Abstract
An allosecurinine alkaloid was assayed against spore germination of some saprophytic and pathogenic fungi e.g., Alternaria alternata, A. solani, A. brassicicola, A. brassicae, Curvularia lunata, C. pallescens, C. maculans, Curvularia species, Colletotrichum species, C. musae, C. gloeosporioides, Erysiphe pisi, Fusarium udum, Helminthosporium echinoclova, H. pennisetti, H. spiciferum, and Heterosporium sp. It inhibited mild spore germination of all the fungi tested. Curvularia lunata, Curvularia sp., Collectotrichum sp., C. musae and Heterosporium sp. were most sensitive as complete inhibition of spore germination was observed at very low concentrations.
Keywords: Allosecurinine, Antifungal activity, Spore germination
Several synthetic fungicides are being used successfully for the control of various fungal diseases of crop plants. This has resulted into human health hazards, resistance in pathogen and environmental pollution. Recent awareness of these negative effects warrants the use of environmentally safe alternative methods of disease control. Some of the approaches currently pursued are: biological control, genetic engineering for evolving resistant varieties and use of induced resistance by biotic and abiotic means (Lyon et al., 1995). The use of biodegradable plant products specially from medicinal plants is another aspect gaining importance in plant disease control.
Many workers has used crude extracts in vitro, in glasshouse and field conditions against several plant pathogens (Asthana et al., 1987). Various active principles isolated from the plants were proved effective against several plant pathogenic fungi in vitro (Goel et al., 2002; Pandey et al., 2002; Sahni et al., 2005; Singh et al., 1990) in glasshouse (Reimers et al., 1993; Singh et al., 1995) and also in the field (Sarma et al., 1999). Although use of plant products under field conditions is rare and usually cost-prohibitive, neemazal, a product of neem (Azadirachta indica) and ajoene, a constituent of garlic (Allium sativum) have recently been used successfully against powdery mildew (Erysiphe pisi) of pea under field conditions (Singh et al., 1995).
Several alkaloids are known to affect biological functions at very low concentrations. Among such alkaloids many are known as antimicrobial (Srivastava et al., 1994; Atta-ur-Rahman et al., 1997; Singh et al., 2000). However, the effect of the present compound on spore germination has not been reported so far. We report the antifungal activity of this compound for the first time.
The fungi were isolated on Potato Dextrose Agar (PDA) (peeled potato 250 g, dextrose 20 g, agar 15 g, distilled water 1 l) from their respective hosts collected from the experimental farm of the Banaras Hindu University, Varanasi, India.
The cultures were purified by single spore isolation technique on PDA slants and maintained by periodic transfer on the same medium for further experiments. Seven to 10 day - old cultures were used in this experiment. The spores of obligate parasitic fungi were directly picked up from their respective hosts.
Phyllanthus amarus Linn. (Family: Euphorbiaceae) is small plant which grows throughout the hotter parts of India during the Mansoon season. The root of the plant is said to be remedy for jaundice; leaves are stomachic, fruit is bitter, useful for tubercular ulcers, scabies and ring worm (Kirtikar and Basu, 1935; Chopra et al., 1956). The whole plant was collected from Mirzapur district, Uttar Pradesh, India.
The air dried powdered whole plant (4 kg) was successively extracted with MeOH in a Soxhlet extractor which on removal of solvent in reduced pressure gave semi solid gummy mass (86 g). The methanolic extract was stirred with 7% aqueous citric acid several times. The acidic solution was basified with ammonia and extracted with CHCl3 which furnished the crude base fraction (15 g). The crude base fraction was chromatographed over SiO2 gel column eluting with a mixture of CHCl3 and MeOH. The eluants collected from CHCl3-MeOH (1 : 4) on crystallisation from MeOH furnished the pure alkaloid (25 mg) (1) as colourless granules, m.p. 136~138℃, C13H15NO2 (MS: m/z 217 [M]+). The pure alkaloid exhibited UV absorption maxima at 257 nm and IR absorption maxima at 1860 and 1760 cm-1 for extended α,β-unsaturated-γ-lactone moiety. The 300MHz 1H NMR spectrum in CDCl3 showed the presence of six proton multiplets at δ 1.00 to 1.80, one proton doublet at δ 1.90, one proton quartet at δ 2.60, one proton singlet at δ 2.85 and two proton multiplets at δ 6.50~7.00. These spectral data favoured the structure of the alkaloid as allosecurinine (Manske, 1977).
