Abstract
The pneumonia severity index (PSI) and CURB-65 are widely used tools for the prediction of community-acquired pneumonia (CAP). This study was conducted to evaluate validation of severity scoring system including the PSI and CURB-65 scores of Korean CAP patients. In the prospective CAP cohort (participated in by 14 hospitals in Korea from January 2009 to September 2011), 883 patients aged over 18 yr were studied. The 30-day mortalities of all patients were calculated with their PSI index classes and CURB scores. The overall mortality rate was 4.5% (40/883). The mortality rates per CURB-65 score were as follows: score 0, 2.3% (6/260); score 1, 4.0% (12/300); score 2, 6.0% (13/216); score 3, 5.7% (5/88); score 4, 23.5% (4/17); and score 5, 0% (0/2). Mortality rate with PSI risk class were as follows: I, 2.3% (4/174); II, 2.7% (5/182); III, 2.3% (5/213); IV, 4.5% (11/245); and V, 21.7% (15/69). The subgroup mortality rate of Korean CAP patients varies based on the severity scores and CURB-65 is more valid for the lower scores, and PSI, for the higher scores. Thus, these variations must be considered when using PSI and CURB-65 for CAP in Korean patients.
Keywords: Pneumonia, Prognosis, Severity Index
INTRODUCTION
Community-acquired pneumonia (CAP) is the leading cause of death from infectious disease in the world and is a major burden on healthcare resources (1, 2). In the assessment and management of CAP, disease severity assessment is crucial because it guides therapeutic options such as the need for hospital or intensive care unit (ICU) admission, the suitability of discharge to the home, the extent of the investigation, and the choice and route of the antimicrobial agent (3, 4). A number of studies suggest, however, that routine clinical judgment is often insufficient for assessing the severity of CAP. Clinical judgment alone may underestimate its severity (5) and may lead to variations in the rates of admission to a hospital (6, 7) or ICU (8). In addition, the decision to admit a patient to the ICU based on clinical judgment alone has been found to be suboptimal (9).
The Pneumonia Severity Index (PSI) developed by Fine et al. (10) in the USA provides a means of stratifying groups of patients according to their risk of mortality and features in recently published North American guidelines. Unfortunately, it is difficult to use because it requires computation of a score based on 20 variables. Thus, it may not be practical for routine application in busy hospital emergency departments or in a primary care setting. In addition, it is best validated for assessing patients with a low mortality risk who may be suitable for home management rather than those with severe CAP at the time of their hospital admission (11).
An international study conducted in Europe (5, 12) proposed a new clinical prediction rule, the CURB-65 score (confusion, urea>7 mM/L [19 mg/dL], respiratory rate≥30/min, systolic blood pressure<90 mmHg or diastolic blood pressure≤60 mmHg, and age≥65 yr). It uses a six-point scale that ranges from 0 to 5. It has limitations, however. For example, by stratifying patients into only two groups (severe or non-severe), it does not identify patients who have a low risk of mortality and who might be suitable for early hospital discharge or home management (11). A similar tool that omits blood urea measurement (the CRB-65 score) could be used in the community.
This study was conducted to compare the mortality rates of three cohort groups that include Korean cases that were assessed using the PSI and CURB-65 scores, and to evaluate the compatibility of these severity scores with community-acquired pneumonia.
