Abstract
This study was carried out to determine the effect of allergic inflammation on the airway response to increasing airway temperature. Our results showed the following: 1) In Brown-Norway rats actively sensitized by ovalbumin (Ova), isocapnic hyperventilation with humidified warm air (HWA) for 2 min raised tracheal temperature (Ttr) from 33.4 ± 0.6°C to 40.6 ± 0.1°C, which induced an immediate and sustained (>10 min) increase in total pulmonary resistance (Rl) from 0.128 ± 0.004 to 0.212 ± 0.013 cmH2O·ml−1·s (n = 6, P < 0.01). In sharp contrast, the HWA challenge caused the same increase in Ttr but did not generate any increase in Rl in control rats. 2) The increase in Rl in sensitized rats was reproducible when the same HWA challenge was repeated 60–90 min later. 3) This bronchoconstrictive effect was temperature dependent: a slightly smaller increase in peak Ttr (39.6 ± 0.2°C) generated a significant but smaller increase in Rl in sensitized rats. 4) The HWA-induced bronchoconstriction was not generated by the humidity delivered by the HWA challenge alone, because the same water content delivered by saline aerosol at room temperature had no effect. 5) The HWA-evoked increase in Rl in sensitized rats was not blocked by atropine but was completely prevented by pretreatment either with a combination of neurokinin (NK)-1 and NK-2 antagonists or with formoterol, a β2 agonist, before the HWA challenge. This study showed that increasing airway temperature evoked a pronounced and reversible increase in airway resistance in sensitized rats and that tachykinins released from the vagal bronchopulmonary C-fiber endings were primarily responsible.
Keywords: vagus, reflex, hyperthermia, extravasation, asthma
body temperature increases as a result of elevated metabolic rate or hindered heat dissipation. Although the lungs are enclosed in the thoracic chamber and constantly exposed to body temperature, an increase in lung temperature can occur under both normal and pathophysiological conditions. For example, body core temperature exceeding 41°C has been reported in healthy humans and animals during exertional exercise (7, 30). Body temperature higher than 40.5°C occurs frequently in patients suffering from severe fever or heatstroke (5). In addition, tissue inflammation can lead to an increase in local temperature in the inflamed area (16, 38). Indeed, a recent report showed that the end-expiratory temperature plateau was 2.7°C higher in mild allergic asthmatic children than in healthy children, and the difference was closely correlated with the exhaled nitric oxide concentration as well as the sputum eosinophil percentage, suggesting an involvement of local tissue inflammation (37).
A recent study in our lab reported that an increase in intrathoracic temperature to above a threshold of ∼39.2°C activated vagal pulmonary C-fiber endings in anesthetized rats (39). A follow-up study further demonstrated a similar stimulatory effect of increasing temperature in isolated rat vagal pulmonary sensory neurons (35). These studies clearly indicated that an increase in temperature within the physiological range can stimulate vagal bronchopulmonary C fibers, and, more importantly, activation of these afferents is known to elicit bronchoconstriction mediated through both cholinergic reflex pathways and local release of tachykinins (10, 21, 24, 28, 41). Indeed, we recently reported that an increase in airway temperature by hyperventilation with hot humid air for 4 min triggered an immediate and transient bronchoconstriction in patients with mild asthma but not in healthy individuals (17). The bronchoconstriction was accompanied by cough and prevented by pretreatment with ipratropium, a muscarinic receptor antagonist, suggesting an involvement of airway sensory nerves. However, the specific type of airway nerves involved could not be determined (17). In a parallel study utilizing an animal model of allergic asthma [Brown-Norway rats actively sensitized by ovalbumin (Ova)], we demonstrated that chronic airway inflammation enhanced the sensitivity of the bronchopulmonary C-fiber afferents to capsaicin, but whether the temperature sensitivity of these afferents was also elevated is not known (47).
To answer these questions, this study was carried out to investigate whether an increase in airway temperature evokes a more intense bronchoconstriction in Ova-sensitized Brown-Norway rats and, if so, to elucidate the possible underlying mechanism(s).
MATERIALS AND METHODS
The procedures described below were performed in accordance with recommendations from the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were also approved by the University of Kentucky Institutional Animal Care and Use Committee.
Animal Sensitization
Young male pathogen-free Brown-Norway rats were divided into two groups (control and sensitized groups). Sensitized animals received an initial intraperitoneal injection of a suspension containing 2 mg of Ova in 1 ml of Imject Alum as adjuvant. Three days later, rats were exposed to Ova aerosol for 15 min three times per week (M/W/F) for 3 wk, following the protocol established by previous investigators (13, 47). During the exposure, the unanesthetized rat was placed in a Plexiglas restrainer (University of Kentucky Center for Manufacturing) and breathed spontaneously and continuously through a nose cone connected to a free stream of air-aerosol mixture under a negative-pressure exhaust hood. Ova solution (wt/vol concentration: 1.25% in saline) was nebulized and delivered by an ultrasonic nebulizer (model 099HD; Devilbiss, Somerset, PA) at a droplet size ranging from 0.5 to 5 μm. Control animals received an intraperitoneal injection and aerosol inhalation of the vehicle (isotonic saline), following the identical procedures.
Animal Preparation
One day after the last Ova or saline exposure, animals were anesthetized with an intraperitoneal injection of α-chloralose (100 mg/kg; Sigma-Aldrich, St. Louis, MO) and urethane (500 mg/kg; Sigma-Aldrich) dissolved in a 2% borax solution. During the experiments, supplemental doses of the same anesthetics were injected intravenously to maintain the abolition of the pain reflex elicited by paw pinch. Animals were placed in a supine position and ventilated mechanically with a respirator (model 683; Harvard, South Natick, MA) via a short tracheal cannula inserted just below the larynx after a tracheotomy. Body temperature was maintained at ∼36°C by means of a heating blanket placed under the animal. A polyethylene catheter was inserted into the jugular vein until its tip was close to the right atrium for bolus injections of drugs. The right and left femoral arteries were cannulated for measurements of arterial blood gas and arterial blood pressure (ABP), respectively. Respiratory frequency was set at 60 breaths/min, and tidal volume (Vt) was adjusted between 6 and 7 ml/kg in each animal to maintain the end-tidal CO2 concentration (model 1260; Novametrix, Wallingford, CT) between 4.6% and 5.0%.
Measurement of Lung Mechanics
One day after the last saline or Ova exposure, control and sensitized animals were anesthetized and artificially ventilated in the same manner. A catheter was inserted into the right intrapleural cavity between the fifth and sixth ribs for measuring intrapleural pressure (Pip). Pneumothorax was then corrected by briefly opening the intrapleural catheter to ambient air during a held hyperinflation (3 × Vt). Transpulmonary pressure was measured as the difference between the tracheal pressure and Pip with a differential pressure transducer. Respiratory flow was measured with a heated pneumotachograph and a differential pressure transducer. These signals were analyzed by an online computer (Biocybernetics TS-100, Taipei, Taiwan) for measurements of total pulmonary resistance (Rl), dynamic lung compliance (Cdyn), ABP, and heart rate (HR). Results obtained from the computer were routinely checked by hand calculation for accuracy.