It was finally identified as allosecurinine (Fig. 1) by direct comparison with authentic sample (mixed m.p., co-TLC and superimposable IR). This is the first report of the occurrence of this alkaloid in P. amarus.
Fig. 1.
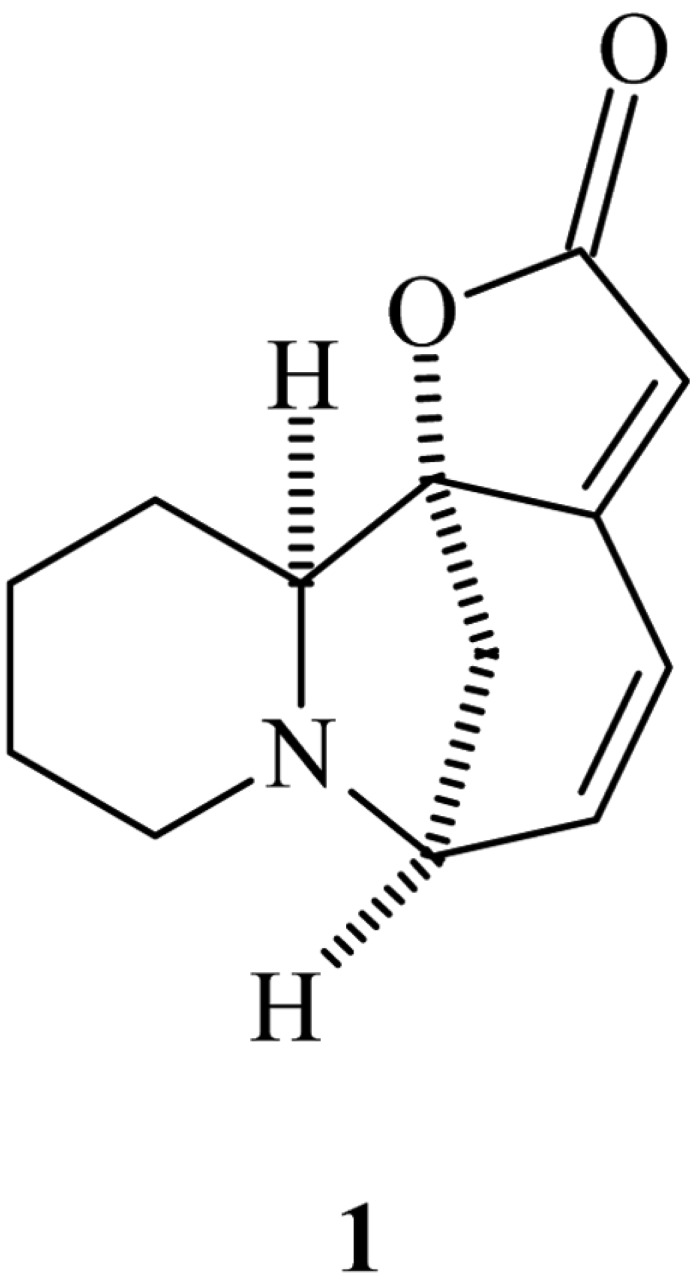
Structure of Allosecurinine.
