Abstract
The recent developments of new devices and advances in anesthesiology have greatly improved the utility and accuracy of intraoperative neurophysiological monitoring (IOM). Herein, we review the basic principles of the electrophysiological methods employed under IOM in the operating room. These include motor evoked potentials, somatosensory evoked potentials, electroencephalography, electromyography, brainstem auditory evoked potentials, and visual evoked potentials. Most of these techniques have certain limitations and their utility is still being debated. In this review, we also discuss the optimal stimulation/recording method for each of these modalities during individual surgeries as well as the diverse criteria for alarm signs.
Keywords: Intraoperative Neurophysiologic Monitoring, Evoked Potential, Guideline
INTRODUCTION
Intraoperative neurophysiological monitoring (IOM) is now an integral part of many surgical procedures. The first use of intraoperative neurophysiological testing dates back to the 1930s, when direct cortical stimulation was performed in order to identify the motor cortex of patients with epilepsy (1); however, it was only with the development of the commercial IOM machine in the early 1980s that the technique became widely used. (2) The 1990s saw transcranial motor evoked potentials (Tc-MEPs) popularized as a method for monitoring corticospinal tract activity (3) as well as for predicting postoperative motor deficits (4). Technological advances in the last 15 yr have allowed monitoring techniques to greatly evolve. The widespread availability of computer networks and integrated communication systems have allowed IOM to be performed from a remote site; this evolution has increased the potential application of the technique and contributed further to the popularity of IOM (5).
BASIC CONCEPT OF IOM
IOM has been utilized in an attempt to minimize neurological damage during surgery, to identify important neural structures in the operative field, and thus to avoid and/or limit significant postoperative impairments. IOM employs a wide variety of modalities, each with a very specific application, with several modalities often being used together in the same surgery. These modalities include motor evoked potentials (MEPs), somatosensory evoked potentials (SSEPs), electroencephalography (EEG), electromyography, brainstem auditory evoked potentials (BAEPs), and visual evoked potentials (VEPs); these are discussed in detail below.
MODALITIES IN IOM
Motor evoked potentials
Transcranial electrical stimulation (TES), rather than the closely related technique of transcranial magnetic stimulation (TMS), is usually employed for the generation of MEPs (and hence stimulation of the corticospinal tract) in the context of IOM, primarily because TES is more resistant to anesthesia than TMS (6). A short train of 5-7 electrical pulse stimuli with high frequency of > 200 Hz are usually used for TES (7). In anesthetized patients, this stimulation technique is advantageous than the single-pulse stimulation technique, because it can generate action potential more easily through the summation of the excitatory postsynaptic potentials (8).
Based on the site of stimulation or recording, intraoperative electrical MEPs can be further classified. For example, MEPs can be recorded over muscle (Tc-mMEP) or over the spinal cord (D and I waves). Of these, Tc-mMEP seems to be the most widely adopted approach because of the relative simplicity of generating and recording MEPs in this manner; however, it does have some disadvantages. These are as follows: 1) although Tc-mMEP can be monitored under the standard total intravenous anesthesia (TIVA) protocol, it is highly vulnerable to inhalation anesthesia, and even a low dose of halogenated inhalation anesthetics can abolish or significantly interfere with Tc-mMEP recording (6); 2) recording of Tc-mMEP requires the transmission of a neural signal through the neuromuscular junction, and the use of muscle relaxants (neuromuscular blockers) during surgical procedures can significantly affect the amplitude of Tc-mMEP (9); and 3) the amplitude of Tc-mMEP has a high intertrial variability in nature (10). This variability makes it difficult to set a clear cut-off value in terms of amplitude changes for warning signals of neural damage. The activation of different pyramidal cells in the brain and motor neurons in the spinal cord is thought to be responsible for this phenomenon (10). Diverse criteria for potential intraoperative neural damage (alarm criteria) using Tc-mMEP have been suggested to overcome this limitation. These include an absence of Tc-mMEP (4), changes in amplitude (11), an increase in MEP stimulation threshold (12), and either changes in amplitude or an increase in the MEP stimulation threshold (13). However, there are still some debates on the optimal criteria for Tc-mMEP (12).
In our laboratory, we adapted individualized criteria for an "alarm sign" according to the types of surgery. Only the absence of mMEP per se is used as an "alarm sign" for spinal surgery (12); however, an amplitude decrement > 50% is also used as a potential alarm sign for brain surgery (13).
Because of the limitations of Tc-mMEP noted above, some studies have advocated the use of D-waves, obtained from TES to the brain, with recordings made from the spinal cord by using an epidural electrode (14). A previous study on intramedullary tumor surgery proposed that the D-wave was more specific to postoperative motor deficits than was Tc-mMEP (4); This study compared the results of D-waves with those of Tc-mMEP recorded from the tibialis anterior (TA) muscle only (4). However, it should be noted that the specificity and sensitivity of Tc-mMEP can vary greatly depending on the type of muscle analyzed. Indeed, in our previous study, we demonstrated that Tc-mMEP recorded from the TA has a relatively low specificity for predicting postoperative motor deficits (15), probably due to the low level of corticospinal fiber innervation of the TA muscle (16). Therefore, a precise comparison of the diagnostic accuracy of Tc-mMEP and D-wave perhaps requires a future study using Tc-mMEP recorded from muscles with greater corticospinal fiber innervations, and thus with higher specificity for postoperative motor deficits, e.g., the abductor hallucis (15, 16). Of note, D-waves also have several disadvantages; these include requiring the use of an epidural electrode (14), a high false-positive rate in scoliosis surgery (17), the inability to monitor spinal motor neurons (18), and a relatively low MEP generation rate (2).
Somatosensory evoked potential (SSEP)
Stimulation for the SSEP is commonly achieved in the median nerve (median nerve SSEP, MNSEP) and in the posterior tibial nerve (posterior tibial SSEP, PTSEP). Although SSEP can be recorded from either Erb's point, spine, brainstem, primary cortical somatosensory area, or all of these areas together, those recorded in the cortical somatosensory area can often provide the most crucial information regarding intraoperative neural damage, with relative ease in recording (Fig. 1) (19).
Fig. 1.
