs are 0.5 and 14%, and lower lobar air-trapping contributions (AirT*) are 24.7 and 36.8% for the normal and severely asthmatic subjects, respectively.
Fig. 9.
Frontal (A and B), left lateral (C and D), and right lateral views (E and F) of air-trapped regions captured by CT intensity at FRC image less than −856 Hounsfield units from Apollo (Vida Diagnostics). A, C, and E: AirT% of a normal subject: total, 0.5%; LUL, 0.9%; LLL, 0.2%; RUL, 0.6%; RML, 1.3%; RLL, 0.3% (AirT*: LUL, 37.6%; LLL, 7.8%; RUL, 20.7%; RML, 17.0%; RLL, 16.9%). B, D, and F: AirT% of a severely asthmatic subject: total, 14%; LUL, 14.4%; LLL, 5.0%; RUL, 9.3%; RML, 39.9%; RLL, 14.9% (AirT*: LUL, 24.1%; LLL, 7.8%; RUL, 11.6%; RML, 27.5%; RLL, 29.0%). Lobes are color-coded: LUL (green), LLL (blue), RUL (red), RML (purple), and RLL (orange).
Tissue fraction.
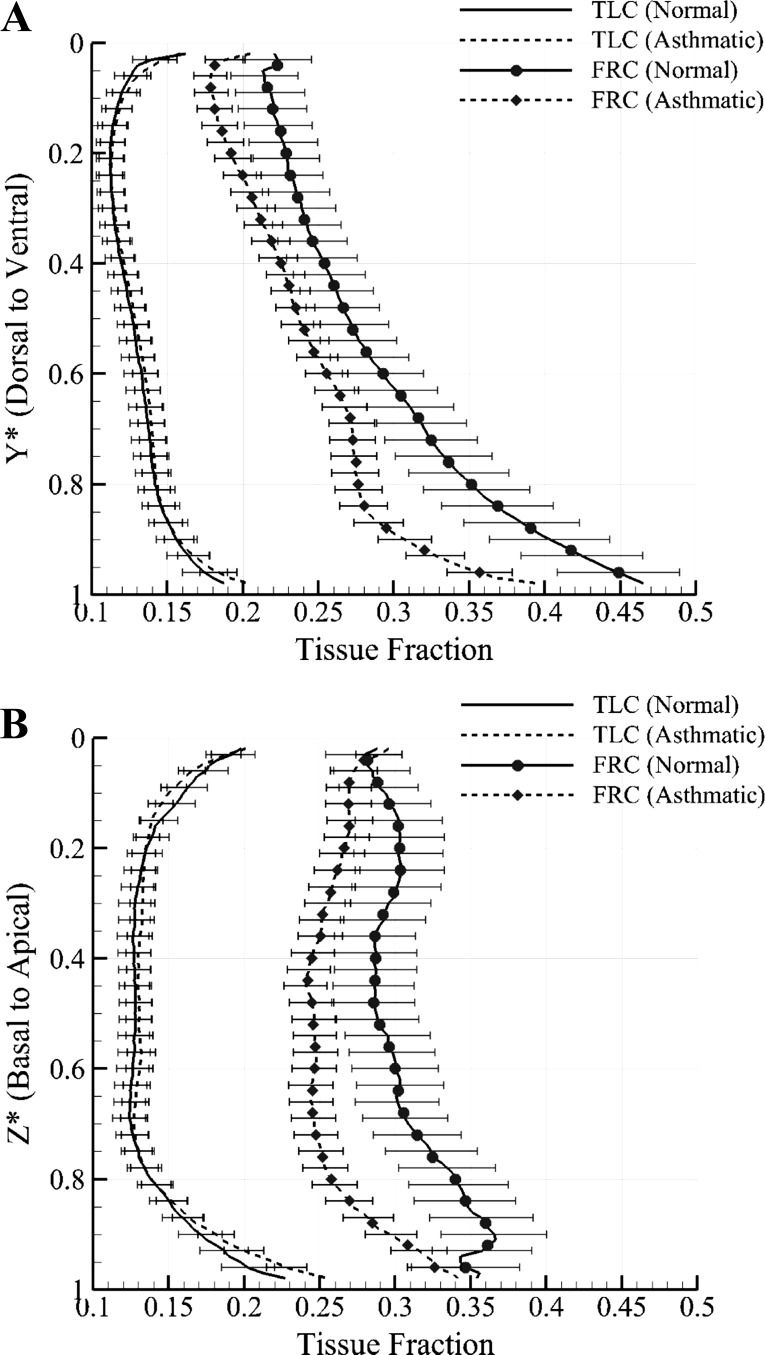
The tissue fractions (βtissue; Eq. 1) are averaged with medians of 14 normal subjects and 30 severe asthmatics along ventral-dorsal and apical-basal axes to observe the differences of tissue fraction between normal subjects and severe asthmatics. Figure 10, A and B, show that tissue at TLC is almost uniformly distributed, and there seems to be little difference between normal subjects and severe asthmatics at TLC. In contrast, at FRC, tissue fraction of severe asthmatics on both axes decreases at all vertical levels