Abstract
Preliminary evidence supports an association between obstructive sleep apnea (OSA) and thoracic aortic dilatation, although potential causative mechanisms are incompletely understood; these may include an increase in aortic wall transmural pressures, induced by obstructive apneas and hypopneas. In patients undergoing cardiac catheterization, mean blood pressure (MBP) in the thoracic aorta and esophageal pressure was simultaneously recorded by an indwelling aortic pigtail catheter and a balloon-tipped esophageal catheter in randomized order during: normal breathing, simulated obstructive hypopnea (inspiration through a threshold load), simulated obstructive apnea (Mueller maneuver), and end-expiratory central apnea. Aortic transmural pressure (aortic MBP minus esophageal pressure) was calculated. Ten patients with a median age (range) of 64 (46–75) yr were studied. Inspiration through a threshold load, Mueller maneuver, and end-expiratory central apnea was successfully performed and recorded in 10, 7, and 9 patients, respectively. The difference between aortic MBP and esophageal pressure (and thus the extra aortic dilatory force) was median (quartiles) +9.3 (5.4, 18.6) mmHg, P = 0.02 during inspiration through a threshold load, +16.3 (12.8, 19.4) mmHg, P = 0.02 during the Mueller maneuver, and +0.4 (−4.5, 4.8) mmHg, P = 0.80 during end-expiratory central apnea. Simulated obstructive apnea and hypopnea increase aortic wall dilatory transmural pressures because intra-aortic pressures fall less than esophageal pressures. Thus OSA may mechanically promote thoracic aortic dilatation and should be further investigated as a risk factor for the development or accelerated progression of thoracic aortic aneurysms.
Keywords: obstructive sleep apnea, aortic dilatation, transmural aortic pressure
obstructive sleep apnea (OSA) is characterized by repetitive episodes of upper airway collapse during sleep, concomitant oxygen desaturations, and sleep disruption. OSA is associated with a substantial risk of developing cardiovascular disease (10) and has been proven to be an independent risk factor for arterial hypertension (4). It has been postulated that repetitive intrathoracic pressure changes due to increased respiratory effort against partially (hypopnea) or completely (apnea) occluded upper airways may contribute to the development of cardiovascular disease by leading to extra radial forces on intrathoracic blood vessels, including the aorta (7). The potential underlying mechanism is thought to be that, during obstructive apnea, thoracic aortic blood pressure does not fall as much as intrathoracic pressure and therefore the extra distending transmural aortic pressure (mean aortic blood pressure minus intrathoracic pressure) increases. This attenuation of aortic pressure oscillations compared with intrathoracic oscillations would be predicted to occur because the cardiovascular system attempts to defend the peripheral perfusion of critical organs, i.e., by increases in stroke volume and effects of the baroreceptor on maintaining blood pressure. Thus, in OSA, hundreds of episodes of increased transmural aortic wall pressure every night may contribute to increased thoracic aortic size, and thus potentially promote thoracic aneurysms.
However, data in humans, investigating the acute physiological effects of OSA on transmural thoracic aortic pressures, are not available. Breathing through an inspiratory threshold load (simulating hypopnea) and the Mueller maneuver (involving a voluntary forced inspiration against an occluded airway simulating apnea) have been used to initiate sudden changes of pleural pressure and study physiological effects on hemodynamics and heart function (2, 9, 12, 16, 17).
In 20 healthy volunteers, simulated obstructive apnea and hypopnea led to an acute increase in proximal aortic root diameter assessed by echocardiography (16). In this study, the difference between mean peripheral blood pressure (−10 mmHg) and mouth pressure, during inspiration through a threshold load (−30 mmHg), was ∼20 mmHg, suggesting that aortic transmural pressure would, in theory, increase by this amount during simulated obstructive hypopnea (16). However, this was merely assumed from the indirect observations on peripheral blood pressure and mouth pressure changes.