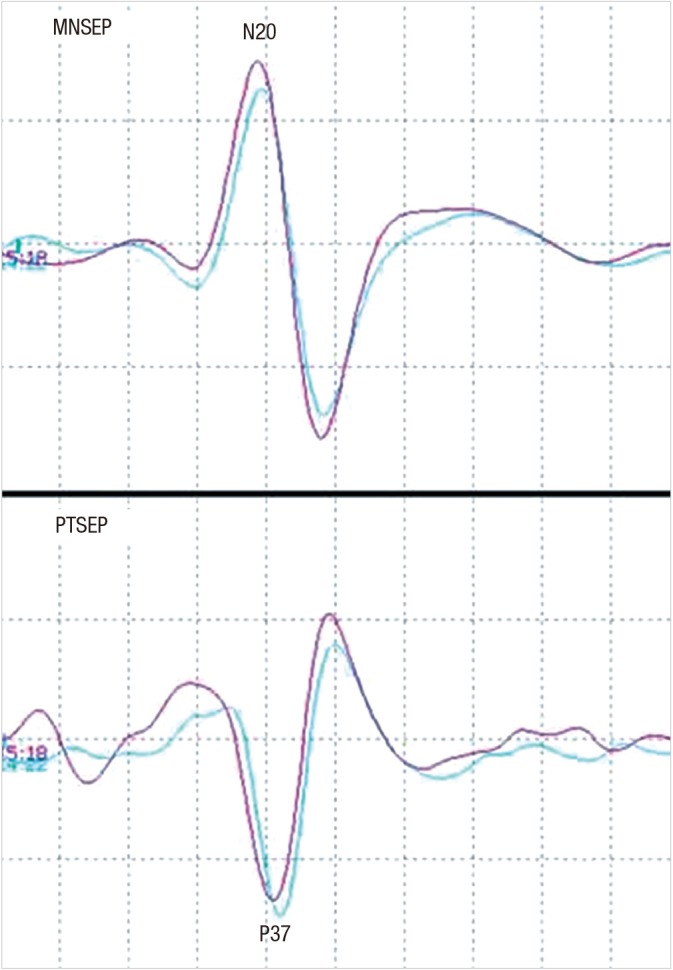
Waves of median nerve somstosensory evoked potential (MNSEP) and posterior tibial nerve somatosensory evoked potential (PTSEP). Electrical stimulation at the median nerve and posterior tibial nerve can evoke generation of the cortical waves of N20 and P37, respectively.
SSEP has long been used for IOM. However, it is essentially only a test for the sensory pathways that ascend through the dorsal column of the spinal cord. This poses the potential risk of small intraoperative injuries in the motor tract not being detected. Despite this limitation, it is now commonly used during surgery, both as a main IOM modality, and as a complement to other modalities such as EEGs or MEPs. The use of SSEP is advantageous for the following reasons: it does not provoke unwanted movement of the patient during surgery; it is easily quantifiable; and it has a relatively low average intertrial variability compared to MEP (2, 20). A decrease in SSEP amplitude of more than 50% and/or an increase in SSEP latency of more than 10% of baseline are generally accepted as the "alarm criteria" for intraoperative neural damage (20).
Electroencephalography
EEG generally records the electrical activity from the scalp. It was first adapted for IOM in the 1960s for the safe performance of carotid endarterectomy (CEA), and it is still widely used for assessing the degree of cerebral perfusion during vascular surgery (21), and for monitoring the depth of anesthesia (22) and the degree of hypothermia for neuroprotection (23). However, it should be noted that while conventional scalp EEG is generally useful for detecting diffuse hypoperfusion states during CEA, it might be less sensitive than the transcranial Doppler (TCD) technique for detecting microemboli during CEA (24). Furthermore, it might be less sensitive than cortical EEG or SSEP for identifying intraoperative changes during cerebral aneurysm surgery (25).
Electromyography
Electromyography (EMG) enables the recording of electrical activity produced by skeletal muscles. The basic techniques include free-running EMG, stimulated EMG, and intraoperative nerve conduction studies (NCS).
Free-running EMG detects mechanical and/or metabolic irritation of the nerve (26). It can be recorded in the innervated muscles without electrical stimulation of the nerve. Two types of discharge, each with different clinical significance, can be observed using free-running EMG monitoring: tonic discharge and phasic discharge. Tonic discharge consists of repetitive and steady episodes of activity from grouped motor units that can last from several seconds to minutes; it can be observed in nerve ischemia due to traction, heat spread from electrocautery, or irrigation with saline (27, 28). In contrast, phasic (burst) discharge is a short and relatively synchronous burst of motor unit potentials, which is mostly associated with blunt mechanical trauma (27, 28).
Stimulated EMG, used for motor NCS, is performed by electrically stimulating the nerves and recording the resulting compound muscle action potentials in the innervated muscle. Stimulated EMG is advantageous for the following reasons: operating surgeons can be informed of the anatomical variation of the motor nerves in individual patients; functions of the nerves can be identified, i.e., motor or sensory; and the proximity of the surgical procedures or device, such as a pedicle screw of the spine, to the endangered nerves can be observed (29).
Brainstem auditory evoked potential
Brainstem auditory evoked potentials (BAEPs) are small electrical potentials generated in response to acoustic stimuli. These potentials are relatively resistant to surgical anesthesia and can be useful for monitoring auditory structures (29). Interpretation of BAEP can be usually made by the latency and/or amplitude of its first 5 negative potentials, i.e., waves I-V. Prolongation of latency more than 1 ms and/or decrease in amplitude more than 50% have been empirically used as alarm criteria for intraoperative neural damage and subsequent postoperative hearing loss (30). However, some researchers have also proposed that only permanent loss of wave V during surgery was associated with significant postoperative hearing loss in the non-cerebellopontine angle (CPA) tumor surgery, but only a decrease in the amplitude of wave V can be important in CPA tumor surgery (31). Therefore, further studies are needed for determining the exact alarm criteria of intraoperative BAEP changes.
