Abstract
Deep vein thrombosis (DVT) and subsequent pulmonary embolism (PE) remain significant causes of morbidity, mortality in patients with spinal cord injury (SCI). Since incidence of DVT after SCI in Korean population has not been much studied, we retrospectively analyzed the medical records of 185 SCI patients admitted for acute rehabilitation unit to investigate the incidence of DVT. Color Doppler ultrasonography was performed to screen for the occurrence of DVT at the time of initial presentation to acute rehabilitation unit. Primary study outcome was the incidence of DVT. Possible risk factors for DVT including the epidemiologic characteristics, completeness of motor paralysis, cause of injury, spasticity, surgery, and active cancer were analyzed. The incidence of DVT after SCI was 27.6%. In multiple logistic regression analysis, absence of spasticity was a significant independent risk factor (P<0.05) for occurrence of DVT. Symptomatic pulmonary embolism was evident in 7 patients without an episode of sudden death. Therefore, it is concluded that the incidence of DVT after SCI in Korean patients is comparable with that in Western populations. This result suggests that pharmacologic thromboprophylaxis should be considered in Korean patients with SCI.
Keywords: Deep Vein Thrombosis, Incidence, Spinal Cord Injuries, Rehabilitation Unit
INTRODUCTION
Deep vein thrombosis (DVT) is common in patients after spinal cord injury (SCI). DVT and subsequent pulmonary embolism (PE) remain significant causes of morbidity, mortality and substantial healthcare costs in patients with SCI. Venous thromboembolism remains the third leading cause of death after SCI and the incidence of PE has been estimated as 4.6% (1).
The incidence of DVT varies significantly among different ethnic/racial groups. While Asian patients have been reported to have a significantly lower incidence of idiopathic and secondary venous thromboembolism, as compared to Western patients (2-4), several recent studies have challenged this view (5, 6). The Assessment of the Incidence of Deep-vein thrombosis in Asia (AIDA) multicenter multinational prospective study reported that the rate of venographic thrombosis in the absence of thromboprophylaxis after major joint surgery in Asian patients was similar to that previously reported in Western patients (5). The Surgical Multinational Asian Registry in Thrombosis (SMART) venography prospective observational study reported that the incidence of asymptomatic and symptomatic venous thromboembolism after major orthopedic surgery was 36.5% (6). Therefore, the incidence of venous thromboembolism after major orthopedic surgery in Asian patients may not be low. It may be consistent with the rates observed in Western countries.
In case of SCI, the incidence of DVT also varies from population-to-population and from country-to-country. In previous studies involving Western SCI patients without pharmacologic thromboprophylaxis, the reported rates of DVT varied widely, from 14%-100% (7-10). Although the real incidence of DVT in Western SCI patients is not known due to different diagnostic methods and inhomogeneity of the subject, it was quite high in some of reports. The incidence of DVT in patients with acute SCI without pharmacologic thromboprophylaxis was reported as 21% in Japan and 3% in India (11, 12). The prevalence of DVT in patients with SCI following the Pakistan earthquake of October 2005 was as low as 4.8% (13). In Korea, overall incidence of DVT after SCI patients were 5.7% in a retrospective study (14). However, in a recent prospective study for Korean population, it was high enough to come close to that in Western patients. In this study, incidence of DVT was reported 43% (4). Discrepancy between above two studies suggests that the incidence of DVT after SCI in Korean population has not been studied sufficiently.
A few studies have evaluated the incidence of DVT after SCI in the acute rehabilitation setting. In a prior Western-based study, the incidence of DVT was 6.6% for first rehabilitation with pharmacologic thromboprophylaxis (15). Another study reported an incidence of 16.4% without pharmacologic thromboprophylaxis at admission to rehabilitation (16). However, the rate in Asian countries is unclear. In addition, most of studies on the incidence of DVT were usually limited to a traumatic origin. So, it is difficult to know the exact incidence of DVT after SCI from all possible cause without pharmacologic thromboprophylaxis.
The aim of this study was to evaluate the incidence of DVT for Korean patients without pharmacologic thromboprophylaxis after SCI in the acute rehabilitation setting and to determine the risk factors for DVT.
MATERIALS AND METHODS
Subjects
We retrospectively reviewed the medical records of SCI patients admitted to acute rehabilitation unit in one tertiary referral hospital from January 2002 to July 2011. The inclusion criteria were traumatic or non-traumatic SCI with an American Spinal Injury Association Impairment Scale (AIS) category of A, B, C, or D with who were not able to ambulate with bipedal locomotion. All patients underwent color Doppler ultrasound (US) as described below to screen for the occurrence of DVT in both lower extremities at the time of initial presentation for acute rehabilitation. This routine screening process for early detection of DVT was one of the standard initial assessing processes for patients with SCI in the rehabilitation department of our hospital. Patients with a history of venous thromboembolism before SCI or before admittance to the acute rehabilitation unit, patients with a known abnormality of the coagulative system, or patients receiving pharmacologic thromboprophylaxis such as unfractionated heparin, low-molecular-weight heparin (LMWH), heparinoids or vitamin K antagonists before acute rehabilitation unit were excluded. Although pharmacologic thromboprophylaxis procedures were not performed, all patients wore gradient compression stocking and engaged in passive range of motion exercise to prevent thromboembolic events. In addition, intermittent pneumatic compression was routinely applied to paralyzed lower extremities since 2010. Spasticity was defined as an Ashworth Scale score of 1 or greater. We defined categories of AIS A and B as completeness of motor paralysis.
