Abstract
Systemic effect of two plant growth-promoting rhizobacterial (PGPR) strains,viz., Pseudomonas fluorescens (Pf4) and P. aeruginosa (Pag), was evaluated on pea (Pisum sativum) against the powdery mildew pathogen Erysiphe pisi. Foliar spray of the two PGPR strains was done on specific nodal leaves of pea and conidial germination of E. pisi was observed on other nodal leaves,distal to the treated ones. Conidial germination was reduced on distant leaves and at the same time,specific as well as total phenolic compounds increased in the leaves distal to those applied with PGPR strains,thereby indicating a positive correlation. The strains induced accumulation of phenolic compounds in pea leaves and the amount increased when such leaves were get inoculated with E. pisi conidia. Between the two strains, Pag was found to be more effective than Pf4 as its effect was more persistent in pea leaves. Foliar application of PGPR strains for the control of powdery mildew of pea is demonstrated in vitro while correlating it with the increased accumulation of plant phenolics.
Keywords: Erysiphe pisi, Foliar spray, Induced resistance, Pseudomonas aeruginosa, Pseudomonas fluorescens
Powdery mildew of pea (Pisum sativum) is incited by Erysiphe pisi and causes infection in all aerial green parts of the plant. Plant growth-promoting rhizobacteria (PGPR) are known to induce systemic resistance (ISR) in plants and restrict establishment of infection by the pathogens in the host (van Peer et al., 1991; Wei et al., 1991). Pseudomonas spp. are known to protect plants from pathogens through various mechanisms, viz., induced systemic resistance in the host (Maurhofer et al., 1994; van Peer et al., 1991), antibiotic production (Thomashaw and Weller, 1988; Maurhofer et al., 1995), growth promotion (Schippers et al., 1987) and competition for nutrients (Duijff et al., 1993; Leeman et al., 1996).
Phenolic compounds are natural constituents of all plants and antibiotic phenols have been implicated in plant defense mechanisms (Elgersma and Liem, 1989; Kuc, 1995; Nicholson and Hammerschmidt, 1992; Baker et al., 2005). Among them, some occur constitutively and function as preformed inhibitors associated with non-host resistance (Millar and Higgins, 1970; Stoessl, 1983), while others are formed in response to pathogen ingress as part of an active defense response (Nicholson and Hammerschmidt, 1992). Matta et al. (1988) noted the activation of phenol metabolism in xylem vessels of tomato during pathogen attack. This observation was late supported by the results of Cooper et al. (1996) who found two phenolic compounds, a triterpenoid, and elemental sulfur in cells associated with vascular pathogens in resistance genotypes of Theobroma cacao. Accumulation of phenolic compounds in carnation by a Pseudomonas sp. and decrease in Fusarium wilt has been reported by van Peer et al. (1991). Similarly, successful protection of pea from Erysiphe pisi through foliar application of PGPR has been demonstrated by Singh et al. (2000). Looking into the antifungal activity of phenolic acids, the present study was conducted to investigate the systemic accumulation of phenolic compounds in pea plant parts following foliar application of two PGPR strains. Induction of resistance in the pea plants following PGPR application was assessed through inhibition of conidial germination of E. pisi.
Materials and Methods
Experimental set up
Ten pea seeds (var. Arkel) were sown in single plastic pots (20 cm diameter) containing sterilized sandy loam soil (pH 7.6) and allowed to grow in a glasshouse for 20 days. PGPR strains Pseudomonas fluorescens strain Pf4 and P. aeruginosa strain Pag (Singh et al., 2003) were grown on King's B (KB) agar (proteose peptone 20 g, K2HPO4·3H2O 1.908 g, MgSO4·7H2O 1.5 g, glycerol 15 ml, Bacto agar 15 g, distilled water 985 ml) medium for 48 h at 25 ± 2℃. Individual as well as mixed suspension of both the strains of approximately 108 cells per ml (OD620 nm 0.8~0.9) in 0.1% carboxy methyl cellulose (CMC) was prepared separately. Five pots of pea plants comprised one set, and each set was sprayed with the bacterial suspensions with a hand atomizer separately. Another set of pea plants was inoculated with only E. pisi conidia by tapping severely infected pea leaves (Singh et al., 2000a). For evaluation of the effect of PGPR in the presence of E. pisi, conidia of the pathogen were tapped on pea leaves after 24 h of foliar spray of the PGPR strains. A set of pea plants was left uninoculated (neither with PGPR nor with E. pisi) in a separate chamber in the glasshouse served as control. The above-mentioned treatments will be referred as T1 = Control; T2 = pea plants inoculated only with E. pisi; T3 = pea plants sprayed only with Pf4 suspension; T4 = pea plants sprayed only with Pag suspension; T5 = pea plants sprayed with mixed suspension of Pf4 + Pag; T6 = pea plants sprayed with suspension of Pf4 followed by inoculation with E. pisi after 24 h; T7 = pea plants sprayed with suspension of Pag followed by inoculation with E. pisi after 24 h; T8 = pea plants sprayed with mixed suspension of Pf4 and Pag followed by inoculation with E. pisi after 24 h.