Challenge with Humidified Warm Air
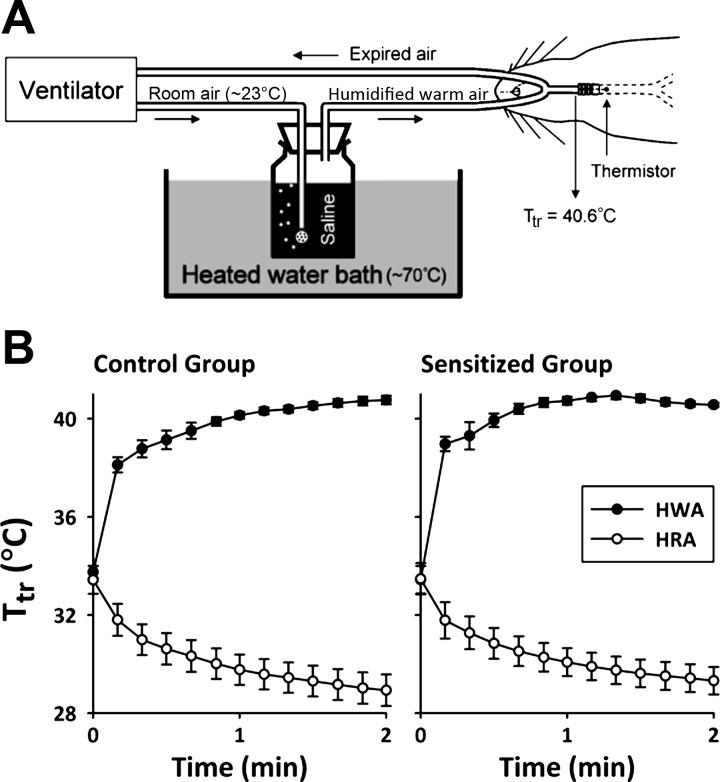
The method for generating humidified warm air (HWA) in this study was described previously (26). Briefly, the outlet of the respirator inspiratory line was connected to an air stone that was immersed in isotonic saline contained in a bottle placed in a heated water bath (Fig. 1A); isotonic saline was used for generating humidity because inhalation of distilled water or hypotonic saline aerosol has been shown to evoke reflex bronchoconstriction in patients with asthma (40). HWA was then delivered directly into the lung via the tracheal tube. Humidified room air (HRA) was delivered in the same manner except that the water bath was kept at room temperature (∼23°C). During HWA or HRA hyperventilation challenges, minute ventilation was increased to ∼375% of the baseline (Vt and respiratory frequency were set to 150% and 250% of the baseline, respectively) for 2 min. To prevent hypocapnia and alkalosis produced by hyperventilation, a gas mixture containing 3.2–4.5% CO2, 21% O2 with the remaining percentage as N2 was administrated via the respirator. A miniature thermometer (model IT-18; Physitemp, Clifton, NJ) was inserted into the tracheal tube and positioned near the thoracic entry (∼0.5 cm distal to the tip of the tracheal tube) to continuously measure the air temperature in the trachea (Ttr; Fig. 1A) before, during, and after the HWA and HRA challenges. We determined in our pilot experiments that average Ttr can be elevated to ∼40.6°C by maintaining the heated water bath temperature at ∼70°C (Fig. 1A). The amount of water content in HWA and HRA measured in this study was 77.6 ± 5.9 and 15.2 ± 0.3 mg/l of air, respectively.
Fig. 1.
A: schematic drawing of the experimental setup for delivery of humidified warm air (HWA) into the lung of anesthetized Brown-Norway rats. Ttr, air temperature in trachea. B: change in Ttr during the 2-min hyperventilation with HWA and humidified room air (HRA; water bath was kept at room temperature ∼23°C) in control (left) and ovalbumin (Ova)-sensitized (right) rats. Data are means ± SE for 6 rats.
Experimental Protocols
Eight series of experiments were carried out in this study.
Series 1.
To determine whether HWA hyperventilation induced bronchoconstriction in Ova-sensitized rats and, if so, whether the effect was generated by the increase in Ttr, in each rat Rl and Cdyn were measured continuously on a breath-by-breath basis for 1 min before (as the baseline) and for 15 min immediately after the hyperventilation. The responses to hyperventilation with HWA were then compared between control and Ova-sensitized rats. To determine the effect of HWA, the responses to hyperventilation with HWA and HRA were then compared in the same rat; the sequence of HWA and HRA challenges was altered between animals to achieve a balanced design, and 60–90 min elapsed between two challenges for recovery.
Series 2.
To determine whether the response induced by HWA in Ova-sensitized rats was temperature dependent, animals were challenged with humidified air of three different temperatures that resulted in three different levels of end-tidal Ttr, high (40.6°C), intermediate (39.6°C), and room air (29.4°C), and 60–90 min was allowed to elapse between two challenges.
Series 3.
To determine whether the HWA-induced responses in Ova-sensitized rats were reproducible, the same HWA challenge was repeated after 60–90 min in the same rat.
Series 4.
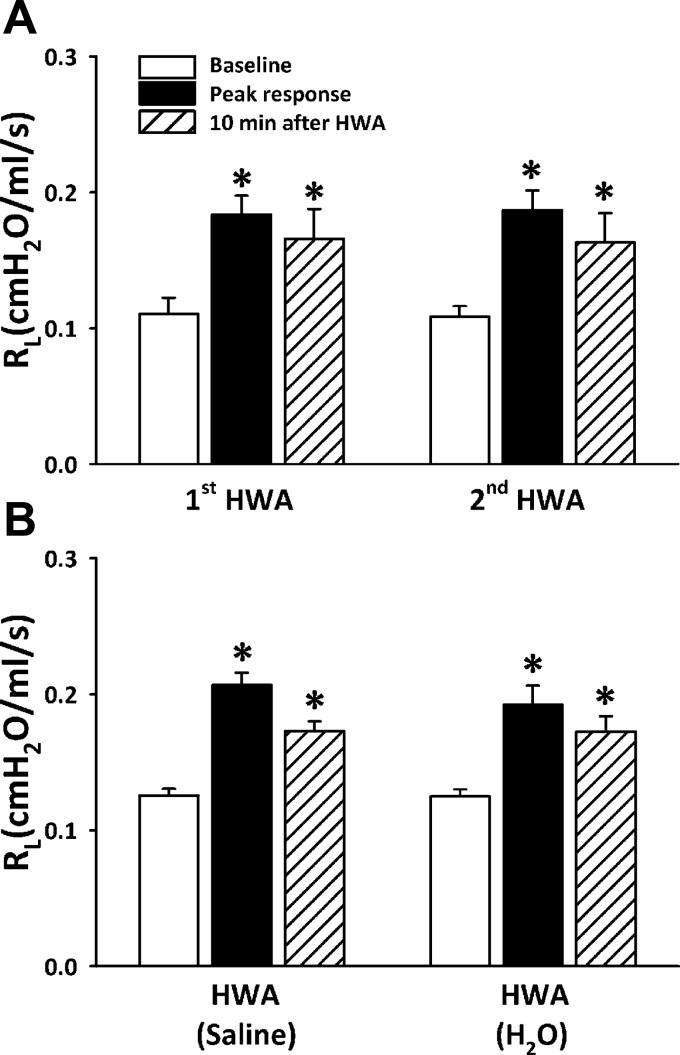
Responses to HWA challenges were compared in the Ova-sensitized rat when the humidity in HWA was generated from isotonic saline and distilled water.
Series 5.
To evaluate the influence of humidity alone in the HWA-induced responses, responses to hyperventilation with HWA and saline aerosol were compared in the same Ova-sensitized rat; the same amount of water content (∼80 mg/l of air) was generated by aerosol (Aeroneb Pro; Aerogen Nektar, Galway, Ireland) and delivered in the identical manner as that in the HWA challenge, except that the saline aerosol was administered at room temperature (∼23°C).
Series 6.
