Abstract
We examined the neuroprotective and neurotrophic effects of Tremella fuciformis. The neurotrophic effects of the hot water extract of T. fuciformis was evaluated by microscopically monitoring its potency to induce neurite outgrowth in PC12h cells. The hot water extract of T. fuciformis promoted neurite outgrowth in PC12h cells in this study, superior to other natural substances which was reported previously. When cells were treated with the hot water extract of T. fuciformis prior to β-amyloid peptide treatment (active domain of A peptide 25~35 treated), toxicity was significantly diminished (p<0.01). These results suggest that T. fuciformis might potentially be used as a precautionary agent in neurodegenerative disease, such as Alzheimer's disease, etc.
Keywords: Neuroprotective effect, Neurotrophic effect, PC12h cell, Tremella fuciformis
Alzheimer's disease (AD) is an age-related progressive neurodegenerative disorder. It is the most common form of dementia affecting approximately 5% of adults over 65 years. There are currently more than 4 million AD patients in the USA, with AD having the 4th highest mortality rate. Patients with AD are expected to increase by 15 million in the next ten years. In Korea, the growing senior population now accounts for 7 percent of the population, with the prevalence of AD growing accordingly (Suh, 1992).
As a result, the economical and social problems related to AD are expected to grow dramatically in the future. Therefore much global effort has been made to find compounds with preventative or therapeutic effects toward AD. Through these efforts, nerve growth factor (NGF), a product having a therapeutic effect on AD, was characterized representatively (Brinton and Yamazaki, 1998; Fukunaga and Miyamoto, 1998). However, the NGF exposed a number of problems in clinical study, such as delivery, a short half-life and poor penetration through the blood brain barrier. Therefore, the search for new compounds with neurotrophic activity has been going continuously. (Lee et al., 2002; Jinghua et al., 2000; Hur et al., 2001; Yamazaki et al., 1996).
In recent study, Liu et al. (2003) reported the neuritogenic effect of the alkaloids obtained from Codonopsis pilosula (Franch) Nannf and Kawagishi et al. (1991) also reported the Hericenone group which stimulate the synthesis of NGF was derived from Hericium erinaceum.
β-Amyloid protein, a 40~42 amino acid peptide proteolytically derived from a larger β-amyloid precursor protein, assembles into insoluble aggregates forming plaques and then accumulate as insoluble extracellular deposits in the senile plaques of AD patients. It is generally accepted that the β-amyloid peptide is potentially important role for in the pathogenesis of AD. The molecular mechanisms of β-amyloid peptide cytotoxicity are also closely involved in the generation of oxidative stress (Goodman and Mattson, 1994).
Species of Tremella belong to the so-called jelly mushrooms, which form gelations fruit bodies. Tremella fuciformis, belonging to the order of the Tremellales and the family of the Tremellaceae, has been appreciated as an edible mushroom. Especially the fruit body of T. fuciformis is a common food and traditional drug used clinically in China as a tonic (Li, 1973). In recent years, the chemical structure of the polysaccharides obtained from fruit body of T. fuciformis has been found. It is built up of α-(1→3)-linked D-mannan backbone chain to which β-(1→2)-linked D-xylose residues are attached at the C-2 position (Kakuta et al., 1979; Yui et al., 1995; De Baets and Vandamme, 2001). The polysaccharide fractions from fruit body of T. fuciformis displayed many physiological activities. Gao et al. (1996a, 1996b) reported that it has the ability to induce human monocytes to produce interleukines (IL-1 and IL-6) and tumor necrosis factor in vitro. Together with this immunomodulatory effect, the polysaccharide fraction composed of acidic and neutral heteroglycans has several pharmacological activites, such as hypoglycemic effect (Kiho et al., 1994), anti-tumor activity (Ukai et al., 1972; Gao et al., 1997) and hypocholesterolemic activity (Cheung, 1996).
Therefore, in this study to confirm usefulness as a precautionary agent for a new functional food on Alzheimr's disease of T. fuciformis extract, the hot water extract of T. fuciformis was tested for nerve growth factor (NGF)-like activity and protection form neurotoxicity induced by β-amyloide protein in PC12h cells.
Materials and Methods
Materials
Nerve growth factor (NGF, 7S, isolated from mouse submandibular gland) was obtained from Sigma Chemical Co. (St. Louis, MO, USA) and dissolved in phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin. Dulbecco's modified minimum essential medium (DMEM) was purchased from Nissui (Tokyo, Japan), and horse serum, fetal calf serum and kanamycin from Gibco BRL (Grand Island, N.Y. U.S.A). Aβ 25~35 and all other chemicals were obtained from Sigma Chemical Co. (St. Louis. Mo. USA).