Foliar spray of bacterial suspension
In a separate experiment, bacterial suspension of Pf4, prepared as above was sprayed with a hand atomizer on a set of 20-d-old pea plants. While spraying Pf4 suspension on I and II nodal leaves, III and IV nodal leaves were protected by covering with polythene sheets. After 24 h E. pisi conidia were inoculated on III and IV nodal leaves by tapping over the pea plants as above. During conidial inoculation I and II nodal leaves were covered by polythene sheet. Similarly, a second set of pea plants was sprayed with Pf4 suspension on III and IV nodal leaves while I and II nodal leaves were covered with polythene sheets. Conidia of E. pisi were inoculated on I and II nodal leaves as described earlier while III and IV nodal leaves were covered with polythene sheets. A third set of pea plants were treated only with E. pisi conidia and a fourth set of pea plants left un-inoculated (neither with PGPR nor E. pisi) in a separate chamber to serve as control. Percent conidial germination on pea leaves treated with Pf4 and/or inoculated with E. pisi along with control were determined after 24 and 48 h of treatments (Singh et al., 2000a). For estimation of phenolic compounds a fifth set of pea plants sprayed with Pf4 suspension without inoculation of E. pisi were left as such.
The conidia of the pea powdery mildew were applied in such as way that each leaf received 200~300 conidia per mm2. At least five leaves per treatment were excised and processed by the method of Carver and Adaigbe (1990). The leaves were placed on a pad of filter paper with adaxial side up containing fixative. Leaves were fixed for 48 h to remove chlorophyll completely and then placed on lacto-phenol cotton blue for 24 h. The germination of conidia were recorded at different time intervals after inoculation by counting 100 conidia per leaf using light microscope.
Extraction of phenolic compounds
Randomly selected 6 plants from 3 pots of a single treatment of the different treatments as described above were harvested and pooled together to make one sample each of leaves, collars and roots to extract phenolic compounds after 24, 48 and 96 h of treatments. At least three samples were prepared from each treatment. Phenolic compounds were extracted from leaves, stems and roots according to Sarma et al. (2002).
For the estimation of total phenolics, 1.0 g fresh plant part was extracted separately in 50% aqueous methanol thrice. The supernatant was evaporated to dryness and finally dissolved in 1.0 ml distilled water and samples prepared in triplicate were analyzed spectrophotometrically using Prussian blue method as modified by Graham (1992). Absorbance of the colour was recorded at 700 nm using an UV-VIS spectrophotometer (Bausch and Lomb, USA). Standard curve of gallic acid was prepared and TPC was calculated in terms of gallic acid equivalents. Analytical grade reagents were used throughout the experiment.
HPLC analysis of phenolic acids
Quantitative analysis of the samples was performed as per the method of Singh et al. (2002). The HPLC system (Shimadzu Corporation, Kyoto, Japan) was equipped with two Shimadzu LC-10 ATVP reciprocating pumps, a variable Shimadzu SPD-10 AVP UV-VIS detector and a Rheodyne Model 7725 injector with a loop size of 20 µl. Peak area was calculated with Winchrom integrator. Reverse phase chromatographic analysis was carried out in isocratic conditions using C-18 reverse phase column (250 × 4.6 mm id, particle size 5 µm Luna 5 µ C-18 (2), Phenomenex, USA) at 25℃. Running conditions included injection volume 5 µl, mobile phase methanol: 0.4% acetic acid (80 : 20 v/v), flow rate 1 ml/min, and detection at 290 nm. Samples were filtered through membrane filter (pore size 0.45 µm, E-Merck, Germany) prior to injection in sample loop. Tannic, gallic, vanillic, caffeic, ferulic, o-coumaric, chlorogenic, cinnamic and synapic acids were used as internal and external standards. Phenolic acids present in the samples were identified by comparing chromatographic peaks with the retention time (Rt) of individual standards and further confirmed by co-injection with isolated standards.
Statistical analysis
Each experiment was conducted thrice and the data were subjected to statistical analysis using the software ORIGIN 5.0. In all the treatments, the data were subjected to ANOVA and results were subjected for statistical significance by Student's t test at p ≤ 0.05.