To evaluate the role of cholinergic reflex in the HWA-evoked change in Rl, responses to HWA in Ova-sensitized rats were compared between before and after pretreatment with atropine sulfate (0.1 mg/kg iv), a muscarinic receptor antagonist.
Series 7.
To test whether the effect of HWA was caused by smooth muscle contraction, responses to HWA in Ova-sensitized rats were compared between before and after pretreatment with formoterol (10 μg/kg iv), a selective β2 agonist.
Series 8.
To investigate a possible involvement of the endogenous tachykinins, the HWA-induced responses in Ova-sensitized rats were compared between before and after pretreatment with a combination of neurokinin (NK) receptor type 1 and 2 (NK-1 and NK-2) antagonists L-732138 (6 mg/kg iv) and SR-48968 (0.3–1 mg/kg iv), respectively. The same doses of SR-48968 and L-732138 almost completely prevented the increase in Rl evoked by bolus intravenous injection of neurokinin A (NKA; 0.2 μM, 0.15 ml) and substance P (SP; 0.2–0.5 μM, 0.15 ml) in anesthetized rats: the peak ΔRl values (above the baseline) after NKA injection were 137 ± 31% before and 24 ± 4% (P < 0.01, n = 5) after the pretreatment with SR-48968 and L-732138, and the peak ΔRl values after SP injection were 29 ± 3% before and 16 ± 2% (P < 0.05, n = 5) after the pretreatment with SR-48968 and L-732138. These results are in agreement with the effective blocking doses of these NK receptor antagonists reported in the literature (25, 45).
Statistical Analysis
In each experiment, baseline Rl and Cdyn were averaged over 1 min (i.e., 60 breaths) before HWA or HRA challenge; the peak responses were averaged over 60 consecutive breaths within the first 3 min after HWA or HRA challenge. Data were compared with a paired t-test or a two-way repeated-measures analysis of variance (ANOVA). When the ANOVA showed a significant interaction, pairwise comparisons were made with a post hoc analysis (Fisher's least significant difference). P values of <0.05 were considered significant. Data are reported as means ± SE.
Materials
Solution of Ova (Sigma-Aldrich) was prepared daily at a concentration of 1.25% (wt/vol) in saline. Imject Alum as adjuvant was purchased from Pierce Biotechnology (Rockford, IL). Stock solutions of chemical agents were prepared as follows: L-732138 (Tocris, Ellisville, MO) was dissolved in DMSO (Sigma-Aldrich) at a concentration of 25 mg/ml; SR-48968 (Sanofi Recherche, Montpellier, France) in polyethylene glycol (average mol wt 200; Sigma-Aldrich) at a concentration of 10 mg/ml; formoterol (Sigma-Aldrich) in DMSO at a concentration of 1 mg/ml; and atropine sulfate (Sigma-Aldrich) in isotonic saline at a concentration of 10 mg/ml. Solutions of these chemical agents at the desired concentrations for injections were then prepared daily by dilution with saline based on the animal's body weight.
RESULTS
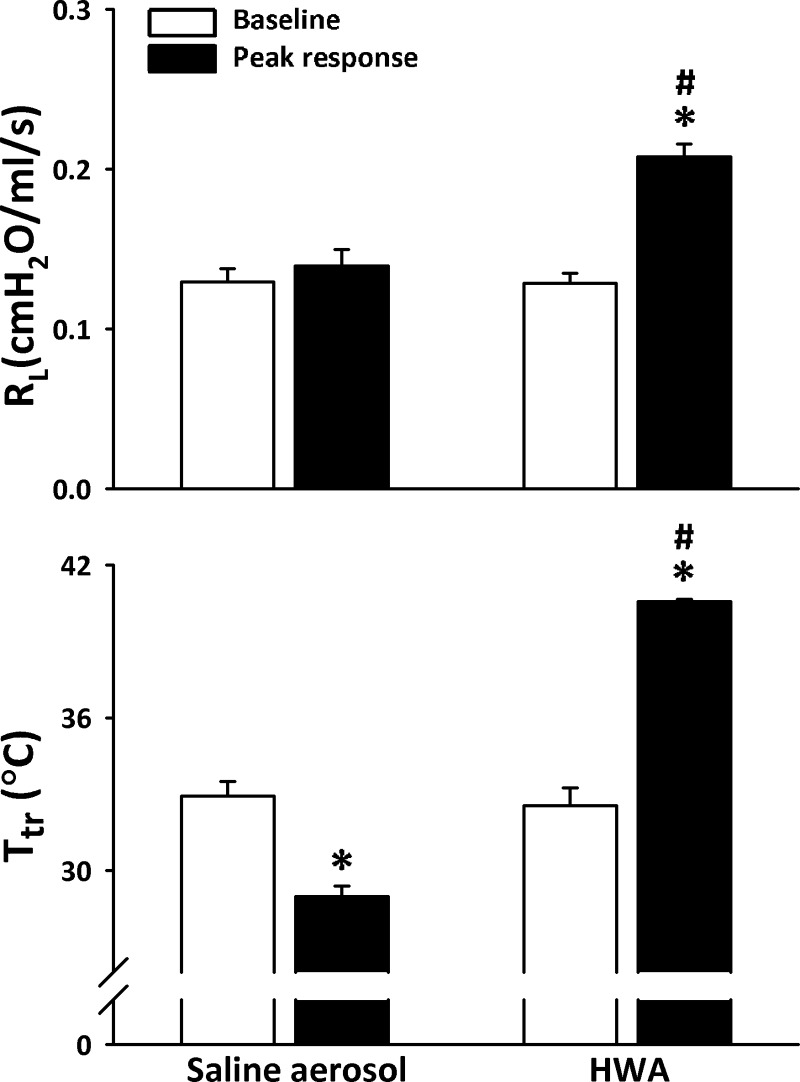
This study was carried out in a total of 41 Brown-Norway rats with an average body weight of 270 ± 5 g. Some of the animals were used in more than one series of experiments. Hyperventilation with HWA led to an increase in peak Ttr from 33.4 ± 0.6°C to 40.6 ± 0.1°C (n = 6; P < 0.001) in the sensitized group and from 33.8 ± 0.2°C to 40.8 ± 0.2°C (n = 6; P < 0.001) in the control group (Fig. 1B). In contrast, hyperventilation with HRA caused a slight decrease in Ttr (from 33.5 ± 0.6°C to 29.3 ± 0.6°C in sensitized group and from 33.4 ± 0.6°C to 28.9 ± 0.6°C in control group). In seven rats, the baseline arterial pH, Po2, and Pco2 were 7.50 ± 0.03, 95.3 ± 4.3 mmHg, and 36.8 ± 1.7 mmHg, respectively, and they did not change when measured during the last 30 s of hyperventilation with HWA in the same animals: arterial pH, Po2, and Pco2 were 7.51 ± 0.02 (P > 0.5), 98.4 ± 3.8 mmHg (P > 0.5), and 34.5 ± 1.0 mmHg (P > 0.05), respectively.