Preparation of hot water extract of T. fuciformis
The fruiting body of T. fuciformis was imported form Fujian Province, China. The dried fruiting body (10 g) was ground into powder and then the fifty volumes of distilled water was poured in precipitate and extracted at 100℃ for 12 hrs. The liquid residue was separated by centrifuge at 3,000 rpm for 10 min. After lyophilizing the filtrate, approximately 1.5 g of the water extracts of fruiting body of T. fuciformis were obtained.
Cell culture
PC12h cells, a subclone of PC12 cells isolated by Dr. Hatanaka (Hatanaka, 1981) and kindly donated by Dr. Chiba (Hokuriku University, Japan), were grown in DMEM medium supplemented with 5% (v/v) horse serum and 5% (v/v) fetal bovine serum in a 100 mm petri dish under 10% CO2 at 37℃.
PC12h cells also undergo certain NGF-responsive cellular events, including neurite outgrowth and the induction of tyrosine hydroxylase activity. PC12h cells are much more sensitive to NGF than PC12 cells (Hatanaka, 1983).
Assay for neuritogenic activity in PC12h cells
For morphological studies, cells were plated in 35 mm culture dishes coated with collagen (Type I, Sigma, U.S.A.) at a density of 5×104 cells in 2 ml medium per dish. After 24 hrs of culture, the medium was replaced with serumfree DMEM/Ham's F12 (1 : 1) medium supplemented with sodium selenate, transferrin, insulin, progesterone, and the vehicle or test compound. After 48 hrs, the neuritogenic activity was evaluated by measuring the length of the longest neurite of individual cells using an image processor system (Leica Qwin, Germany) attached to a phase-contrast microscope. One hundred cells in at least 10 random fields in two culture dishes were measured, with the values averaged.
Anti neurotoxicity induced by β-amyloid protein of T. fuciformis in PC12h cells
Cells were plated in 96 well plates coated with collagen (Type I, Sigma, U.S.A.) at a density of 1 × 104 cells per 0.1 ml growth medium. After 24 hrs of culture, the cells were exposed to low-serum containing media (0.5% horse serum and 0.5% fetal bovine serum) for 24 hrs, either with or without the test compound (the hot water extract of T. fuciformis). The test compound was used at concentrations range of 0.1~100 µg/ml.
5 µM of β-amyloid peptide 25~35 (diluted in phosphate buffered saline) was added to the culture media, which was then incubated for 60 hrs. The viability of PC12h cells was determined using the MTT assay. To assess cell viability, β-amyloid peptide 25~35 containing media was aspirated, and the PC12h cells were incubated with 3-[4, 5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT, 1 mg/ml) for 4 hrs at 37℃. The MTT solution was then aspirated, and the formazan (MTT reduction product) was dissolved in 0.1 ml of dimethyl sulfoxide and quantified spectrophotometrically at 570 nm (reference 650 nm). The viability of the living cells, from three independent experiments, was expressed as a percentage of the control.
Statistical analysis
The data were analyzed using analysis of variance (ANOVA) with the SAS statistical program and differences among the means were compared using Ducan's multiple range tests. All results were expressed as the mean ± S.E. of triplicate determination, with each experiment repeated 3 times.