Visual evoked potential
Visual evoked potential (VEP) refers to the electrical potentials induced by brief visual stimuli, which are recorded from the scalp over the visual cortex. IOM of the visual pathway is necessary in diverse types of surgeries, including transsphenoidal surgery, aneurysm clipping of the posterior circulation, and removal of tumors that lie near the optic radiation. Recently, it has gained attention with advances in both monitoring and anesthetic techniques (32, 33). However, the usefulness of intraoperative VEP has only been demonstrated in a limited number of studies (32, 33), and there seems to be some debate regarding the correlation of intraoperative VEP changes and postoperative visual outcomes (34, 35). Further studies on the standard method and the clinical utility of intraoperative VEP are required.
SPECIAL CONSIDERATIONS FOR IOM
IOM team
The IOM team is composed of the surgeon, clinical neurophysiologist, anesthesiologist, and monitoring technologist (36). Clinical neurophysiologists require training and experience in the general field of clinical neurophysiology, including in-depth knowledge of evoked potentials (EPs), EEGs, EMGs, and NCS, as well as experience in neurophysiology in the operating room (OR). This level of training and experience is necessary as the neurophysiologist needs to be able to interpret electrophysiological changes during surgery, discriminate and reduce artifacts, identify peak waves, train the technologists, and integrate diverse monitoring modalities.
Level of supervision and remote monitoring
In 2001, the health care financing administration (HCFA) of the USA set a revised 3-level protocol for physician supervision during a diagnostic test: general, direct, and personal supervision (37). Briefly, general supervision means that a technologist trained by a supervising physician can perform the procedure; however, the physician does not need to be present during procedure. Direct supervision states that the supervising physician needs to be available somewhere near, but it does not require the physician to be physically present in the room during procedure. Lastly, personal supervision means that the supervising physician must be in the room during procedure.
An important issue in IOM is which level of supervision is required from the clinical neurophysiologist. Several guidelines and books have suggested that the answer to this depends on the type of neurophysiological procedure being carried out. Generally, there are 2 types of neurophysiological procedures that should be taken into consideration when determining the level of supervision; these can be distinguished according to their aims.
The first type is neurophysiological testing to identify specific neural structures, for example, localization of the language or motor area of the brain, which requires a high level of knowledge and experience for the interpretation of the test results. To perform this neurophysiological testing, clinical neurophysiologists are needed in the OR in order to identify the function of stimulated neural tissues and to communicate personally with the operating surgeons. Therefore, it is recommended that this type of intraoperative neurophysiological testing should be performed under personal supervision (2).
The other neurophysiological procedure is neurophysiological monitoring, including routine intraoperative monitoring of the brain and spinal cord (2). This type of procedure does not require continuous personal supervision by the clinical neurophysiologist inside the OR (2, 38). Rather, a technologist can perform the routine monitoring under the direct supervision of a clinical neurophysiologist who can either be in the OR or nearby (2, 39, 40).
The 2001 HCFA also approved routine intraoperative monitoring performed by real time monitoring from a remote site (remote monitoring) (2, 37). Since then, remote IOM has become more widespread and popular (5, 40), and most of the recently commercial available IOM machines have built-in remote monitoring capabilities.
Anesthesia and IOM
The results of diverse IOM modalities, especially those of MEP, can be significantly affected by the type and depth of anesthesia. Currently, one of the most commonly used anesthetic regimens for IOM is TIVA, with a combination of propofol and opioids (2). Propofol, which is thought to act on GABA receptors, is rapidly metabolized and can be easily titrated to levels that enable MEP generation (41). However, despite its usefulness for IOM, high doses can still interfere with the generation of MEPs (6). Another potential disadvantage is that it can suppress EEG in a dose-dependent manner (42). Halogenated inhalation agents can easily abolish MEP (Fig. 2) (6); therefore, they are not routinely recommended for MEP IOM. Lastly, one of the oldest intravenous anesthetic agents, ketamine, has a tendency to increase the amplitude of EPs (43).
Fig. 2.
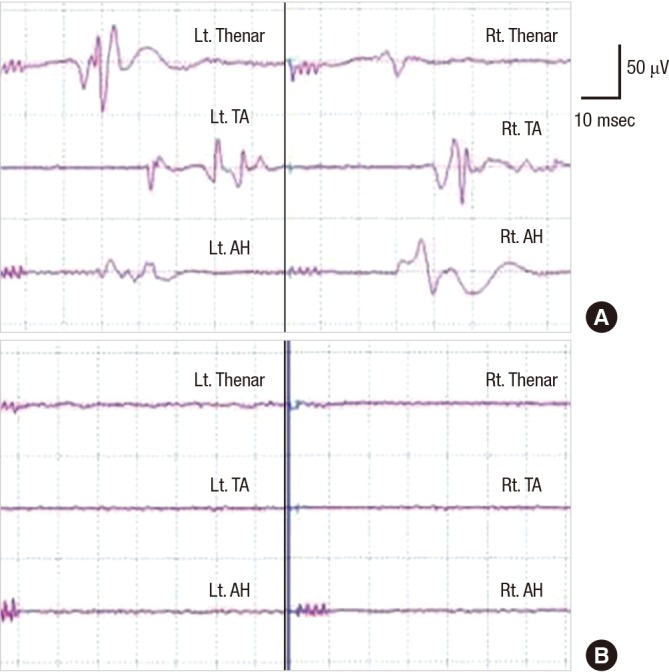
Halogenated anesthetics can abolish the generation of motor evoked potential (MEP). MEP is generated well in a patient underwent surgery with total venous anesthesia (A). The MEP in this patient completely disappears on using halogenated gas anesthetics (B). This patient had no post-operative neural deficit.
The use of muscle relaxants such as rocuronium or vecuronium for endotracheal intubation or during surgery can also affect signal transmission across the neuromuscular junction and can therefore decrease the amplitude or even abolish MEPs or EMG. Two types of tests can be used for assessing the degree of neuromuscular blockage: the first compares the amplitude of compound muscle action potentials obtained by stimulation of the peripheral motor nerves, before and after the injection of a muscle relaxant; the second, called the train of four (TOF), is more commonly used because of its convenience. Briefly, the TOF technique delivers 4 supramaximal electrical stimulations to the peripheral nerve, with a frequency of 2 Hz. When TOF produces more than 1 to 2 twitches, generally the degree of neuromuscular blockage obtained is regarded as acceptable to record MEP (6); however, it is evident that the more number of twitches obtained with TOF, the higher the generation rate of MEP would be (Fig. 3). This is important because in a considerable number of patients, especially those with significant preoperative motor weakness, MEPs cannot be generated (15).
