Abstract
Chronic inflammation is thought to be the leading cause of colorectal cancer, and interleukin-10 (IL10) has been identified as a potent immunomodulatory cytokine that regulates inflammatory responses in the gastrointestinal tract. Although several single nucleotide polymorphisms (SNPs) in IL10 have been associated with the risk of colorectal cancer, their prognostic significance has not been determined. Two hundred and eighty-two colorectal cancer patients were genotyped for two candidate cancer-associated SNPs in IL10. The associations of these SNPs with distant metastasis-free survival and overall survival were evaluated by Kaplan-Meier analysis and Cox regression model. The minor homozygote GG genotype of IL10 rs3021094 was significantly associated with a 3.30-fold higher risk of death compared with the TT+TG genotypes (P=0.011). The patients with IL10 rs3021094 GG genotype also had a poorer overall survival in Kaplan-Meier analysis (log-rank P=0.007) and in multivariate Cox regression model (P=0.044) adjusting for age, gender, carcinoembryonic antigen levels, tumor differentiation, stage, lymphovascular invasion, and perineural invasion. In conclusion, our results suggest that IL10 rs3021094 might be a valuable prognostic biomarker for colorectal cancer patients.
Keywords: Colorectal Neoplasms, Inflammation, Interleukin-10, Polymorphism, Survival
INTRODUCTION
Chronic inflammation is a well-established risk factor for colorectal cancer. However, the mechanisms involved in this process are still poorly understood. The current opinion is that there is an overreaction of the immune system toward antigens of the gut microbiota leading to chronic inflammation (1). Chronic accumulation of activated immune cells is accompanied by the release of proinflammatory cytokines. These cytokines can lead to the generation of oxygen and nitrogen reactive species, which are known to induce dysplasia by inducing DNA modifications in intestinal epithelial cells (2). These processes have been associated with altered expression of genes contributed to tumor growth such as p53, APC, and K-ras (3). However, activation of the immune system has also been shown to cause dysplastic cell elimination and preventing tumor progression under certain conditions (4).
The action of cytokines can be crudely described as pro-inflammatory (e.g., interleukin [IL]-6 and tumor necrosis factor-α) or anti-inflammatory (e.g., IL10 and transforming growth factor-β). The main biological function of IL10 is to limit inflammatory responses and is crucial for maintaining the immune homeostasis of the gastrointestinal tract (5). Animal studies showed that IL10-deficient mice spontaneously developed colorectal cancer when infected with enteric bacteria. Administration of exogenous IL10 could prevent colorectal cancer development in this model, indicating that IL10 is pivotal in the control of inflammation and inflammation-related colorectal cancer (6). IL10 gene is polymorphic and common single nucleotide polymorphisms (SNPs) of IL10 have been associated with colorectal cancer risk (7-9). Here, we investigated the prognostic significance of two candidate haplotype tagging SNPs (tSNPs) in IL10 on distant metastasis-free and overall survival in a cohort of 282 colorectal cancer patients.
MATERIALS AND METHODS
Patient recruitment and data collection
This cohort was generated from the tumor tissue bank at China Medical University Hospital, Taiwan. All patients seen at China Medical University Hospital, Taiwan with a diagnosis of cancers were approached to participate. Two hundred and eighty-two patients with histopathologically confirmed colorectal cancer were identified between 2001 and 2007; had consented to provide information and tissue; and had undergone blood collection for research purposes. The clinical data and outcomes were obtained from patients' clinical records and pathological reports. Among patients receiving curative surgery (stage I-III, n=233), distant metastasis-free survival was defined as the time from surgery to the date of distant metastases or when censored at the latest date. Overall survival was defined as the time from diagnosis (n=282) to the date of death from any cause or when censored at the latest date if patients were still alive. The survival data were updated most recently in 2008.
SNP selection and genotyping
Two candidate tSNPs in IL10 were selected based on the evidence of association with risk of colorectal cancer (9). The tSNP rs3024496 within the 3' untranslated region of IL10 captures 6 SNPs, rs1800890, rs2222202, rs3024491, rs3024493, rs3024495, and rs3024505, located across promoter, intron 1, intron 3, intron 4, and 3' downstream in linkage disequilibrium according to HapMap CHB (Han Chinese in Beijing, China) population data (10). The tSNP rs3021094 locates within the first intron of the IL10.