MATERIALS AND METHODS
Study institutions and subjects
In the prospective cohort of CAP (participated in 14 hospitals in Korea from January 2009 to September 2011), consecutive 883 people in over 18-yr-old patients were studied. Thirteen participating study hospitals were teaching centers and one was a secondary hospital. The ethical approval was obtained from local hospital ethics committees. In all studies, CAP was defined as an acute respiratory tract illness associated with radiographic shadowing on an admission chest radiograph or computed tomography in 48 hr after admission and showing new infiltration or consolidation or pleural effusion consistent with pneumonia. The following exclusion criteria applied: 1) hospital-acquired pneumonia, 2) hospitalization over 72 hr previous 14 days, or 3) patients with tuberculosis, 4) secondary pneumonia (e.g., pulmonary seeding from primary bacteremia), 5) conditions likely to cause diagnostic confusion or where chest radiograph changes were equivocal, 6) immunocompromised patients, neutropenia (absolute neutrophil count<500/µL), leukemia, lymphoma, HIV infection, and splenectomy. Co-morbid illness was defined as the presence of any of the following conditions for which the patient was under active medical supervision or was receiving treatment at the time of hospital admission: alcoholism, chronic obstructive lung disease, bronchiectasis, obstruction of bronchus, smoking (over 10 pack-years), pulmonary aspiration, deteriorated mental status, influenza, nutritional deficiency, malignancy, cardiac disease (ischemic heart disease, cardiac failure, hypertension, atrial fibrillation), cerebrovascular disease (including previous transient ischemic attacks), diabetes mellitus, chronic liver disease, chronic renal disease. The main outcome measure was 30-day mortality. Laboratory findings were also collected. AST and ALT were documented of their highest value in the clinical course. Severity scoring system for CAP was performed for all patients including CURB-65, and PSI score. Hospitalized days defined from admission date to discharge date. Admission to an intensive care unit (ICU) was also investigated for outcome estimation. Follow up days defined from admission date to last outpatient date or discharge date in dead patients. For comparing cohort study group for 30-day mortality according to PSI score, we reviewed Medisgroups derivation cohort (10) (n=14,199) in 1989, Medisgroups validation cohort (n=38,039) (13) in 1991, Pneumonia PORT validation cohort (n=2,287) (14) in 1994. For comparing cohort study group for 30-day mortality according to CURB-65 score, we reviewed Lim et al. (11) derivation cohort (n =718) and Lim et al. (11) validation (n=214) in 2003, Capelastegui et al. (12) cohort study (n=1,776) in 2006.
Statistical analysis
The data were analyzed using SPSS version 18.0 for PASW 18.0 (SPSS Korea Datesolution, Inc., Seoul, Korea). The PSI score was calculated based on 20 criteria and classified into five risk classes (I to V). The CURB-65 scores were calculated based on five criteria (confusion, urea>7 mM/L [19 mg/dL], respiratory rate≥30/min, systolic blood pressure<90 mmHg or diastolic blood pressure≤60 mmHg, and age≥65 yr). They used a six-point scale that ranged from 0 to 5. Each potential predictor variable, each of the components of the CURB score and the PSI score, was analyzed via a frequency test and compared with the previous cohort study groups. A chi-square test and a Student t-test were performed on the demographic factors and clinical characteristics. The results were expressed with P values. The distributions of the PSI scores, CURB-65 scores, and subgroup mortality rates were analyzed with a chi-square test among the compared study groups. The ICU admission was also analyzed with a chi-square test. A two-tailed P value<0.05 was considered statistically significant.
Ethics statement
This study was approved by Kyungpook National University Hospital's institutional review board (KNUH_09-1069) and the institutional review boards of 13 other hospitals. The subjects' informed consent was waived by the boards due to the observation design of this study.
RESULTS
Patients' characteristics
A total of 883 patients, 882 inpatients (99.9%) and 1 outpatients (0.1%), were included in this study. Of these, the CURB-65 score and the PSI score was evaluated in all the patients. The comparison of the patients based on their PSI scores showed that in the Medisgroup study (13), the patients were younger in our study and the PORT validation cohort study (14) included more younger than our patients. Nursing-home residents were fewer than in the other two studies. Most of the co-existing conditions had a lower prevalence in our study than in the other two study groups, but neoplastic disease was more prevalent than in the Medisgroup derivation cohort study (10), and liver disease was the highest prevalence of all in our study. There were fewer abnormal findings in the most of the physical examination in our study than in the Medisgroup cohort study (13) and the PORT validation study (14). The demographic and clinical characteristics of the CAP patients who were compared based on the Pneumonia Severity Index (PSI) are shown in Table 1. The comparison of the patients based on their CURB-65 scores showed that the patients in our study, age more than 65 yr and males were intermediate prevalence among three study groups but nursing home residents were less than in the Capelastegui et al. (12) study. There were fewer abnormal physical examination findings in this study than in the cohort study of Lim et al. (11). The demographic and clinical characteristics of the CAP patients who were compared with CURB-65 are shown in Table 2.
Table 1.