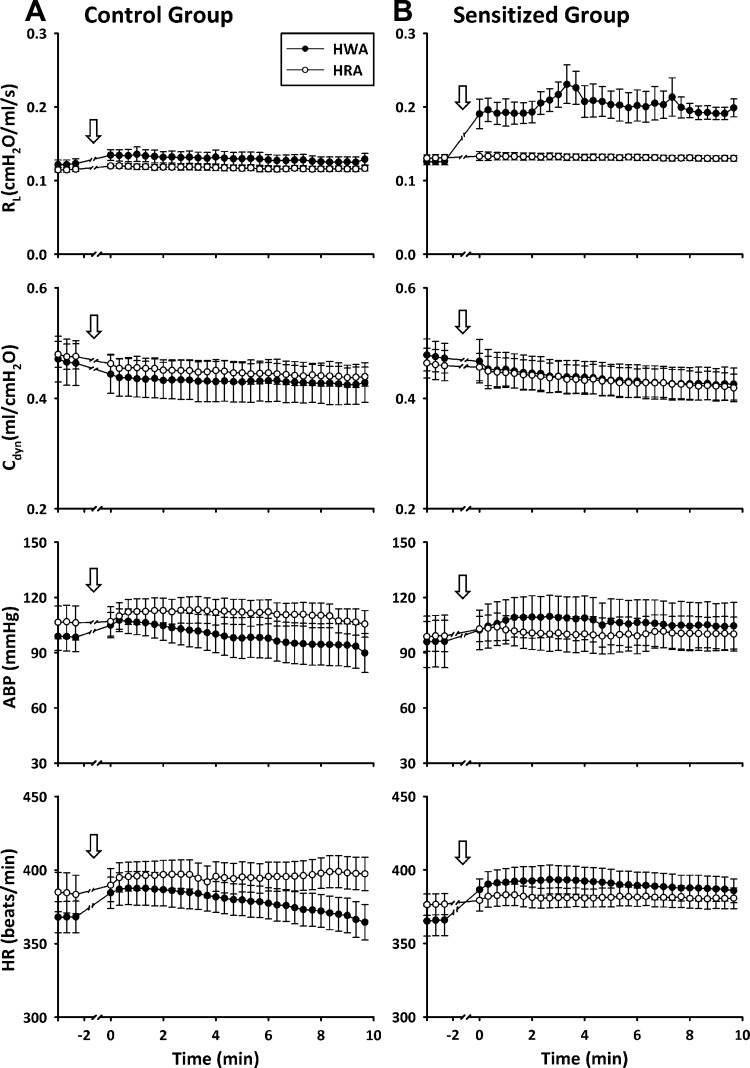
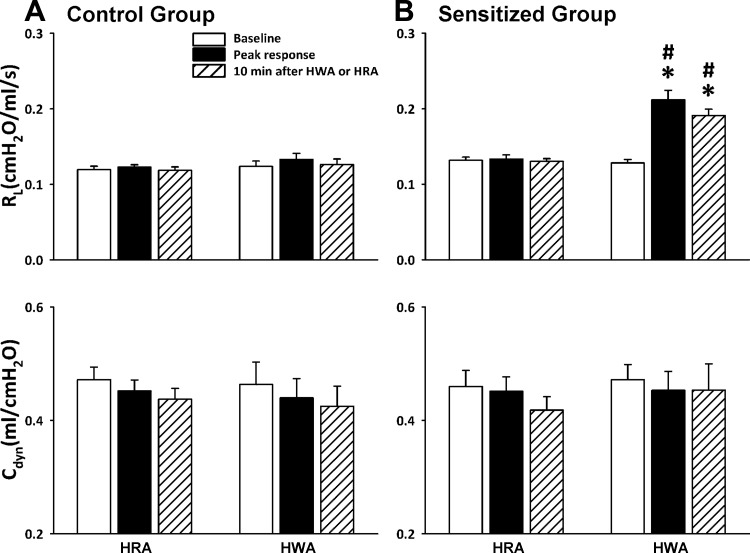
Hyperventilation with HWA induced an increase in Rl in Ova-sensitized rats but not in control rats. The response occurred immediately after the HWA challenge and lasted for >10 min (Figs. 2 and 3). Rl increased from 0.128 ± 0.004 cmH2O·ml−1·s (60-breath average) before to 0.212 ± 0.013 cmH2O·ml−1·s (60-breath average) after HWA (P < 0.01, n = 6; Fig. 3). In sharp contrast, the same HWA challenge did not generate any detectable changes in Rl in control rats (from 0.124 ± 0.007 to 0.133 ± 0.008 cmH2O·ml−1·s, P > 0.05, n = 6; Fig. 3). Hyperventilation with HRA did not cause any significant change in Rl in either control or sensitized rats (Figs. 2 and 3). Furthermore, there was no significant change in Cdyn after either HWA or HRA challenge in either sensitized or control rats (Figs. 2 and 3).
Fig. 2.
Effect of hyperventilation with HWA and HRA on total pulmonary resistance (Rl), dynamic lung compliance (Cdyn), arterial blood pressure (ABP), and heart rate (HR) in control (A) and Ova-sensitized (B) rats. Responses were not recorded during hyperventilation (arrows), which was administered between −2 and 0 min. Data before time −2 min represent baseline values; 60–90 min was allowed to elapse between 2 challenges. Each data point was averaged over 20 consecutive breaths. Data are means ± SE for 6 rats.
Fig. 3.
Comparison of the responses of Rl and Cdyn to hyperventilation with HWA and HRA in control (A) and Ova-sensitized (B) rats. Open bars, baseline data averaged over 1 min before; filled bars, peak responses averaged over 1 min after; hatched bars, responses 10 min after HWA or HRA challenge; 60–90 min was allowed to elapse between 2 challenges. Data are means ± SE for 6 rats. *Significantly different from baseline (P < 0.05); #significant difference when corresponding data between HWA and HRA were compared (P < 0.05).
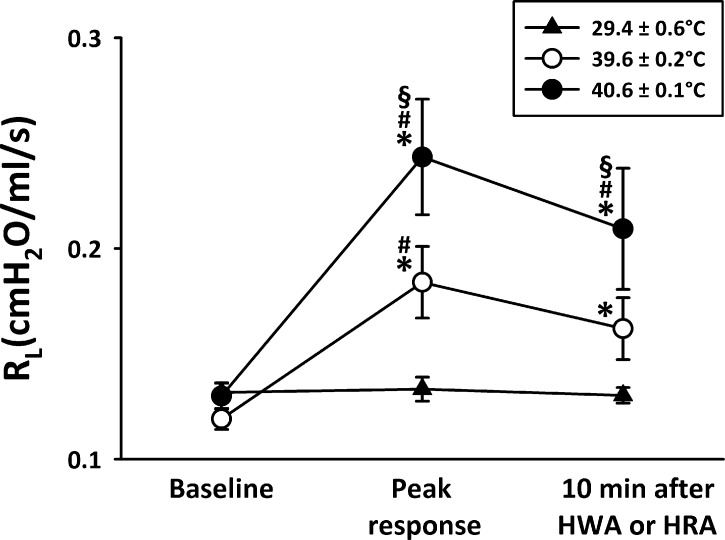
The increase in Rl was temperature dependent; a slightly smaller increase in Ttr (39.6 ± 0.2°C) generated a smaller but significant and consistent increase in Rl (from 0.119 ± 0.005 to 0.184 ± 0.017 cmH2O·ml−1·s) in sensitized rats (Fig. 4).
Fig. 4.
Temperature-dependent effect of HWA challenge on Rl in Ova-sensitized rats. Three different levels of Ttr were maintained during the test: high (40.6°C), intermediate (39.6°C), and room air (29.4°C); 60–90 min was allowed to elapse between 2 challenges. Data are means ± SE for 6 rats. *Significantly different from baseline (P < 0.05); #significantly different from response to room air temperature (P < 0.05); §significantly different from response to intermediate temperature (P < 0.05).
The increases in Rl were reproducible when the same HWA challenge was repeated after 60–90 min in the same rats (Fig. 5A); there were no significant differences between the responses to the first and second HWA challenges (P > 0.4, n = 5).
Fig. 5.