Genomic DNA was extracted from peripheral blood using the QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA, USA) and stored at -80℃ until the time of study. Genotyping was performed as described previously (11-16) using Sequenom iPLEX matrix-assisted laser desorption/ionization-time of flight mass spectrometry technology at the National Center for Genome Medicine, Academia Sinica, Taiwan. The average genotype call rate for these SNPs was 99.6% and the frequencies of the SNPs were in Hardy-Weinberg equilibrium (P>0.01).
Statistical analysis
Patient clinicopathologic characteristics were summarized as number and percentage of patients or median and interquartile range (IQR) of values. Age was dichotomized at the median value within the cohort. Carcinoembryonic antigen (CEA) level was dichotomized at 5 µg/L because of its correlation with an increasing stage of the colorectal cancer (17). The associations of IL10 tSNPs and clinical characteristics with distant metastasis-free and overall survival were assessed using the Cox proportional hazards regression model and Kaplan-Meier analysis with log-rank test. We tested different genetic models, dominant, recessive, and additive models for each tSNP. The model with the most significant P value was considered the best-fitting model. Multivariate analyses to determine the interdependency of genotypes and other known prognostic factors, such as age at diagnosis, gender, CEA levels, tumor differentiation, stage, lymphovascular invasion, perineural invasion, and lymph node involvement, were carried out using Cox proportional hazards regression model. Statistical Package for the Social Sciences software version 19.0.0 (IBM, Armonk, NY) was used for other statistical analyses. A two-sided P value of <0.05 was considered statistically significant.
Ethic statement
This study was approved by the institutional review board of the China Medical University Hospital (DMR98-IRB-210). Written informed consent was obtained from all patients.