Results
Foliar application of the two PGPR strains resulted in the induction of individual phenolic acids as well as total phenolics in different parts of pea. Based on retention time as well as co-injection with standards, five phenolic compounds, viz., gallic (Rt 2.92 min), caffeic (Rt 3.18 min), vanillic (Rt 3.32 min), ferulic (Rt 3.56 min) and cinnamic (Rt 4.45 min) acids were identified. Among phenolic acids, gallic and cinnamic acids were consistently detected in varied amounts in all the parts of pea plants in all treatments including control. Maximum accumulation of gallic acid was observed in leaves that had received foliar application of Pag at 48 h while at 96 h its maximum accumulation was observed in T7 (Pag + E. pisi). The amount of gallic acid was maximum in leaves at 48 and 96 h but it gradually increased in roots as well in other treatments at 96 h. (Table 1). In contrast to gallic acid, maximum accumulation of cinnamic acid was observed in roots at 48 h (5.1 µg/g fresh wt.) in Pf4 (T3) treated plants. Except T6, the amount of cinnamic acid was higher in roots as compared to stem and leaves at 48 h (Table 1). At 96 h maximum amount of cinnamic acid was observed in leaves of Pf4 (T3) treated plants (1.8 µg/g fresh wt.) while almost in all other treatments its content was higher in roots (Table 1).
Table 1.
Effect of foliar spray of plant growth-promoting rhizobacteria on the induction of phenolic compounds in pea (Pisum sativum) after 48 and 96 h
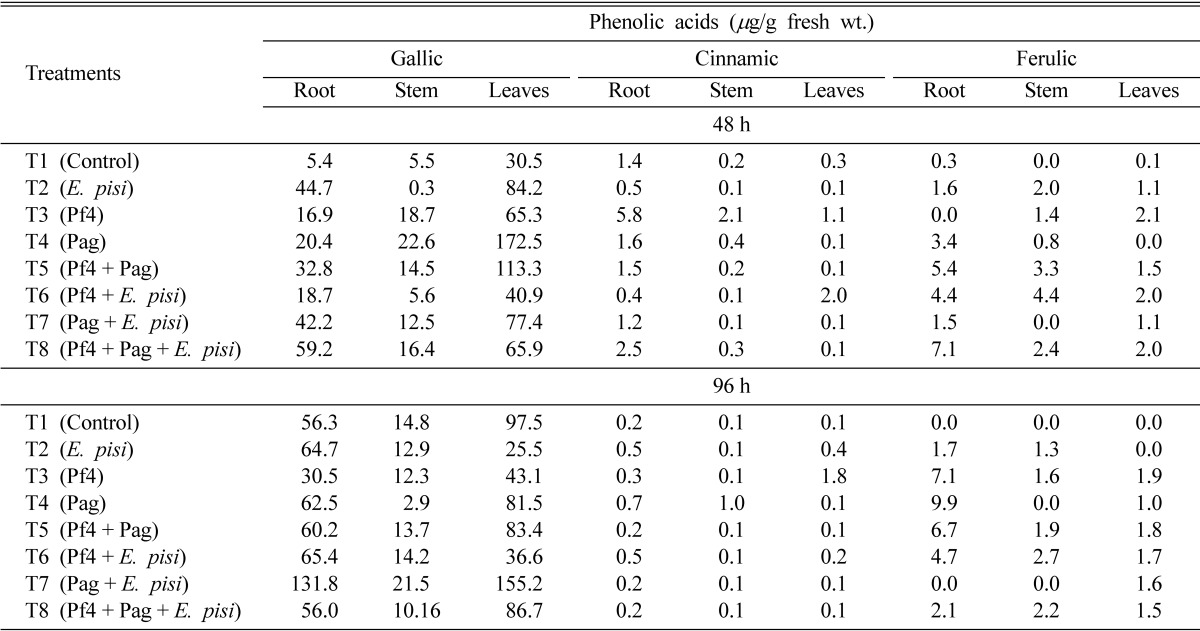
Consistent occurrence of ferulic acid was noticed in different parts of pea. In leaves, ferulic acid was detected at 48 and 96 h in most of the treatments, being maximum in Pf4 (T3) treated plants followed by Pf4 and E. pisi (T6) treated plants (Table 1). In stem maximum ferulic acid accumulation was observed in Pf4 and E. pisi (T6) treated plants after 48 and 96 h. In roots, maximum accumulation of ferulic acid was found in Pf4 + Pag + E. pisi (T8) treated plants after 48 h but after 96 h, root of only Pag (T4) treated plants showed maximum accumulation (Table 1).
