Abstract
Although cats that have been spinalized can also be trained to stand and step with full weight support, directionally appropriate long-latency responses to perturbations are impaired, suggesting that these behaviors are mediated by distinct neural mechanisms. However, it remains unclear whether these responses reflect an attenuated postural response using the appropriate muscular coordination patterns for balance or are due to fundamentally different neural mechanisms such as increased muscular cocontraction or short-latency stretch responses. Here we used muscle synergy analysis on previously collected data to identify whether there are changes in the spatial organization of muscle activity for balance within an animal after spinalization. We hypothesized that the modular organization of muscle activity for balance control is disrupted by spinal cord transection. In each of four animals, muscle synergies were extracted from postural muscle activity both before and after spinalization with nonnegative matrix factorization. Muscle synergy number was reduced after spinalization in three animals and increased in one animal. However, muscle synergy structure was greatly altered after spinalization with reduced direction tuning, suggesting little consistent organization of muscle activity. Furthermore, muscle synergy recruitment was correlated to subsequent force production in the intact but not spinalized condition. Our results demonstrate that the modular structure of sensorimotor feedback responses for balance control is severely disrupted after spinalization, suggesting that the muscle synergies for balance control are not accessible by spinal circuits alone. Moreover, we demonstrate that spinal mechanisms underlying weight support are distinct from brain stem mechanisms underlying directional balance control.
Keywords: EMG, kinetics, motor control, posture, spinal cord injury
the vertebrate spinal cord is capable of producing locomotor and other functional behaviors (Rossignol et al. 1996), and evidence from amphibians and reptiles suggests that the modular organization of muscle activity underlying these behaviors (Tresch et al. 1999) is retained after spinalization. Spinalized mammals can generate robust locomotor patterns via spinal central pattern generators (CPGs) (McCrea and Rybak 2008; Rossignol et al. 2006), which are modulated by sensory feedback pathways within the spinal cord (Forssberg et al. 1980; Rossignol et al. 2008). Work in amphibians suggests that the modular control of muscles in groups called muscle synergies for locomotion and other spinal reflexive behaviors is encoded in spinal interneuronal networks (Giszter et al. 2007; Hart and Giszter 2010; Stein and Daniels-McQueen 2002) and remains intact after deafferentation (Cheung et al. 2005; Kargo et al. 2010) as well as decerebration or spinalization (Roh et al. 2011). In humans, common muscle synergies underlie locomotion and reactive balance control, suggesting they may be encoded in the spinal cord and recruited from different pathways (Chvatal and Ting 2012, 2013). Furthermore, muscle synergies underlie generation of locomotor and perturbed locomotor patterns (Cappellini et al. 2006; Chvatal and Ting 2012; Oliveira et al. 2012) and may be preserved after cortical stroke, albeit with impaired recruitment (Cheung et al. 2009b, 2012; Clark et al. 2010).
However, there is debate about the capacity of spinal cord circuitry for balance control (Mori 1987), as coordinated postural responses may require supraspinal input. Brain stem neurons are active during balance control (Schepens et al. 2008; Stapley and Drew 2009), and disrupting the connectivity between the brain stem and spinal cord impairs balance control and responses to perturbations (Deliagina et al. 2008; Honeycutt et al. 2009). It is well known that animals can step on a treadmill after spinal cord transection although they are lacking lateral balance control (Barbeau and Rossignol 1987; Carter and Smith 1986; Grillner 1975; Rossignol et al. 1996). Although some weight-bearing capacity may remain after spinal cord injury (SCI) and improve with rehabilitation in both humans and animals (De Leon et al. 1998; Edgerton et al. 2001; Nooijen et al. 2009), the ability to maintain postural equilibrium and balance does not recover (Barbeau and Rossignol 1987; Lyalka et al. 2009), suggesting that these behaviors are mediated by distinct neural mechanisms. Weight support and balance capabilities may be dissociated by imposing perturbations during standing. Directionally appropriate muscular activation patterns in long-latency responses to balance perturbations are impaired after spinalization (Macpherson and Fung 1999). However, it remains unclear whether these responses reflect an attenuated postural response using the appropriate muscular coordination patterns for balance or are due to fundamentally different neural mechanisms such as increased muscular cocontraction or short-latency stretch responses.