RESULTS
The clinicopathologic characteristics of 282 colorectal cancer patients are summarized in Table 1. Twenty-five (10.7%) patients developed distant metastasis after curative surgery with a median follow-up of 24.8 months. Eighteen (6.4%) patients died after a median follow-up of 28.2 months. Younger age, higher stage, positive lymphovascular invasion, and lymph node involvement had a significantly higher risk of developing distant metastasis (P≤0.033). Gender, CEA levels, stage, and lymph node involvement were significantly associated with overall survival (P≤0.046).
Table 1.
Demographic and clinical characteristics of colorectal cancer patients
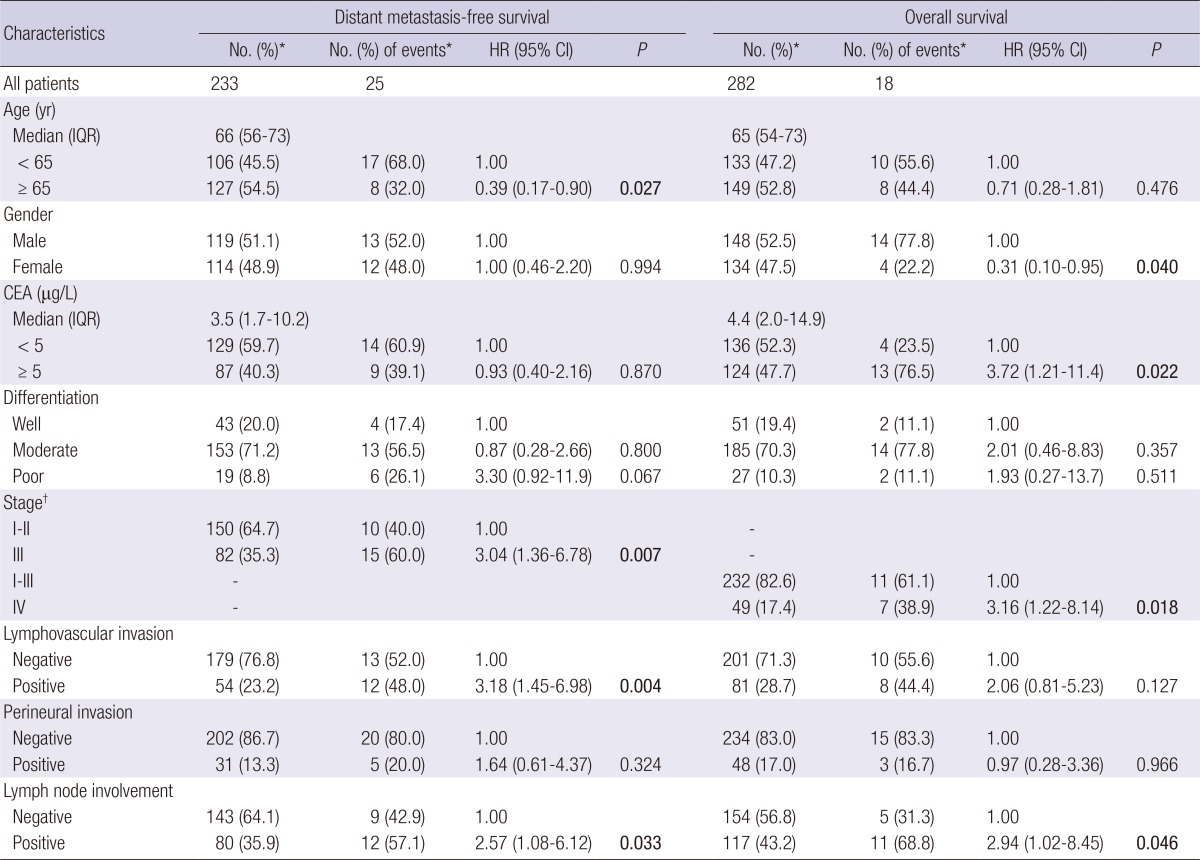
*Column subtotals do not sum to No. of patients and No. of events due to missing data; †According to the American Joint Committee on Cancer - Cancer Staging Manual (version 6.0). P<0.05 are in boldface. CEA, carcinoembryonic antigen; IQR, interquartile range; HR, Hazard ratio; CI, confidence interval.
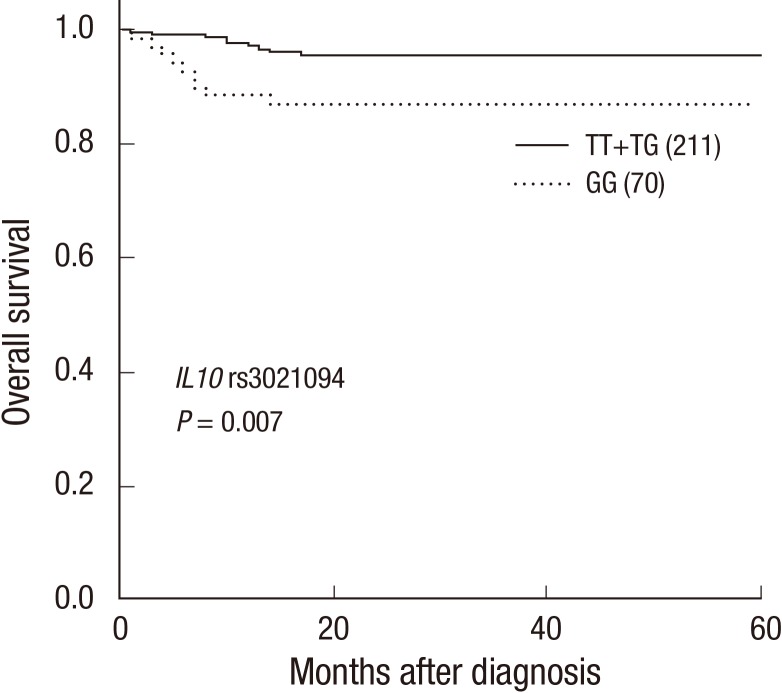
We assessed the association of IL10 tSNPs with distant metastasis-free and overall survival using a univariate Cox model (Table 2). We identified that the IL10 rs3021094 GG genotype had a 3.30-fold higher risk of death compared with the TT+TG genotypes (P=0.011). Kaplan-Meier survival curves and log-rank test also showed that the IL10 rs3021094 GG genotype was significantly associated with poorer overall survival compared with the TT+TG genotypes (P=0.007; Fig. 1).
Table 2.
Association of IL10 polymorphisms with distant metastasis-free survival and overall survival
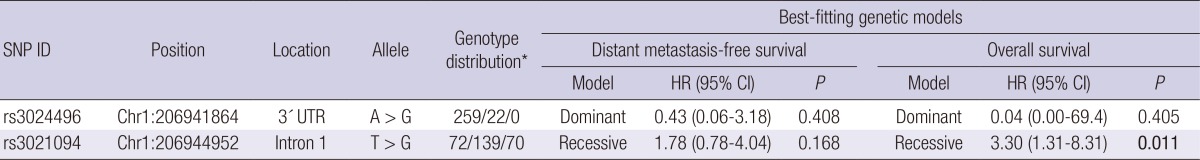
*The number represents major allele homozygotes, heterozygotes, and minor allele homozygotes, respectively. P<0.05 are in boldface. SNP, single nucleotide polymorphism; HR, Hazard ratio; CI, confidence interval; UTR, untranslated region.