Results and Discussion
Neuritogenic and neuroprotective effect of T. fuciformis
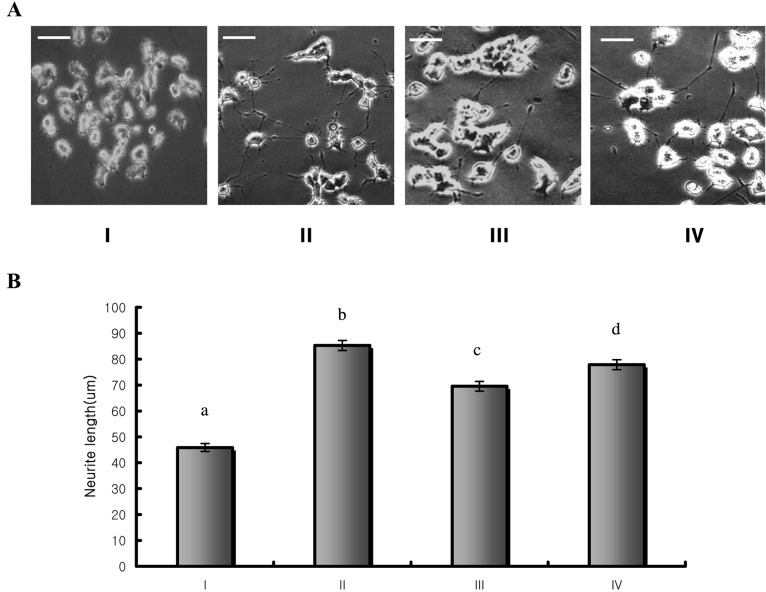
NGF has been reported to induce morphological changes in PC12h cells by improving neurite outgrowth (Hatanaka, 1983). NGF was shown to improve the differentiation of PC12h cells in a dose-dependent manner, therefore longer neurite outgrowth was observed as the treatment dose increased (Fig. 1). After treatment of PC12h cells with the hot water extract of T. fuciformis at a concentration range of 0.1 and 1 µg/ml for 48 hrs, the longest neurite length of each cell was measured. The average neurite length of cells treated with the test compounds are 69.51 ± 2.3 µm and 77.84 ± 0.92 µm respectively (Fig. 2). They were significant increase in differentiation (p < 0.01) compared to the neurite length of the control. When we treated with the hot water extract of T. fuciformis less than concentration of 0.1 µg/ml on PC12h cells, there was no significant effect (Data not shown). In present study, we investigated the neuritogenic effect of T. fuciformis on PC12h cells. The hot water extract of T. fuciformis exerts neuronal differentiation activity in PC12h cells by inducing neurite outgrowth at low concentration (1 µg/ml) compared to other natural substances, such as Angelica gigas, Glycyrrhiza uralensis or Lycium chinese (Data not shown).
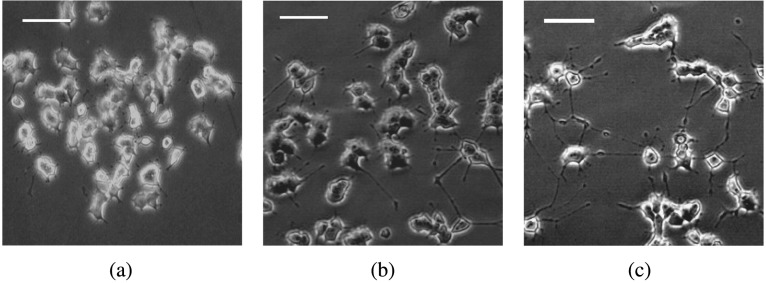
Fig. 1.
Neuritogenesis induced by NGF in PC12h cells (× 200). Scale bar = 50 µm. (a) Control (negative vehicle); (b) NGF 10 ng/ml; (c) NGF 100 ng/ml.
We reported the neuritogenic activity of genipin containing fraction from Korean gardenia fruit previously (Park et al., 2006). When PC12h cells were treated with 5 µg/ml of genipin containing fraction from Korean gardenia fruit, we observed similar neuritogenic activity compared to 1 µg/ml of hot water extract of T. fuciformis (Data not shown).
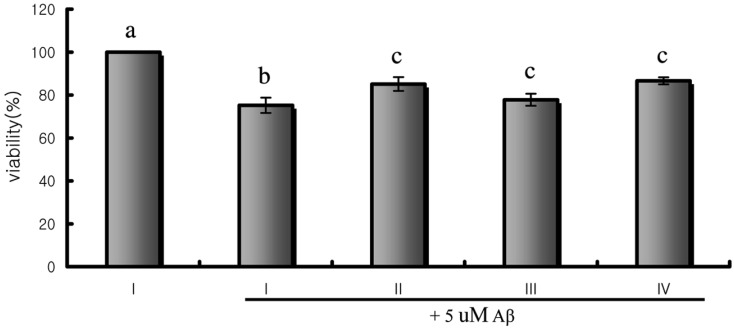
When PC12h cells were exposed to β-amyloid peptide 25~35 for 60 hrs, the MTT reducing activity was significantly inhibited. Pretreatment of cells with 0.1~100 µg/ml of the hot water extract of T. fuciformis for 24 hrs significantly reduced the degree of β-amyloid peptide-induced inhibition of MTT reduction compared to the non-treated control at concentrations of 100, 10 and 1 µg/ml (Fig. 3). As mentioned above, the oxidative stress induced by β-amyloid was found to be one of the major causes of AD pathology. Therefore the free radical scavengers and antioxidants can reduce the β-amyloid induced neurotoxicity on neuronal cells. Tsai et al. (2003) reported the superoxide-scavenging ability of aqueous extract of Tremella fuciformis.