Fig. 3.
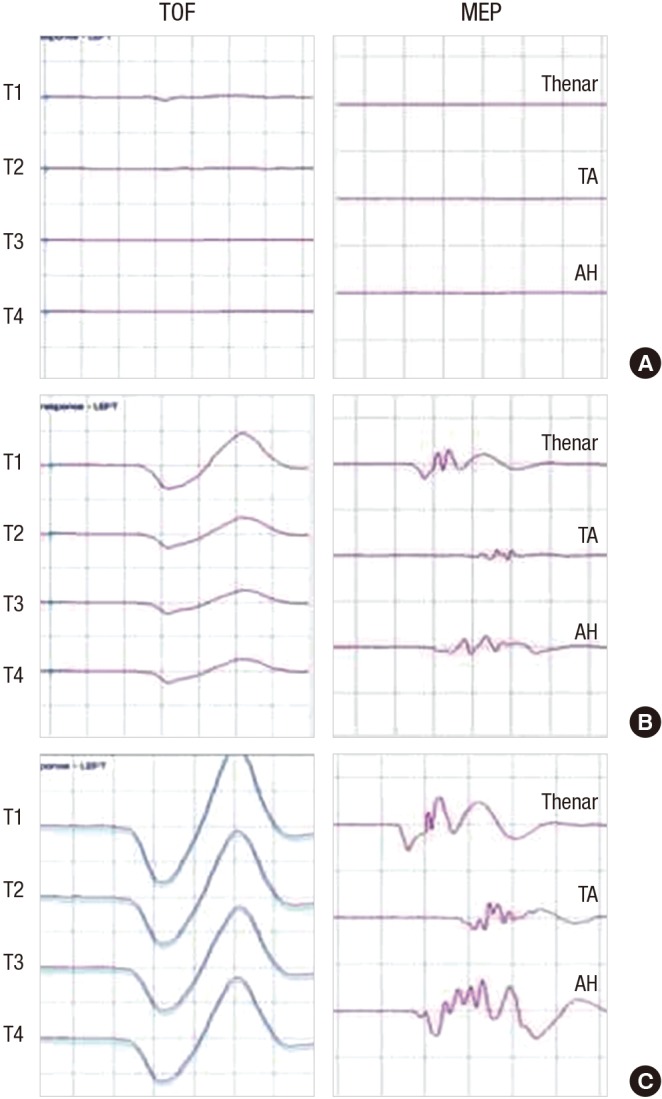
Train of four (TOF) can monitor the degree of neuromuscular blockage in IOM with muscle motor evoked potential (mMEP). Both number and amplitude of muscle twitch, obtained with four consecutive electrical stimulation at the motor nerve (TOF), represent the degree of neuromuscular blockage. No muscle twitch is observed with the TOF and no mMEP are generated (A). When 4 muscle twitches were observed with TOF but the amplitude fourth muscle twitch (T4) were relative small, compared with that of first muscle twitch (T1), mMEP with small amplitude are generated (B). When four muscle twitches with constant amplitude are observed with TOF, mMEP with large amplitude are observed (C).
Various causes of IOM wave changes other than the neural damages
In practice, all of the wave changes that occur during IOM do not represent neural damage; rather, many of the changes are associated with malfunctions of the IOM machine, inappropriate settings, artifact due to the surgical procedure, changes in anesthesia, and changes in hemodynamic state. Artifact can be commonly observed during IOM by electrocautery, hammering, electrical stimulation, electrical artifact of the heart (electrocardiography artifact), movement of respiratory muscles of patients, contact of cable with operating table, influence of electrical power lines or plug, or diverse surgical procedures. Though automated filters and artifact rejection program can be partly useful in reducing artifacts, the role of neurophysiologist is still crucial in discriminating such artifact from real IOM changes and also in reducing it. For this reason, it is essential that the clinical neurophysiologist is trained in basic electrophysiological principles and gains experience in IOM.
IOM FOR INDIVIDUAL SURGERIES
Cerebral tumor surgery
As was mentioned earlier in this article, IOM for cerebral tumors can be performed by routine monitoring of neural integrity using MEP and SSEP, as well as by neurophysiological testing to identify the exact location of eloquent areas, such as the motor or language cortex. This neurophysiological testing is generally performed using direct electrical stimulation (DES) of the eloquent areas during surgery. DES of the motor area can induce involuntary movement under general anesthesia, while DES of the language area can induce transient disturbances in language of awake patients, both of which in turn can enable the IOM team to identify the location of the motor or language areas (44, 45).
Brainstem surgery
In brainstem tumor surgery, routine motoring of brainstem function can be achieved by BAEP, SSEP, and MEP. In addition, stimulated or free-run EMG for the endangered cranial nerve (CN) or nucleus can be useful. To record the signals from the cranial motor nucleus or nerve, recording electrodes can be placed in the masseter muscle (CN V), facial muscles (CN VII), stylopharyngeal muscle (CN IX), muscles in the vocal cord and larynx (CN X), trapezius (CN XI), or tongue (CN XII) (46). Of those mentioned above, monitoring of CN X has traditionally required the additional use of a laryngoscope and some technical skills since the muscles innervated by this CN are located deep in the larynx. However, diverse types of endotracheal tube-based surface electrodes for vocal cord muscles (CN X) have recently been developed, which in turn have enabled simple EMG recordings of the vocal cord muscles (47, 48).
Another important use of IOM in brainstem surgery is the monitoring of lateral spread response (LSR) during microvascular decompression (MVD) of hemifacial spasm (HFS). LSR is an abnormal response of the facial muscles and is thought to be associated with the hyperexcitability of these muscles in patients with HFS (49, 50). Although there have been some debates on the exact mechanism of LSR generation, a recent study proposed that it is likely mediated by trigeminal afferent inputs, because it has similar latency to blink reflex (50). Several studies have shown that IOM for the disappearance of LSR during MVD can predict the surgical outcome both at the end of surgery and in the long term (49, 51).