Fig. 1.
Kaplan-Meier curves of overall survival by the genotypes at IL10 rs3021094. Numbers in parentheses indicate the number of patients. Subtotal does not sum to 282 patients due to missing data.
To determine the interdependency of IL10 rs3021094 with overall survival, a multivariate Cox proportional hazards model, including age, gender, CEA levels, tumor differentiation, stage, lymphovascular invasion, perineural invasion, lymph node involvement, and IL10 rs3021094, was performed (Table 3). None of the factors reached significance when all clinical factors were included in the multivariate analysis (P>0.05; Model 1). However, IL10 rs3021094 remained as a significant predictor for overall survival without adjusting for lymph node involvement (P=0.044; Model 2) because of a weak association between IL10 rs3021094 and lymph node involvement (P=0.107; data not shown). We did not observe any other noteworthy association between IL10 tSNPs and other clinical characteristics listed in Table 1.
Table 3.
Multivariate analysis of factors associated with overall survival
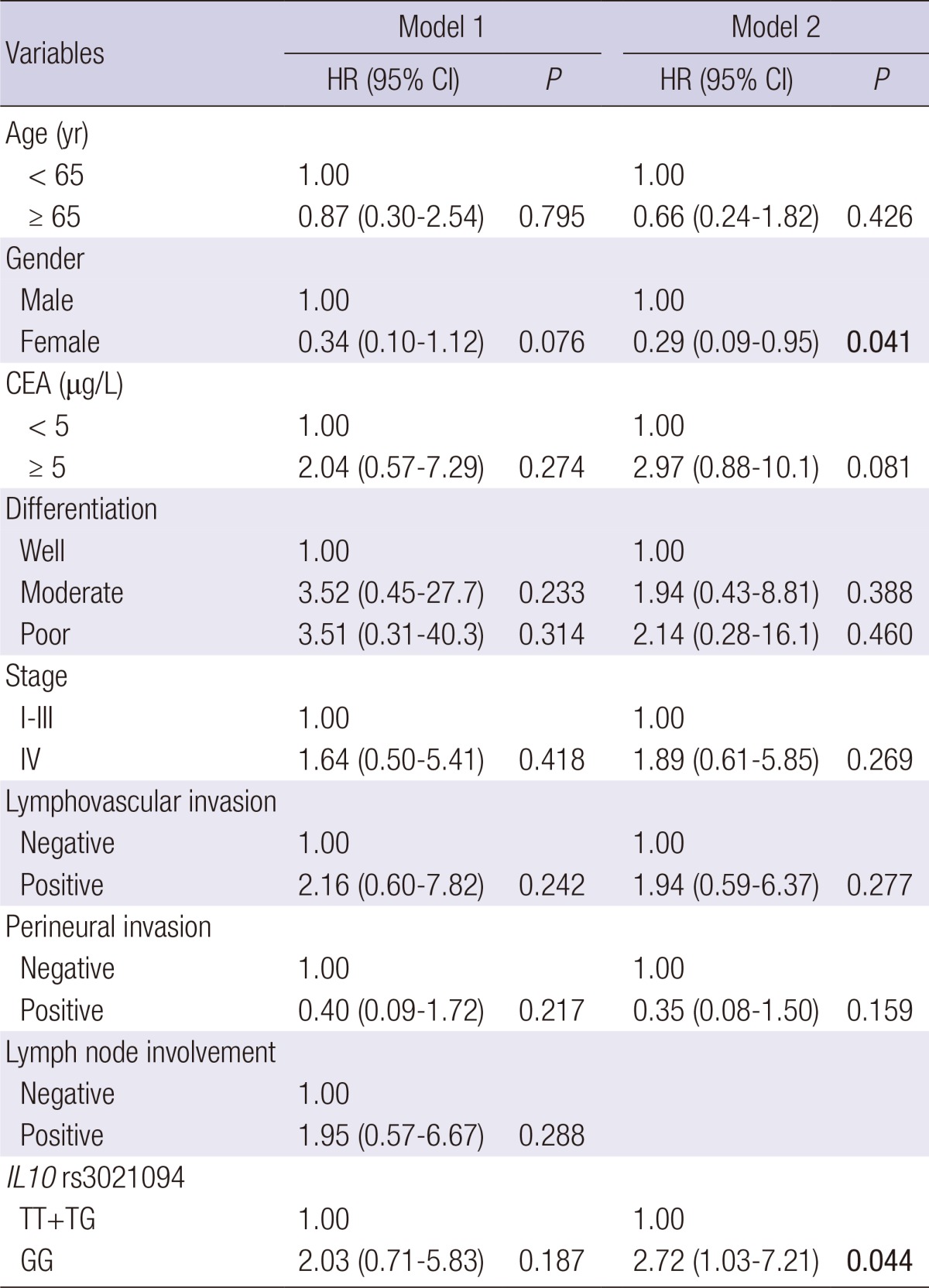
P<0.05 are in boldface. CEA, carcinoembryonic antigen; HR, Hazard ratio; CI, confidence interval; Multivariate models included age, gender, CEA levels, tumor differentiation, stage, lymphovascular invasion, perineural invasion, IL10 rs3021094, or lymph node involvement.