Fig. 3.
Protective effects of the hot water extract of T. fuciformis on Aβ protein-induced cytotoxicity in PC12h cells. Cells were pretreated with or without various concentrations (1~100 µg/ml) of the hot water extract of T. fuciformis for 24 hrs and then incubated with Aβ protein (5 uM) for 60 hrs. Cell viability was assessed by MTT reduction assay. I) Control (negative vehicle); II) Hot water extract of T. fuciformis 100 µg/ml; III) Hot water extract of T. fuciformis 10 µg/ml; IV) Hot water extract of T. fuciformis 1 µg/ml. The data are the means ± S.E. of three separate experiments performed in triplicate. Means with different letters are significantly different (p < 0.01).
These results suggest that T. fuciformis might potentially be used as a precautionary agent in neurodegenerative disease, such as Alzheimer's disease, etc. However, further studies are required to determine the mechanism by which compound induce neurite extension and inhibit Aβ toxicity in PC12h cells.
Fig. 2.
T. fuciformis induced neuronal differentiation in PC12h cells. (A) Morphological changes induced by NGF and hot water extract of T. fuciformis in PC12h cells. Scale bar = 50 µm. (B) Effect of the hot water extract of T. fuciformis on PC12h cells neuritogenesis (n = 150). The neurite length was measured as described in the materials and methods. Means with different letters are significantly different (p < 0.01). Cells were cultured with vehicle (nc), 0.1 µg/ml of NGF or two concentrations of test compound (0.1 and 1 µg/ml) for 48 hrs. I) Control (negative vehicle); II) NGF 0.1 µg/ml; III) Hot water extract of T. fuciformis 0.1 µg/ml; IV) Hot water extract of T. fuciformis 1 µg/ml.
References
- 1.Diaz Brinton R, Yamazaki RS. Advances and challenges in the prevention and treatment of Alzheimer's disease. Pharm Res. 1998;15:386–398. doi: 10.1023/a:1011963929012. [DOI] [PubMed] [Google Scholar]
- 2.Cheung PCK. The hypocholesterolemic effect of two edible mushroom: Auricularia auricular (tree-ear) and Tremella fuciformis (white jelly-leaf) in hypercholesterolemic rats. Nutr Res. 1996;16:1721–1725. [Google Scholar]
- 3.De Baets S, Vandamme EJ. Extrcellular Tremella polysacchardies: structure, properties and applications. Biotechnol Lett. 2001;23:1361–1366. [Google Scholar]
- 4.Fukunaga K, Miyamoto E. Role of MAP kinase in neurons. Mol Neurobiol. 1998;16:79–95. doi: 10.1007/BF02740604. [DOI] [PubMed] [Google Scholar]
- 5.Gao QP, Jiang R, Chen H, Jensen E, Seljejid R. Characterization and cytokine stimulating activities of heteroglycans form Tremella fuciformis. Planta Med. 1996a;62:297–302. doi: 10.1055/s-2006-957888. [DOI] [PubMed] [Google Scholar]
- 6.Gao Q, Seljejid R, Chen H, Jiang R. Characterisation of acidic heterolycans from Tremella fuciformis Berk with cytokine stimulating activity. Carbohydr Res. 1996b;288:135–142. doi: 10.1016/s0008-6215(96)90789-2. [DOI] [PubMed] [Google Scholar]
- 7.Gao Q, Killie MK, Chen H, Jiang R, Seljejid R. Characterization and cytokine-stimulating activities of acidic heteroglycans form Tremella fuciformis. Planta Med. 1997;63:457–460. doi: 10.1055/s-2006-957733. [DOI] [PubMed] [Google Scholar]
- 8.Goodman Y, Mattson MP. Secreted forms of β-amyloid precursor protein protect hippocampal neurons against amyloid β-peptided induced oxidative injury. Exp Neurol. 1994;128:1–12. doi: 10.1006/exnr.1994.1107. [DOI] [PubMed] [Google Scholar]
- 9.Hatanaka H. Nerve growth factor-mediated stimulation of tyrosine hydroxylase activity in clonal rat pheochromocytoma cell line. Brain Res. 1981;222:225–233. doi: 10.1016/0006-8993(81)91029-5. [DOI] [PubMed] [Google Scholar]
