Abstract
Hypolipidemic effect of biopolymers extracted from culture broth (CP), mycelia (MP), and fruiting bodies (FP) of Auricularia auricula-judae was investigated in dietary-induced hyperlipidemic rats. The experimental animals were administrated (100 mg/kg body weight) with different biopolymers, daily for 4 weeks. Hypolipidemic effects were achieved in all the experimental groups, however, FP was proved to be the most potent one. The administration of the FP reduced the plasma triglyceride, total cholesterol, low-density lipoprotein cholesterol, and atherogenic index by 24.3, 28.5, 36.4, and 40.9%, respectively, while increased the high-density lipoprotein cholesterol level (9.0%), when compared to the saline (control) administered group.
Keywords: Auricularia auricula-judae, Biopolymer, Hypolipidemic effect
Hyperlipidemia (mainly increased level of cholesterol or low density lipoprotein cholesterol) is an important risk factor in the initiation and progression of atherosclerotic lesions (Goldstein et al., 1973; Harrison et al., 2003). The beneficial effect of lowering elevated serum cholesterol level in the prevention of coronary heart disease is well established (Lipid Research Clinics Program, 1984; Simons, 2002). Several dietary fibers significantly decrease serum cholesterol and, thereby, reduce the risk for coronary heart disease. The search for natural substances are capable of lowering blood cholesterol is ongoing in the field of nutrition and many of dietary factors, which include plant proteins, unsaturated fatty acids, calcium and flavonoids, have been reported for their hypolipidemic potential (Kang and Song, 1997).
Edible mushrooms are the ideal dietetic materials for the prevention of atherosclerosis due to their high content of fiber, proteins, microelements and their low fat content (Bae et al., 2000; Crisan and Sands, 1978; Kurasawa et al., 1982). Auricularia auricular-judae is known to have potent hypocholesterolemic effect in plasma (Kaneda and Tokuda, 1966). The fruiting bodies of A. auricula-judae have long been used in food and for medicinal purposes. The polymer of this mushroom also has various biological activities: such as anti-tumor (Misaki et al., 1981), hypoglycemic (Yuan et al., 1998), anticoagulant (Yoon et al., 2003), and anti-complement activity (Jeong et al., 2004).
In the present investigation, hypolipidemic effects of biopolymers extracted from CP, MP, and FP of A. auricula-judae in dietary-induced hyperlipidemic rats have been reported.
Materials and Methods
Strains and preparation of CP, MP and FP
A. auricula-judae was obtained from the Rural Development Administration in South Korea, while the fruiting body of this mushroom was obtained from the local market. The experimental organism was maintained on potato dextrose agar (PDA, Difco) slant at 4℃ and subcultured in every 3 months. The seed culture was grown (25℃/130 rpm/approx. 7 d) in 250-ml Erlenmeyer flasks containing 100-ml of potato dextrose broth (pH 5.0) medium. One hundred ml of the medium with mycelial pellets was then homogenized aseptically in a Sorvall omni-mixer for 3 min in an ice bath (Song et al., 1998) and inoculated in the fermentation media (4%, v/v) for submerged cultivation. The mushroom complete medium (MCM) of the following composition (g/l): Glucose 20, MgSO4·7H2O 0.5, KH2PO4 0.46, K2HPO4 1.0, yeast extract 2.0, and peptone 2.0, with pH 5.0 was used to perform submerged mycelia culture for the production of biopolymers.