A: reproducibility of the responses of Rl to 2 consecutive HWA challenges in the same group of Ova-sensitized rats. B: responses of Rl to HWA were compared in the same group of Ova-sensitized rats when humidity was generated from distilled water and isotonic saline. Sixty to ninety minutes were allowed to elapse between 2 challenges. Data are means ± SE; n = 5 in A and n = 6 in B. *Significantly different from baseline (P < 0.05).
The responses to HWA generated from distilled water and isotonic saline were compared in the same Ova-sensitized rats; the sequences of these two tests were alternated between animals. There were no significant differences between the responses of Rl to HWA generated from distilled water and isotonic saline (P > 0.5, n = 6; Fig. 5B).
The increase in Rl was not caused by the humidity in the HWA alone because the same water content as in HWA delivered by saline aerosol for 2 min did not generate any increase in Rl (Fig. 6), while the aerosol inhalation caused a small decrease in Ttr (from a baseline of 32.9 ± 0.6°C to 29.0 ± 0.4°C after challenge).
Fig. 6.
Comparison of responses of Rl (top) and Ttr (bottom) to hyperventilation with HWA and with saline aerosol in the Ova-sensitized rats. The same amount of water content (77.6 ± 5.9 mg/l of air) was delivered by aerosol and by HWA in the same manner in the same rats, but the saline aerosol was generated by a nebulizer and delivered at room temperature (∼23°C); 60–90 min was allowed to elapse between 2 challenges. Data are means ± SE for 6 rats. *Significantly different from baseline (P < 0.05); #significant difference when corresponding data between HWA and saline aerosol were compared (P < 0.05).
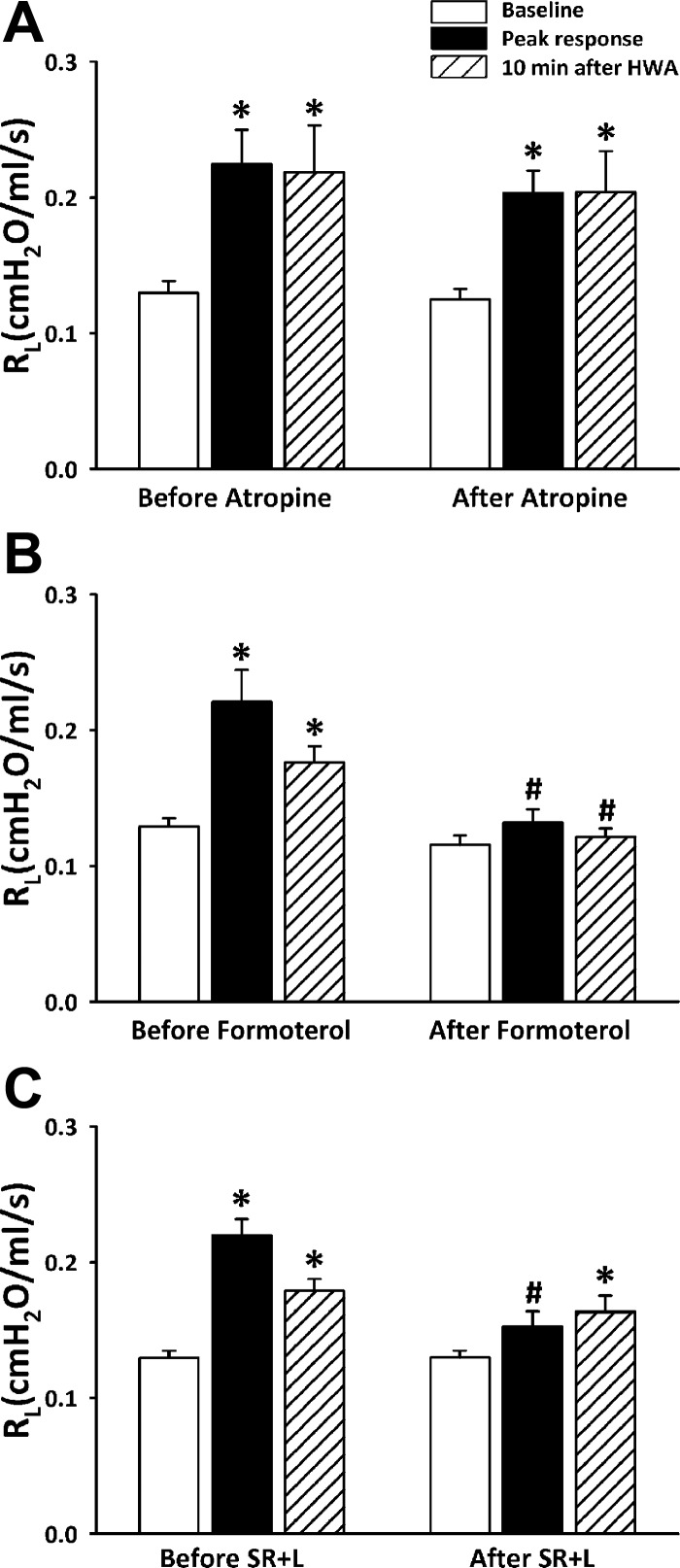
Pretreatment with atropine sulfate did not reduce the HWA-induced increase in Rl (Fig. 7A); the increases in Rl after HWA challenge were 0.095 ± 0.023 and 0.078 ± 0.011 cmH2O·ml−1·s before and after atropine, respectively, in Ova-sensitized rats (P > 0.5, n = 5). In a separate study, we verified that pretreatment with the same dose of atropine (0.1 mg/kg iv) completely prevented a pronounced increase in Rl evoked by inhalation of aerosolized acetylcholine chloride (200 mM; 5 μl delivered by respirator in 3 or 4 consecutive tidal breaths) in anesthetized rats prepared in the identical manner; before atropine ACh inhalation increased Rl from 0.09 ± 0.01 to 0.40 ± 0.11 cmH2O·ml−1·s (P < 0.001, n = 4), and after atropine the same ACh challenge did not change Rl (from 0.09 ± 0.01 to 0.10 ± 0.01 cmH2O·ml−1·s; P > 0.05, n = 4).
Fig. 7.
A: comparison of the responses of Rl to hyperventilation with HWA before and after pretreatment with atropine sulfate (0.1 mg/kg iv). B: comparison between before and after pretreatment with formoterol (10 μg/kg iv). C: comparison between before and after pretreatment with a combination of L-732138 (L; 6 mg/kg iv) and SR-48968 (SR; 0.3–1.0 mg/kg iv). Sixty to ninety minutes was allowed to elapse between 2 challenges. Data are means ± SE for 6 rats. *Significantly different from baseline (P < 0.05); #significant difference when corresponding data between before and after pretreatment were compared (P < 0.05).
Pretreatment with formoterol completely abolished the increase in Rl caused by the HWA challenge in sensitized rats (Fig. 7B); the peak increases in Rl were 0.092 ± 0.024 and 0.016 ± 0.011 cmH2O·ml−1·s before and after formoterol, respectively (P < 0.05, n = 6).
Pretreatments with L-732138 and SR-48968 also completely abolished the increase in Rl caused by the HWA challenge in sensitized rats (Fig. 7C); the immediate increases in Rl after the HWA challenge were 0.090 ± 0.014 and 0.023 ± 0.007 cmH2O·ml−1·s before and after the combined treatments with L-732138 and SR-48968, respectively (P < 0.01, n = 6). A slight increase in Rl (P < 0.05, n = 6) was detected at 10 min after the HWA challenge (Fig. 7C).