Color doppler US for screening of the occurrence of DVT
DVT of the lower extremities and pelvis were assessed by an experienced examiner using an ACUSON Antares US system (Siemens Medical Solution, Mountain View, CA, USA) or an IU 22 US system (Philips, Best, The Netherlands). Examinations were performed at the time of initial presentation to the acute rehabilitation unit regardless of symptoms and signs of DVT. Patients were performed color Doppler US during rehabilitation in case of clinical symptom of DVT. Deep venous system of both lower extremities was examined from the inguinal ligaments to the ankle. Compatible findings with DVT in color Doppler US were the presence of visible embolus or dilated incompressible vessel with loss of phasic pattern or absence of Doppler velocity signal. DVT was classified as proximal (thrombosis at the level of popliteal veins or above) or distal (thrombosis affecting the calf veins). Visible swelling or increased calf girth circumference (>3 cm) compared to the contralateral limb were accepted as a clinical symptom of DVT as detected by color Doppler US. PE was evaluated by multi-slice spiral computed tomography pulmonary angiography (CTPA). Regardless of the presence of DVT by initial screening color Doppler US, PE was studied by CTPA in all cases where there was a clinical suspicion of PE, such as sudden-onset dyspnea, tachypnea, chest pain, and oxygen desaturation during the rehabilitation admission period. During the second half of this study, routine CTPA was performed in all patients who had DVT detected by screening US.
Outcome measurement
Primary study outcome was the incidence of DVT detected by color Doppler US at the time of initial presentation to the acute rehabilitation unit, regardless of whether it was symptomatic or not. Risk factor analysis was performed for following possible risk factors of DVT including the epidemiologic characteristics, completeness of motor paralysis, AIS, level of injury, cause of injury, spasticity, surgery (spine surgery that performed under general anesthesia), and active cancer.
Statistical methods