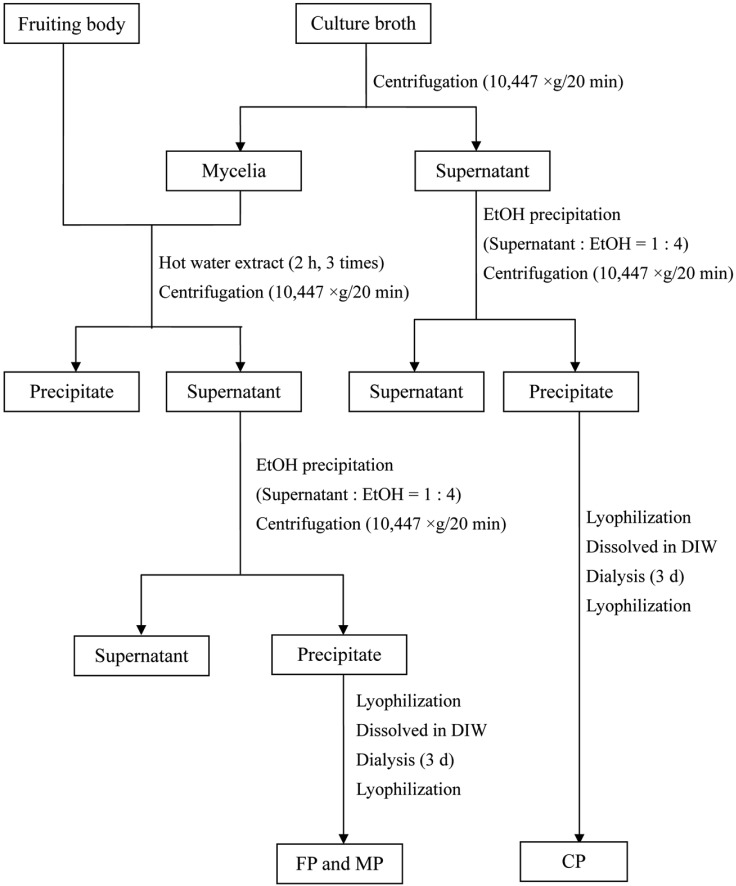
The submerged mycelia cultures were carried out in 500-ml Erlenmeyer flasks containing 250-ml of MCM media on a rotary shaker (130 rpm/pH 5/25℃). The recovery procedure of the CP, MP, and FP of A. auricula-judae is shown in Fig. 1.
Fig. 1.
A schematic diagram depicting the recovery process of biopolymers extracted from culture broth (CP), mycelia (MP), and fruiting bodies (FP) of Auricularia auricula-judae.
Animal experiments
Sprague-Dawley male rats (80~100 g) obtained from Daehan Biolink Co., Ltd. (Seoul, Korea) were housed individually in stainless steel cages in a room with controlled temperature (22 ± 2℃), humidity (55 ± 5%), and a 12 h cycle of light and dark. The rats were fed with a modified AIN-76 (American Institute of Nutrition, 1977) high fat diet for six weeks (Table 1).
Table 1.
Composition of high fat diet
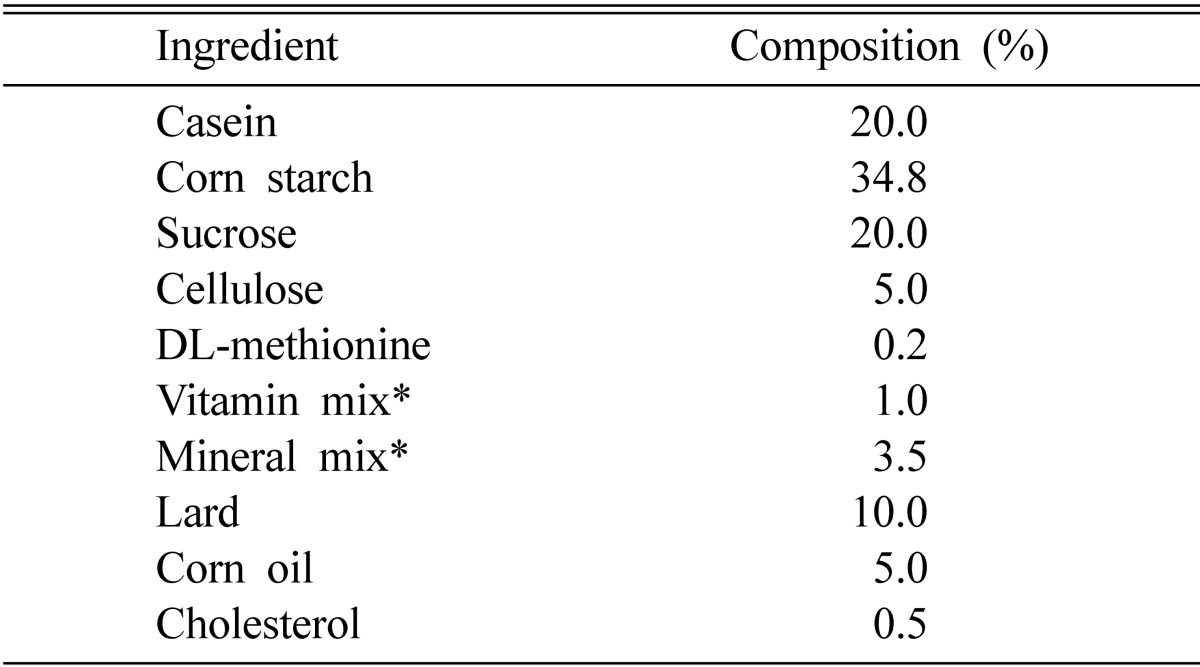
*Mineral and vitamin (g/kg) mixture (AIN-76).