DISCUSSION
This study showed that an increase in Ttr to 40.6°C after hyperventilation with HWA for 2 min triggered a significant and sustained increase in airway resistance in Ova-sensitized rats. This bronchoconstrictive effect of HWA was reversible and reproducible in the same rats. In sharp contrast, the same HWA hyperventilation challenge did not induce any bronchoconstriction in control rats, despite the same increase in Ttr. The intensity of the HWA-induced bronchoconstriction was temperature dependent; a slightly smaller increase in Ttr (39.6°C) caused a smaller increase in airway resistance in Ova-sensitized rats. This effect was not caused by humidity alone, because the same water content delivered by saline aerosol at room temperature failed to generate any significant bronchoconstriction. Also, the same effect was observed when the humidity was generated from distilled water. These results illustrated that chronic airway inflammation caused by allergen sensitization rendered the airways more vulnerable to airway hyperthermia and induced pronounced bronchoconstriction. This HWA-evoked bronchoconstriction was prevented by pretreatment with a combination of both NK-1 and NK-2 antagonists, indicating that an endogenous release of tachykinins was primarily responsible.
There are three primary types of mammalian tachykinins: SP, NKA, and neurokinin B (NKB). In the respiratory tract, SP and NKA (both derived from the same precursor gene preprotachykinin A) are mainly synthesized in the cell body of nonmyelinated (C fiber) sensory nerves and transported along the axon toward the peripheral terminals, where they are stored in large granular vesicles and released from the sensory endings upon a surge of calcium influx triggered by membrane depolarization. SP and NKA are frequently colocalized and coreleased from the same pulmonary sensory neurons. The actions of these tachykinins are mediated through three distinct types of receptors, NK-1, NK-2, and NK-3, that are expressed in the plasma membrane of various effector cells in the airways and have preferential affinities for the three major types of endogenous tachykinins, SP, NKA, and NKB, respectively (21, 29). Activation of NK-1 receptors by SP causes an increase in vascular permeability to plasma macromolecules, adhesion of leukocytes to the vascular endothelium, and vascular smooth muscle relaxation in the arterioles; these effects together induce vasodilatation in the microvessels and mucosal edema in the airways (21, 31, 33). In addition, the NK-1 receptor is known to be involved in initiating the chemotactic reaction and leukocyte infiltration into the airway and the consequent release of inflammatory mediators and cytokines (31, 33). Indeed, the diverse immunomodulatory effects of tachykinins, mainly mediated through activation of NK-1 receptors, on mast cells, neutrophils, lymphocytes, and macrophages in the respiratory tract have been extensively documented (21). The NK-2 receptor is expressed primarily on the airway smooth muscle, and its activation induces bronchoconstriction (21, 28, 41). The NK-2 receptor is also found on the airway cholinergic ganglion neurons and postganglionic prejunctional cholinergic nerve terminals, which upon activation can facilitate ganglionic transmission and acetylcholine release (28, 41). NKB is rarely found in the lung and airways (20), but the NK-3 receptor, present on the cholinergic ganglion neurons, can be also activated by SP and NKA and facilitate the synaptic transmission (8). Combined effects of these neuropeptides are believed to contribute to the local “axon reflexes” that are generated by stimulation of C-fiber afferents but cannot be abolished by bilateral vagotomy or atropine (21, 28, 41). Sustained and/or intense stimulation of C-fiber afferents can lead to the development of “neurogenic inflammatory reaction” in the airways (2, 21, 32, 41).
The species-dependent variation in the functions of these different NK receptors is well documented; for example, SP induces airway smooth muscle contraction in guinea pig and hamster airways via the activation of both the NK-2 and, to a lesser extent, the NK-1 receptors (21, 28). Thus capsaicin administered by injection or inhalation evokes bronchoconstriction in these species via both the centrally mediated cholinergic reflex pathway and the local axon reflex responses. However, stimulation of vagal bronchopulmonary C-fiber afferents by capsaicin does not generate a conspicuous and consistent bronchoconstriction in rats (43, 44); the lack of response may be related to the fact that SP released from C-fiber endings upon stimulation can activate NK receptors in the epithelial and endothelial cells and trigger the secondary release of bronchodilating mediators such as prostaglandin E2 and nitric oxide from these cells in rat airways (11, 25, 44), which can mask the centrally mediated reflex bronchoconstriction.
Chronic allergic inflammation caused by active sensitization is known to increase the expression of preprotachykinin mRNA in the vagal sensory neurons and the amount of tachykinin synthesis and release in the airways (15). In addition, allergic airway inflammation is known to inhibit the enzyme activity of neutral endopeptidase (NEP) that is present on the membranes of various cell types (including epithelium and nerve fibers) in the airways (9). NEP can cleave tachykinins immediately after their release (19), and therefore the attenuated NEP activity may also contribute to the enhanced bronchoconstrictive effect of endogenous tachykinins. Chronic airway inflammation induced by Ova sensitization can also cause a phenotypic switch of both the tachykinergic innervation and the expression of transient receptor potential vanilloid type 1 receptors (TRPV1) in the airways (34, 46). Under normal conditions, expressions of both tachykinins and TRPV1 are found almost exclusively in capsaicin-sensitive, neurofilament-negative C-fiber afferents innervating the guinea pig airways. However, in Ova-sensitized guinea pigs SP was found in ∼30% of the large-diameter, neurofilament-positive neurons (34). A recent study further demonstrated that chronic allergic inflammation in Brown-Norway rats actively sensitized with Ova induced a significant increase in the expression of TRPV1 in bronchopulmonary neurons in nodose ganglia, mainly in neurofilament-positive (myelinated) neurons (46). Indeed, capsaicin sensitivity was detected in some of the vagal myelinated (A fiber) afferents that normally do not exhibit capsaicin sensitivity (46), and the baseline activity and sensitivities of pulmonary C fibers to both chemical stimulants and lung inflation were also markedly elevated in Ova-sensitized rats (47). The upregulation of both tachykinin and TRPV1 expression in the airway sensory nerves of Ova-sensitized animals may have contributed, to some extent, to the increase in airway resistance after the HWA hyperventilation challenge observed in this study.
Previous investigators have reported the expression of TRPV channels (TRPV2 and TRPV1) in mast cells (4, 42). Thus an increase in airway temperature generated by the HWA challenge may activate TRPVs and trigger mast cell degranulation and release of histamine and other bronchoactive autacoids, which may have contributed to the bronchoconstriction. In addition, previous studies have extensively documented the expression of NK-1 receptors in mast cells and other inflammatory cells (21). Thus in either direct activation of TRPVs by high temperature or indirect activation by SP released from bronchopulmonary C fibers, a possible involvement of mast cell degranulation should be considered in the airway response to HWA. In particular, such an action mediated through mast cells is expected to be enhanced in Ova-sensitized rats. Our observation that HWA-induced bronchoconstriction was completely prevented by pretreatment with NK receptor antagonists seems to support the latter (i.e., involvement of the NK-1 receptor instead of TRPVs). We should also point out that the elevated airway temperature during the HWA challenge in this study was substantially lower than the activation threshold for TRPV2 (>50°C) (3).
The HWA-evoked increase in airway resistance in Ova-sensitized rats was completely prevented by pretreatment with formoterol, a bronchodilator, which seems to suggest that the bronchoconstrictive effect of HWA involved contraction of airway smooth muscles. However, we cannot rule out the possibility that the increase in airway resistance may have also resulted, partially or totally, from the protein extravasation and mucosa edema in the airway walls of sensitized animals. Previous investigators have demonstrated that β2 agonists, such as formoterol, can attenuate the plasma extravasation by reducing the number of endothelial gaps in postcapillary venules, which is probably mediated through an increase in intracellular cAMP resulting from β2-receptor activation in the endothelial cells (1). Furthermore, pretreatment with β2 agonist can also reduce the number of eosinophils and neutrophils adhered to the vascular endothelium and thus diminish the inflammatory reaction and the resulting bronchoconstriction (6).