relative to normal subjects. The difference is more evident near the dorsal regions (Y* = 0.7–1; Fig. 10A) and near the basal regions (Z* = 0.7–1.0; Fig. 10B). Since the summation of both air and tissue fractions is equal to unity, the decrease in tissue fraction implies an increase in air fraction.
Fig. 10.
Means ± SE of tissue fraction in TLC and FRC on dorsal-ventral axis (A) and on basal-apical axis (B) of both normal subjects and severe asthmatics. The TLC curves for normal subjects and severe asthmatics are difficult to distinguish because they are closely juxtaposed.
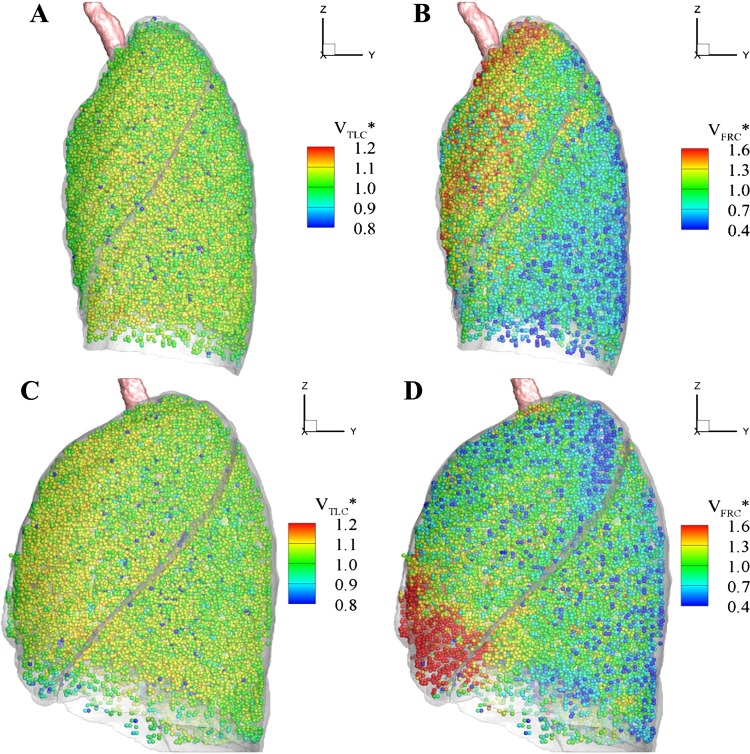
The distributions of air volume at both TLC and warped FRC for the selected subjects are also displayed in Fig. 11 for comparison. The FRC image is warped into the TLC image domain by applying the transform for visual comparison. Similarly to the distributions of tissue fractions at TLC, the air volumes at TLC for both normal and severely asthmatic subjects are distributed uniformly. Meanwhile, the difference in lung shape between normal subjects and severe asthmatics is quantified by the ratio of apical-basal to ventral-dorsal lung extent at TLC. The ratios are 1.56 ± 0.05 and 1.36 ± 0.03 for normal and asthmatic subjects, respectively (P < 0.005). The morphological difference is still observed in both age-controlled and BMI-controlled asthmatic subjects (P < 0.005).