Spinal surgery
In 2012, both the American Academy of Neurology (AAN) and the American Clinical Neurophysiology Society (ACNS) recommended that IOM using SSEPs and Tc-MEP be established as an effective means of predicting an increased risk of adverse outcomes, such as paraparesis, paraplegia, and quadriplegia, in spinal surgery (evidence level A); this was based on the results of several Class I and II studies (52). We have previously shown that Tc-mMEPs were more sensitive in the tibialis anterior muscles, but more specific in the abductor hallucis muscle for perioperative neural damage. Therefore, multi-muscle recording may be required to achieve a higher level of accuracy of IOM in spinal surgery (15).
In addition to SSEP and Tc-MEP, stimulated or free-run EMG can give the IOM team additional information, such as the proximity of procedures to the nerve root, the anatomical location of a motor rootlet, or irritation or traction of nerve root. Moreover, the correct placement of a pedicle screw can be screened simply by measuring electronic conductivity between the pedicle screw and the nerve root by using stimulated EMG (53, 54).
Vascular surgery
During CEA, up to 15% of patients experience cerebral hypoperfusion after carotid cross clamping (55). This hypoperfusion leads to changes in electrophysiological signals in the brain, such as a decrease in fast EEG activities and a decrease in the amplitude or increase in the latencies of SSEP. Thus, IOM can help detect patients who will eventually require a selective shunt during the CEA procedure (56). IOM can also predict the risk of postoperative neurological deficits in cerebral aneurysm surgery (25) and thoracic abdominal aneurysm surgery (11).
Thyroid, parathyroid, and esophagus surgery
In thyroid and parathyroid surgery, paralysis of the vocal cord due to injury of the recurrent laryngeal nerve (RLN) has been reported in up to 3% of cases. This can lead to hoarseness, voice loss, and even to airway emergency in severe cases (57). The RLN originates from the vagus nerve (CN X), descends into the thorax, and rises up to the neck to innervate muscles around the vocal cord and larynx. Therefore, monitoring of the RLN can be performed by recording the spontaneous EMG signals of the vocal cord muscles, as well as by recording simulated EMG signals evoked by electrical stimulation of the vagus nerve (CN X) or RLN itself (Fig. 4). (47) A recent randomized clinical trial demonstrated that IOM using RLN stimulation during thyroidectomy could significantly reduce postoperative RLN injury (58). Conventionally, EMG signals from the vocal cord were recorded using needle electrodes inserted with a laryngoscope. However, the use of needle electrodes poses the following risks: trauma, hematoma, laceration, infections inside the airway due to the misplacement or fracture of needle, need for re-intubation, and needle dislodgement (47). Recently developed endotracheal tube-based surface electrodes are more advantageous than the conventional needle electrodes because they do not cause airway trauma and can be easily manipulated in case of electrode misplacement during surgery. Together with the continuous vagal nerve stimulation techniques, the use of endotracheal tube-based surface electrodes has contributed to the wide-spread use of IOM during thyroidectomy (47, 59).
Fig. 4.
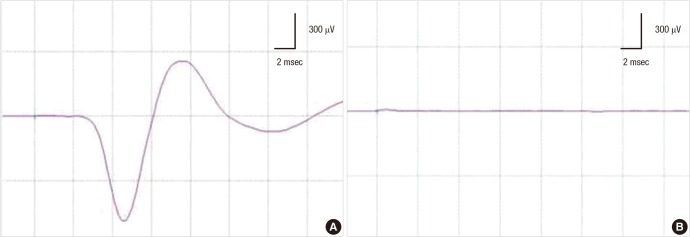
Monitoring for the recurrent laryngeal nerve injury in thyroid surgery. During thyroidectomy, compound muscle action potential (CMAP) is recorded at the vocal cord muscles, using continuous nerve stimulation technique (A). The CMAP is abruptly lost during surgery and does not recover at the end of surgery (B). This patient experienced post-operative complication of the vocal cord paralysis.
RLN palsy can also be found in patients who underwent esophageal cancer surgery. A recent study reported that IOM during esophagectomy can significantly reduce the incidence of postoperative RLN palsy (60). However, further successive studies focusing on the exact utility of IOM during esophagectomy and as a standard technical method are needed.
Peripheral nerve (including plexus)
Surgeries for tumors involving the peripheral nerves or nerve sheaths carry the risk of potential postoperative nerve damage. IOM of the peripheral nerve can be achieved by measuring nerve action potentials with DES on the peripheral nerve, as well as routine MEP and SSEP monitoring. This DES of the peripheral nerve enables mapping of the nerve fibers around the nerve sheath tumors, the identification of motor axons, and the stable monitoring of the motor nerve systems (61, 62).