DISCUSSION
This translational study discovered that the IL10 tSNP, rs3021094, might serve as a potential prognostic determinant for colorectal cancer. The result remained significant after adjusting for most of the major clinicopathologic characteristics. To our knowledge, this study might provide the first indication that a tSNP of IL10, a key cytokine involved in immune homeostasis, is significantly associated with the clinical outcomes in colorectal cancer patients.
IL10 has a pivotal role in the immunity of the gastrointestinal tract, and IL10-deficient mice have been shown to develop colitis and colorectal cancer (6). A recent genome-wide association study implicated a 3' downstream IL10 SNP, rs3024505 (captured by rs3024496 in this study), to the susceptibility of ulcerative colitis, a subtype of inflammatory bowel disease (18). However, we did not observed significant association between IL10 rs3024496 and clinical outcomes in colorectal cancer, suggesting that IL10 might play different roles during colorectal cancer development and progression. Another candidate tSNP, rs3021094, is located in the first intron of the IL10 gene, and is predicted to alter a putative transcription factor binding site for SP3 transcription factor, according to the SNPInfo analysis (19). It has been demonstrated that IL10 expression is regulated by the constitutively and ubiquitously expressed SP1 and SP3 as key transcription factors (20). Therefore, it is possible that IL10 rs3021094 might influence IL10 expression by altering the consensus transcription factor binding site for SP3. Further fine-mapping and functional analyses are required to provide molecular biology evidence of how IL10 rs3021094 translates into physiologic processes and affects colorectal cancer progression.
In conclusion, we observed a statistically significant association between a SNP, rs3021094, in the anti-inflammatory cytokine IL10 and survival in colorectal cancer patients. We are cautious in our interpretation of these data, because the study is limited by its small size, short follow-up period, and homogeneous Chinese Han population. However, we view this finding as exploratory and given the emerging evidence on chronic inflammation in colorectal carcinogenesis. Further independent external studies are warranted and might lead to new intervention or prevention strategies for colorectal cancer.
ACKNOWLEDGEMENTS
We thank National Genotyping Center of National Research Program for Genomic Medicine, National Science Council, Taiwan, for their technical support.
Footnotes
This work was supported by the National Science Council (NSC), Taiwan (grant number: NSC-98-2320-B-039-019-MY3, NSC-100-2314-B-039-009-MY3 and NSC-102-2628-B-039-005-MY3), and China Medical University (grant number: CMU98-N1-21 and CMU98-C-12).