The present invasive examinations quantitate simultaneous changes in esophageal pressure (as a surrogate of intrapleural/intrathoracic pressure) and mean thoracic aortic blood pressure during simulated apnea and hypopnea to determine the magnitude of transmural aortic pressure changes in patients referred for cardiac catheterization. We investigated the hypothesis that simulated obstructive apnea and hypopnea induce a fall in aortic blood pressure that is less than the simultaneous fall in esophageal pressure and therefore increases transmural aortic wall pressures thereby potentially promoting thoracic aortic dilatation.
METHODS
Subjects
This study was conducted at the cardiac catheterization laboratory of the University Hospital of Zurich, Switzerland. Subjects were eligible if they were between 18 and 75 yr old and excluded if previously diagnosed with OSA, aortic disease, or psychiatric illness precluding informed consent. The study was approved by the University of Zurich research ethics committee (EK-1672). Written informed consent was obtained from all participants.
Measurements
Anthropometrics.
Weight, height, waist, and hip circumference were measured in all participants.
Breathing maneuvers.
All maneuvers were performed with the patients lying supine immediately following cardiac catheterization. Before the measurements, participants were instructed in the performance of the different breathing maneuvers. A nose clip was placed before the maneuvers. To generate an inspiratory threshold load, a negative pressure valve was incorporated into the mouthpiece. This valve contained an inspiratory resistance that could not be overcome unless a threshold pressure of −30 mmHg was generated at the mouth. For the Mueller maneuver, an occluded mouthpiece was used, with a small air leak to prevent complete closure of the glottis during the maneuver. At the end of expiration, inspiration was carried out against the mouthpiece. Mean thoracic aortic blood pressure and esophageal pressure were measured in randomized order during 1) steady-state normal breathing, 2) inspiration through the inspiratory threshold load, 3) Mueller maneuver, and 4) expiratory apnea (without respiratory effort). The patients were instructed to perform each maneuver for 20 s with breaks of at least 1 min between each maneuver.
Mean thoracic aortic blood pressure.
Room temperature and lighting were set at the same level for all measurements. The participants first underwent left heart cardiac catheterization. At the end of cardiac catheterization, the femoral arterial access was used to place an indwelling Pigtail catheter in the thoracic aorta. The reference level for blood pressure measurement was the midaxillary line in the supine patient. The tip of the catheter was placed under radiographic control at the level of the tracheal bifurcation, connected to a pressure transducer (Infinity HemoMed Pod; Draeger Medical Switzerland), and linked to a corresponding patient monitor (Draeger infinity delta; Draeger Medical Switzerland). Pressure was sampled at a rate of 1/s with a pressure sensitivity of 5 mV·V−1·mmHg−1.
Esophageal pressure measurements.
A balloon-tipped esophageal catheter inflated with 0.5–1.0 ml of air (Microtek Medical, Zutphen, Netherlands) was used for the measurement of esophageal pressure. Pressure calibration was performed with a pressure transducer tester (Veri-Cal; Utah Medical Products). Before the measuring device was introduced into the esophagus, a topical anesthetic (viscous lidocaine 4%) was administered in the nostrils. Furthermore, the topical anesthetic was applied to the balloon of the catheter. Thereafter, the catheter was carefully inserted through the nostril, and the patient was asked to drink water through a straw followed by further insertion of the catheter. After deep insertion, the catheter was slowly withdrawn from the stomach until a negative pressure deflection during inspiration could be identified. Next, the catheter was withdrawn 10 cm for correct positioning in the middle esophagus, usually at a distance of 35–45 cm from the nares, and fixed by tape. At its final position, the pressure recorded had to be negative on inspiration and positive on expiration.
Data Analysis
To analyze changes of mean aortic blood pressure and esophageal pressure induced by the respiratory maneuvers, we recorded 10 s immediately before (baseline) the maneuver, 20 s during the maneuver, and the 20 s immediately after termination of the maneuver. The maximum change in pressure induced by the different breathing maneuvers, compared with normal breathing, was used for further analysis.