The effect of neural lesions on muscle synergy organization for locomotion or other behaviors has not yet been examined in mammals. Here we used muscle synergy analysis on the data previously collected in J. M. Macpherson's lab (Macpherson and Fung 1999) to explore differences in the spatial organization of muscle activity for balance within the same animal before and after spinalization. Previously, conclusions about multimuscle coordination were limited by the greatly reduced magnitude and increased latency of muscle recruitment in all flexors and some extensors. However, because the ground-reaction forces looked qualitatively similar to those found in intact animals, it was unclear whether the evoked muscle activity following a perturbation to balance was fundamentally reorganized or simply an attenuated but appropriately coordinated muscular response. To overcome these limitations, we used muscle synergy analysis to compare the functional organization of muscle activity for balance, independent of the individual muscle amplitude and timing. We hypothesized that the modular organization of muscle activity for balance control is disrupted by spinal cord transection. We predict that one of several possible outcomes will occur, which would suggest different neural mechanisms of impairment. First, if the same muscle synergies are identified before and after spinalization, it would suggest that the balance response is intact but attenuated, suggesting that it could be improved by upregulating spinal pathways. Second, as shown by prior research in stroke, muscle synergies could be decreased in number and have a merged structure, indicating a loss of independent recruitment of spinal muscle synergies due to loss of supraspinal input. Third, some of the intact muscle synergies could be retained after spinalization, suggesting that some neural pathways for balance are within the spinal cord whereas others require supraspinal input and/or modulation. Finally, the postspinalization muscle synergies could differ entirely from the intact muscle synergies, suggesting a complete disruption of the neural pathways for balance by spinalization.
METHODS
Experimental setup.
We analyzed previously collected postural responses to investigate the role of the spinal cord in recruiting coordinated, functional muscle synergies for balance control in the cat. Postural responses were elicited by multidirectional support-surface translation perturbations before and after spinal cord transection at the T6 level (Macpherson and Fung 1999). Muscle synergies were extracted from postural muscle activity before and after spinalization, and their composition and recruitment within each animal were compared. A brief overview of the experimental setup and data collection procedure is presented here, and further details of the experimental and training procedures were described previously (Fung and Macpherson 1999). The original protocol was approved by the Institutional Animal Care and Use Committee of the Oregon Health and Science University and conformed to National Institutes of Health guidelines regarding the care and treatment of animals.
Four adult male cats [cats Ni (4.8 kg), Go (4.36 kg), Re (3.8 kg), and Ru (3.9 kg)] were trained to stand quietly with one foot on each of four triaxial force plates that were mounted on a moveable platform (Macpherson et al. 1987). Once trained, each animal was implanted with chronically indwelling electrodes and electromyographic (EMG) activity was recorded from 8–11 hindlimb muscles on the left side of the body (Table 1). After each animal had recovered from the implantation surgery, control data were collected. Each animal was then spinalized at the level of the sixth thoracic vertebra [see Fung and Macpherson (1999) for details of the transection]. Data collection resumed when each animal was able to support its own weight on all four limbs. Kinematic data were recorded from body segments at 100 Hz with an Optotrak system (Northern Digital). Ground-reaction forces were recorded from each of the four force plates. EMGs were band-pass filtered (200 Hz and 2 kHz), full-wave rectified, and low-pass filtered (35 Hz) before sampling at 500 Hz.
Table 1.