Fisher's exact test, chi-square test, and Mann-Whitney U test were used to determine significant differences in occurrence of DVT with respect to age, sex, completeness of motor paralysis, AIS, level of injury, cause of injury, spasticity, surgery, and active cancer. Multiple logistic regression analysis was performed to confirm independent predictive factors. All statistical analysis was performed using IBM SPSS statistical software version 19.0. P values<0.05 were regarded as significant.
Ethics statement
The study protocol was approved by the institute review board of Samsung Medical Center (IRB No. 2011-12-075). Informed consent was waived.
RESULTS
One hundred and eighty-five patients were enrolled in this study. There were 133 men and 52 women with an average age 49.1 yr (range, 17-83 yr) at the time of injury. Forty-eight patients were AIS grade A, 32 were grade B, 69 were grade C, and 36 were grade D. Eighty SCI patients had non-traumatic causes and 105 had traumatic causes (Table 1).
Table 1.
Summary of demographics and etiologies
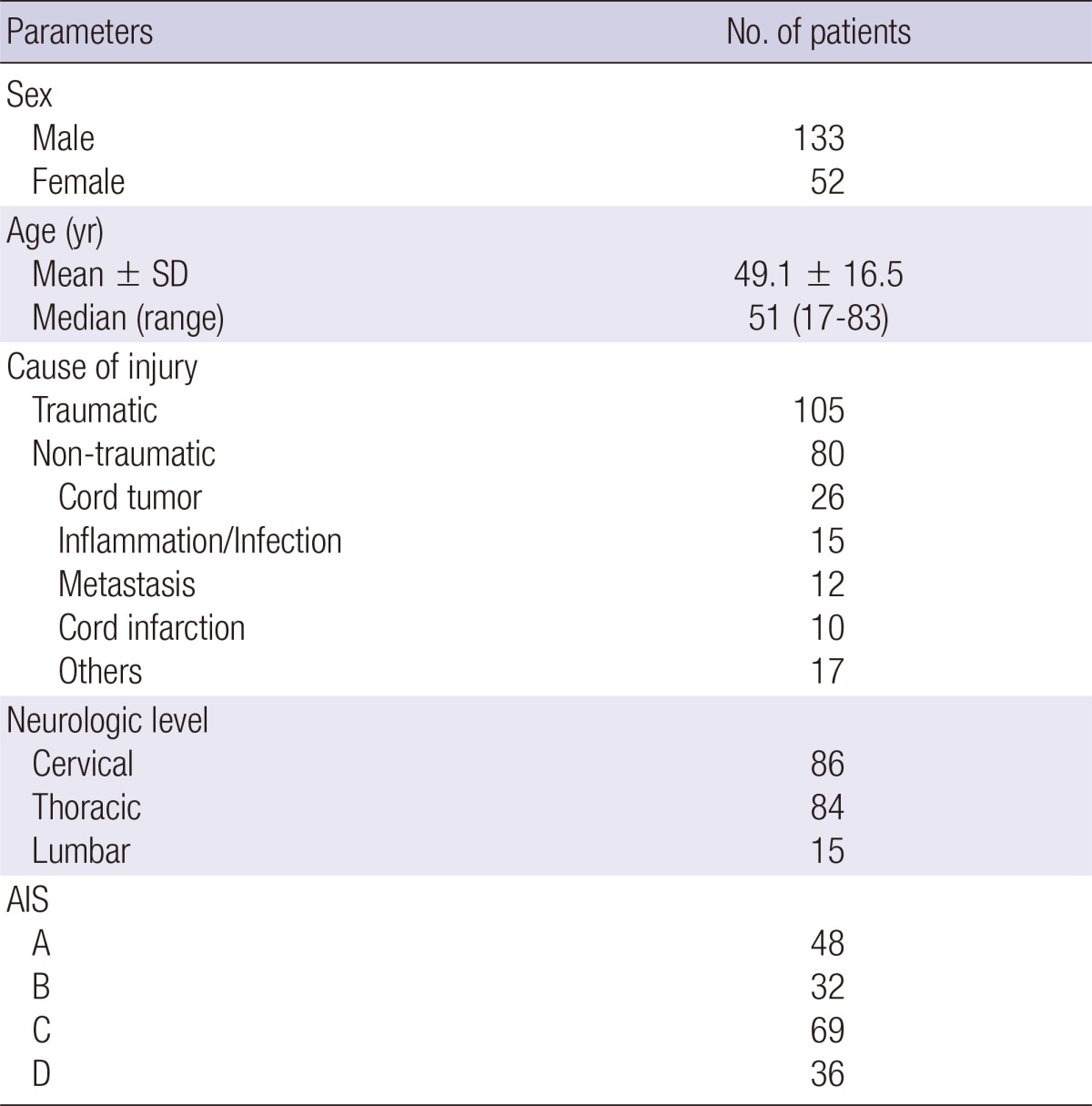
SD, standard deviation; AIS, American Spinal Injury Association Impairment Scale.
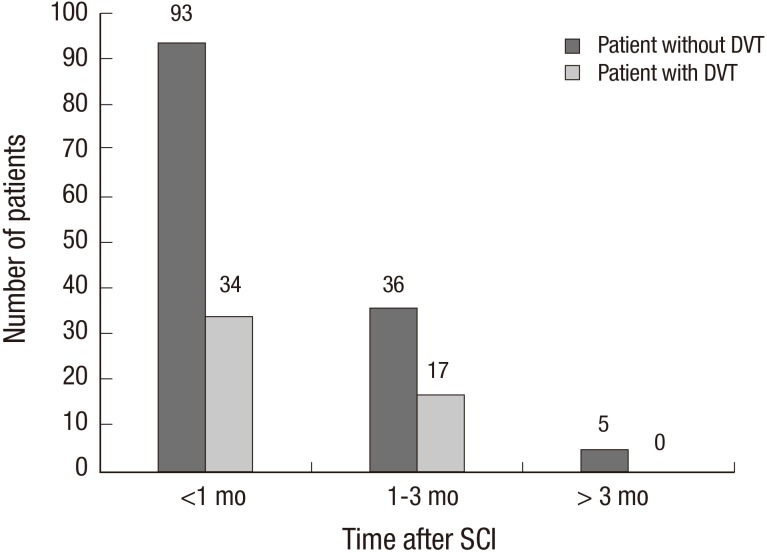
DVT was detected in 51 of the 185 patients. The incidence of DVT after SCI was 27.6% (185 patients, 95% confidence interval [CI] 21.1-34.0) at the time of initial presentation to the acute rehabilitation unit. During rehabilitation two cases of DVT were newly detected by color Doppler US. The mean interval from to injury to initial color Doppler US screening was 31.7 days (range, 3-345 days). Ten of the 185 patients (5.4%, 95% CI 2.1-8.7) had symptomatic DVT. Forty-one of 175 asymptomatic patients (23.4%, 95% CI 20.3-26.5) had DVT. Thirty-four of 127 patients (26.8%) patients displayed thrombosis within 1 month after SCI, and 17 of 53 patients (32.1%) displayed thrombosis 1-3 months after SCI. No patients displayed thrombosis more than 3 months after SCI (Fig. 1).
Fig. 1.
Time distribution on the detection of DVT by color Doppler US. DVT, deep vein thrombosis; Mo, month; SCI, spinal cord injury; US, ultrasound.