The amount of total phenolics (TP) varied in different parts of pea plants in different treatments. Maximum amount of TP was observed in leaves at 48 and 96 h in Pf4 (T3) treated plants (73.5 and 57.6 mg/g fresh wt., respectively) (Table 2). In stem and roots, Pag (T4) treated plants showed maximum accumulation of TP after 48 h. However, stem and roots of plants treated with Pag + E. pisi (T7) accumulated maximum TP after 96 h (23.6 and 51.3 mg/g fresh wt., respectively) (Table 2).
Table 2.
Total phenolic content in pea (Pisum sativum) after foliar spray with plant growth-promoting rhizobacteria
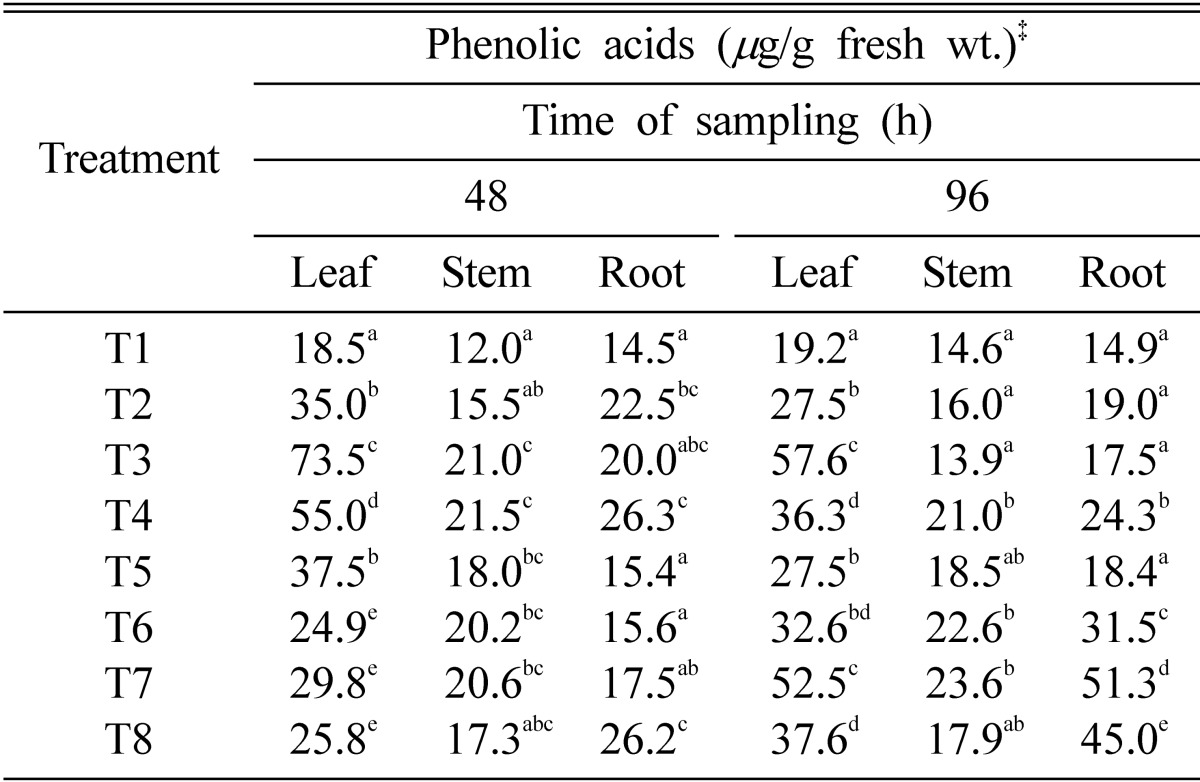
CD = (6.04) (5.86) (5.78) (5.45) (6.13) (5.53) T1 = Control; T2 = Erysiphe pisi; T3 = Pf4; T4 = Pag; T5 = Pf4 + Pag; T6 = Pf4 + E. pisi; T7 = Pag + E. pisi; T8 = Pf4 + Pag + E. pisi.
‡Column data superscript with similar letters vary significantly at P ≤ 0.05 by Student-t test.
It is evident from Table 3 that germination of E. pisi conidia was severely affected in both the treatments as compared to control (conidial germination on healthy plants). Foliar application of Pf4 on I and II nodal (lower) leaves of pea caused significant reduction in percent conidial germination on III and IV nodal (upper) leaves and vice-versa. In another set of conditions, where whole aerial part of the plants were sprayed with Pf4 followed by E. pisi inoculation after 24 h, conidial germination reduced to several fold as compared to healthy plants (Table 3).