All values are presented as medians unless otherwise stated. All statistical analyses were performed with Statistica version 8.0 (StatSoft, Tulsa, OK). Statistical significance of pressure changes induced by simulated maneuvers was explored by nonparametric testing (Wilcoxon matched-pairs test). Statistical significance was assumed at P < 0.05.
RESULTS
Subjects
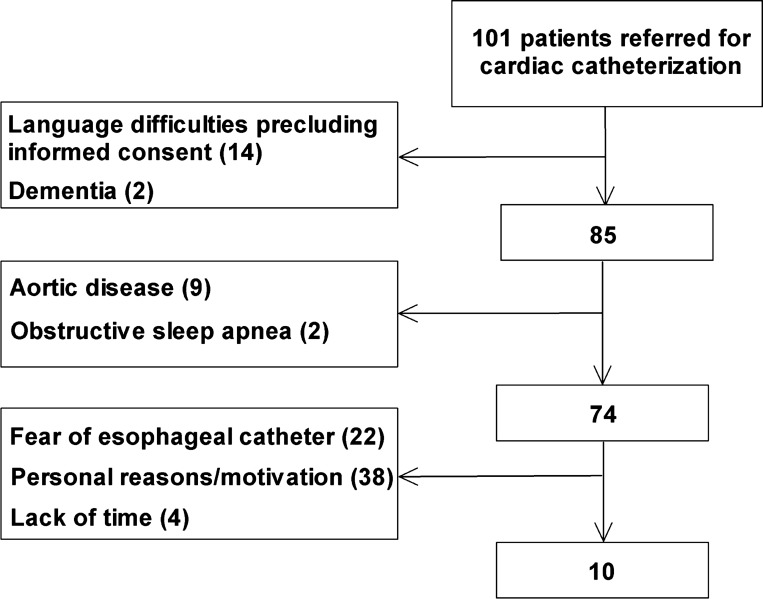
One hundred one patients were screened and asked to participate in the study. The study flow is displayed in Fig. 1. Ten male patients were finally included in the study, and inspiration through a threshold load, Mueller maneuver, and end-expiratory central apnea was successfully performed and recorded in 10, 7, and 9 patients, respectively.
Fig. 1.
Study flow.
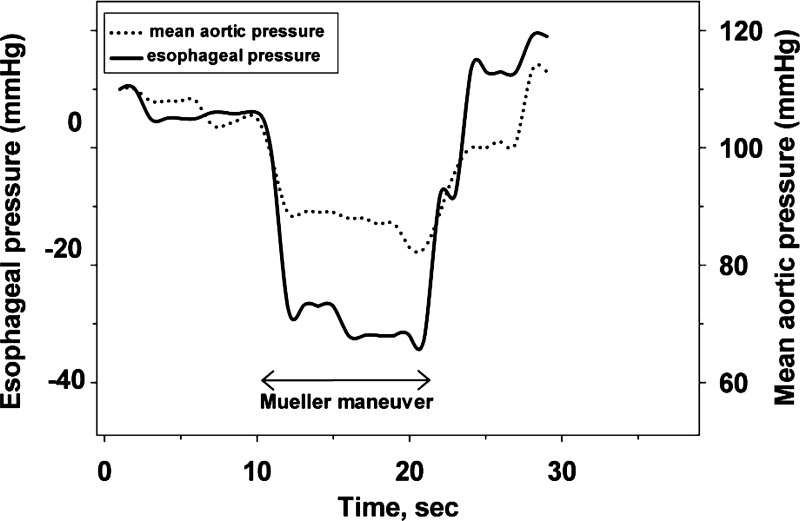
The characteristics of the participants are shown in Table 1. Cardiac catheterization revealed coronary artery disease in nine patients and cardiomyopathy in one patient. Typical changes in mean aortic blood pressure and esophageal pressure induced by a Mueller maneuver in a patient are illustrated in Fig. 2. Note that, with the onset of the Mueller maneuver, the fall in aortic pressure was lower than the concomitant fall in esophageal pressure, indicating an increase in transmural aortic wall pressure.