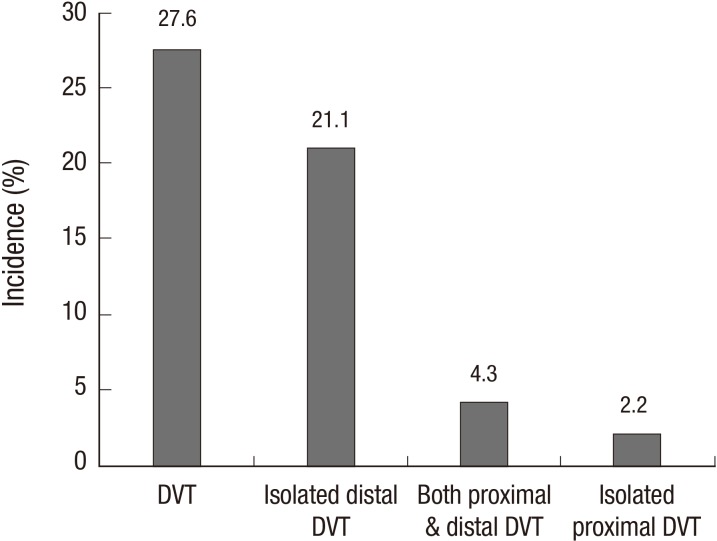
Proximal DVT was present in 12 of the 185 patients (6.5%, 95% CI 2.9-10.0). Four patients had an isolated proximal DVT, 8 patients had both proximal and distal DVT, and 39 patients had an isolated distal DVT. DVT in the iliofemoral vein was observed in nine of the 185 patients (4.9%, 95% CI 1.8-7.9) (Fig. 2).
Fig. 2.
The incidence and location of DVT. DVT, deep vein thrombosis.
CTPA was performed on 32 patients with DVT and 21 patients without DVT. PE was conformed in 13 patients. Nine of the 32 patients with DVT and four of the 21 patients without DVT had PE. Seven of the 185 patients (3.8%, 95% CI 1.0-6.5) had symptomatic PE. No death was occurred during the rehabilitation period due to venous thromboembolism.
Age, sex, completeness of motor paralysis, AIS, level of injury, cause of injury, surgery, and active cancer were not significantly associated with occurrence of DVT. In univariate analysis, absence of surgery and absence of spasticity were significantly associated with the primary study outcome (Table 2). Multivariate analysis confirmed that absence of spasticity was a significant independent risk factor (P<0.05) for occurrence of DVT, with an odds ratio of 3.28 (95% CI 1.148-9.372) (Table 3).
Table 2.
Univariate analyses of risk factors of DVT
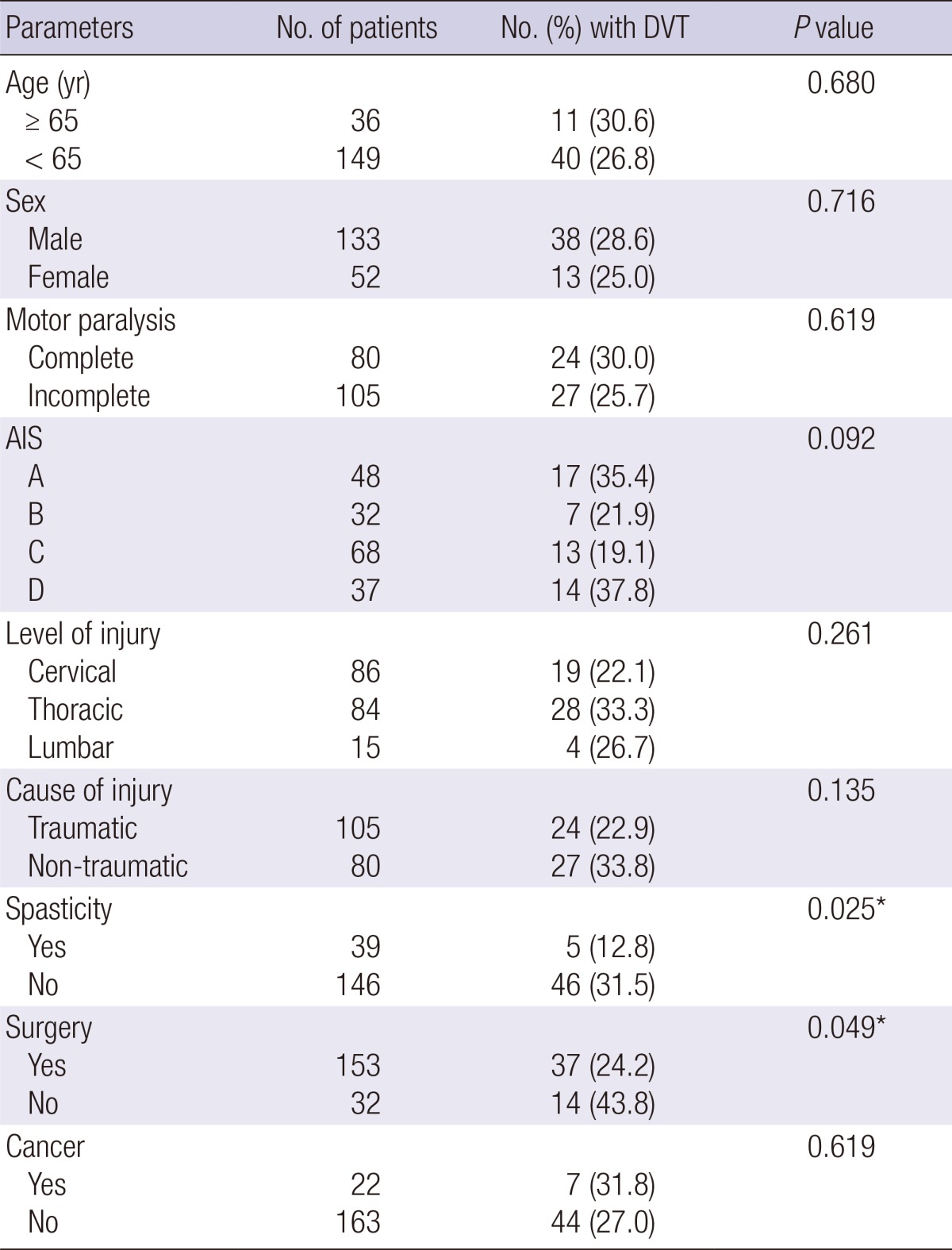
*P<0.05. DVT, deep vein thrombosis; AIS, American Spinal Injury Association Impairment Scale.