The animals were provided daily with the diets and water. After two weeks of acclimatization in the growth room, they were brought into the experimental conditions. Based on their body weights, the rats were divided into four experimental group of nine. Each group of nine dietary-induced hyperlipidemic rats was orally administered either with 0.9% saline (control) or CP, MP and FP (100 mg/kg BW: body weight) daily for 4 weeks (Table 2).
Table 2.
Experimental group for identifying hypolipidemic activity
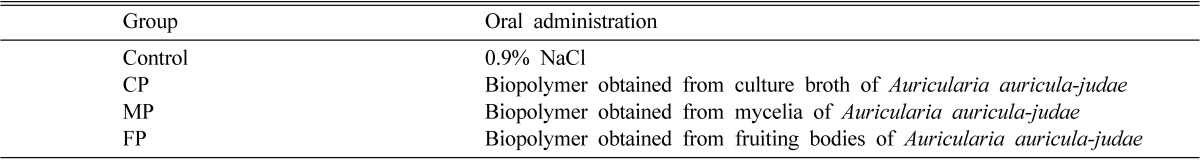
Group of 9 hyperlipidemic rats. The rats in each experimental group were orally administered with either saline (control), CP, MP, and FP at 100 mg/kg body weight daily for 4 weeks.
The food intake and body weights were recorded every alternate and every day, respectively. At the end of the 4 weeks experiment, the rats were fasted for 12 h and immediately sacrificed, following an abdominal incision under light ether anesthesia. Later, the blood was collected from the main artery into heparinized tubes. Plasma was prepared by centrifugation (1,110×g, 10 min) after keeping the samples at room temperature for 2 h.
Chemical analysis
The level of triglyceride, total cholesterol, and high-density lipoprotein (HDL) cholesterol in plasma were determined enzymatically using commercial kits (Asan Pharm. Co., Ltd., Chungnam, Korea) based on the methods of glycerol kinase (Bucolo and David, 1973), cholesterol oxidase-DAOS (Allain et al., 1974), and phosphotungstic acid-Mg2+ precipitation (Finley et al., 1978), respectively. Low-density lipoprotein (LDL) cholesterol and atherogenic index of plasma were calculated by the following equation: LDL cholesterol = total cholesterol - HDL cholesterol - (triglyceride/5) (Friedewald et al., 1972), Atherogenic index = (total cholesterol - HDL cholesterol)/HDL cholesterol (Haglund et al., 1991).
Statistical analysis
Each data value is expressed as the mean ± S.E. The group means were compared using a one-way analysis of variance and Duncan's multiple-range test (Duncan, 1957). The statistical differences were considered significant at p < 0.05.
Results and Discussion
Growth response
The CP, MP, and FP of A. auricula-judae were orally administered to dietary-induced hyperlipidemic rats, daily for 4 weeks. All the biopolymers tested, FP group significantly reduced body weight gain, while, there was no significant effect on food intake and food efficiency ratio in the all experimental group (Table 3). The reduction of body weight observed in the present investigation clearly shows the hypolipidemic potential of FP. The increased body weight observed in hyperlipidemic rats (control group) may be due to accumulation of an excess amount of lipid in the liver tissues (Gurr and Harwood, 1991). The oral administration of CP, MP and FP caused no changes in gross behavior of rats, which shows that there were no harmful effects and moreover, all the rats remained healthy in all the experiment. Several workers while working on Ganoderma lucidum (Yang et al., 2002) and Hericium erinaceus (Yang et al., 2003) biopolymers have also reported similar results.