Table 1.
Patient characteristics
| Subjects (n = 10) | |
|---|---|
| Clinical characteristics | |
| Age, yr | 64 (46–75) |
| Body mass index, kg/m2 | 28.3 (23.9–34.8) |
| Waist-to-hip ratio | 1.03 (0.96–1.09) |
| Systolic blood pressure, mmHg | 136 (123–154) |
| Diastolic blood pressure, mmHg | 80 (65–100) |
| Pulse rate, 1/min | 71 (56–84) |
| Left ventricular ejection fraction, % | 59 (30–65) |
| Arterial hypertension, N | 6 |
| Diabetes, N | 4 |
| Medication | |
| ACE-inhibitor, N | 7 |
| β-Blocker, N | 6 |
| Calcium antagonist, N | 2 |
| Statin, N | 7 |
Values are medians (range) or absolute values (N).
Fig. 2.
Mean aortic blood pressure and esophageal pressure before, during, and after performing the Mueller maneuver to simulate obstructive apnea in a representative subject. With onset of the Mueller maneuver, the decrease in esophageal pressure was greater than the concomitant fall in mean thoracic aortic blood pressure, indicating an increase in dilating aortic wall pressure.
Effects of Simulated Apnea and Hypopnea on Esophageal Pressure
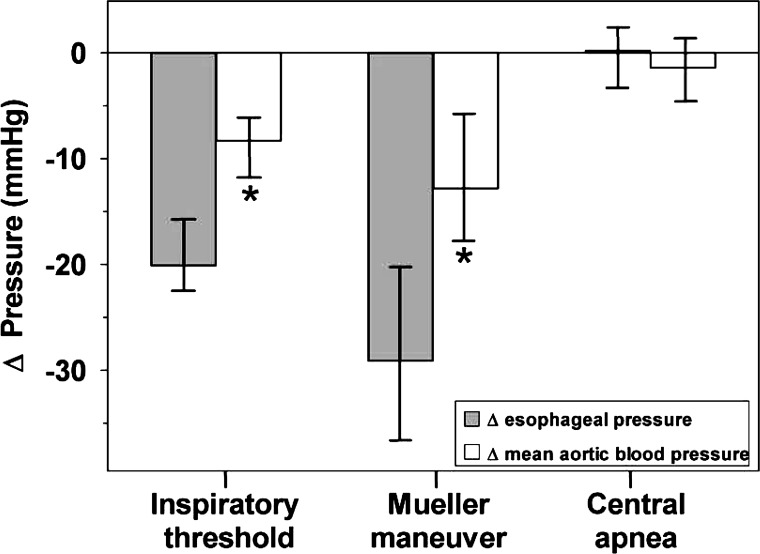
Compared with normal breathing, the esophageal pressure median (quartiles) decreased significantly during inspiration through a threshold load [−20.1 (−21.9, −15.0) mmHg, P < 0.01], during the Mueller maneuver [−29.1 (−38.4, −20.6) mmHg, P = 0.02], but not during end-expiratory central apnea [0.1 (−2.9, 2.0) mmHg, P = 0.88]. The changes compared with normal breathing are illustrated in Fig. 3.
Fig. 3.
Median changes (quartiles) of esophageal pressure and mean aortic blood pressure compared with normal breathing during inspiration through a threshold load, the Mueller maneuver, and end-expiratory central apnea. *P < 0.05 vs. Δesophageal pressure.
Effects of Simulated Apnea and Hypopnea on Mean Aortic Blood Pressure
Mean thoracic aortic blood pressure fell significantly during inspiration through a threshold load [−8.3 (−12.3, −6.4) mmHg, P < 0.01] and during the Mueller maneuver [−12.8 (−17.5, −5.4) mmHg, P = 0.02], whereas there was no significant change in mean aortic blood pressure induced by simulated end-expiratory central apnea.