Demographic and clinical characteristics of the community acquired pneumonia patients who were compared based on the Pneumonia Severity Index (PSI)
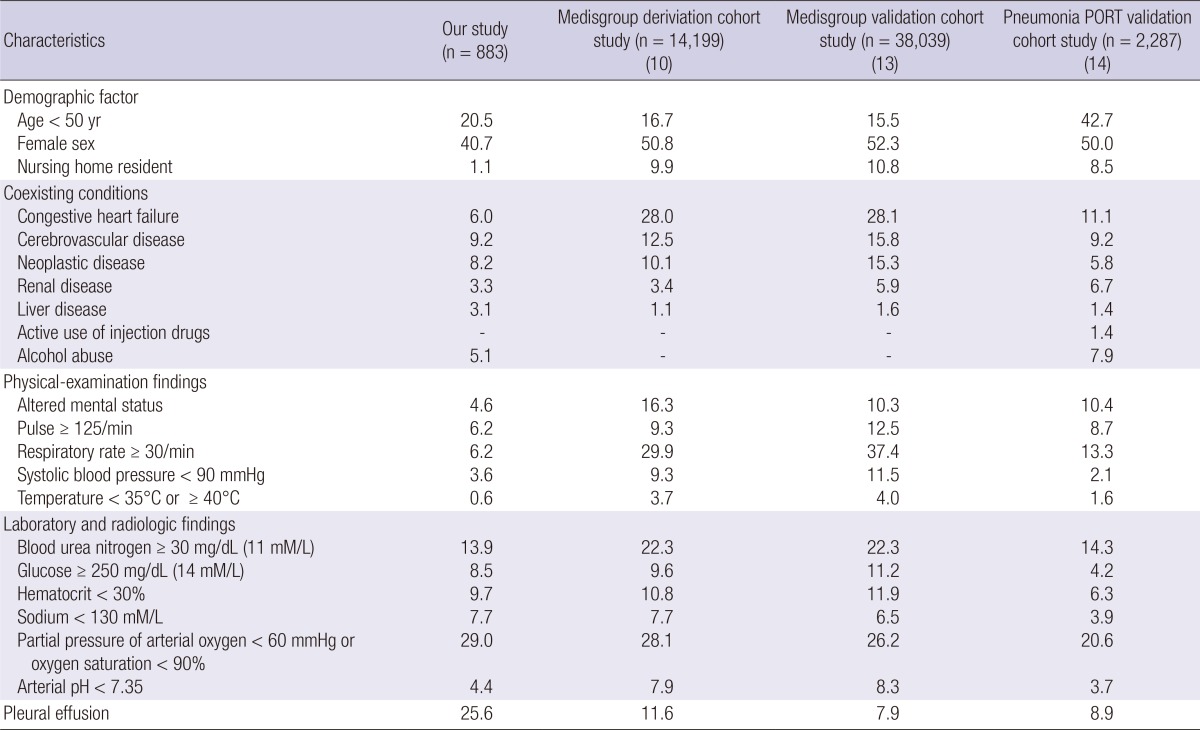
Data are % of patients, unless otherwise indicated.
Table 2.
Demographic and clinical characteristics of the community acquiredpneumonia patients who were compared with CURB-65
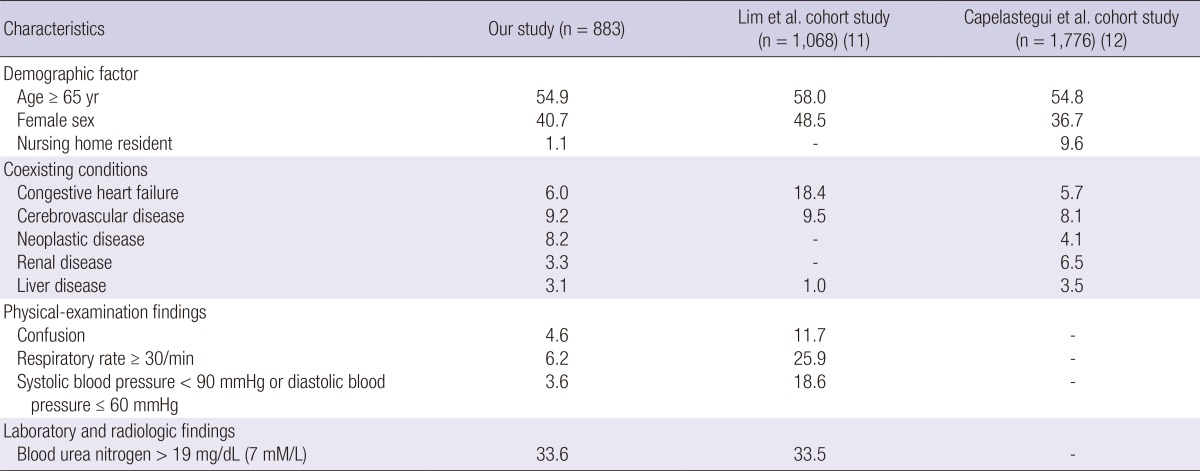
Data are % of patients, unless otherwize indicated.