Table 3.
Effect of biopolymers extracted from culture broth (CP), mycelia (MP), and fruiting bodies (FP) of Auricularia auricula-judae on growth parameters in dietary-induced hyperlipidemic rats for 4 weeks
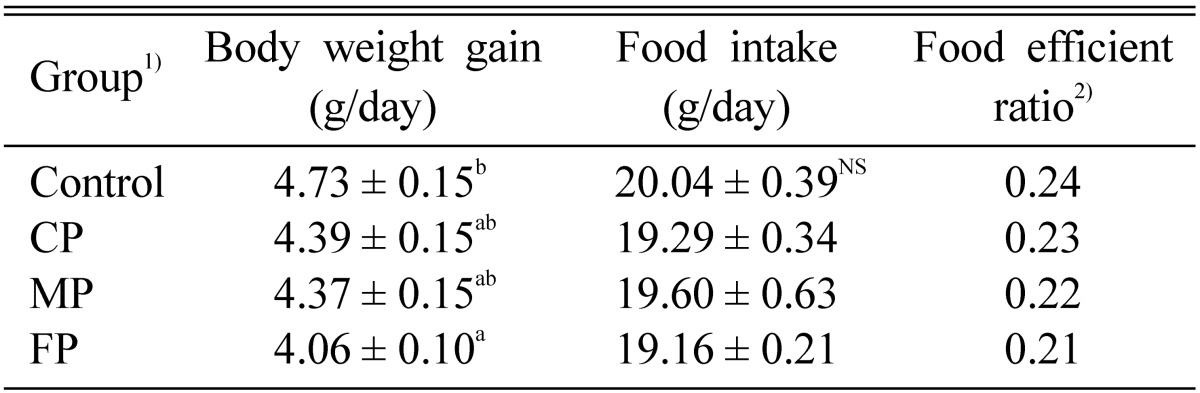
1)See Table 2.
2)Body weight gain/Food intake.
NSNot significant.
Each values are means ± S.E. (n = 9).
a,bValues with different superscript letters in the same column significantly different among the group at p < 0.05.
Hypolipidemic effect
The effects of CP, MP, and FP on plasma triglyceride and total cholesterol have been shown in Table 4. In the present investigation, the FP (24.3%) and MP (16.3%) group significantly decreased the level of plasma triglyceride, as compared to control group. Some recent studies (Davignon and Cohn, 1996) have indicated that decreased plasma triglyceride concentration was associated with a lower risk of coronary heart disease. The reduction in triglyceride level due to dietary fiber as observed in the present studies may be due to the direct interference of triglyceride absorption as well as increased excretion of triglyceride via fecal fat (Miettinen, 1987). Yang et al. (2002) while working with biopolymers of Ganoderma lucidum, have also reported that their polymers significantly decreased the concentration of triglyceride when administered orally in dietary induced hyperlipidemic rats.
Table 4.
Effect of biopolymers extracted from culture broth (CP), mycelia (MP), and fruiting bodies (FP) of Auricularia auricula-judae on plasma total cholesterol and triglyceride in dietary-induced hyperlipidemic rats for 4 weeks
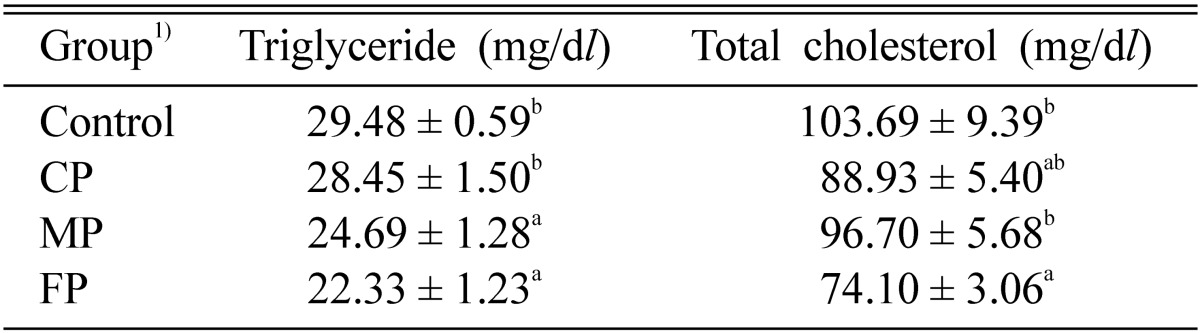
1)See Table 2.