Stock solution (2000 ppm) was prepared by dissolving 10 mg of chemical initially with a few drops of methanol in a test tube. After the chemical was completely dissolved, approx. 5 ml of distilled water was added. The methanol was then evaporated on water bath. The required concentration (200, 400, 600, 800, and 1000 ppm) of the chemical were prepared from the stock solution by diluting with distilled water. A drop (30~40 µl) of the chemical solution was placed on a grease-free glass slide. Fungal spores (about 200~300) were mixed in the solution with the help of a sterile inoculation needle. Erysiphe pisi conidia were directly picked up from diseased plants and mixed in the solution. The slides were later placed in moist chamber made by placing two sterile moist filter papers on the lid and base of petri plates. The spores were then incubated at 25 ± 2℃ for 24 h for germination. The germination of the spores was observed after staining with cotton blue prepared in lactophenol under a binocular light microscope (Nikon, Japan). All the experiments were conducted in triplicate.
The effect of allosecurinine on spore germination of some plant pathogenic fungi was seen (Table 1). The sensitivity of different fungi to this chemical varied considerably. Curvularia lunata, Curvularia sp., Colletotrichum sp., Colletotrichum musae and Heterosporium sp. were the most sensitive as complete inhibition of germination was observed in all the concentrations (200, 400, 600, 800, 1000 ppm) of the chemical. However, only 1000 ppm was effective against spore germination of Helminthosporium echinoclova, H. pennisetti and H. spiciferum. The chemical inhibited mild spore germination of all other fungi at lower concentration.
Table 1.
Effect of allosecurinine on spore germination of some fungi
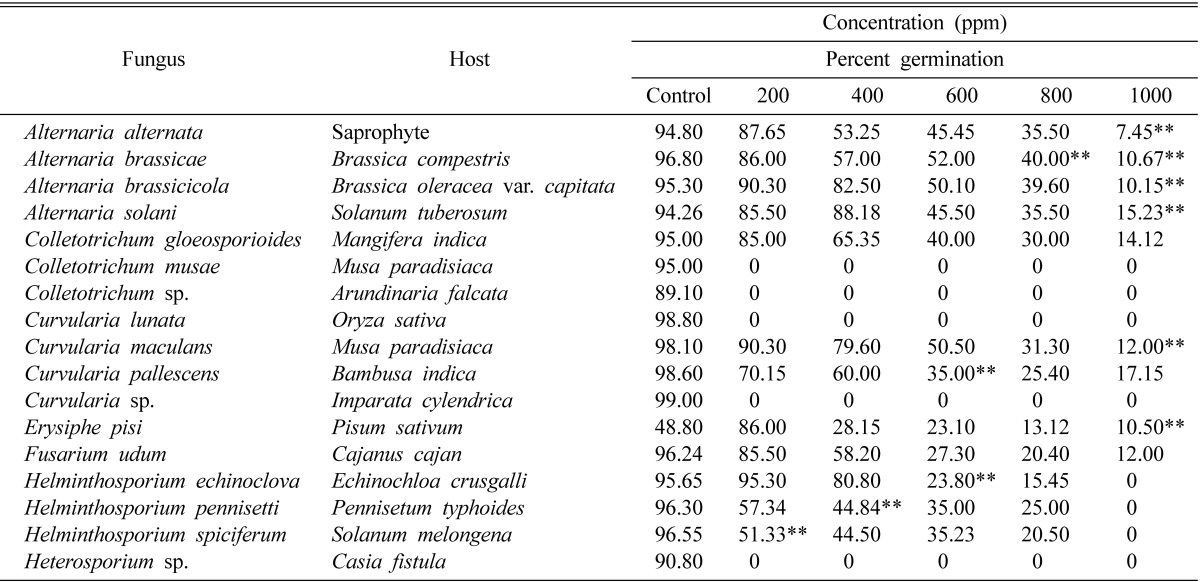
aValues with double asterisk are significantly different from corresponding control values at p ≤ 0.01 based on the Student t-test. C.D. at 1% = 45.45.