30-day mortality
The total 30-day mortality of our study patients was 4.5% (40/883) and lowest among PSI study groups (Table 3 and Table 4). The subgroup 30-day mortality had a statistically significant increase with the increase of PSI (P<0.001) and CURB-65 score (P= 0.002) by linear by linear association in our study. At the PSI risk class I, the 30-day mortality in this study was 2.3%; in the Medisgroup derivation cohort study (10), 0.4% (P=0.012); in the Medisgroup validation cohort study (13), 0.1% (P<0.001); and in the PORT validation cohort study (14), 0.1% (P=0.005). At the PSI risk class II, the 30-day mortality in this study was 2.7%; in the Medisgroup derivation cohort study (10), 0.7% (P=0.004); in the Medisgroup validation cohort study (13), 0.6% (P<0.001); and in the PORT validation cohort study (14), 0.6% (P=0.044). At the PSI risk class IV, the 30-day mortality in this study was 4.5%; in the Medisgroup derivation cohort study (10), 8.5% (P=0.027); in the Medisgroup validation cohort study (13), 8.2% (P=0.035); and in the PORT validation cohort study (14), 9.3% (P=0.021). At the PSI risk class III and V, the 30-day mortality showed no statistically significant differences with other group (Table 3).
Table 3.
Distribution of the PSI scores and the subgroup mortality (chi-square test) of the community acquired pneumonia patients
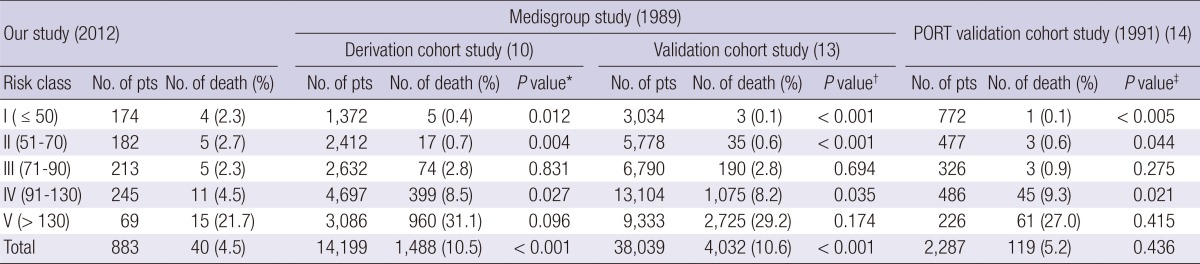
*The chi-square test was conducted between the Medisgroup (derivation cohort) study and our study for each risk class and total number of death; †The chi-square test was conducted between the Medisgroup (validation cohort) study and our study for each risk class and total number of death; ‡The chi-square test was conducted between the PORT validation cohort study and our study for each risk class and total number of death.
Table 4.