Each values are means ± S.E. (n = 9).
a,bValues with different superscript letters in the same column significantly different among the group at p < 0.05.
Though administration of CP, MP, and FP in the dietary-induced hyperlipidemic rats lowered the total cholesterol concentration (14.2%, 6.7% and 28.5%) in plasma, respectively, however, FP group was found to be best among others. The hypolipidemic effect exerted by these biopolymers may be due to its high viscous nature (Ebihara and Schneeman, 1989), or/and might raise the secretion of pancreatic juice in rats (Ikegami et al., 1990). As observed in the present studies, perhaps for this property, it could lower the triglyceride and total cholesterol adsorption by inhibiting the formation of micelles in the small intestine and by altering the physical characteristics of the intestinal mucosa of rats (Chen and Anderson, 1986).
Table 5 shows the effects of the A. auricula-judae CP, MP, and FP on the plasma HDL cholesterol, LDL cholesterol, and atherogenic index. As compared to the control group, the FP group reduced the LDL cholesterol levels and atherogenic index by as much as 36.4% and 40.9%, respectively. Similar results have also been recorded by several workers (Yang et al., 2002). The plasma HDL cholesterol level in the FP group (9.0%) showed the highest among the groups.
Table 5.
Effect of biopolymers extracted from culture broth (CP), mycelia (MP), and fruiting bodies (FP) of Auricularia auricula-judae on plasma HDL cholesterol, LDL cholesterol, and the level of atherogenic index in dietary-induced hyperlipidemic rats for 4 weeks
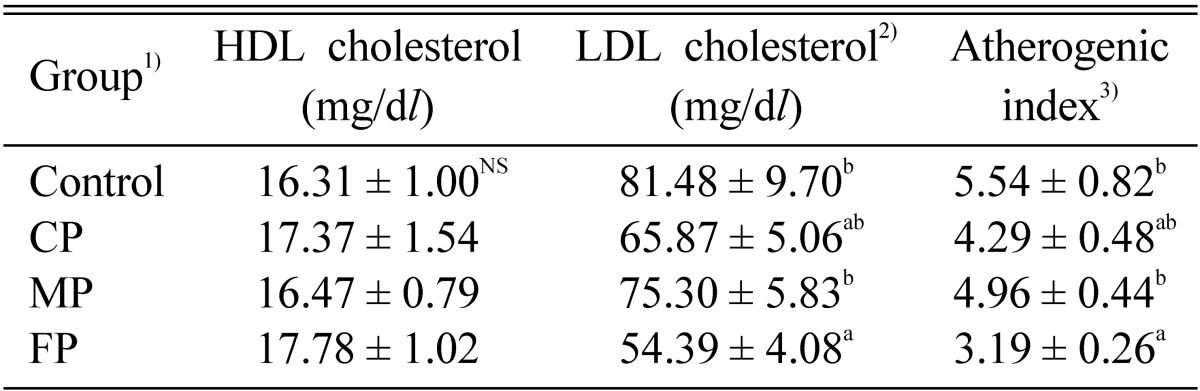
1)See Table 2.
2)Total cholesterol - HDL cholesterol - (Triglyceride/5).
3)(Total cholesterol - HDL cholesterol)/HDL cholesterol.
NSNot significant.
Each values are means ± S.E. (n = 9).
a,bValues with different superscript letters in the same column significantly different among the group at p < 0.05.