Table 3.
Conidial germination of Erysiphe pisi on the leaves of 20 day old pea plants treated with rhizobacteria and inoculated with E. pisi after 24 h of inoculation
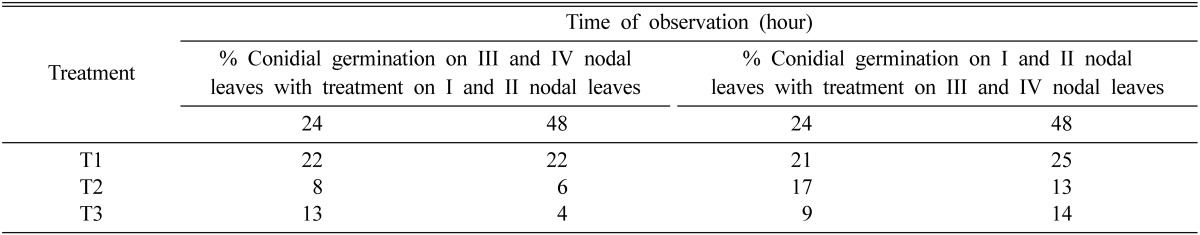
T1 = Control, T2 = Pf4 treated plants, T3 = Whole plants sprayed with Pf4 after 24 h of Erysiphe pisi inoculation; number of replications-three; 100 conidia were counted in each replication.
Tannic, gallic and ferulic acids were detected consistently in pea leaves treated with Pf4 and/or inoculated with E. pisi. Treatment with Pf4 on lower nodal leaves (I and II) followed by inoculation with E. pisi on upper leaves (T4) caused maximum accumulation of ferulic acid in upper (III and IV) and lower leaves (I and II) (3.8 and 17.9 µg/g fresh wt. after 24 h respectively). However, spraying with Pf4 on the whole plants followed by inoculation with E. pisi (T5) caused maximum accumulation of ferulic acid in I and II (2.7 µg/g fresh wt.) and III and IV (20.8 µg/g fresh wt.) after 48 h (Table 4).
Table 4.
Phenolic acid content in pea leaves after 24 and 48 h treatment with Pseudomonas fluorescens strain Pf4 and Erysiphe pisi
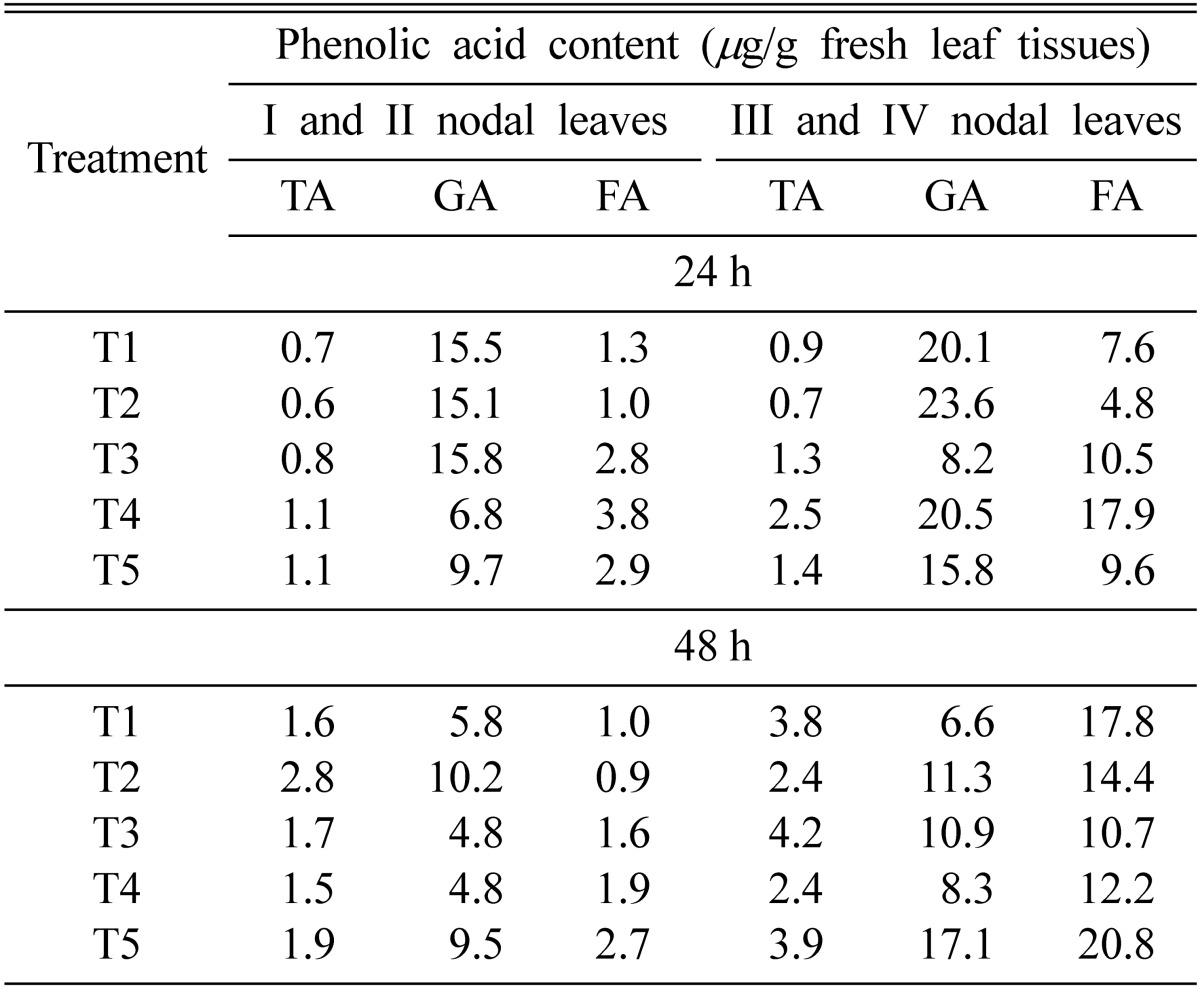
T1 = Healthy, T2 = Erysiphe pisi inoculated, T3 = Erysiphe pisi inoculated I and II nodal leaves and Pf4 treated III and IV nodal leaves, T4 = Pf4 treated I, II nodal leaves and Erysiphe pisi inoculated III and IV nodal leaves, T5 = Pf4 sprayed on whole plant followed by E. pisi inoculation after 24 h; TA-tannic, GA-gallic acid and FA-ferulic acid.
Discussion
Foliar application of PGPR strains namely P. fluorescens (Pf4) and P. aeruginosa (Pag) either alone or in different combinations, on different nodal leaves of pea plants, greatly affected the accumulation of specific as well as total phenolics systemically in leaves distal to the application as well as in other parts of pea plants. Observations in the present investigation that the induced distal accumulation of phenolic compounds, away from the site of application following foliar application of PGPR strains and subsequent reduction in conidial germination of the pathogen at those sites confirms systemic nature of the effect caused due to these rhizobacteria. It is interesting to note that whole plants when sprayed with PF4 following inoculation of E. pisi after 24 h (treatment T3 in Table 3 and T5 in Table 4) not only caused reduced conidial germination as compared to control, but also showed greater accumulation of antifungal