Fig. 11.
Distribution of air volume normalized with the respective mean of TLC (A), warped FRC image of a normal subject (B), TLC (C), and warped FRC image of a severely asthmatic subject (D). For 3-dimensional visualization at TLC domains, we define ∼30,000 parenchymal cubical units to approximate lumped acini. Each cube consists of about 1,000 voxels given the current image resolutions.
DISCUSSION
Comparison of CT- and PFT-based volumes.
In both normal subjects and severe asthmatics, the CT-based TLV (CT) and AV (CT) are significantly correlated with the PFT-based volumes at both TLC and FRC (see Fig. 1). In addition, TLVs (CT) are in similar ranges (∼90%) with PFT volumes (see Table 3), being consistent with the studies of Brown and colleagues (5, 6). For air volume AV (CT), about 20 and 40% reductions from PFTs are measured at TLC and FRC, respectively, in both normal subjects and severe asthmatics (see Table 3).
It is known that supine CT-based air volumes are smaller than upright PFT measurements for several reasons. For example, the plethysmographic PFT includes dead space and gas in the abdomen, but CT includes only segmented lung regions. In addition, coaching TLC is difficult, and the change of body posture from upright to supine can decrease lung volumes (6, 14, 52). The effect of body posture from upright to supine is particularly significant at FRC, resulting in about 30% reduction in PFT-based volumes (34, 42). Therefore, our analysis is consistent with previous studies and further shows that the effect of body posture on air volume is uniform in both normal and severely asthmatic groups.
Characteristics of ΔVair*, J*, and ADI* in normal lungs.
Existing studies (31, 32, 55) indicate that ventilation of the dependent region of normal lungs is higher than that of the nondependent region in the supine posture (known as vertical gradient) due to the gravity. In addition to this gradient, several researchers (3, 4, 19, 21) have reported an apical-to-basal (horizontal) gradient of ventilation existing in the supine posture. Our quantitative analysis also shows both vertical and horizontal gradients of air volume change (ΔVair*) and volume change (J*) in the deep breathing of normal lungs. In fact, the gradient of air fraction shall inversely correlate with the gradient of tissue fraction. As shown in Fig. 10, the gradient of tissue fraction at FRC is greater than at TLC along both dorsal-to-ventral and basal-to-apical axes for normal subjects, consistent with the findings of others (31, 32, 46). Therefore, air volume change (ΔVair*) and volume change (J*) could depend on air volume distribution at FRC as well as lobar size distribution at TLC because air volume at TLC is distributed almost uniformly (see Fig. 11).
Amelon et al. (2) demonstrated that the eigenvector corresponding to the principal eigenvalue (see Eq. 7) orients mainly to the apical-basal axis, being approximately normal to the diaphragm plane. Therefore, the increased anisotropy (ADI*) near basal regions may reflect the directionality of diaphragm movement, as shown in Fig. 7C. In summary, in the case of normal subjects, relatively large volume change (ΔVair* and J*) and anisotropic deformation are observed in the basal and dependent regions, whereas relatively small volume change and isotropic deformation are observed in apical and nondependent regions.
Characteristics of ΔVair*, J*, and ADI* in severely asthmatic lungs.
The air volume change (ΔVair*) in normal lungs increases gradually from the apex to the base (from 20 to 80%) and from the ventral to dorsal lung regions, whereas it becomes fairly uniformly distributed in severely asthmatic subjects (see Figs. 5, 7, and 8). Quantitatively, the lobar air volume change ratio of U/(M + L) ∣ v = 0.78 in severe asthmatics is much higher than the air volume change ratio of 0.62 in normal subjects, as shown in Fig. 3B (P < 0.0005). Furthermore, the air volume change and volume change (J*) in severe asthmatics increase in the upper lobes but decrease in the lower lobes compared with normal subjects. Accordingly, we demonstrated that reduced air volume change in severe asthmatics occurs mainly in the lower lobes, which could be substantiated by comparing the contours of air volume change capturing nearly 80% of the apical-to-basal distance, as demonstrated in Figs. 7A and 8A. The relatively small anisotropic deformation (ADI*) of the severely asthmatic subject near the basal region (80% in Fig. 8C) might be due to the reduced directionality of diaphragm movement (see Table 1) in the asthmatic group. The geometric shape of the severe asthmatic lung at TLC exhibits reduced lung height (apical-basal) and increased lung depth (ventral-dorsal), which is also consistent with reduced ADI* in basal regions.