A strong relationship has been documented between the plasma total cholesterol, triglyceride, LDL cholesterol and atherogenic index (Gurr and Harwood, 1991). A substantial reduction of total cholesterol, triglyceride, LDL cholesterol and atherogenic index in plasma observed in the present case may be due to a reduced production of cholesterol by the liver tissues (by inhibiting HMG-CoA reductase) and/or efficient removal of the LDL cholesterol by various tissues without subsequent renewal (Yang et al., 2003). Plasma LDL values may be affected by modifications of very-low-density lipoprotein (VLDL) metabolism, including the rate of conversion of VLDL to LDL. Basically, nascent VLDL is converted to mature VLDL and to LDL through the loss of triglyceride by the action of lipoprotein lipase. Shen et al. (1998) suggested that the LDL cholesterol lowering effect of Psyllium is due to slow conversion of VLDL to LDL.
The plasma LDL may arise physiologically from the catabolism of VLDL (Bilheimer et al., 1972; Sigurdsson et al., 1975) which documents an inter-relationship of these two lipoprotein classes and hence of plasma cholesterol and triglyceride, suggesting a possible metabolic site for the lesion in combined hyperlipidemia.
The plasma HDL cholesterol, an indicator of anti-atherosclerosis, is generally involved in the transport of excess cholesterol to the liver for reprocessing. It has been reported by several workers (Kang and Song, 1997; Kim et al., 1992) that some dietary fibers can elevate the plasma HDL cholesterol level. The increased HDL value in this investigation may be due to the lower conversion of HDL to LDL or by some other means. Kinnunen et al. (1983) have demonstrated that the triglyceride lowering effect in plasma may be due to the elevated lipoprotein lipase activity. Shen et al. (1998) and Dietschy et al. (1993) also reported that a reduced plasma LDL cholesterol level in dietary-induced hyperlipidemic animals in an improper lipid metabolism leading to an enhanced level of hepatic acetyl-CoA, which in turn participates in lipogenesis and is accumulated as lipid in the liver tissue. Present studies suggest that the risk of atherosclerosis may dependent on more the plasma LDL level than the total cholesterol level in the body system. The level of HDL cholesterol found to be inversely related to the risk of atherosclerosis (Ganong, 1987).
The present investigation demonstrated the potential of A. auricular-judae FP in reducing the level of cholesterol rich-LDL (which is quantitatively the most significant lipoprotein class in the control of serum cholesterol levels) and preserving the HDL at relatively high level. Such effects can help in reducing the risk of atherosclerosis. It is possible that the hypocholesterolemic effect of A. auricula-judae FP appeared to be due to the reduced cholesterol synthesis in the liver. Also, it can not be ruled out the possibility of combined effects (the inhibition of cholesterol absorption, and/or the inhibition of biosynthesis of LDL and acceleration of their fractional turnover) (Tokuda et al., 1974; Vahouny et al., 1987).
In the present study, the CP, MP and FP in A. auricula-judae were screened for their hypolipidemic activity. Among other experimental groups, the FP group was shown to have the potency to combat hyperlipidemia in dietary-induced hyperlipidemic rats. These results indicate the necessity to perform a dose-dependent experiment. Moreover, further comprehensive chemical and pharmacological investigations may help in elucidating the exact mechanism of hypolipidemic effects. Isolation of active principles of this mushroom may be helpful in preventive and therapeutic purposes to alleviate the hyperlipidemic status.
Acknowledgments
This work was supported by the RIC program of MOIEC.