The authors have no conflicts of interest to disclose. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 2.Meira LB, Bugni JM, Green SL, Lee CW, Pang B, Borenshtein D, Rickman BH, Rogers AB, Moroski-Erkul CA, McFaline JL, et al. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J Clin Invest. 2008;118:2516–2525. doi: 10.1172/JCI35073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie J, Itzkowitz SH. Cancer in inflammatory bowel disease. World J Gastroenterol. 2008;14:378–389. doi: 10.3748/wjg.14.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 5.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 6.Berg DJ, Davidson N, Kühn R, Müller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunter MJ, Canzian F, Landi S, Chanock SJ, Sinha R, Rothman N. Inflammation-related gene polymorphisms and colorectal adenoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1126–1131. doi: 10.1158/1055-9965.EPI-06-0042. [DOI] [PubMed] [Google Scholar]
- 8.Macarthur M, Sharp L, Hold GL, Little J, El-Omar EM. The role of cytokine gene polymorphisms in colorectal cancer and their interaction with aspirin use in the northeast of Scotland. Cancer Epidemiol Biomarkers Prev. 2005;14:1613–1618. doi: 10.1158/1055-9965.EPI-04-0878. [DOI] [PubMed] [Google Scholar]
- 9.Tsilidis KK, Helzlsouer KJ, Smith MW, Grinberg V, Hoffman-Bolton J, Clipp SL, Visvanathan K, Platz EA. Association of common polymorphisms in IL10, and in other genes related to inflammatory response and obesity with colorectal cancer. Cancer Causes Control. 2009;20:1739–1751. doi: 10.1007/s10552-009-9427-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frazer KA, Ballinger DG, Cox DR, Hinds DA, Stuve LL, Gibbs RA, Belmont JW, Boudreau A, Hardenbol P, Leal SM, et al. A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007;449:851–861. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bao BY, Pao JB, Huang CN, Pu YS, Chang TY, Lan YH, Lu TL, Lee HZ, Chen LM, Ting WC, et al. Significant associations of prostate cancer susceptibility variants with survival in patients treated with androgen-deprivation therapy. Int J Cancer. 2012;130:876–884. doi: 10.1002/ijc.26091. [DOI] [PubMed] [Google Scholar]
- 12.Bao BY, Pao JB, Huang CN, Pu YS, Chang TY, Lan YH, Lu TL, Lee HZ, Juang SH, Chen LM, et al. Polymorphisms inside microRNAs and microRNA target sites predict clinical outcomes in prostate cancer patients receiving androgen-deprivation therapy. Clin Cancer Res. 2011;17:928–936. doi: 10.1158/1078-0432.CCR-10-2648. [DOI] [PubMed] [Google Scholar]
- 13.Bao BY, Pao JB, Lin VC, Huang CN, Chang TY, Lan YH, Lu TL, Lee HZ, Chen LM, Ting WC, et al. Individual and cumulative association of prostate cancer susceptibility variants with clinicopathologic characteristics of the disease. Clin Chim Acta. 2010;411:1232–1237. doi: 10.1016/j.cca.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 14.Huang CN, Huang SP, Pao JB, Chang TY, Lan YH, Lu TL, Lee HZ, Juang SH, Wu PP, Pu YS, et al. Genetic polymorphisms in androgen receptor-binding sites predict survival in prostate cancer patients receiving androgen-deprivation therapy. Ann Oncol. 2012;23:707–713. doi: 10.1093/annonc/mdr264. [DOI] [PubMed] [Google Scholar]
- 15.Huang CN, Huang SP, Pao JB, Hour TC, Chang TY, Lan YH, Lu TL, Lee HZ, Juang SH, Wu PP, et al. Genetic polymorphisms in oestrogen receptor-binding sites affect clinical outcomes in patients with prostate cancer receiving androgen-deprivation therapy. J Intern Med. 2012;271:499–509. doi: 10.1111/j.1365-2796.2011.02449.x. [DOI] [PubMed] [Google Scholar]
- 16.Huang SP, Lan YH, Lu TL, Pao JB, Chang TY, Lee HZ, Yang WH, Hsieh CJ, Chen LM, Huang LC, et al. Clinical significance of runt-related transcription factor 1 polymorphism in prostate cancer. BJU Int. 2011;107:486–492. doi: 10.1111/j.1464-410X.2010.09512.x. [DOI] [PubMed] [Google Scholar]
- 17.Wanebo HJ, Rao B, Pinsky CM, Hoffman RG, Stearns M, Schwartz MK, Oettgen HF. Preoperative carcinoembryonic antigen level as a prognostic indicator in colorectal cancer. N Engl J Med. 1978;299:448–451. doi: 10.1056/NEJM197808312990904. [DOI] [PubMed] [Google Scholar]
- 18.Franke A, Balschun T, Karlsen TH, Sventoraityte J, Nikolaus S, Mayr G, Domingues FS, Albrecht M, Nothnagel M, Ellinghaus D, et al. Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nat Genet. 2008;40:1319–1323. doi: 10.1038/ng.221. [DOI] [PubMed] [Google Scholar]
- 19.Xu Z, Taylor JA. SNPinfo: integrating GWAS and candidate gene information into functional SNP selection for genetic association studies. Nucleic Acids Res. 2009;37:W600–W605. doi: 10.1093/nar/gkp290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tone M, Powell MJ, Tone Y, Thompson SA, Waldmann H. IL-10 gene expression is controlled by the transcription factors Sp1 and Sp3. J Immunol. 2000;165:286–291. doi: 10.4049/jimmunol.165.1.286. [DOI] [PubMed] [Google Scholar]