CONCLUSION
Despite a long history of more than 30 yr, the use of IOM has only recently become widespread with the development of the commercial IOM machine and the availability of educational programs. The knowledge and experience of the clinical neurophysiologist in basic electrophysiology and electrophysiology in the operating room, comprehensive training of the monitoring technologist, active communication between members of the IOM team, and a tailored approach suited to the aim of IOM in specific surgeries will contribute greatly to the accuracy of IOM.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- 2.Nuwer MR. Intraoperative monitoring of neural function. Amsterdam: Elsevier; 2008. [Google Scholar]
- 3.Kothbauer K, Deletis V, Epstein FJ. Intraoperative spinal cord monitoring for intramedullary surgery: an essential adjunct. Pediatr Neurosurg. 1997;26:247–254. doi: 10.1159/000121199. [DOI] [PubMed] [Google Scholar]
- 4.Kothbauer KF, Deletis V, Epstein FJ. Motor-evoked potential monitoring for intramedullary spinal cord tumor surgery: correlation of clinical and neurophysiological data in a series of 100 consecutive procedures. Neurosurg Focus. 1998;4:e1. doi: 10.3171/foc.1998.4.5.4. [DOI] [PubMed] [Google Scholar]
- 5.Greiner A, Mess WH, Schmidli J, Debus ES, Grommes J, Dick F, Jacobs MJ. Cyber medicine enables remote neuromonitoring during aortic surgery. J Vasc Surg. 2012;55:1227–1232. doi: 10.1016/j.jvs.2011.11.121. [DOI] [PubMed] [Google Scholar]
- 6.Sloan TB, Heyer EJ. Anesthesia for intraoperative neurophysiologic monitoring of the spinal cord. J Clin Neurophysiol. 2002;19:430–443. doi: 10.1097/00004691-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Rohde V, Krombach GA, Baumert JH, Kreitschmann-Andermahr I, Weinzierl M, Gilsbach JM. Measurement of motor evoked potentials following repetitive magnetic motor cortex stimulation during isoflurane or propofol anaesthesia. Br J Anaesth. 2003;91:487–492. doi: 10.1093/bja/aeg224. [DOI] [PubMed] [Google Scholar]
- 8.Kalkman CJ, Ubags LH, Been HD, Swaan A, Drummond JC. Improved amplitude of myogenic motor evoked responses after paired transcranial electrical stimulation during sufentanil/nitrous oxide anesthesia. Anesthesiology. 1995;83:270–276. doi: 10.1097/00000542-199508000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Calancie B, Harris W, Broton JG, Alexeeva N, Green BA. "Threshold-level" multipulse transcranial electrical stimulation of motor cortex for intraoperative monitoring of spinal motor tracts: description of method and comparison to somatosensory evoked potential monitoring. J Neurosurg. 1998;88:457–470. doi: 10.3171/jns.1998.88.3.0457. [DOI] [PubMed] [Google Scholar]
- 10.Woodforth IJ, Hicks RG, Crawford MR, Stephen JP, Burke DJ. Variability of motor-evoked potentials recorded during nitrous oxide anesthesia from the tibialis anterior muscle after transcranial electrical stimulation. Anesth Analg. 1996;82:744–749. doi: 10.1097/00000539-199604000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs MJ, Meylaerts SA, de Haan P, de Mol BA, Kalkman CJ. Strategies to prevent neurologic deficit based on motor-evoked potentials in type I and II thoracoabdominal aortic aneurysm repair. J Vasc Surg. 1999;29:48–57. doi: 10.1016/s0741-5214(99)70349-6. [DOI] [PubMed] [Google Scholar]
- 12.Calancie B, Molano MR. Alarm criteria for motor-evoked potentials: what's wrong with the "presence-or-absence" approach? Spine (Phila Pa 1976) 2008;33:406–414. doi: 10.1097/BRS.0b013e3181642a2f. [DOI] [PubMed] [Google Scholar]
- 13.Szelényi A, Hattingen E, Weidauer S, Seifert V, Ziemann U. Intraoperative motor evoked potential alteration in intracranial tumor surgery and its relation to signal alteration in postoperative magnetic resonance imaging. Neurosurgery. 2010;67:302–313. doi: 10.1227/01.NEU.0000371973.46234.46. [DOI] [PubMed] [Google Scholar]
- 14.Deletis V, Sala F. Intraoperative neurophysiological monitoring of the spinal cord during spinal cord and spine surgery: a review focus on the corticospinal tracts. Clin Neurophysiol. 2008;119:248–264. doi: 10.1016/j.clinph.2007.09.135. [DOI] [PubMed] [Google Scholar]
- 15.Kim SM, Yang H, Park SB, Han SG, Park KW, Yoon SH, Hyun SJ, Kim HJ, Park KS, Lee KW. Pattern-specific changes and discordant prognostic values of individual leg-muscle motor evoked potentials during spinal surgery. Clin Neurophysiol. 2012;123:1465–1470. doi: 10.1016/j.clinph.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 16.Jankowska E, Padel Y, Tanaka R. Projections of pyramidal tract cells to alpha-motoneurones innervating hind-limb muscles in the monkey. J Physiol. 1975;249:637–667. doi: 10.1113/jphysiol.1975.sp011035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ulkatan S, Neuwirth M, Bitan F, Minardi C, Kokoszka A, Deletis V. Monitoring of scoliosis surgery with epidurally recorded motor evoked potentials (D wave) revealed false results. Clin Neurophysiol. 2006;117:2093–2101. doi: 10.1016/j.clinph.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 18.Dong CC, MacDonald DB, Janusz MT. Intraoperative spinal cord monitoring during descending thoracic and thoracoabdominal aneurysm surgery. Ann Thorac Surg. 2002;74:S1873–S1876. doi: 10.1016/s0003-4975(02)04137-1. [DOI] [PubMed] [Google Scholar]