References
- 1.Allain CC, Poon LS, Chan CSG, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- 2.American Institute of Nutrition. Report of the American Institute of Nutrition Ad hoc committee on standards for nutritional studies. J Nutr. 1977;107:1340–1348. doi: 10.1093/jn/107.7.1340. [DOI] [PubMed] [Google Scholar]
- 3.Bae JT, Sinha J, Park JP, Song CH, Yun JW. Optimization of submerged culture conditions for exo-biopolymer production by Paecilomyces japonica. J Microbiol Biotechnol. 2000;10:482–487. [Google Scholar]
- 4.Bilheimer DW, Eisenberg S, Levy RI. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972;260:212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- 5.Bucolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973;19:476–482. [PubMed] [Google Scholar]
- 6.Chen WL, Anderson JW. Hypocholesterolemic effects of soluble fiber. In: Vahouny GV, Kritchevsky D, editors. Dietary Fiber; Basic and Clinical Aspects. Plenum Press: New York; 1986. pp. 275–286. [Google Scholar]
- 7.Crisan EV, Sands A. Nutritional Value. In: Chang ST, Hayes WA, editors. The Biology and Cultivation of Edible Mushroom. New York: Academic Press; 1978. pp. 137–168. [Google Scholar]
- 8.Davignon J, Cohn JS. Triglycerides: A risk factor for coronary heart disease. Atherosclerosis. 1996;124:S57–S64. doi: 10.1016/0021-9150(96)05858-3. [DOI] [PubMed] [Google Scholar]
- 9.Dietschy JM, Turley SD, Spady DK. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J Lipid Res. 1993;34:1637–1659. [PubMed] [Google Scholar]
- 10.Duncan DB. Multiple range tests for correlated and heteroscedastic means. Biometrics. 1957;13:164–176. [Google Scholar]
- 11.Ebihara K, Schneeman BO. Interaction of bile acids, phospholipids, cholesterol and triglyceride with dietary fibers in the small intestine of rats. J Nutr. 1989;119:1100–1106. doi: 10.1093/jn/119.8.1100. [DOI] [PubMed] [Google Scholar]
- 12.Finley PR, Schifman RB, Williams RJ, Lichti DA. Cholesterol in high-density lipoprotein: use of Mg2+/dextran sulfate in its enzymic measurement. Clin Chem. 1978;24:931–933. [PubMed] [Google Scholar]
- 13.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 14.Ganong WF. Review of Medical Physiology. 13th ed. Norwalk, Conn, U.S.A: Appleton & Lange; 1987. pp. 103–276. [Google Scholar]
- 15.Goldstein JL, Schrott HG, Hazzard WR, Bierman EL, Motulsky AG. Hyperlipidaemia in coronary heart disease II. Genetic analysis of lipid levels in 176 families and delineation of a new inherited disorder, combined hyperlipidemia. J Clin Invest. 1973;52:1544–1568. doi: 10.1172/JCI107332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gurr MI, Harwood JL. Lipid Biochemistry:An Introduction. 4th ed. London: Chapman & Hall; 1991. pp. 163–245. [Google Scholar]
- 17.Haglund O, Luostarinen R, Wallin R, Wibell L, Saldeen T. The effects of fish oil on triglycerides, cholesterol, fibrinogen and malondialdehyde in humans supplemented with vitamin E. J Nutr. 1991;121:165–169. doi: 10.1093/jn/121.2.165. [DOI] [PubMed] [Google Scholar]
- 18.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91:7A–11A. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 19.Ikegami S, Tsuchihashi F, Harada H, Tsuchihashi N, Nishide E, Innami S. Effect of viscous indigestible polysaccharides on pancreatic-biliary secretion and digestive organs in rats. J Nutr. 1990;120:353–360. doi: 10.1093/jn/120.4.353. [DOI] [PubMed] [Google Scholar]
- 20.Jeong SC, Cho SP, Yang BK, Gu YA, Jang JH, Huh TL, Song CH. Production of an anti-complement exo-polymer produced by Auricularia auricula-judae in submerged culture. Biotechnol Lett. 2004;26:923–927. doi: 10.1023/b:bile.0000025904.21519.3a. [DOI] [PubMed] [Google Scholar]
- 21.Kaneda T, Tokuda S. Effect of various mushroom preparations on cholesterol levels in rats. J Nutr. 1966;90:371–376. doi: 10.1093/jn/90.4.371. [DOI] [PubMed] [Google Scholar]