Table 3.
Multivariate analyses of significant risk factors for DVT after SCI
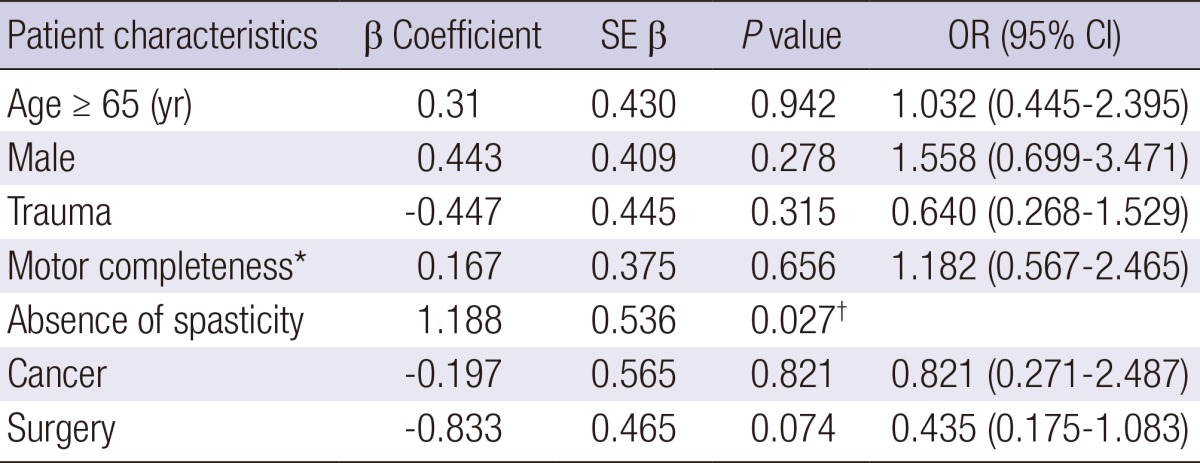
R2=0.101, *American Spinal Injury Association Impairment Scale A and B, †P<0.05. DVT, deep vein thrombosis; SCI, spinal cord injury; CI, confidence interval; OR, odds ratio; SE β, standard error of β coefficient.
DISCUSSION
In this study of Korean patients with SCI, DVT was detected in 51 of the 185 patients (27.6%) without pharmacologic thromboprophylaxis after SCI at the time of initial presentation to the acute rehabilitation unit. Although international consensus statements recommend routine pharmacologic thromboprophylaxis for all patients after acute SCI (3), pharmacologic thromboprophylaxis is not usually performed in Asian patients because of the presumed markedly low incidence of DVT after SCI (11, 13). A recent prospective study of the incidence of DVT for a small number of Korean patients reported a similar value as Western patients did (4). In the study, 16 of 37 patients (43%) with acute SCI routinely given mechanical prophylaxis without anticoagulation had DVT, based on the findings of color Doppler US. The findings of the previous and current studies provide evidence that the incidence of DVT in Korean populations without pharmacologic prophylaxis after SCI may not be appreciably lower than those in Western populations, but instead may be similar. If so, this has profound implications for pharmacologic thromboprophylaxis.
There are several explanations for the comparable incidence of DVT after SCI in Korean populations with Western populations. In some previous Asian-based studies, Doppler US was carried out only upon clinical suspicion of DVT (13, 14). Thus, asymptomatic DVT might have been missed in those studies. On the other hand, five of the 18 Korean patients (27.7%) had DVT when duplex US performed routinely for every SCI patients (14). There is a possibility that the actual incidence would be higher if all patients had been screened with Doppler US. This speculation is supported by the results of a recent prospective study in Korea (4). In this study, all patients were routinely checked for DVT by color Doppler US within 1 week of injury. The incidence of DVT after SCI was higher than previous studies for Asian populations. Another possible reason may be an environmental factor. With growing industrialization in Asian countries, lifestyle patterns, including diet, has changed, which has led to a higher consumption of a fat-rich diet and urban life style. Risk factors for occurrence of DVT such as obesity and heart disease are increasing in Asia countries (17, 18).