Distribution of CURB-65 score and subgroup mortality (chi-square test) of the community acquired pneumonia patients
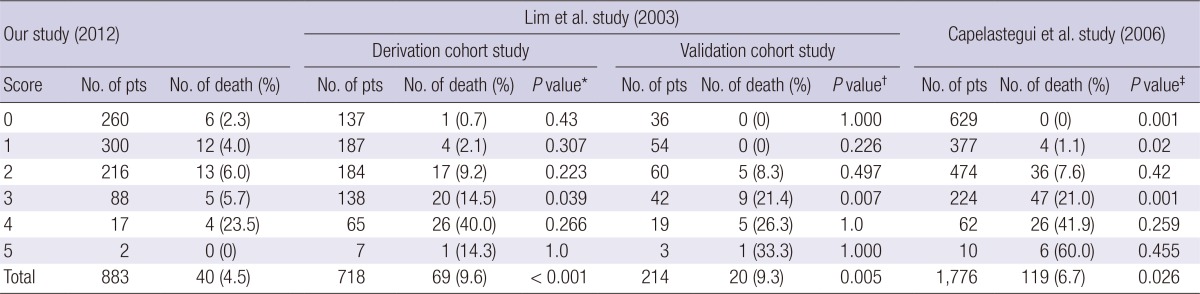
*Chi-square test was conducted between the Lim et al. (derivation cohort) study (11) and our study for each risk class and total number of death; †Chi-square test was conducted between the Lim et al. (validation cohort) study (11) and our study for each risk class and total number of death; ‡Chi-square test was conducted between the Capelastegui et al. study (12) and our study for each risk class and total number of death.
At the CURB-65 score of 0, the 30-day mortality in this study was 2.3%; 0.7% in the Lim et al. (11) derivation cohort study (P=0.43); 0% in the Lim et al. (11) validation cohort study (P=1.0); and 0% in the cohort study of Capelastegui et al. (P=0.001) (12). At the CURB-65 score 1, the 30-day mortality rate in this study was 4.0%; 2.1% in the Lim et al. (11) derivation cohort study (P=0.307); 0% in the Lim et al. (11) validation cohort study (P=0.226); and 1.1% in the Capelastegui et al. (12) (P=0.02) study. At the CURB-65 score of 3, the 30-day mortality in this study was 5.7%; in the Lim et al. (11) derivation cohort study, 14.5% (P=0.039); in the Lim et al. (11) validation cohort study, 21.4% (P=0.007); and in the Capelastegui et al. (12) cohort study, 21.0% (P=0.001). At the CURB-65 score of 4, 5, there was no statistically significant difference in the 30-day mortality with other studies (Table 4).
The further analysis showed that the causes of the death of 35 of the 40 patients who died were reviewed. Among the four patients at PSI risk class I, one died due to a non-infectious cause, and at PSI risk class II, one died due to a non-infectious cause and another, due to an unknown cause. At the CURB-65 score of 0, one patient died due to a non-infectious cause and two, due to unknown causes. At the CURB-65 score of 1, three died due to non-infectious causes and one, due to an unknown cause.
ICU admission
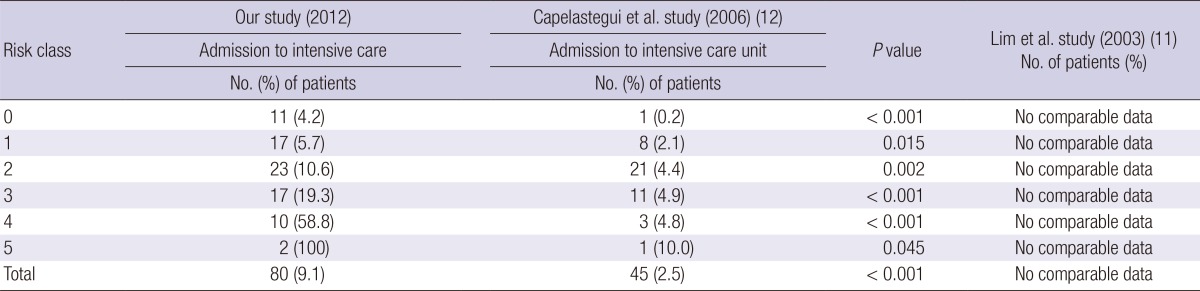
The admission to the ICU of all the patients and of the PSI subgroup in this study was compared with that in the PORT validation cohort study (14) and with that in the Capelastegui et al. (12) at the CURB-65 score. Such data could not be acquired in the Medisgroup study (13) and the Lim et al. (11) study. More patients had to be admitted to the ICU, according to the PSI in this study, among all the patients (P<0.001) than the PORT validation cohort study (14) (Table 5). ICU admission was also more common in this study according to the CURB-65 score, among all the patients (P<0.001) than Capelastegui et al. (12) (Table 6).