Effects of Simulated Apnea and Hypopnea on Transmural Aortic Pressure
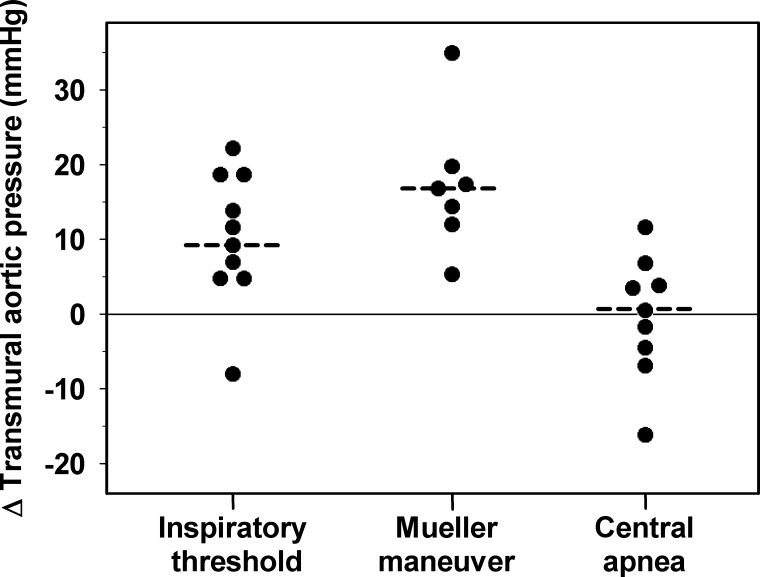
As demonstrated in Fig. 3, the decrease in esophageal pressure was greater than the concomitant decrease in mean aortic blood pressure, indicating an increase in transmural aortic pressure. Individual changes from baseline of transmural aortic pressure during the breathing maneuvers are presented in Fig. 4. The median difference of changes (quartiles) between mean thoracic aortic blood pressure and esophageal pressure (and thus the extra aortic dilatory force) increased significantly during simulated obstructive hypopnea [+9.3 (5.4, 18.6) mmHg, P = 0.02] and apnea [+16.3 (12.8, 19.4) mmHg, P = 0.02] but remained almost unchanged during simulated central apnea [+0.4 (−4.5, 4.8) mmHg, P = 0.80].
Fig. 4.
Individual changes of transmural aortic pressure during inspiration through a threshold load, the Mueller maneuver, and end-expiratory central apnea. The medians are indicated by the dashed lines.
DISCUSSION
We found that simulated apnea and hypopnea were associated with a fall in mean thoracic aortic blood pressure that was significantly lower than the concomitant fall in esophageal pressure, indicating an increase in transmural aortic wall pressure, and thus an extra dilatory force on the thoracic aorta. This finding is of clinical importance because the increase in transmural aortic wall pressure, potentially recurring several hundred times night after night in patients with OSA, may be one underlying factor for accelerated thoracic aortic dilatation.
Data in humans on the acute physiological effects of OSA on transmural aortic pressure are currently not available. However, in an animal model, Peters et al. experimentally induced obstructive apnea in anesthetized dogs that led to distension (increase in diameter) of the intrathoracic aorta of about 6.5% (13, 14). To our knowledge, only one study performed esophageal and aortic measurements to investigate effects of the Mueller maneuver in humans (9). In three subjects, esophageal pressure, aortic systolic pressure, and pulmonary wedge pressure were measured during normal breathing and the Mueller maneuver (9). During the Mueller maneuver, the investigators observed an increase in transmural aortic pressure indicating an increase of dilatatory force (9).
In a limited number of previous studies in humans, it has been hypothesized that OSA might be one underlying factor for accelerated aortic dilatation. Approximately one-third of patients with the Marfan syndrome, an inherited disorder that affects the connective tissue, had evidence for OSA (5, 6). Event-free survival was significantly shorter in Marfan patients with OSA compared with patients without OSA, suggesting that OSA may be a risk factor for aortic root dilatation (6).