Our study enrolled only Korean populations, so the incidence of DVT after SCI in this study may not be generalizable to other Asian populations. However, two recent prospective studies revealed a high incidence of DVT after major orthopedic surgery without pharmacologic thromboprophylaxis in Asian patients (5, 6). Considering these previous findings along with the present results, it seems likely that the incidence of DVT after SCI may be higher than previously estimated, not only in Korean populations but also in other Asian populations. Further research to explore the reason for the increasing prevalence of DVT in Asian countries is necessary.
Although most DVT events occur within 2-3 weeks after SCI, several studies have shown the occurrence of DVT within the rehabilitation phase or more than 3 months after SCI (15, 16, 19). Several studies have investigated the incidence of DVT at rehabilitation unit. Presently, the incidence of DVT at acute rehabilitation unit was 27.6%, which is comparable to studies conducted in Western countries and other Asian countries (Table 4). The present mean interval from to injury to initial US screening (31.7 days, range 3-345 days) is similar to reported values from Western countries. Therefore, results of our study suggest that pharmacologic thromboprophylaxis and routine surveillance for DVT should be considered before entry of rehabilitation unit and during rehabilitation in Korea.
Table 4.
Studies investigated the incidence of DVT at rehabilitation unit
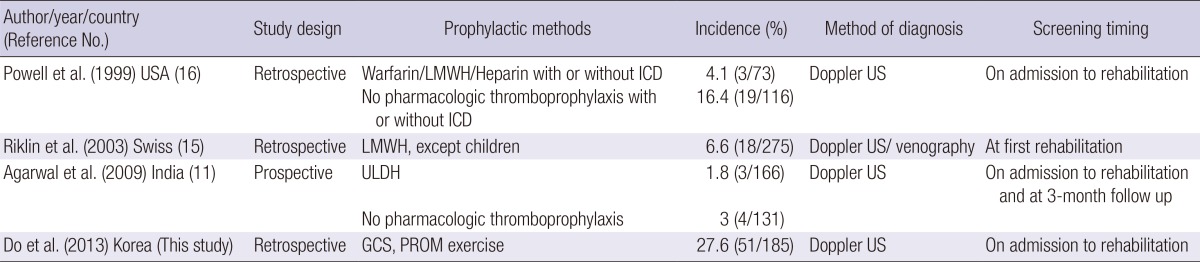
DVT, deep vein thrombosis; ICD, intermittent compression device; US, ultrasound; LMWH, low molecular weight heparin; ULDH, unfractionated low-dose heparin; GCS, gradient compression stocking; PROM, passive range of motion.
In clinical practice proximal DVT is reported to be more important than distal DVT because DVT of the proximal lower extremities is usual source of PE. Although the incidence of PE is reported to be higher in proximal DVT than distal DVT, DVT of the distal lower extremities can cause secondary complications other than PE, such as post-thrombotic phlebitis, venous valvular insufficiency. The proportion of distal DVT was 71.4% in previous study for incidence of DVT in patients with SCI (20). This result is similar with our finding of a high proportion of distal DVT (76.5%). Distal DVT should not be overlooked in routine surveillance of DVT in patients with SCI.
Frequency of PE in the SCI population receiving pharmacologic thromboprophylaxis varies from 0%-5.2% with a mean pooled frequency of 4.4% with fatal PE in 0%-5% of patients with SCI in Western countries (21-23). A previous Western-based study reported that the incidence of PE in patients after SCI with pharmacologic thromboprophylaxis was 1.5% for first rehabilitation (15). Some studies reported that prevalence of PE in Asian patients was lower than that in Western patients. They explained the reasons for the low incidence of PE in Asian patients could be genetic factors, such as a more active or efficient fibrinolytic system or inherited resistance to thrombosis formation. In our study, 7 of 185 patients (3.8%, 95% CI 1.0-6.5) had symptomatic PE, although no sudden death due to PE occurred. These results indicate that symptomatic PE is not a rare condition in Korean SCI subjects if no pharmacologic thromboprophylaxis is carried out.
In this study, the absence of spasticity was a significant independent risk factor (P<0.05) for occurrence of DVT, with an odds ratio of 3.28 (95% CI 1.148-9.372). Whether spasticity is associated with occurrence of DVT is a contentious issue (24-26). Lower limb spasticity could prevent venous stasis and contribute to more effective emptying of lower extremity veins by improving the efficacy of the calf muscle pump. Physiological evidence for this hypothesis has not been presented. Further study is needed to define the ability of spasticity after SCI patients to affect venous blood flow.
Although it is not significant, AIS-D patients who were not able to ambulate with bipedal locomotion has the high incidence (37.8%) of DVT. One of the possible reason for failure of bipedal locomotion in AIS-D patients in our study is that combined medical problem such as pneumonia, urinary tract infection. These factors may contribute to patient's immobility and occurrence of DVT.