Table 5.
Medical outcomes according to the Pneumonia Severity Index of the community acquired pneumonia patients
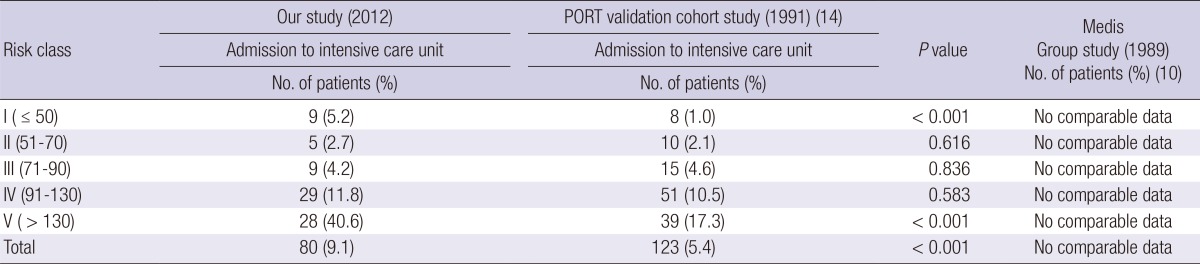
Table 6.
Medical outcomes according to CURB-65 score of the community acquired pneumonia patients
DISCUSSION
Several years have passed since tools for estimating the severity of CAP were devised. Overestimation or underestimation was the common problem with the PSI and CURB-65 scores since they were first devised.
The limitations of the severity scores are next discussed along with recent attempts to improve predictive tools, with the development of new biomarkers and alternative scoring systems (15). The study of Park et al. (16) was performed to investigate the clinical aspects of patients who satisfy the minor severity criteria of the Infectious Disease Society of America/American Thoracic Society (IDSA/ATS), focusing on their treatment response to empirical antibiotics. The minor severity criteria (≥3) were significantly associated with treatment failure (odds ratio, 2.838; 95% confidence interval, 1.216-6.626) (16). For predicting the treatment failure, the value of the area under the receiver operating characteristic curve for the minor criteria was 0.731, which was similar to that in other established scoring methods (16). The SEPAR/IDSA guidelines improved the following process-of-care indicators: appropriateness of treatment, unjustified hospital re-admission (39.4% in 2006 vs 8.5% in 2007 [P< 0.001], and 17.2% in 2008 [P=0.005]), and early treatment. Moreover, the mortality rates of the patients at risk classes IV-V, in which the PSI had been estimated, were lower than those that were measured using the SEQ/ATS guidelines (22.7%; P=0.003) (17). This study showed that PSI and CURB-65 score were still convenient and good predictors of mortality in CAP patients, but the subgroup mortality and pattern were different from the previous studies. Thus, there is a need to develop a more accurate and sensitive scoring system for mild to moderate CAP.
The total 30-day mortality of our study patients was 4.5% (40/883) and lowest among PSI study groups (our study, Medisgroup Derivation Cohort Study, Medisgroup Validation Cohort Study, and PORT Validation Cohort Study, Table 3). These results have the following implications. First, the improvement of the treatment antibiotics and the medical environment might significantly decrease the total mortality of community-acquired pneumonia. And we may assume more improved clinical practice with following formally published guidelines for CAP patients. Second, nursing-home residents were fewer in our study than in the other studies. Nursing-home residence is a known risk factor of morbidity and mortality (18). Other risk factors related to mortality might have a positive effect on mortality. Co-existing conditions were the criteria for the PSI score and are generally accepted as important risk factors (12). The comparison of the PSI scores showed that most of the co-existing conditions were less prevalent than in the other studies. These fewer rates of nursing-home residents and co-existing conditions might be contributed to the lowest total 30-day mortality. Third, the most laboratory findings were better in our study than in the Medisgroup studies (13) but worse than in the PORT validation study (14) in terms of the PSI scores, but the laboratory findings were only truly investigated in this study and used from tables or descriptions in other studies, a thorough comparison was not possible. Fewer abnormal findings from the physical examinations showed in our study than the most of the other studies. The lower prevalence of abnormal findings in the laboratory and physical examinations might be related to the lowest total 30-day mortality than in the other studies. This must not be concluded easily, however, because there were slight increases and decreases in each of the factors and study groups.