Relationships between air trapping, PFT, ΔVair*, and tissue fractions.
Air-trapping percentage (AirT%) of severe asthmatics increases significantly compared with normal subjects (P < 0.005), being consistent with existing studies (7). The result is also substantiated with the tissue fractions at FRC in severe asthmatics, which are much smaller than in normal subjects on both dorsal-ventral and basal-apical axes, as shown in Fig. 10. In addition to overall AirT%, the lobar distributions of air-trapping fractions (AirT*) at FRC are different between normal and severely asthmatic subjects (see Table 6). More specifically, air-trapping fraction in the lower lobes of the severely asthmatic subjects increases compared with normal subjects (see AirT* in Table 6), being consistent with the finding of Fain et al. (20). Several imaging studies (1, 28–30, 50) have reported that the areas of ventilation defect are observed mostly in the dependent and basal regions. Figure 10, A and B, shows that tissue fractions of severe asthmatics are much smaller than those of normal subjects in gravitational-dependent and basal regions, thus implying air trapping and reduced air volume change. The results are consistent with existing studies for ventilation defects, qualitatively.
The PFT analysis of the severe asthmatics demonstrated that air trapping (RV/TLC) is correlated with airflow obstruction (FEV1/FVC). Thus, increased air trapping in the lower lobes of severely asthmatic subjects may be correlated with reduced air volume change. The PFT results shown in Table 1 demonstrate that TLC and FRC volumes for both normal and severely asthmatic subjects are close to the predicted values, and CT-based lung volumes are not different between normal subjects and severe asthmatics. Therefore, reduced air volume change in the lower lobes is compensated by increased air volume change from upper lung regions. Figures 4A and 5A suggest that reduced air volume change in the lower lung may be correlated with relatively increased air volume change of upper lobes, resulting in elevated volume change and anisotropic deformation in the upper lobes. A recent study based on registration of three lung CT images acquired at different inflation levels (60) demonstrated that air volume change depends much more on the lower lobes than the upper lobes at the beginning of expiration from TLC. Since FEV1 is measured for 1 s at the beginning of expiration from TLC, the reduced air volume change of lower lobes observed in severe asthmatics may contribute to the reduced FEV1 measured in PFT.
Characteristics of RML.
Among the five lobes, RML has the smallest air volume change and volume change and the highest ADI in both normal and severely asthmatic subjects. In addition, significant air trapping is also observed in RML. Intuitively, RML has less freedom for deformation because it is bounded with both upper and lower lobes, resulting in smaller air volume change and volume change. The same constraint can also lead to more stretching and shearing, reflecting in a high anisotropic deformation. As noted before, J and ADI are independent measurements.
In conclusion, air volume change and lung deformation of severely asthmatic lungs were studied and compared with those of normal lungs. In the case of normal subjects, air volume change, volume change, and anisotropic deformation of lower lobes are higher than those of severely asthmatic subjects. As a result, the dependence of air volume change on lower lobes is greater than upper lobes. In contrast, in the case of severely asthmatic subjects, deformation of lower lobes is limited, as suggested by decreased volume change and reduced anisotropic deformation, resulting in increased volume change and enhanced anisotropic deformation in the upper lobes. This study also established some correlations between existing variables, such as PFT and an air-trapping measurement from a single CT image and registration-based quantities derived from two CT images, such as lobar fraction of air volume change, ΔVair, J, and ADI. These new variables may potentially serve as sensitive measurements for the study of asthmatic lungs.
Limitations and future study directions.