phenolics namely ferulic and gallic acids. In general, among individual phenolic acids, gallic acid content was vary high as compared to the ferulic and cinnamic acids, indicating that these compounds are minor constituents of the phenolic cascade. Gallic acid accumulation was found to be maximum in leaves after 48 and 96 h of treatment. Cinnamic acid accumulation was not very consistent although it increased after 96 h in several treatments. Lesser content of ferulic acid in leaves after 96 h and increased accumulation in roots at the same time, in almost all treatments may result from the downward transmission of the compound from leaves to roots. However, further experiments are required to verify these data.
These results are further supported by several workers who have successfully demonstrated rhizobacteria-mediated induction of phenolic compounds against phytopathogens. van Peer et al. (1991) showed that Pseudomonas sp. strain WCS417r protected carnation from Fusarium oxysporum f. sp. dianthi infection through induction of phenolic compounds. Similarly, Benhamou et al. (2000) recently demonstrated that phenolic compounds were key components of the structural resistance response induced by Serratia plymuthica, an endophytic bacteria, in cucumber plants against Pythium ultimum. Daaye et al. (2003) also showed that induction of phenolic acids in cucumber plants formed the basis of resistance of host against powdery mildew infection. Antifungal activity of ferulic acid was reported by several workers (Demyttenaere et al., 1997; Sarma and Singh, 2003). Similarly, gallic acid not being antifungal, is converted into gallotannins, which along with other tannins, is also known to provide protection to the hosts from bacterial and fungal infections (Salisbury and Ross, 1986). Singh et al. (2000b) have shown successful control of powdery mildew of pea through the application of P. fluorescens (strains Pf1, Pf3, Pf5) and P. aeruginosa (Pag). Hence, the report of the induction of antifungal phenolic acids in pea in the present investigation following foliar application of the two PGPR strains provides a biochemical basis of resistance in pea against powdery mildew by PGPR. Reduction of conidial germination in both upper and lower leaves further shows that the systemic effect progresses in both upward and downward directions. However, involvement of more than one mechanism in inducing resistance in pea in the present investigation may not be ruled out.