This study showed a lower 30-day mortality rate than that in the previous study at the PSI risk class IV and CURB-65 scores of 3, but a higher 30-day mortality rate at the PSI risk classes I and II and in some cases of CURB-65 scores 0 and 1. The all-cause mortality rate was calculated when the mortality rate was analyzed, so the mortality rates of the mild CAP patients might have been higher than those in previous studies. Some of the deaths due to non-infectious and unknown causes were included in the mild CAP patients, and this might result in higher mortality with the mild CAP patients.
The cases of the patients who had to be admitted to the ICU due to poorer medical outcomes were also reviewed (12). In this study, the need for ICU admission was more common than in the previous study, and was found to be an important factor that reflects the medical outcome and the CAP severity. This also means that this study did not have more clinically mild or moderate patients than the previous studies.
This study had some limitations. First, it had fewer patients than the previous study, which means a large-scale study is further needed. Second, the data from the previous studies were not perfect, and the numbers or percentages that were used were taken from published articles, so there were some difficulties in accurately comparing the clinical data. Third, most of the patients were inpatients, and outpatients were nearly not included. This might be a bias of this study. Fourth, the hospitals in this study were not distributed equally. The study of Chong et al. (19) on the bacterial etiology of CAP in Korea was conducted in secondary and tertiary hospitals and was revealed as representative of the epidemiology in Korea. Most of the hospitals that participated in this study were tertiary hospitals, and primary physicians were not included in it. Fourth, some of the deaths due to non-infectious and unknown causes were included in the mild CAP patients, and this might result in higher mortality with the mild CAP patients. This study was a multicenter study of community-acquired pneumonia in Korea, and is representative of the country. The PSI score and CURB-65 score were very useful and sensitive tools for estimating the severity of community-acquired pneumonia, but the appropriateness of their application to Koreans will be further studied.
In summary, the mortalities of CAP in this study, especially of severe CAP, which were obtained by comparing the PSI and CURB-65 scores, were lower than in those previously reported. However, the subgroup mortality varied in the severity score that was calculated with PSI or CURB 65. There was a more consistent trend in the lower scores with CURB-65 and in the higher scores with PSI in this study. Therefore, the use of PSI and CURB-65 for CAP in Korean patients should be considered.
Footnotes
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health & Welfare, Republic of Korea (A102065).
The authors have no conflicts of interest to disclose.
References
- 1.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM, Jr, Musher DM, Niederman MS, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44:S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song JH, Jung KS, Kang MW, Kim DJ, Pai H, Suh GY, Shim TS, Ahn JH, Ahn CM, Woo JH, et al. Treatment guidelines for community-acquired pneumonia in Korea: an evidence-based approach to appropriate antimicrobial therapy. Infect Chemother. 2009;41:133–153. [Google Scholar]
- 3.Bartlett JG, Dowell SF, Mandell LA, File TM, Jr, Musher DM, Fine MJ. Practice guidelines for the management of community-acquired pneumonia in adults: Infectious Diseases Society of America. Clin Infect Dis. 2000;31:347–382. doi: 10.1086/313954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mandell LA, Marrie TJ, Grossman RF, Chow AW, Hyland RH. Canadian guidelines for the initial management of community-acquired pneumonia: an evidence-based update by the Canadian Infectious Diseases Society and the Canadian Thoracic Society: the Canadian Community-Acquired Pneumonia Working Group. Clin Infect Dis. 2000;31:383–421. doi: 10.1086/313959. [DOI] [PubMed] [Google Scholar]