The above assessments of our results suggest that HWA challenge elevated the activity of vagal bronchopulmonary C-fiber afferents in Ova-sensitized rats. Indeed, results obtained from a parallel study carried out in our lab support this hypothesis. Using the same preparations and animal model, we recorded “single-fiber” activities of vagal bronchopulmonary C-fiber afferents before and after HWA hyperventilation challenge and compared them between control and Ova-sensitized rats (27). The study showed that an increase in Ttr by HWA challenge similar to that in this study markedly elevated the baseline activity of these afferents and further amplified their hypersensitivity to chemical stimulation in Ova-sensitized rats but not in control rats (27).
The mechanism(s) involved in the HWA-induced C-fiber hypersensitivity is not fully understood, but an increase in the temperature sensitivity of these C-fiber afferents is probably involved. The primary sensors for detecting warm and hot temperature in mammalian species are TRPV channels. TRPVs are a family of ion channels containing six transmembrane domains that form nonselective, non-voltage-gated cationic channels (36). Each of the four subtypes of TRPVs (TRPV1–4) is activated in a different temperature range (3, 12). In light of our earlier electrophysiological study in isolated rat vagal pulmonary sensory neurons (35), the increase in Ttr generated by HWA challenge in the present study is likely to activate more than one type of these TRPV channels. Among them, TRPV1, generally considered as a reliable biomarker for the C-fiber sensory nerves in rat lung (18, 23), accounts for approximately half of the total current evoked by a similar increase in temperature in isolated pulmonary sensory neurons (35).
A recent study conducted in our laboratory reported that hyperventilation with HWA triggered an immediate and reversible bronchoconstriction in patients with mild and stable asthma but not in healthy subjects (17). The degree and pattern of bronchoconstriction were similar to those observed in the Ova-sensitized rats in the present study. Accompanying the bronchoconstriction, breathing HWA also triggered coughs in these patients, suggesting an involvement of activation of airway sensory nerves (17). Although we hypothesized that the HWA-induced increase in Ttr activated bronchopulmonary C fibers, direct evidence could not be obtained in these patients. The bronchoconstriction in these patients was completely prevented by pretreatment with ipratropium, indicating a dominant role of cholinergic reflex, which is a sharp contrast to our finding in this study that pretreatment with atropine in the Ova-sensitized rats did not significantly diminish the HWA-induced response (Fig. 7A). Several factors are probably involved in causing this clear discrepancy. A major factor to be considered is the well-documented variance between different species (especially between large mammals and rodents) in the relative contributions of centrally mediated cholinergic and local tachykininergic mechanisms to the airway responses to C-fiber stimulation (8, 14, 21, 22), as described previously. In addition, in our earlier study of patients with asthma the airway responses to HWA were measured in awake subjects, whereas in the present study rats were anesthetized during the HWA challenge; it is well recognized that reflex responses mediated through the central nervous system are generally suppressed during anesthesia. Another contributing factor may be related to the differences in the nature, stage, and severity of the pathological features between this animal model of allergic asthma and the chronic disease of human asthma.
In conclusion, an increase in airway temperature within the physiological range evoked an immediate and sustained increase in airway resistance in Ova-sensitized rats but not in control rats. Tachykinins released from the vagal bronchopulmonary C-fiber endings in response to airway hyperthermia appear to be primarily responsible for generating this bronchoconstrictive effect.
GRANTS
This study was supported in part by National Heart, Lung, and Blood Institute Grant HL-96914 (L.-Y. Lee), US Department of Defense DMRDP/USAMRMC/TATRC Award W81XWH-10-2-0189 (L.-Y. Lee), and ROC NSC98-2917-I-038-101 Fellowship (C.-C. Hsu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.-C.H. and R.-L.L. performed experiments; C.-C.H. and R.-L.L. analyzed data; C.-C.H., R.-L.L., and Y.S.L. prepared figures; C.-C.H., Y.S.L., and L.-Y.L. drafted manuscript; Y.S.L. and L.-Y.L. interpreted results of experiments; Y.S.L. and L.-Y.L. edited and revised manuscript; L.-Y.L. conception and design of research; L.-Y.L. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Michelle Lim and Chayse Martin for their technical assistance.
REFERENCES
- 1.Baluk P, McDonald DM. The beta2-adrenergic receptor agonist formoterol reduces microvascular leakage by inhibiting endothelial gap formation. Am J Physiol Lung Cell Mol Physiol 266: L461–L468, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Barnes PJ, Lundberg JM. Airway neuropeptides and asthma. In: Asthma: Its Pathology and Treatment, edited by Kaliner MA, Barnes PJ, Persson CG. New York: Dekker, 1991 [Google Scholar]
- 3.Benham CD, Gunthorpe MJ, Davis JB. TRPV channels as temperature sensors. Cell Calcium 33: 479–487, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Bíró T, Maurer M, Modarres S, Lewin NE, Brodie C, Acs G, Acs P, Paus R, Blumberg PM. Characterization of functional vanilloid receptors expressed by mast cells. Blood 91: 1332–1340, 1998 [PubMed] [Google Scholar]
- 5.Bouchama A, Parhar RS, el-Yazigi A, Sheth K, al-Sedairy S. Endotoxemia and release of tumor necrosis factor and interleukin 1alpha in acute heatstroke. J Appl Physiol 70: 2640–2644, 1991 [DOI] [PubMed] [Google Scholar]
- 6.Bowden JJ, Sulakvelidze I, McDonald DM. Inhibition of neutrophil and eosinophil adhesion to venules of rat trachea by beta2-adrenergic agonist formoterol. J Appl Physiol 77: 397–405, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Brooks GA, Hittelman KJ, Faulkner JA, Beyer RE. Tissue temperatures and whole-animal oxygen consumption after exercise. Am J Physiol 221: 427–431, 1971 [DOI] [PubMed] [Google Scholar]
- 8.Canning BJ. Neurokinin3 receptor regulation of the airways. Vascul Pharmacol 45: 227–234, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Capaz FR, Ruffie C, Lefort J, Manzini S, Vargaftig BB, Pretolani M. Effect of active sensitization on the bronchopulmonary responses to tachykinins in the guinea pig. Modulation by peptidase inhibitors. J Pharmacol Exp Ther 266: 812–819, 1993 [PubMed] [Google Scholar]
- 10.Coleridge JC, Coleridge HM. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol 99: 1–110, 1984 [DOI] [PubMed] [Google Scholar]
- 11.Devillier P, Acker GM, Advenier C, Marsac J, Regoli D, Frossard N. Activation of an epithelial neurokinin NK-1 receptor induces relaxation of rat trachea through release of prostaglandin E2. J Pharmacol Exp Ther 263: 767–772, 1992 [PubMed] [Google Scholar]
- 12.Dhaka A, Viswanath V, Patapoutian A. Trp ion channels and temperature sensation. Annu Rev Neurosci 29: 135–161, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Elwood W, Lotvall JO, Barnes PJ, Chung KF. Characterization of allergen-induced bronchial hyperresponsiveness and airway inflammation in actively sensitized brown-Norway rats. J Allergy Clin Immunol 88: 951–960, 1991 [DOI] [PubMed] [Google Scholar]