- 10.Hatanaka H. Nerve growth factor-mediated differentiation of a nerve cell line cultured in a hormone-supplementd serum-free medium. Dev Brain Res. 1983;6:243–250. doi: 10.1016/0165-3806(83)90063-9. [DOI] [PubMed] [Google Scholar]
- 11.Hur JY, Kim BH, Suk KH, Sohn NW, Kim HC, Kwon HC, Lee KR, Kim SY. Neuroprotective and neurotrophic effects of Quinic acids from Aster scaber in PC12 cells. Biol Pharm Bull. 2001;24:921–924. doi: 10.1248/bpb.24.921. [DOI] [PubMed] [Google Scholar]
- 12.Xu J, Yukihisa M, Kinzo M, Michihisa T, Hiroshi W, Shaohui Z, Qinghai Y, Jia S. Protective effect of Oren-gedoku-to (Huang-Lian-Jei-Du-Tang) against impairment of learning and memory induced by transient cerebral ischemia in mice. J Ethnopharmacol. 2000;73:405–413. doi: 10.1016/s0378-8741(00)00303-2. [DOI] [PubMed] [Google Scholar]
- 13.Kakuta M, Sone Y, Umeda T, Misaki A. Comparative structural studies on acidic heteropolysaccharides isolated form 'Shirokikurage', fruit body of Tremella fuciformis Berk, and the growing culture of its yeast-like cells. Agric Biol Chem. 1979;43:1659–1668. [Google Scholar]
- 14.Kawagishi H, Ando M, Sakamoto H, Yoshida S. Hericenones C, D and E, stimulators of nerve growth factor (NGF) synthesis, from the mushroom Hericium erinaceum. Tetrahedron Lett. 1991;32:4561–4564. [Google Scholar]
- 15.Kiho T, Tsujimura Y, Sakushima M, Usui S, Ukai S. Polysaccharides in fungi. XXXIII. Hypoglycemic activity of an acidic polysaccharide (AC) from Tremella fuciformis. Yakugaku zasshi. 1994;114:308–315. doi: 10.1248/yakushi1947.114.5_308. [DOI] [PubMed] [Google Scholar]
- 16.Lee IK, Yun BS, Kim YH, Yoo ID. Two neuroprotective compounds form mushroom Daldinia concentrica. J Microbiol Biotechnol. 2002;12:692–694. [Google Scholar]
- 17.Li SC. In: Chinese medicinal herbs. Smith FP, Stuart GA, translators. San Francisco: Georgetown Press; 1973. p. 272. [Google Scholar]
- 18.Liu JH, Bao YM, Song JJ, Ahn LJ. Codonpsis pilosula (Franch) Nannf total alkaloides potentiate neurite outgrowth induced by nerve growth factor in PC12 cells. Acta Pharmacol Sin. 2003;24:913–917. [PubMed] [Google Scholar]
- 19.Park KJ, Ha HC, Kim HS, Chiba K, Yeo IH, Lee SY. The Neuroprotective and Neurotrophic Effects of Korean Gardenia jasminoides Ellis in PC12h cells. Food Sci Biotechnol. 2006;15:735–738. [Google Scholar]
- 20.Suh YH. The molecular biology for Alzheimer disease (Senile dementia) Biochem News. 1992;12:238–241. [Google Scholar]
- 21.Tsai CH, Chang RC, Chiou JF, Liu TZ. Improved superoxide-generating system suitable for the assessment of the superoxides-scavenging ability of aquous extracts of food constituents using ultraweak chemiluminescence. J Agric Food Chem. 2003;51:58–62. doi: 10.1021/jf020799t. [DOI] [PubMed] [Google Scholar]
- 22.Ukai S, Hirose K, Kiho T, Hara C, Irikura T. Antitumor activity on sarcoma 180 of the polysaccharides from Tremella fuciformis Berk. Chem Pharm Bull. 1972;20:2293–2294. doi: 10.1248/cpb.20.2293. [DOI] [PubMed] [Google Scholar]
- 23.Yamazaki M, Chiba K, Mohri T. Neuritogenic effect of natural iridoid compounds on PC12h cells and its possible relation to signaling protein kinase. Biol Pharm Bull. 1996;19:791–795. doi: 10.1248/bpb.19.791. [DOI] [PubMed] [Google Scholar]
- 24.Yui T, Ogawa K, Kakuta M, Misaki A. Chiain conformation of a glucuronoxylomannan isolated from fruit body of Tremella fuciformis Berk. J Carbohydr Chem. 1995;14:255–263. [Google Scholar]