There are several limitations in this study. The first is the retrospective design. Although we reviewed data for the previous decade, we lacked information about some risk factors for DVT such as obesity due to insufficient data. A second limitation is the diagnosis method for DVT. Contrast venography is the gold standard method for detection of DVT, although it is considered not suitable for routine assessment of asymptomatic DVT due to its invasive nature (21). However, previous studies have indicated that Doppler US could replace traditional venography as a screening tool of DVT (27, 28). Further studies will be necessary to get more precise data using contrast venography for detecting DVT. A third limitation is that initial screening time for DVT was not standardized. The incidence would have been higher if all patients were screened by Doppler US at a more acute phase after SCI. Follow up Doppler US was not routinely performed. Furthermore, evaluations for PE were not performed routinely. Despite these limitations, the present data represent a more precise incidence of DVT after SCI in Korean populations than previous studies, because of the relatively large sample size and routine US screening protocol for DVT. Larger prospective studies will be needed precisely ascertain the incidence of DVT after SCI in Korean populations and to understand the clinical relevance of these results.
In conclusion, the incidence of DVT (27.6%) at acute rehabilitation in Korean subjects without pharmacologic thromboprophylaxis after SCI is comparable with those in Western populations. Seven patients had symptomatic PE without an episode of sudden death. These results suggest that pharmacologic thromboprophylaxis at the acute rehabilitation unit should be considered in Korean patients with SCI unless there is any ongoing bleeding or severe coagulopathy.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Waring WP, Karunas RS. Acute spinal cord injuries and the incidence of clinically occurring thromboembolic disease. Paraplegia. 1991;29:8–16. doi: 10.1038/sc.1991.2. [DOI] [PubMed] [Google Scholar]
- 2.White RH, Zhou H, Romano PS. Incidence of idiopathic deep venous thrombosis and secondary thromboembolism among ethnic groups in California. Ann Intern Med. 1998;128:737–740. doi: 10.7326/0003-4819-128-9-199805010-00006. [DOI] [PubMed] [Google Scholar]
- 3.Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, Colwell CW American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 4.Chung SB, Lee SH, Kim ES, Eoh W. Incidence of deep vein thrombosis after spinal cord injury: a prospective study in 37 consecutive patients with traumatic or nontraumatic spinal cord injury treated by mechanical prophylaxis. J Trauma. 2011;71:867–870. doi: 10.1097/TA.0b013e31822dd3be. [DOI] [PubMed] [Google Scholar]
- 5.Piovella F, Wang CJ, Lu H, Lee K, Lee LH, Lee WC, Turpie AG, Gallus AS, Planès A, Passera R, et al. Deep-vein thrombosis rates after major orthopedic surgery in Asia: an epidemiological study based on postoperative screening with centrally adjudicated bilateral venography. J Thromb Haemost. 2005;3:2664–2670. doi: 10.1111/j.1538-7836.2005.01621.x. [DOI] [PubMed] [Google Scholar]
- 6.Leizorovicz A SMART Venography Study Steering Committee. Epidemiology of post-operative venous thromboembolism in Asian patients: results of the SMART venography study. Haematologica. 2007;92:1194–1200. doi: 10.3324/haematol.10819. [DOI] [PubMed] [Google Scholar]
- 7.Todd JW, Frisbie JH, Rossier AB, Adams DF, Als AV, Armenia RJ, Sasahara AA, Tow DE. Deep venous thrombosis in acute spinal cord injury: a comparison of 125I fibrinogen leg scanning, impedance plethysmography and venography. Paraplegia. 1976;14:50–57. doi: 10.1038/sc.1976.8. [DOI] [PubMed] [Google Scholar]
- 8.Perkash A, Prakash V, Perkash I. Experience with the management of thromboembolism in patients with spinal cord injury: part I. incidence, diagnosis and role of some risk factors. Paraplegia. 1978;16:322–331. doi: 10.1038/sc.1978.62. [DOI] [PubMed] [Google Scholar]
- 9.Watson N. Anti-coagulant therapy in the prevention of venous thrombosis and pulmonary embolism in the spinal cord injury. Paraplegia. 1978;16:265–269. doi: 10.1038/sc.1978.50. [DOI] [PubMed] [Google Scholar]