- 14.Fahy JV, Wong HH, Geppetti P, Reis JM, Harris SC, Maclean DB, Nadel JA, Boushey HA. Effect of an NK1 receptor antagonist (CP-99,994) on hypertonic saline-induced bronchoconstriction and cough in male asthmatic subjects. Am J Respir Crit Care Med 152: 879–884, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Fischer A, McGregor GP, Saria A, Philippin B, Kummer W. Induction of tachykinin gene and peptide expression in guinea pig nodose primary afferent neurons by allergic airway inflammation. J Clin Invest 98: 2284–2291, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gourine AV, Rudolph K, Korsak AS, Kubatko J, Tesfaigzi J, Kozak W, Kluger MJ. Role of capsaicin-sensitive afferents in fever and cytokine responses during systemic and local inflammation in rats. Neuroimmunomodulation 9: 13–22, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Hayes D, Jr, Collins PB, Khosravi M, Lin RL, Lee LY. Bronchoconstriction triggered by breathing hot humid air in patients with asthma: role of cholinergic reflex. Am J Respir Crit Care Med 185: 1190–1196, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho CY, Gu Q, Lin YS, Lee LY. Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respir Physiol 127: 113–124, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Hsiue TR, Garland A, Ray DW, Hershenson MB, Leff AR, Solway J. Endogenous sensory neuropeptide release enhances nonspecific airway responsiveness in guinea pigs. Am Rev Respir Dis 146: 148–153, 1992 [DOI] [PubMed] [Google Scholar]
- 20.Hua XY, Theodorsson-Norheim E, Brodin E, Lundberg JM, Hokfelt T. Multiple tachykinins (neurokinin A, neuropeptide K and substance P) in capsaicin-sensitive sensory neurons in the guinea-pig. Regul Pept 13: 1–19, 1985 [DOI] [PubMed] [Google Scholar]
- 21.Joos GF, Germonpre PR, Pauwels RA. Role of tachykinins in asthma. Allergy 55: 321–337, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Joos GF, Kips JC, Peleman RA, Pauwels RA. Tachykinin antagonists and the airways. Arch Int Pharmacodyn Ther 329: 205–219, 1995 [PubMed] [Google Scholar]
- 23.Lee LY, Gu Q. Role of TRPV1 in inflammation-induced airway hypersensitivity. Curr Opin Pharmacol 9: 243–249, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee LY, Undem BJ. Bronchopulmonary vagal sensory nerves. In: Advances in Vagal Afferent Neurobiology, edited by Undem BJ, Weinreich D. Boca Raton, FL: CRC, 2005, p. 279–313 [Google Scholar]
- 25.Li PC, Shaw CF, Kuo TF, Chien CT. Inducible nitric oxide synthase evoked nitric oxide counteracts capsaicin-induced airway smooth muscle contraction, but exacerbates plasma extravasation. Neurosci Lett 378: 117–122, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Lin RL, Hayes D, Jr, Lee LY. Bronchoconstriction induced by hyperventilation with humidified hot air: role of TRPV1-expressing airway afferents. J Appl Physiol 106: 1917–1924, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin YJ, Lee LY. Hypersensitivity of bronchopulmonary C-fibers induced by an increase in airway temperature in ovalbumin (Ova)-sensitized Brown Norway rats (Abstract). FASEB J 27: 930.19, 2013. 23195032 [Google Scholar]
- 28.Lundberg JM, Saria A. Polypeptide-containing neurons in airway smooth muscle. Annu Rev Physiol 49: 557–572, 1987 [DOI] [PubMed] [Google Scholar]
- 29.Maggi CA. The mammalian tachykinin receptors. Gen Pharmacol 26: 911–944, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Maron MB, Wagner JA, Horvath SM. Thermoregulatory responses during competitive marathon running. J Appl Physiol 42: 909–914, 1977 [DOI] [PubMed] [Google Scholar]
- 31.McDonald DM. Neurogenic inflammation in the rat trachea. I. Changes in venules, leucocytes and epithelial cells. J Neurocytol 17: 583–603, 1988 [DOI] [PubMed] [Google Scholar]
- 32.McDonald DM, Bowden JJ, Baluk P, Bunnett NW. Neurogenic inflammation: a model for studying efferent actions of sensory nerves. Adv Exp Med Biol 410: 453–462, 1996 [PubMed] [Google Scholar]
- 33.McDonald DM, Mitchell RA, Gabella G, Haskell A. Neurogenic inflammation in the rat trachea. II. Identity and distribution of nerves mediating the increase in vascular permeability. J Neurocytol 17: 605–628, 1988 [DOI] [PubMed] [Google Scholar]
- 34.Myers AC, Kajekar R, Undem BJ. Allergic inflammation-induced neuropeptide production in rapidly adapting afferent nerves in guinea pig airways. Am J Physiol Lung Cell Mol Physiol 282: L775–L781, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Ni D, Gu Q, Hu HZ, Gao N, Zhu MX, Lee LY. Thermal sensitivity of isolated vagal pulmonary sensory neurons: role of transient receptor potential vanilloid receptors. Am J Physiol Regul Integr Comp Physiol 291: R541–R550, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev 87: 165–217, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Piacentini GL, Peroni D, Crestani E, Zardini F, Bodini A, Costella S, Boner AL. Exhaled air temperature in asthma: methods and relationship with markers of disease. Clin Exp Allergy 37: 415–419, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Planas ME, Rodriguez L, Sanchez S, Pol O, Puig MM. Pharmacological evidence for the involvement of the endogenous opioid system in the response to local inflammation in the rat paw. Pain 60: 67–71, 1995 [DOI] [PubMed] [Google Scholar]
- 39.Ruan T, Gu Q, Kou YR, Lee LY. Hyperthermia increases sensitivity of pulmonary C-fibre afferents in rats. J Physiol 565: 295–308, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheppard D, Rizk NW, Boushey HA, Bethel RA. Mechanism of cough and bronchoconstriction induced by distilled water aerosol. Am Rev Respir Dis 127: 691–694, 1983 [DOI] [PubMed] [Google Scholar]
- 41.Solway J, Leff AR. Sensory neuropeptides and airway function. J Appl Physiol 71: 2077–2087, 1991 [DOI] [PubMed] [Google Scholar]
- 42.Stokes AJ, Shimoda LM, Koblan-Huberson M, Adra CN, Turner H. A TRPV2-PKA signaling module for transduction of physical stimuli in mast cells. J Exp Med 200: 137–147, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szarek JL, Spurlock B. Antagonism of cholinergic nerve-mediated contractions by the sensory nerve inhibitory system in rat bronchi. J Appl Physiol 81: 260–265, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Szarek JL, Spurlock B, Gruetter CA, Lemke S. Substance P and capsaicin release prostaglandin E2 from rat intrapulmonary bronchi. Am J Physiol Lung Cell Mol Physiol 275: L1006–L1012, 1998 [DOI] [PubMed] [Google Scholar]
- 45.Yang XX, Powell WS, Xu LJ, Martin JG. Strain dependence of the airway response to dry-gas hyperpnea challenge in the rat. J Appl Physiol 86: 152–158, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Zhang G, Lin RL, Wiggers M, Snow DM, Lee LY. Altered expression of TRPV1 and sensitivity to capsaicin in pulmonary myelinated afferents following chronic airway inflammation in the rat. J Physiol 586: 5771–5786, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang G, Lin RL, Wiggers ME, Lee LY. Sensitizing effects of chronic exposure and acute inhalation of ovalbumin aerosol on pulmonary C fibers in rats. J Appl Physiol 105: 128–138, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]