Inclusive list of muscles recorded from left hindlimb across cats
| Label | Muscle Name | Cat |
|---|---|---|
| ILPS | Iliopsoas | Ni, Ru |
| RFEM | Rectus femoris | Ni, Go, Re, Ru |
| VMED | Vastus medialis | Ni, |
| SRTA | Anterior sartorius | Ni, Go, Re, Ru |
| GRAA | Anterior gracilis | Ru |
| SRTM | Medial sartorius | Ni |
| GLUT | Gluteus medius | Ni, Go, Re, Ru |
| BFMA | Anterior biceps femoris | Ni, Re, Ru |
| BFMP | Posterior biceps femoris | Ni, Ru |
| SEMA | Anterior semimembranosus | Ni, Go, Re |
| SEMP | Posterior semimembranosus | Ni, Re, Ru |
| FDL | Flexor digitorum longus | Ni |
| VLAT | Vastus lateralis | Go, Re |
| BFMM | Medial biceps femoris | Go |
| LGAS | Lateral gastrocnemius | Go, Re |
| ADFM | Adductor femoris | Go |
| STEN | Semitendinosus | Re |
| TIBA | Tibialis anterior | Re |
Perturbations were initiated during quiet standing, consisting of ramp-and-hold linear translations with a mean peak velocity of 16 cm/s. Perturbations were given in 16 directions equally spaced in the horizontal plane. The perturbation amplitude was constant for each direction but scaled from 4 cm along the longitudinal (y) axis to 2.5 cm along the lateral (x) axis. The perturbation duration was adjusted to attain the fixed peak mean velocity for the different amplitudes. The control data were collected over several days during the 2–3 wk prior to spinalization. Each day, 5 trials were collected for each direction of translation, giving a total of 80 trials per session. Trials were blocked by direction, but the order of directions was randomized from day to day. Average EMG and force traces were generated by averaging 10–20 trials per perturbation direction for each cat.
After spinalization, data collection resumed once each animal was able to support its weight, which occurred at day 11 for cats Ni and Ru, day 17 for Re, and day 18 for Go. The cat was placed on the four force plates, and experimenters helped the animal to balance independently for brief periods. When stability was achieved, the experimenter withdrew her hands and the computer operator initiated the translation. If the animal lost its balance at any time, spotters prevented falling. The spinal data analyzed here were collected 15–61 days after spinalization for Ru, 18–72 days after spinalization for Go, 24–72 days after spinalization for Re, and 73–101 days after spinalization for Ni. Average EMG and force traces were generated by averaging 5–20 unsupported trials per perturbation direction for each cat. In addition, several reflex responses were elicited after spinalization to demonstrate that muscles could be activated in activities other than the postural response. Flexion withdrawal reflexes were elicited by pinching toes, and paw shake was elicited by placing tape on the toes.
Data processing.
We generated data vectors consisting of the mean EMG activity during a background period (BK) and during the automatic postural response (APR) across perturbation directions. First, we averaged trials by perturbation direction. We then computed the EMG background (EMGBK) as the mean EMG during a 50-ms window that ended 60 ms prior to perturbation onset. The EMG during the postural response (EMGAPR) was computed as the mean EMG during an 80-ms window beginning 60 ms after perturbation onset (Torres-Oviedo et al. 2006) (Fig. 1). For each animal we obtained data matrices before and after spinalization in which the rows represented muscles and the columns background and postural response periods for each perturbation direction. For display purposes, each muscle's EMG values were initially normalized to the maximum value observed in the prespinalization data matrix so that each value was between 0 and 1. Prior to extraction of muscle synergies, each muscle vector in the control conditions was normalized to have unit variance to ensure equal weighting in the muscle synergy extraction. Postspinalization data were normalized by the same factors as the control data to allow pre- and postspinalization comparisons.
Fig. 1.
Example of differences in postural responses to perturbation during standing in the intact and spinalized conditions in cat Ni. A: response to forward and rightward perturbation of the support surface that loaded the hindlimb. B: response to backward and leftward perturbation of the support surface that unloaded the hindlimb. EMG responses before (black lines) and after (red lines) spinalization are shown, and vertical dashed line indicates onset of platform motion. Mean EMG activity was averaged during the automatic postural response (APR) during a time window beginning 60 ms after perturbation onset and lasting 80 ms, indicated by the gray shaded region. Shown here are iliopsoas (ILPS), vastus medialis (VMED), medial sartorius (SRTM), gluteus medius (GLUT), posterior biceps femoris (BFMP), anterior biceps femoris (BFMA), anterior semimembranosus (SEMA), rectus femoris (RFEM), and anterior sartorius (SRTA) EMG responses from the left hindlimb. Forces under the left hindlimb are also shown. The time window used to analyze ground-reaction forces (gray shaded region) was delayed 60 ms after EMG to account for electromechanical delays. C: muscle and force tuning curves across all perturbation directions. In the intact condition, muscle and force tuning curves varied in magnitude over all perturbation directions, and their shapes varied from muscle to muscle. In the spinal condition, extensor activity was reduced with degraded directional tuning whereas flexor activity was largely absent. However, force tuning curves after spinalization were similar in shape but reduced in magnitude compared with the intact condition.