- 10.Merli GJ, Herbison GJ, Ditunno JF, Weitz HH, Henzes JH, Park CH, Jaweed MM. Deep vein thrombosis: prophylaxis in acute spinal cord injured patients. Arch Phys Med Rehabil. 1988;69:661–664. [PubMed] [Google Scholar]
- 11.Agarwal NK, Mathur N. Deep vein thrombosis in acute spinal cord injury. Spinal Cord. 2009;47:769–772. doi: 10.1038/sc.2009.37. [DOI] [PubMed] [Google Scholar]
- 12.Sugimoto Y, Ito Y, Tomioka M, Tanaka M, Hasegawa Y, Nakago K, Yagata Y. Deep venous thrombosis in patients with acute cervical spinal cord injury in a Japanese population: assessment with Doppler ultrasonography. J Orthop Sci. 2009;14:374–376. doi: 10.1007/s00776-009-1342-y. [DOI] [PubMed] [Google Scholar]
- 13.Rathore MF, Hanif S, New PW, Butt AW, Aasi MH, Khan SU. The prevalence of deep vein thrombosis in a cohort of patients with spinal cord injury following the Pakistan earthquake of October 2005. Spinal Cord. 2008;46:523–526. doi: 10.1038/sj.sc.3102170. [DOI] [PubMed] [Google Scholar]
- 14.Ko HY, Shin YB, Jho SK. Incidence of deep vein thrombosis in spinal cord injury. J Korean Acad Rehabil Med. 2005;29:359–364. [Google Scholar]
- 15.Riklin C, Baumberger M, Wick L, Michel D, Sauter B, Knecht H. Deep vein thrombosis and heterotopic ossification in spinal cord injury: a 3 year experience at the Swiss Paraplegic Centre Nottwil. Spinal Cord. 2003;41:192–198. doi: 10.1038/sj.sc.3101421. [DOI] [PubMed] [Google Scholar]
- 16.Powell M, Kirshblum S, O'Connor KC. Duplex ultrasound screening for deep vein thrombosis in spinal cord injured patients at rehabilitation admission. Arch Phys Med Rehabil. 1999;80:1044–1046. doi: 10.1016/s0003-9993(99)90058-8. [DOI] [PubMed] [Google Scholar]
- 17.Asia Pacific Cohort Studies Collaboration. The burden of overweight and obesity in the Asia-Pacific region. Obes Rev. 2007;8:191–196. doi: 10.1111/j.1467-789X.2006.00292.x. [DOI] [PubMed] [Google Scholar]
- 18.Khoo KL, Tan H, Liew YM, Deslypere JP, Janus E. Lipids and coronary heart disease in Asia. Atherosclerosis. 2003;169:1–10. doi: 10.1016/s0021-9150(03)00009-1. [DOI] [PubMed] [Google Scholar]
- 19.Perkash A, Sullivan G, Toth L, Bradleigh LH, Linder SH, Perkash I. Persistent hypercoagulation associated with heterotopic ossification in patients with spinal cord injury long after injury has occurred. Paraplegia. 1993;31:653–659. doi: 10.1038/sc.1993.105. [DOI] [PubMed] [Google Scholar]
- 20.Germing A, Schakrouf M, Lindstaedt M, Grewe P, Meindl R, Mügge A. Do not forget the distal lower limb veins in screening patients with spinal cord injuries for deep venous thromboses. Angiology. 2010;61:78–81. doi: 10.1177/0003319709333224. [DOI] [PubMed] [Google Scholar]
- 21.Furlan JC, Fehlings MG. Role of screening tests for deep venous thrombosis in asymptomatic adults with acute spinal cord injury: an evidence-based analysis. Spine (Phila Pa 1976) 2007;32:1908–1916. doi: 10.1097/BRS.0b013e31811ec26a. [DOI] [PubMed] [Google Scholar]
- 22.Gündüz S, Oğur E, Möhür H, Somuncu I, Açjksöz E, Ustünsöz B. Deep vein thrombosis in spinal cord injured patients. Paraplegia. 1993;31:606–610. doi: 10.1038/sc.1993.96. [DOI] [PubMed] [Google Scholar]
- 23.Green D, Lee MY, Lim AC, Chmiel JS, Vetter M, Pang T, Chen D, Fenton L, Yarkony GM, Meyer PR., Jr Prevention of thromboembolism after spinal cord injury using low-molecular-weight heparin. Ann Intern Med. 1990;113:571–574. doi: 10.7326/0003-4819-113-8-571. [DOI] [PubMed] [Google Scholar]
- 24.Yelnik A, Dizien O, Bussel B, Schouman-Claeys E, Frija G, Pannier S, Held JP. Systematic lower limb phlebography in acute spinal cord injury in 147 patients. Paraplegia. 1991;29:253–260. doi: 10.1038/sc.1991.36. [DOI] [PubMed] [Google Scholar]
- 25.Green D, Hartwig D, Chen D, Soltysik RC, Yarnold PR. Spinal Cord Injury Risk Assessment for Thromboembolism (SPIRATE Study) Am J Phys Med Rehabil. 2003;82:950–956. doi: 10.1097/01.PHM.0000098043.88979.BA. [DOI] [PubMed] [Google Scholar]
- 26.Bors E, Conrad CA, Massell TB. Venous occlusion of lower extremities in paraplegic patients. Surg Gynecol Obstet. 1954;99:451–454. [PubMed] [Google Scholar]
- 27.Kadyan V, Clinchot DM, Colachis SC. Cost-effectiveness of duplex ultrasound surveillance in spinal cord injury. Am J Phys Med Rehabil. 2004;83:191–197. doi: 10.1097/01.phm.0000113401.47681.a6. [DOI] [PubMed] [Google Scholar]
- 28.Zierler BK. Ultrasonography and diagnosis of venous thromboembolism. Circulation. 2004;109:I9–I14. doi: 10.1161/01.CIR.0000122870.22669.4a. [DOI] [PubMed] [Google Scholar]