In a cross-sectional study reported by Serizawa et al. (15), 150 consecutive patients referred to the sleep clinic underwent respiratory polygraphy, and thoracic aortic diameter was measured from chest computed tomography. In multivariate analysis, AHI, male gender, and age were independent factors positively associated with thoracic aortic diameter. The authors hypothesized that apnea-induced repetitive increases in transmural pressures might have been responsible for their observations. Baguet et al. (1) assessed aortic root size by cardiac ultrasound in 156 patients with OSA but without cardiovascular disease. In univariate analysis, aortic root size was increased in those OSA patients with lower mean nocturnal oxygen saturation and associated with older age and higher diastolic blood pressure. In the study by Lee and colleagues (8), 94 patients admitted for ST elevation myocardial infarction were evaluated by echocardiography and polysomnography, and 64 patients had evidence for OSA. Patients with increased aortic root size were older, had a higher BMI and blood pressure, and were more likely to have OSA. In contrast to the study by Serizawa et al. (15), the latter studies (1, 8) suggest that increased blood pressure may be the major mechanism contributing to thoracic aortic dilatation, since OSA was not related to aortic size after adjustment of covariates. However, comparison of these studies on aortic size in OSA (1, 8, 15) is hampered by marked differences in the studied populations.
In a recent study from Stoewhas et al. (16), it was reported that simulated obstructive hypopnea and apnea both induced significant surges in continuously measured peripheral blood pressure, and simulated obstructive hypopnea lead to an increase in proximal aortic diameter, as assessed by echocardiography. In addition to the preliminary findings by Stoewhas and colleagues that relied on changes in diameter assessed by echocardiography (16), the present invasive experimental set-up allowed direct quantification of intrathoracic and intra-aortic pressure changes. Because it is difficult to detect subtle changes in aortic diameter by echocardiographic measurement during simulated apnea and hypopnea, the current invasive investigations avoid the inaccuracies of measurement that limited the interpretation of the findings by Stoewhas et al. (16).
In addition to the discussed observations concerning the thoracic aorta, a recent observational study demonstrated a high prevalence of OSA in patients with abdominal aortic aneurysms, and OSA severity seemed to be an independent predictor for abdominal aneurysm expansion (11). However, potential mechanisms underlying the evolution of thoracic aortic expansion may not be the same as those influencing the evolution of abdominal aneurysms, because it is unlikely that the same pressure changes induced by apneas are experienced below and above the diaphragm. Although our study was not designed to identify mechanisms other than thoracic pressure surges, additional mechanisms of aneurysm expansion will have to be taken into account in future studies examining the evolution of thoracic and abdominal aneurysms. Furthermore, the known variation in physical structure and elastin/collagen content may contribute to the differences observed in the pathogenesis of thoracic and abdominal aortic aneurysms (3).
One limitation of our study is that no sleep recordings were performed to exclude OSA in the studied population. However, whether experimentally simulated apnea and hypopnea have a different effect on aortic wall transmural pressure in subjects with OSA compared with subjects without OSA is unknown and will have to be investigated in future studies.
In conclusion, the present study has confirmed the initial hypothesis that simulated OSA leads to acute increases in transmural aortic wall pressure, and thus may lead to aortic dilatation in the longer term. Further studies are needed to investigate the effects of sleep-disordered breathing on transmural aortic pressure to define the role in the pathogenesis of aortic dilatation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: C.F.C., C.W., J.R.S., and M.K. conception and design of research; C.F.C., G.C., N.A.S., C.W., and M.K. performed experiments; C.F.C., G.C., N.A.S., C.W., J.R.S., and M.K. analyzed data; C.F.C., G.C., N.A.S., C.W., J.R.S., and M.K. interpreted results of experiments; C.F.C. and M.K. prepared figures; C.F.C., G.C., N.A.S., C.W., J.R.S., and M.K. drafted manuscript; C.F.C., G.C., N.A.S., C.W., J.R.S., and M.K. edited and revised manuscript; C.F.C., G.C., N.A.S., C.W., J.R.S., and M.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Thomas Hafner, Draeger Medical Switzerland, for support with the experimental set-up.