The presence or absence of the pigment(s) in spores does not seem to affect the activity of the chemical. Hyaline spores of Colletotrichum sp. as well as pigmented Curvularia species were sensitive against this chemical. Singh et al. (1990) found that hyaline spores were more sensitive to ajoene as compared to pigmented ones. A number of chemical compounds isolated from plants have already been reported to be antifungal (Srivastava et al., 1994; Sarma et al., 1999; Singh et al., 1990, 2000). Although several alkaloids are already known to be antifungal but the antifungal activity of the present chemical is being reported for the first time. The chemical was equally effective against biotrophic and saprophytic fungi. The efficacy of the chemical is significantly high even at low concentration which indicates a possibility of its use to control plant diseases under field conditions.
References
- 1.Asthana A, Chandra H, Dikshit A, Dixit SN. Volatile fungitoxicants from leaves of some higher plants against Helminthosporium oryzae. Z Planzenkr. 1987;89:475–479. [Google Scholar]
- 2.Atta-ur-Rahman, Nasreen A, Akhtar F, Shekhani MS, Clardy J, Parvez M, Choudhary MI. Antifungal diterpenoid alkaloids from Delphinium denudatum. J Nat Prod. 1997;60:474–475. doi: 10.1021/np960663n. [DOI] [PubMed] [Google Scholar]
- 3.Chopra RN, Nayar SL, Chopra IC. Glossary of Indian Medicinal Plants. New Delhi: CSIR; 1956. p. 191. [Google Scholar]
- 4.Goel M, Maurya S, Pandey VB, Singh VP, Singh AK, Singh UP. Effect of Ent-norsecurinine, an alkaloid, on spore germination of some fungi. Mycobiology. 2002;30:225–227. [Google Scholar]
- 5.Kirtikar KR, Basu BD. Indian Medicinal Plants. (2nd ed) 1935;vol. III:2225. [Google Scholar]
- 6.Lyon GP, Reglinski T, Newton AC. Novel disease control compounds: the potential to immunize plants against infection. Plant Pathol. 1995;44:407–427. [Google Scholar]
- 7.Manske RHF. The Alkaloids. vol. XIV. New York, London: Academic Press; 1977. pp. 425–506. [Google Scholar]
- 8.Pandey MK, Pandey R, Singh VP, Pandey VB, Singh UP. Antifungal activity of 4',7-dimethoxyisoflavone against some fungi. Mycobiology. 2002;30:55–56. [Google Scholar]
- 9.Reimers F, Smolka SE, Wemens S, Schumacher KP, Wagner KG. Effect of azoene, a compound derived from Allium sativum, on phytopathogenic and epiphytic microorganisms. J Plant Dis Prot. 1993;20:622–633. [Google Scholar]
- 10.Sahni S, Maurya S, Singh UP, Singh AK, Singh VP, Pandey VB. Antifungal activity of nor-securinine against some phytopathogenic fungi. Mycobiology. 2005;33:97–103. doi: 10.4489/MYCO.2005.33.2.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarma BK., Pandey VB, Mishra GD, Singh UP. Antifungal activity of berberine iodide, a constituent of Fumaria indica. Folia Microbiol. 1999;44:164–166. [Google Scholar]
- 12.Singh UP, Pandey VN, Wagner KG, Singh KP. Antifungal activity of ajoene, a constituent of garlic (Allium sativum) Can J Bot. 1990;68:1354–1356. [Google Scholar]
- 13.Singh UP, Prithiviraj B, Wagner KG, Schumacher KP. Effect of ajoene, a constituent of garlic (Allium sativum) on powdery mildew (Erysiphe pisi) of pea (Pisum sativum) J Plant Dis Prot. 1995;102:399–406. [Google Scholar]
- 14.Singh UP, Sarma BK, Mishra PK, Ray AB. Antifungal activity of Venenatine, an indol alkaloid isolated from Alstonia venenata. Folia Microbiol. 2000;45:173–176. doi: 10.1007/BF02817419. [DOI] [PubMed] [Google Scholar]
- 15.Srivastava BP, Singh KP, Singh UP, Pandey VB. Effect of some naturally occurring alkaloids on conidial germination of Botrytis cinerea. Bioved. 1994;5:69–72. [Google Scholar]