- 5.Neill AM, Martin IR, Weir R, Anderson R, Chereshsky A, Epton MJ, Jackson R, Schousboe M, Frampton C, Hutton S, et al. Community acquired pneumonia: aetiology and usefulness of severity criteria on admission. Thorax. 1996;51:1010–1016. doi: 10.1136/thx.51.10.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodhead MA, Macfarlane JT, McCracken JS, Rose DH, Finch RG. Prospective study of the aetiology and outcome of pneumonia in the community. Lancet. 1987;1:671–674. doi: 10.1016/s0140-6736(87)90430-2. [DOI] [PubMed] [Google Scholar]
- 7.Almirall J, Bolíbar I, Vidal J, Sauca G, Coll P, Niklasson B, Bartolomé M, Balanzó X. Epidemiology of community-acquired pneumonia in adults: a population-based study. Eur Respir J. 2000;15:757–763. doi: 10.1034/j.1399-3003.2000.15d21.x. [DOI] [PubMed] [Google Scholar]
- 8.Angus DC, Marrie TJ, Obrosky DS, Clermont G, Dremsizov TT, Coley C, Fine MJ, Singer DE, Kapoor WN. Severe community-acquired pneumonia: use of intensive care services and evaluation of American and British Thoracic Society Diagnostic criteria. Am J Respir Crit Care Med. 2002;166:717–723. doi: 10.1164/rccm.2102084. [DOI] [PubMed] [Google Scholar]
- 9.McQuillan P, Pilkington S, Allan A, Taylor B, Short A, Morgan G, Nielsen M, Barrett D, Smith G, Collins CH. Confidential inquiry into quality of care before admission to intensive care. BMJ. 1998;316:1853–1858. doi: 10.1136/bmj.316.7148.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, Coley CM, Marrie TJ, Kapoor WN. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 11.Lim WS, van der Eerden MM, Laing R, Boersma WG, Karalus N, Town GI, Lewis SA, Macfarlane JT. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capelastegui A, España PP, Quintana JM, Areitio I, Gorordo I, Egurrola M, Bilbao A. Validation of a predictive rule for the management of community-acquired pneumonia. Eur Respir J. 2006;27:151–157. doi: 10.1183/09031936.06.00062505. [DOI] [PubMed] [Google Scholar]
- 13.Fine MJ, Singer DE, Hanusa BH, Lave JR, Kapoor WN. Validation of a pneumonia prognostic index using the MedisGroups Comparative Hospital Database. Am J Med. 1993;94:153–159. doi: 10.1016/0002-9343(93)90177-q. [DOI] [PubMed] [Google Scholar]
- 14.Fine MJ, Stone RA, Singer DE, Coley CM, Marrie TJ, Lave JR, Hough LJ, Obrosky DS, Schulz R, Ricci EM, et al. Processes and outcomes of care for patients with community-acquired pneumonia: results from the Pneumonia Patient Outcomes Research Team (PORT) Cohort Study. Arch Intern Med. 1999;159:970–980. doi: 10.1001/archinte.159.9.970. [DOI] [PubMed] [Google Scholar]
- 15.Chalmers JD, Rutherford J. Can we use severity assessment tools to increase outpatient management of community-acquired pneumonia? Eur J Intern Med. 2012;23:398–406. doi: 10.1016/j.ejim.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Park SY, Park S, Lee MG, Kim DG, Suh GY, Kim C, Lee CY, Park YB, Jung KS. Minor criteria of Infectious Disease Society of America/American Thoracic Society for severe community-acquired pneumonia can predict delayed treatment response. J Korean Med Sci. 2012;27:907–913. doi: 10.3346/jkms.2012.27.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delgado M, Alvarez MM, Carrascosa I, Rodríguez-Velasco M, Barrios JL, Canut A. The routine use of the Pneumonia Severity Index in the emergency department: effect on process-of-care indicators and results in community acquired pneumonia. Enferm Infecc Microbiol Clin. 2013;31:289–297. doi: 10.1016/j.eimc.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 18.Naughton BJ, Mylotte JM. Treatment guideline for nursing home-acquired pneumonia based on community practice. J Am Geriatr Soc. 2000;48:82–88. doi: 10.1111/j.1532-5415.2000.tb03034.x. [DOI] [PubMed] [Google Scholar]
- 19.Chong YP, Jung KS, Lee KH, Kim MN, Moon SM, Park S, Hur J, Kim DM, Jeon MH, Woo JH. The bacterial etiology of community-acquired pneumonia in Korea: a nationwide prospective multicenter study. Infect Chemother. 2010;42:397–403. [Google Scholar]