This study shall be extended to investigate the effects of age and BMI and the severity of asthma on registration-derived variables in the future. In addition, B-spline cubic interpolation provides the smoothed displacement field, which may result in more smoothed J* and ADI*. Therefore, the discontinuous effects of lobar slippage and boundary near diaphragm shall be investigated with the lung physiology (18, 59). Furthermore, the trends of the ΔVair* and J* in Fig. 5, A and B, in the range of Z* = 0.85–1 are different, and the number of sample points in that region is much smaller than other regions due to TLC lung geometry. Yin et al. (58) reported that the registration errors in the regions near the diaphragm are greater than other regions. Thus, whether the discrepancy in this region is physiological requires further investigation.
GRANTS
This study was supported in part by NIH Grants R01-HL-094315 and S10-RR-022421.
DISCLOSURES
E. A. Hoffman is a shareholder in VIDA diagnostics, which is commercializing lung image analysis software derived from the University of Iowa lung imaging group.
AUTHOR CONTRIBUTIONS
S.C. and C.-L.L. contributed to the conception and design of the research; S.C. performed the experiments; S.C. and C.-L.L. analyzed the data; S.C., E.A.H., S.E.W., M.H.T., Y.Y., M.C., and C.-L.L. interpreted the results of the experiments; S.C. prepared the figures; S.C. and C.-L.L. drafted the manuscript; S.C., E.A.H., S.E.W., M.H.T., Y.Y., M.C., and C.-L.L. edited and revised the manuscript; S.C., E.A.H., and C.-L.L. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Dr. Jiwoong Choi, E. Burnette, Wang Lu, Dr. Kung-Sik Chan, and Feiran Jiao for assisting with data acquisition and analysis.
REFERENCES
- 1.Altes TA, Powers PL, Knight-Scott J, Rakes G, Platts-Mills TA, de Lange EE, Alford BA, Mugler JP, Brookeman JR. Hyperpolarized 3He MR lung ventilation imaging in asthmatics: preliminary findings. J Magn Reson Imaging 13: 378–384, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Amelon R, Cao K, Ding K, Christensen GE, Reinhardt JM, Raghavan ML. Three-dimensional characterization of regional lung deformation. J Biomech 44: 2489–2495, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amis TC, Jones HA, Hughes JM. Effect of posture on inter-regional distribution of pulmonary ventilation in man. Respir Physiol 56: 145–167, 1984 [DOI] [PubMed] [Google Scholar]
- 4.Bake B, Bjure J, Grimby G, Milic-Emili J, Nilsson NJ. Regional distribution of inspired gas in supine man. Scand J Respir Dis 48: 189–196, 1967 [PubMed] [Google Scholar]
- 5.Brown MS, Kim HJ, Abtin F, Da Costa I, Pais R, Ahmad S, Angel E, Ni C, Kleerup EC, Gjertson DW. Reproducibility of lung and lobar volume measurements using computed tomography. Acad Radiol 17: 316–322, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Brown MS, McNitt-Gray MF, Goldin JG, Greaser LE, Hayward UM, Sayre JW, Arid MK, Aberle DR. Automated measurement of single and total lung volume from CT. J Comput Assist Tomogr 23: 632–640, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Busacker A, Newell JD, Jr, Keefe T, Hoffman EA, Granroth JC, Castro M, Fain S, Wenzel S. A multivariate analysis of risk factors for the air-trapping asthmatic phenotype as measured by quantitative CT analysis. Chest 135: 48–56, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med 344: 350–362, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Campana L, Kenyon J, Zhalehdoust-Sani S, Tzeng YS, Sun Y, Albert M, Lutchen KR. Probing airway conditions governing ventilation defects in asthma via hyperpolarized MRI image functional modeling. J Appl Physiol 106: 1293–1300, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castillo R, Castillo E, Guerra R, Johnson VE, McPhail T, Garg AK, Guerrero T. A framework for evaluation of deformable image registration spatial accuracy using large landmark point sets. Phys Med Biol 54: 1849–1870, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Castillo R, Castillo E, Martinez