Similarly, ferulic acid is highly antifungal and its higher accumulation in the plant is an important correlation with host resistance. The synthesis of ferulic acid does not always take place through the usual phenyl propanoid pathway as it is also reported to be synthesized in the host following pathogen ingress through some alternative pathway (Nicholson and Hammerschmidt, 1992). Its higher accumulation within 48 h in the present investigation suggests involvement of such a phenomenon. The amount of TPC at 96 h decreased after an initial rise at 48 h in the plants following treatments with either PGPR or E. pisi alone. But, interestingly, combined application of PGPR strains with E. pisi resulted into higher accumulation of TPC at 96 h than from their corresponding amounts at 48 h. Although activation of the phenyl propanoid pathway is evident following inoculation with only E. pisi conidia, corresponding higher amount of the same products as well as TPC in the host following co-inoculation of E. pisi with PGPR, further supports the efficacy of the PGPR strains in inducing synthesis of the phenolics in presence of E. pisi.
Both the PGPR strains used in this investigation are effective in inducing phenolic acids in the host applied either singly or in combination. However, the effect of Pag was found to be more persistent as it performed well in presence of E. pisi at 96 h. Survival of PGPR in the phylloplane of pea for a significant time period (Singh et al., 2000b) and their ability to induce synthesis of defence-related compounds systemically in the host further strengthens their use as foliar spray to control powdery mildew of pea.
Acknowledgements
The Department of Science and Technology, New Delhi is gratefully acknowledged for financial support in this work.
References
- 1.Baker CJ, Whitaker BD, Mock NM, Rice C, Deahl KL, Roberts DP, Ueng PP, Averyanov AA. Differential induction of extracellular bioactive phenolics that are redox sensitive. Physiol Mol Plant Pathol. 2005;66:90–98. [Google Scholar]
- 2.Benhamou N, Gagne S, Quere DL, Dehbi L. Bacterial-mediated induced resistance in cucumber: beneficial effect of the endophytic bacterium Serratia plymuthica on the protection against infection by Pythium ultimum. Phytopathology. 2000;90:45–56. doi: 10.1094/PHYTO.2000.90.1.45. [DOI] [PubMed] [Google Scholar]
- 3.Carver TLW, Adaigbe ME. Effects of oat genotype, leaf age and position and incubation, humidity on germination and germling development by Erysiphe graminis f. sp. avenae. Mycol Res. 1990;94:18–26. [Google Scholar]
- 4.Cooper RM, Resende MLV, Flood J, Rowan MG, Beale MH, Potter U. Detection and localization of elemental sulfur in disease resistant genotypes of Theobroma cacao. Nature. 1996;379:159–162. [Google Scholar]
- 5.Daaye F, Ongena M, Boulanger R, Hadrami IE, Belanger RR. Induction of phenolic compounds in two cultivars of cucumber by treatment of healthy and powdery mildew-infected plants with extract of Reynautria sachalinensis. J Chem Ecol. 2000;26:1579–1593. [Google Scholar]
- 6.Demyttenaere JCR, Willemen HM, Carmen Herrera MD, Verhe R. Antifungal properties of essential oil components; Twentieth International Symposium on Essential Oils; Eskisehir, Turkey; 0-1, 1-3 September 1997. 1997. [Google Scholar]
- 7.Duijff BJ, Meijer JW, Bakker PAHM, Schippers B. Siderophore-mediated competition for iron and induced resistance in the suppression of Fusarium wilt of carnation by fluorescent Pseudomonas spp. Nether J Pl Pathol. 1993;99:277–289. [Google Scholar]
- 8.Elgersma DM, Liem JI. Accumulation of phytoalexins in susceptible and near isogenic lines of tomato infected with Verticillium albo-atrum or Fusarium oxysporum f. sp. lycopersici. Physiol Mol Pl Path. 1989;34:545–555. [Google Scholar]
- 9.Graham HG. Stabilization of the Prussian blue color in the determination of polyphenols. J Agri Food Chem. 1992;40:801–805. [Google Scholar]