- 19.Dinner DS, Lüders H, Lesser RP, Morris HH, Barnett G, Klem G. Intraoperative spinal somatosensory evoked potential monitoring. J Neurosurg. 1986;65:807–814. doi: 10.3171/jns.1986.65.6.0807. [DOI] [PubMed] [Google Scholar]
- 20.Nuwer MR, Dawson EG, Carlson LG, Kanim LE, Sherman JE. Somatosensory evoked potential spinal cord monitoring reduces neurologic deficits after scoliosis surgery: results of a large multicenter survey. Electroencephalogr Clin Neurophysiol. 1995;96:6–11. doi: 10.1016/0013-4694(94)00235-d. [DOI] [PubMed] [Google Scholar]
- 21.Thompson JE. Surgery for cerebrovascular insufficiency (stroke) with special emphasis on carotid endarterectomy. Springfield: Thomas; 1968. [Google Scholar]
- 22.Rampil IJ. A primer for EEG signal processing in anesthesia. Anesthesiology. 1998;89:980–1002. doi: 10.1097/00000542-199810000-00023. [DOI] [PubMed] [Google Scholar]
- 23.Akiyama T, Kobayashi K, Nakahori T, Yoshinaga H, Ogino T, Ohtsuka Y, Takeuchi M, Morita K, Sano S, Oka E. Electroencephalographic changes and their regional differences during pediatric cardiovascular surgery with hypothermia. Brain Dev. 2001;23:115–121. doi: 10.1016/s0387-7604(01)00192-9. [DOI] [PubMed] [Google Scholar]
- 24.Jansen C, Moll FL, Vermeulen FE, van Haelst JM, Ackerstaff RG. Continuous transcranial Doppler ultrasonography and electroencephalography during carotid endarterectomy: a multimodal monitoring system to detect intraoperative ischemia. Ann Vasc Surg. 1993;7:95–101. doi: 10.1007/BF02042666. [DOI] [PubMed] [Google Scholar]
- 25.Martin CJ, Sinson G, Patterson T, Zager EL, Stecker MM. Sensitivity of scalp EEG, cortical EGG, and somatosensory evoked responses during surgery for intracranial aneurysms. Surg Neurol. 2002;58:317–320. doi: 10.1016/s0090-3019(02)00881-9. [DOI] [PubMed] [Google Scholar]
- 26.Harper CM, Daube JR. Facial nerve electromyography and other cranial nerve monitoring. J Clin Neurophysiol. 1998;15:206–216. doi: 10.1097/00004691-199805000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Prass RL, Lüders H. Acoustic (loudspeaker) facial electromyographic monitoring: part 1. evoked electromyographic activity during acoustic neuroma resection. Neurosurgery. 1986;19:392–400. doi: 10.1097/00006123-198609000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Yingling CD, Ashram YA. Intraoperative monitoring of cranial nerves in skull base surgery. In: Jackler RK, Brackmann DE, editors. Neurotology. 2nd ed. Philadelphia: Elsevier Mosby; 2005. [Google Scholar]
- 29.Grundy BL, Jannetta PJ, Procopio PT, Lina A, Boston JR, Doyle E. Intraoperative monitoring of brain-stem auditory evoked potentials. J Neurosurg. 1982;57:674–681. doi: 10.3171/jns.1982.57.5.0674. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe E, Schramm J, Strauss C, Fahlbusch R. Neurophysiologic monitoring in posterior fossa surgery: II. BAEP-waves I and V and preservation of hearing. Acta Neurochir (Wien) 1989;98:118–128. doi: 10.1007/BF01407337. [DOI] [PubMed] [Google Scholar]
- 31.James ML, Husain AM. Brainstem auditory evoked potential monitoring: when is change in wave V significant? Neurology. 2005;65:1551–1555. doi: 10.1212/01.wnl.0000184481.75412.2b. [DOI] [PubMed] [Google Scholar]
- 32.Kodama K, Goto T, Sato A, Sakai K, Tanaka Y, Hongo K. Standard and limitation of intraoperative monitoring of the visual evoked potential. Acta Neurochir (Wien) 2010;152:643–648. doi: 10.1007/s00701-010-0600-2. [DOI] [PubMed] [Google Scholar]
- 33.Sasaki T, Itakura T, Suzuki K, Kasuya H, Munakata R, Muramatsu H, Ichikawa T, Sato T, Endo Y, Sakuma J, et al. Intraoperative monitoring of visual evoked potential: introduction of a clinically useful method. J Neurosurg. 2010;112:273–284. doi: 10.3171/2008.9.JNS08451. [DOI] [PubMed] [Google Scholar]
- 34.Torres CV, Pastor J, Rocío E, Sola RG. Continuous monitoring of cortical visual evoked potentials by means of subdural electrodes in surgery on the posterior optic pathway: a case report and review of the literature. Rev Neurol. 2012;55:343–348. [PubMed] [Google Scholar]
- 35.Chung SB, Park CW, Seo DW, Kong DS, Park SK. Intraoperative visual evoked potential has no association with postoperative visual outcomes in transsphenoidal surgery. Acta Neurochir (Wien) 2012;154:1505–1510. doi: 10.1007/s00701-012-1426-x. [DOI] [PubMed] [Google Scholar]
- 36.American Association of Neuromuscular and Electrodiagnostic Medicine. AANEM POSITION STATEMENT: the role of the intraoperative monitoring team. [accessed on 16 September 2008]. Available at http://www.aanem.org/getmedia/44fbb8e3-27db-44e8-90df-81797109be2f/IOMMonitoringTeam_000.pdf.aspx.
- 37.Health Care Financing Administration. Physician supervision of diagnostic tests. [accessed on 19 April 2001]. Available at http://www.aarc.org/members_area/advocacy/federal/md_supervision_tests.pdf.
- 38.Nuwer JM, Nuwer MR. Neurophysiologic surgical monitoring staffing patterns in the USA. Electroencephalogr Clin Neurophysiol. 1997;103:616–620. doi: 10.1016/s0013-4694(97)00065-5. [DOI] [PubMed] [Google Scholar]
- 39.American Academy of Neurology. Principles of Coding for Intraoperative Neurophysiologic Monitoring (IOM) and Testing Model Policy. [accessed on 10 February 2012]. Available at http://www.aan.com/uploadedFiles/Website_Library_Assets/Documents/3.Practice_Management/1.Reimbursement/1.Billing_and_Coding/5.Coverage_Policies/Coverage%20Policies%20-%20IONM.pdf.