J, Guerrero T. Ventilation from four-dimensional computed tomography: density versus Jacobian methods. Phys Med Biol 55: 4661–4685, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Castro M, Fain SB, Hoffman EA, Gierada DS, Erzurum SC, Wenzel S; National Heart, Lung, and Blood Institute's Severe Asthma Research Program. Lung imaging in asthmatic patients: the picture is clearer. J Allergy Clin Immunol 128: 467–478, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi Y, Lee S. Injectivity conditions of 2D and 3D uniform cubic B-spline functions. Graphical Models 62: 411–427, 2000 [Google Scholar]
- 14.Clausen J. Measurement of absolute lung volumes by imaging techniques. Eur Respir J 10: 2427–2431, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Coxson H, Mayo J, Behzad H, Moore B, Verburgt L, Staples C, Pare P, Hogg J. Measurement of lung expansion with computed tomography and comparison with quantitative histology. J Appl Physiol 79: 1525–1530, 1995 [DOI] [PubMed] [Google Scholar]
- 16.de Lange EE, Altes TA, Patrie JT, Battiston JJ, Juersivich AP, Mugler JP, 3rd, Platts-Mills TA. Changes in regional airflow obstruction over time in the lungs of patients with asthma: evaluation with 3He MR imaging. Radiology 250: 567–575, 2009 [DOI] [PubMed] [Google Scholar]
- 17.de Lange EE, Altes TA, Patrie JT, Parmar J, Brookeman JR, Mugler JP, 3rd, Platts-Mills TA. The variability of regional airflow obstruction within the lungs of patients with asthma: assessment with hyperpolarized helium-3 magnetic resonance imaging. J Allergy Clin Immunol 119: 1072–1078, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Ding K, Yin Y, Cao K, Christensen GE, Lin CL, Hoffman EA, Reinhardt JM. Evaluation of lobar biomechanics during respiration using image registration. Med Image Comput Comput Assist Interv 5761: 739–746, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engel LA, Prefaut C. Cranio-caudal distribution of inspired gas and perfusion in supine man. Respir Physiol 45: 43–53, 1981 [DOI] [PubMed] [Google Scholar]
- 20.Fain SB, Peterson ET, Sorkness RL, Wenzel S, Castro M, Busse WW. Severe asthma research program-phenotyping and quantification of severe asthma. Imaging Decisions MRI 13: 24–27, 2009 [Google Scholar]
- 21.Froese AB, Bryan AC. Effects of anesthesia and paralysis on diaphragmatic mechanics in man. Anesthesiology 41: 242–255, 1974 [DOI] [PubMed] [Google Scholar]
- 22.Fuld MK, Easley RB, Saba OI, Chon D, Reinhardt JM, Hoffman EA, Simon BA. CT-measured regional specific volume change reflects regional ventilation in supine sheep. J Appl Physiol 104: 1177–1184, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Galbán CJ, Han MK, Boes JL, Chughtai KA, Meyer CR, Johnson TD, Galbán S, Rehemtulla A, Kazerooni EA, Martinez FJ, Ross BD. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med 18: 1711–1715, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gevenois PA, De Vuyst P, De Maertelaer V, Zanen J, Jacobovitz D, Cosio MG, Yernault J. Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med 154: 187–192, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Halaweish AF, Hoffman EA, Thedens DR, Fuld MK, Sieren JP, van Beek EJ. Effect of Lung Inflation Level on Hyperpolarized 3He Apparent Diffusion Coefficient Measurements in Never-Smokers. Radiology. Published online before print April 16, 2013, 10.1148/radiol.13120005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med 159: 179–187, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Harris RS, Fujii-Rios H, Winkler T, Musch G, Vidal Melo MF, Venegas JG. Ventilation defect formation in healthy and asthma subjects is determined by lung inflation. PLoS One 7: e53216, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris RS, Winkler T, Musch G, Vidal Melo MF, Schroeder T, Tgavalekos N, Venegas JG. The prone position results in smaller ventilation defects during bronchoconstriction in asthma. J Appl Physiol 107: 266–274, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris RS, Winkler