- 10.Kuc J. Induced systemic resistance-an overview. In: Hammerschmidt R, Kuc J, editors. Induced resistance to disease in plants. Amsterdam, The Netherlands: Kluwer Publishers; 1995. pp. 169–175. [Google Scholar]
- 11.Leeman M, Den Ouden FM, Van Pelt A, Dirkx FPM, Stejil H, Bakker PAHM, Schippers B. Iron availability affects induction of systemic resistance to Fusarium wilt of radish by Pseudomonas fluorescens. Phytopathology. 1996;86:149–155. [Google Scholar]
- 12.Matta A, Ferraris L, Abbattista GI. Variations of phenoloxidase activities and the consequence of stress induced resistance to Fusarium wilt of tomato. Phytopathology. 1988;122:45–53. [Google Scholar]
- 13.Maurhofer M, Hase C, Meuwly P, Metraux JP, Defago G. Induction of systemic resistance of tobacco to tobacco necrosis virus by the root-colonizing Pseudomonas fluorescens strain CHA0: Influence of the gacA gene and of pyoverdine production. J Phytopathol. 1994;84:139–146. [Google Scholar]
- 14.Maurhofer M, Keel C, Haas D, Defago G. Influence of plant species on disease suppression by Pseudomonas fluorescens CHA0 with enhanced antibiotic production. Plant Pathol. 1995;44:44–50. [Google Scholar]
- 15.Millar RL, Higgins HJ. Association of cyanide with infection birdsfoot trefoil by Stemphylium loti. Phytopathol. 1970;60:104–110. [Google Scholar]
- 16.Nicholson RL, Hammerschmidt R. Phenolic compounds and their role in disease resistance. Ann Rev Phytopathol. 1992;30:369–389. [Google Scholar]
- 17.Salisbury FB, Ross CW. Plant physiology. Delhi: CBS Publishers & Distributors; 1986. Lipids and other natural products; pp. 268–287. [Google Scholar]
- 18.Sarma BK, Mehta S, Singh HB, Singh UP. Plant growth-promoting rhizobacteria elicited alteration in phenolic profile of chickpea (Cicer arietinum) infected by Sclerotium rolfsii. Phytopathol J. 2002;150:277–282. [Google Scholar]
- 19.Sarma BK, Singh UP. Ferulic acid may prevent infection by Sclerotium rolfsii in Cicer arietinum. World J Microbiol Biotechnol. 2003;19:123–127. [Google Scholar]
- 20.Schippers B, Bakker AW, Bakker PAHM. Interactions of deleterious and beneficial rhizosphere microorganisms and the effect of cropping practices. Ann Rev Phytopathol. 1987;25:339–358. [Google Scholar]
- 21.Singh UP, Prithiviraj B. Neemazal, a product of neem (Azadirachta indica) induces resistance in pea (Pisum sativum) against Erysiphe pisi. Phys Mol Pl Path. 1997;51:181–194. [Google Scholar]
- 22.Singh UP, Prithiviraj B, Sarma BK. Development of Erysiphe pisi on some pea (Pisum sativum) cultivars and on non-hosts. J Plant Dis Prot. 2000a;107:53–58. [Google Scholar]
- 23.Singh UP, Prithiviraj B, Singh KP, Sarma BK. Control of powdery mildew (Erysiphe pisi) of pea (Pisum sativum) by combined application of plant growth-promoting rhizobacteria and Neemazal™. J Plant Dis Prot. 2000b;107:59–66. [Google Scholar]
- 24.Singh UP, Sarma BK, Singh DP. Effect of plant growth-promoting rhizobacteria and culture filtrate of Sclerotium rolfsii on phenolic and salicylic acid contents in chickpea (Cicer arietinum L.) Curr Microbiol. 2003;46:131–140. doi: 10.1007/s00284-002-3834-2. [DOI] [PubMed] [Google Scholar]
- 25.Stoessl A. The dynamics of host defence. In: Bailey JA, Daverall BJ, editors. Secondary plant metabolites in preinfectional and postinfectional resistance. Academic Press: New York; 1983. pp. 71–122. [Google Scholar]
- 26.Thomashow LS, Weller DM. Role of phenazine antibiotic from Pseudomonas fluorescens in biological control of Gaeumannomyces graminis var. tritici. J Bacteriol. 1988;170:3499–3508. doi: 10.1128/jb.170.8.3499-3508.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van peer R, Nieman GJ, Schippers B. Induced resistance and phytoalexin accumulation in biological control of Fusarium wilt of carnation by Pseudomonas WCS417r. Phytopathology. 1991;81:728–734. [Google Scholar]
- 28.Wei G, Kloepper JW, Tuzun S. Induction of systemic resistance to cucumber to Colletotrichum orbiculare by selected strains of plant growth-promoting rhizobacteria. Phytopathology. 1991;81:1508–1512. [Google Scholar]