- 40.Emerson R. Remote monitoring. In: Husain AM, editor. A practical approach to neurophysiologic intraoperative monitoring. New York: Demos; 2008. [Google Scholar]
- 41.Scheufler KM, Zentner J. Total intravenous anesthesia for intraoperative monitoring of the motor pathways: an integral view combining clinical and experimental data. J Neurosurg. 2002;96:571–579. doi: 10.3171/jns.2002.96.3.0571. [DOI] [PubMed] [Google Scholar]
- 42.Huotari AM, Koskinen M, Suominen K, Alahuhta S, Remes R, Hartikainen KM, Jäntti V. Evoked EEG patterns during burst suppression with propofol. Br J Anaesth. 2004;92:18–24. doi: 10.1093/bja/aeh022. [DOI] [PubMed] [Google Scholar]
- 43.Schubert A, Licina MG, Lineberry PJ. The effect of ketamine on human somatosensory evoked potentials and its modification by nitrous oxide. Anesthesiology. 1990;72:33–39. doi: 10.1097/00000542-199001000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere: an electrical stimulation mapping investigation in 117 patients. J Neurosurg. 1989;71:316–326. doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- 45.Duffau H. Lessons from brain mapping in surgery for low-grade glioma: insights into associations between tumour and brain plasticity. Lancet Neurol. 2005;4:476–486. doi: 10.1016/S1474-4422(05)70140-X. [DOI] [PubMed] [Google Scholar]
- 46.Møller AR. Intraoperative neurophysiologic monitoring. United Kingdom: Harwood Academic; 1995. [Google Scholar]
- 47.Randolph GW, Dralle H, Abdullah H, Barczynski M, Bellantone R, Brauckhoff M, Carnaille B, Cherenko S, Chiang FY, Dionigi G, et al. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope. 2011;121:S1–S16. doi: 10.1002/lary.21119. [DOI] [PubMed] [Google Scholar]
- 48.Brennan J, Moore EJ, Shuler KJ. Prospective analysis of the efficacy of continuous intraoperative nerve monitoring during thyroidectomy, parathyroidectomy, and parotidectomy. Otolaryngol Head Neck Surg. 2001;124:537–543. doi: 10.1067/mhn.2001.115402. [DOI] [PubMed] [Google Scholar]
- 49.Neves DO, Lefaucheur JP, de Andrade DC, Hattou M, Ahdab R, Ayache SS, Le Guerinel C, Keravel Y. A reappraisal of the value of lateral spread response monitoring in the treatment of hemifacial spasm by microvascular decompression. J Neurol Neurosurg Psychiatry. 2009;80:1375–1380. doi: 10.1136/jnnp.2009.172197. [DOI] [PubMed] [Google Scholar]
- 50.Ishikawa M, Ohira T, Namiki J, Ajimi Y, Takase M, Toya S. Abnormal muscle response (lateral spread) and F-wave in patients with hemifacial spasm. J Neurol Sci. 1996;137:109–116. doi: 10.1016/0022-510x(95)00308-o. [DOI] [PubMed] [Google Scholar]
- 51.Kong DS, Park K, Shin BG, Lee JA, Eum DO. Prognostic value of the lateral spread response for intraoperative electromyography monitoring of the facial musculature during microvascular decompression for hemifacial spasm. J Neurosurg. 2007;106:384–387. doi: 10.3171/jns.2007.106.3.384. [DOI] [PubMed] [Google Scholar]
- 52.Nuwer MR, Emerson RG, Galloway G, Legatt AD, Lopez J, Minahan R, Yamada T, Goodin DS, Armon C, Chaudhry V, et al. Evidence-based guideline update: intraoperative spinal monitoring with somatosensory and transcranial electrical motor evoked potentials: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the American Clinical Neurophysiology Society. Neurology. 2012;78:585–589. doi: 10.1212/WNL.0b013e318247fa0e. [DOI] [PubMed] [Google Scholar]
- 53.Ovadia D, Korn A, Fishkin M, Steinberg DM, Wientroub S, Ofiram E. The contribution of an electronic conductivity device to the safety of pedicle screw insertion in scoliosis surgery. Spine (Phila Pa 1976) 2011;36:E1314–E1321. doi: 10.1097/BRS.0b013e31822a82ec. [DOI] [PubMed] [Google Scholar]
- 54.Glassman SD, Dimar JR, Puno RM, Johnson JR, Shields CB, Linden RD. A prospective analysis of intraoperative electromyographic monitoring of pedicle screw placement with computed tomographic scan confirmation. Spine (Phila Pa 1976) 1995;20:1375–1379. [PubMed] [Google Scholar]
- 55.Guérit JM, Witdoeckt C, de Tourtchaninoff M, Ghariani S, Matta A, Dion R, Verhelst R. Somatosensory evoked potential monitoring in carotid surgery: I. relationships between qualitative SEP alterations and intraoperative events. Electroencephalogr Clin Neurophysiol. 1997;104:459–469. doi: 10.1016/s0168-5597(97)00022-1. [DOI] [PubMed] [Google Scholar]
- 56.Ackerstaff RG, Moons KG, van de Vlasakker CJ, Moll FL, Vermeulen FE, Algra A, Spencer MP. Association of intraoperative transcranial doppler monitoring variables with stroke from carotid endarterectomy. Stroke. 2000;31:1817–1823. doi: 10.1161/01.str.31.8.1817. [DOI] [PubMed] [Google Scholar]
- 57.Thomusch O, Sekulla C, Machens A, Neumann HJ, Timmermann W, Dralle H. Validity of intra-operative neuromonitoring signals in thyroid surgery. Langenbecks Arch Surg. 2004;389:499–503. doi: 10.1007/s00423-003-0444-9. [DOI] [PubMed] [Google Scholar]
- 58.Barczyński M, Konturek A, Cichoń S. Randomized clinical trial of visualization versus neuromonitoring of recurrent laryngeal nerves during thyroidectomy. Br J Surg. 2009;96:240–246. doi: 10.1002/bjs.6417. [DOI] [PubMed] [Google Scholar]
- 59.Friedrich C, Ulmer C, Rieber F, Kern E, Kohler A, Schymik K, Thon KP, Lamadé W. Safety analysis of vagal nerve stimulation for continuous nerve monitoring during thyroid surgery. Laryngoscope. 2012;122:1979–1987. doi: 10.1002/lary.23411. [DOI] [PubMed] [Google Scholar]
- 60.Zhong D, Zhou Y, Li Y, Wang Y, Zhou W, Cheng Q, Chen L, Zhao J, Li X, Yan X. Intraoperative recurrent laryngeal nerve monitoring: a useful method for patients with esophageal cancer. Dis Esophagus. 2012 doi: 10.1111/j.1442-2050.2012.01414.x. doi: 10.1111/j.1442-2050.2012.01414.x. [DOI] [PubMed] [Google Scholar]
- 61.Kwok K, Davis B, Kliot M. Resection of a benign brachial plexus nerve sheath tumor using intraoperative electrophysiological monitoring. Neurosurgery. 2007;60:316–320. doi: 10.1227/01.NEU.0000255375.34475.99. [DOI] [PubMed] [Google Scholar]
- 62.Hickey C, Gugino LD, Aglio LS, Mark JB, Son SL, Maddi R. Intraoperative somatosensory evoked potential monitoring predicts peripheral nerve injury during cardiac surgery. Anesthesiology. 1993;78:29–35. doi: 10.1097/00000542-199301000-00006. [DOI] [PubMed] [Google Scholar]