T, Tgavalekos N, Musch G, Melo MF, Schroeder T, Chang Y, Venegas JG. Regional pulmonary perfusion, inflation, and ventilation defects in bronchoconstricted patients with asthma. Am J Respir Crit Care Med 174: 245–253, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heckscher T, Bass H, Oriol A, Rose B, Anthoniesen NR, Bates DV. Regional lung function in patients with bronchial asthma. J Clin Invest 47: 1063–1070, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffman EA. Effect of body orientation on regional lung expansion: a computed tomographic approach. J Appl Physiol 59: 468–480, 1985 [DOI] [PubMed] [Google Scholar]
- 32.Hoffman EA, Ritman EL. Effect of body orientation on regional lung expansion in dog and sloth. J Appl Physiol 59: 481–491, 1985 [DOI] [PubMed] [Google Scholar]
- 33.Hoffman EA, Simon BA, McLennan G. State of the Art. A structural and functional assessment of the lung via multidetector-row computed tomography: phenotyping chronic obstructive pulmonary disease. Proc Am Thorac Soc 3: 519–532, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ibanez J, Raurich J. Normal values of functional residual capacity in the sitting and supine positions. Intensive Care Med 8: 173–177, 1982 [DOI] [PubMed] [Google Scholar]
- 35.Jarjour NN, Erzurum SC, Bleecker ER, Calhoun WJ, Castro M, Comhair SA, Chung KF, Curran-Everett D, Dweik RA, Fain SB, Fitzpatrick AM, Gaston BM, Israel E, Hastie A, Hoffman EA, Holguin F, Levy BD, Meyers DA, Moore WC, Peters SP, Sorkness RL, Teague WG, Wenzel SE, Busse WW; NHLBI Severe Asthma Research Program (SARP) Severe asthma: lessons learned from the National Heart, Lung, and Blood Institute Severe Asthma Research Program. Am J Respir Crit Care Med 185: 356–362, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King GG, Harris B, Mahadev S. V/Q SPECT: utility for investigation of pulmonary physiology. Semin Nucl Med 40: 467–473, 2010 [DOI] [PubMed] [Google Scholar]
- 36a.Lawrence MA. ez: Easy Analysis and Visualization of Factorial Experiments. R package version 4.1-1, 2012. [The software is available at http://CRAN.R-project.org/package=ez].
- 37.Mase GT, Smelser R, Mase GE. Continuum Mechanics for Engineers (Series: Computational Mechanics and Applied Analysis). Boca Raton, FL: CRC, 2009 [Google Scholar]
- 38.Mathew L, Wheatley A, Castillo R, Castillo E, Rodrigues G, Guerrero T, Parraga G. Hyperpolarized (3)He magnetic resonance imaging: comparison with four-dimensional x-ray computed tomography imaging in lung cancer. Acad Radiol 19: 1546–1553, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, Calhoun WJ, Castro M, Chung KF, Clark MP, Dweik RA, Fitzpatrick AM, Gaston B, Hew M, Hussain I, Jarjour NN, Israel E, Levy BD, Murphy JR, Peters SP, Teague WG, Meyers DA, Busse WW, Wenzel SE; National Heart, Lung, Blood Institute's Severe Asthma Research Program Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol 119: 405–413, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, D'Agostino R, Jr, Castro M, Curran-Everett D, Fitzpatrick AM, Gaston B, Jarjour NN, Sorkness R, Calhoun WJ, Chung KF, Comhair SA, Dweik RA, Israel E, Peters SP, Busse WW, Erzurum SC, Bleecker ER; National Heart, Lung, and Blood Institute's Severe Asthma Research Program Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med 181: 315–323, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moreno F, Lyons HA. Effect of body posture on lung volumes. J Appl Physiol 16: 27–29, 1961 [DOI] [PubMed] [Google Scholar]
- 43.Musch G, Layfield JD, Harris RS, Melo MF, Winkler T, Callahan RJ, Fischman AJ, Venegas JG. Topographical distribution of pulmonary perfusion and ventilation, assessed by PET in supine and prone humans. J Appl Physiol 93: 1841–1851, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Newman KB, Lynch DA, Newman LS, Ellegood D, Newell JD., Jr Quantitative computed tomography detects air trapping due to asthma. Chest 106: 105–109, 1994 [DOI] [PubMed] [Google Scholar]