- 22.Kang HJ, Song YS. Dietary fiber and cholesterol metabolism. J Korean Soc Food Sci Nutr. 1997;26:358–369. [Google Scholar]
- 23.Kim GJ, Kim HS, Chung SY. Effects of varied mushroom on lipid compositions in dietary hypercholesterolemic rats. J Korean Soc Food Nutr. 1992;21:131–135. [Google Scholar]
- 24.Kinnunen PKJ, Virtanen JA, Vainio P. Lipoprotein lipase and hepatic endothelial lipase. Ateroscler Rev. 1983;11:65–71. [Google Scholar]
- 25.Kurasawa S, Sugahara T, Hayashi J. Studies on dietary fiber of mushrooms and edible wild plants. Nutr Rep Int. 1982;26:167–173. [Google Scholar]
- 26.Lipid Research Clinics Program. The lipid research clinics coronary primary prevention trial results. I. Reduction in incidence of coronary heart disease. JAMA. 1984;251:351–364. doi: 10.1001/jama.1984.03340270029025. [DOI] [PubMed] [Google Scholar]
- 27.Miettinen TA. Dietary fiber and lipids. Am J Clin Nutr. 1987;45:1237–1242. doi: 10.1093/ajcn/45.5.1237. [DOI] [PubMed] [Google Scholar]
- 28.Misaki A, Kakuta M, Sasaki T, Tanaka M, Miyaji H. Studies on interrelation of structure and antitumor effects of polysaccharides: antitumor action of periodate modified, branched (1→3)-β-D-glucan of Auricularia auricula-judae, and other polysaccharides containing (1→3)-glycosidic linkages. Carbohydr Res. 1981;92:115–129. doi: 10.1016/s0008-6215(00)85986-8. [DOI] [PubMed] [Google Scholar]
- 29.Shen H, He L, Price RL, Fernandez ML. Dietary soluble fiber lowers plasma LDL cholesterol concentrations by altering lipoprotein metabolism in female guinea pigs. J Nutr. 1998;128:1434–1441. doi: 10.1093/jn/128.9.1434. [DOI] [PubMed] [Google Scholar]
- 30.Sigurdsson G, Nicoll A, Lewis B. Conversion of very low density lipoprotein to low density lipoprotein: a metabolic study of apolipoprotein B kinetics in human subjects. J Clin Invest. 1975;56:1481–1490. doi: 10.1172/JCI108229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simons LA. Additive effect of plant sterol-ester margarine and cerivastatin in lowering low-density lipoprotein cholesterol in primary hypercholesterolemia. Am J Cardiol. 2002;90:737–740. doi: 10.1016/s0002-9149(02)02600-0. [DOI] [PubMed] [Google Scholar]
- 32.Song CH, Yang BK, Ra KS, Shon DH, Park EJ, Go GI, Kim YH. Hepatoprotective effect of extracellular polymer produced by submerged culture of Ganoderma lucidum WK-003. J Microbiol Biotechnol. 1998;8:277–279. [Google Scholar]
- 33.Tokuda S, Tagiri A, Kano E, Sugawara Y, Suzuki S, Sato H, Kaneda T. Reducing mechanism of plasma cholesterol by shiitake. Mushroom Sci. 1974;9:445–462. [Google Scholar]
- 34.Vahouny GV, Khalafi R, Satchithanandam S, Watkins DW, Story JA, Cassidy MM, Kritchevsky D. Dietary fiber supplementation and fecal bile acids, neutral steroids and divalent cations in rats. J Nutr. 1987;117:2009–2015. doi: 10.1093/jn/117.12.2009. [DOI] [PubMed] [Google Scholar]
- 35.Yang BK, Park JB, Song CH. Hypolipidemic effect of exo-biopolymer produced from a submerged mycelial culture of Hericium erinaceus. Biosci Biotechnol Biochem. 2003;67:1292–1298. doi: 10.1271/bbb.67.1292. [DOI] [PubMed] [Google Scholar]
- 36.Yang BK, Jeong SC, Song CH. Hypolipidemic effect of exo- and endo-biopolymers produced from submerged mycelial culture of Ganoderma lucidum in rats. J Microbiol Biotechnol. 2002;12:872–877. [Google Scholar]
- 37.Yuan Z, He P, Cui J, Takeuchi H. Hypoglycemic effect of water-soluble polysaccharide from Auricularia auricula-judae Quel. on genetically diabetic KK-Ay mice. Biosci Biotechnol Biochem. 1998;62:1898–1903. doi: 10.1271/bbb.62.1898. [DOI] [PubMed] [Google Scholar]
- 38.Yoon SJ, Yu MA, Pyun YR, Hwang JK, Chu DC, Juneja LR, Mourao PAS. The nontoxic mushroom Auricularia auricula contains a polysaccharide with anticoagulant activity mediated by antithrombin. Thrombosis Research. 2003;112:151–158. doi: 10.1016/j.thromres.2003.10.022. [DOI] [PubMed] [Google Scholar]