REFERENCES
- 1.Baguet JP, Minville C, Tamisier R, Roche F, Barone-Rochette G, Ormezzano O, Levy P, Pepin JL. Increased aortic root size is associated with nocturnal hypoxia and diastolic blood pressure in obstructive sleep apnea. Sleep 34: 1605–1607, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen RC, Que CL, Yan S. Introduction to a new inspiratory threshold loading device. Eur Respir J 12: 208–211, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Guo DC, Papke CL, He R, Milewicz DM. Pathogenesis of thoracic and abdominal aortic aneurysms. Ann NY Acad Sci 1085: 339–352, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Haentjens P, Van Meerhaeghe A, Moscariello A, De Weerdt S, Poppe K, Dupont A, Velkeniers B. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med 167: 757–764, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Kohler M, Blair E, Risby P, Nickol AH, Wordsworth P, Forfar C, Stradling JR. The prevalence of obstructive sleep apnoea and its association with aortic dilatation in Marfan's syndrome. Thorax 64: 162–166, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Kohler M, Pitcher A, Blair E, Risby P, Senn O, Forfar C, Wordsworth P, Stradling JR. The impact of obstructive sleep apnea on aortic disease in Marfan's Syndrome. Respiration (September 20, 2012). 10.1159/000340008 [DOI] [PubMed] [Google Scholar]
- 7.Kohler M, Stradling JR. Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol 7: 677–685, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Lee LC, Torres MC, Khoo SM, Chong EY, Lau C, Than Y, Shi DX, Kailasam A, Poh KK, Lee CH, Yeo TC. The relative impact of obstructive sleep apnea and hypertension on the structural and functional changes of the thoracic aorta. Sleep 33: 1173–1176, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magder SA, Lichtenstein S, Adelman AG. Effect of negative pleural pressure on left ventricular hemodynamics. Am J Cardiol 52: 588–593, 1983 [DOI] [PubMed] [Google Scholar]
- 10.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 365: 1046–1053, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Mason RH, Ruegg G, Perkins J, Hardinge M, Amann-Vesti B, Senn O, Stradling JR, Kohler M. Obstructive sleep apnea in patients with abdominal aortic aneurysms: highly prevalent and associated with aneurysm expansion. Am J Respir Crit Care Med 183: 668–674, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Orban M, Bruce CJ, Pressman GS, Leinveber P, Romero-Corral A, Korinek J, Konecny T, Villarraga HR, Kara T, Caples SM, Somers VK. Dynamic changes of left ventricular performance and left atrial volume induced by the mueller maneuver in healthy young adults and implications for obstructive sleep apnea, atrial fibrillation, and heart failure. Am J Cardiol 102: 1557–1561, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters J, Kindred MK, Robotham JL. Transient analysis of cardiopulmonary interactions. I Diastolic events J Appl Physiol 64: 1506–1517, 1988 [DOI] [PubMed] [Google Scholar]
- 14.Peters J, Kindred MK, Robotham JL. Transient analysis of cardiopulmonary interactions. II Systolic events J Appl Physiol 64: 1518–1526, 1988 [DOI] [PubMed] [Google Scholar]
- 15.Serizawa N, Yumino D, Takagi A, Gomita K, Kajimoto K, Tsurumi Y, Hagiwara N. Obstructive sleep apnea is associated with greater thoracic aortic size. J Am Coll Cardiol 52: 885–886, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Stoewhas AC, Namdar M, Biaggi P, Russi EW, Bloch KE, Stradling JR, Kohler M. The effect of simulated obstructive apnea and hypopnea on aortic diameter and BP. Chest 140: 675–680, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Warley AR, Fontes F, Wilson M, Raine AE, Stradling JR. Lack of effect of an inspiratory threshold load on plasma atrial natriuretic peptide levels. Clin Sci (Lond) 78: 311–313, 1990 [DOI] [PubMed] [Google Scholar]