- 45.Reinhardt JM, Ding K, Cao K, Christensen GE, Hoffman EA, Bodas SV. Registration-based estimates of local lung tissue expansion compared to xenon CT measures of specific ventilation. Med Image Anal 12: 752–763, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robinson PJ, Kreel L. Pulmonary tissue attenuation with computed tomography: comparison of inspiration and expiration scans. J Comput Assist Tomogr 3: 740–748, 1979 [PubMed] [Google Scholar]
- 47.Sorkness RL, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Chung KF, Curran-Everett D, Erzurum SC, Gaston BM, Israel E, Jarjour NN, Moore WC, Peters SP, Teague WG, Wenzel SE; National Heart, Lung, and Blood Institute Severe Asthma Research Program Lung function in adults with stable but severe asthma: air trapping and incomplete reversal of obstruction with bronchodilation. J Appl Physiol 104: 394–403, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Stocks J, Quanjer PH. Reference values for residual volume, functional residual capacity and total lung capacity. Eur Respir J 8: 492–506, 1995 [DOI] [PubMed] [Google Scholar]
- 49.Tzeng YS, Hoffman E, Cook-Granroth J, Gereige J, Mansour J, Washko G, Cho M, Stepp E, Lutchen K, Albert M. Investigation of hyperpolarized 3He magnetic resonance imaging utility in examining human airway diameter behavior in asthma through comparison with high-resolution computed tomography. Acad Radiol 15: 799–808, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Venegas JG, Schroeder T, Harris S, Winkler RT, Melo MF. The distribution of ventilation during bronchoconstriction is patchy and bimodal: a PET imaging study. Respir Physiol Neurobiol 148: 57–64, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Venegas JG, Winkler T, Musch G, Vidal Melo MF, Layfield D, Tgavalekos N, Fischman AJ, Callahan RJ, Bellani G, Harris RS. Self-organized patchiness in asthma as a prelude to catastrophic shifts. Nature 434: 777–782, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Wanger J, Clausen J, Coates A, Pedersen O, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, Van Der Grinten C. Standardisation of the measurement of lung volumes. Eur Respir J 26: 511–522, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Washko G, Parraga G, Coxson H. Quantitative pulmonary imaging using computed tomography and magnetic resonance imaging. Respirology 17: 432–444, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wenzel SE, Busse WW; National Heart, Lung, and Blood Institute's Severe Asthma Research Program Severe asthma: lessons from the severe asthma research program. J Allergy Clin Immunol 119: 14–21, 2007 [DOI] [PubMed] [Google Scholar]
- 55.West JB. Respiratory Physiology: the Essentials. Philadelphia, PA: Lippincott Williams & Wilkins, 2008 [Google Scholar]
- 56.Yin Y, Choi J, Hoffman EA, Tawhai MH, Lin CL. Simulation of pulmonary air flow with a subject-specific boundary condition. J Biomech 43: 2159–2163, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yin Y, Hoffman EA, Lin CL. Local tissue-weight-based nonrigid registration of lung images with application to regional ventilation. Proc of SPIE 7262: 72620C, 2009 [Google Scholar]
- 58.Yin Y, Hoffman EA, Lin CL. Mass preserving nonrigid registration of CT lung images using cubic B-spline. Med Phys 36: 4213–4222, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yin Y, Hoffman EA, Lin CL. Lung lobar slippage assessed with the aid of image registration. Med Image Comput Comput Assist Interv 6362: 578–585, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yin Y, Choi J, Hoffman EA, Tawhai MH, Lin CL. A multiscale MDCT image-based breathing lung model with time-varying regional ventilation. J Comput Phys 244: 168–192, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zapke M, Topf HG, Zenker M, Kuth R, Deimling M, Kreisler P, Rauh M, Chefd'hotel C, Geiger B, Rupprecht T. Magnetic resonance lung function—a breakthrough for lung imaging and functional assessment? A phantom study and clinical trial. Respir Res 6: 106, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]