Background forces (FBK) during quiet stance were computed as the mean ground-reaction force under the left hindlimb in the same period as EMGBK. The active force during the postural response (FAPR) was computed as the change in force from background levels during an 80-ms window that began 60 ms after EMGAPR onset or 120 ms after perturbation onset (Jacobs and Macpherson 1996) to accommodate excitation-contraction coupling time (Fig. 1). This definition of active force was used in our previous work in which the change in force from background was considered (Jacobs and Macpherson 1996; Macpherson 1988; Ting and Macpherson 2005; Torres-Oviedo et al. 2006).
Extraction of muscle synergies.
We extracted muscle synergies from EMG data matrices by nonnegative matrix factorization (NNMF) (Lee and Seung 1999; Tresch et al. 1999), which has previously been used for muscle synergy analysis (Chvatal and Ting 2012; Ting and Macpherson 2005; Torres-Oviedo and Ting 2007). Here we assume each muscle activation pattern, M, at a given time can be represented by a linear combination of a few muscle synergies, Wi, that are each recruited by a synergy recruitment coefficient, ci:
Each component of Wi represents the contribution of one particular muscle to that synergy, and an individual muscle may contribute to multiple synergies. The muscle synergy vectors Wi are constant across directions, but their scalar recruitment coefficient, ci, may vary as a function of time and/or perturbation direction. After muscle synergies were extracted, the unit variance scaling was removed from data so that each muscle ranged from 0 and 1 to permit data inspection and interpretation.
The goodness of fit of the data reconstruction using the muscle synergies was quantified by the variability accounted for (VAF), defined as 100 × uncentered Pearson's correlation coefficient (Torres-Oviedo et al. 2006; Zar 1999). The number of muscle synergies that best described the data (Nsyn) was determined by global and local criteria: 1) total VAF > 90%, 2) VAF across muscles > 75%, and 3) VAF across perturbation directions > 75% [see Chvatal et al. (2011)]. This combination of both global and local variability criteria ensured that each pattern of muscle activation measured for a given perturbation direction, and each muscle tuning curve over all directions, was well-reconstructed.
To validate Nsyn we compared the overall reconstruction VAF using the identified muscle synergies to the overall VAF using muscle synergies extracted from shuffled data. We estimated 99% bootstrap confidence intervals (CIs) for the overall reconstruction VAF using muscle synergies extracted from a shuffled data set by bootstrapping with replacement (Cheung et al. 2009a; Efron 1993) to resample each data matrix. In the shuffled version of the original data matrix, each muscle's data were shuffled independently; therefore, this shuffled data matrix contained the same values, range, and variance for each muscle, but the relationships between muscle activations were removed. The overall reconstruction VAF using either the muscle synergies extracted from the original data or the muscle synergies extracted from shuffled data was calculated for 500 resampled data sets, and 99% CI bounds were estimated by selecting the 0.5 and 99.5 percentiles of the VAF distribution. The VAF CI found using the muscle synergies extracted from the original data set was compared to the VAF CI found using muscle synergies from shuffled data. We expected VAF confidence intervals for original and shuffled data not to overlap for the selected Nsyn value.
Data analysis.
The identified muscle synergies before (Wintact) and after (Wspinal) spinalization were used to reconstruct the recorded EMG tuning curves from which they were extracted, and similarity between measured and reconstructed data was quantified by r2 and VAF (Torres-Oviedo et al. 2006; Zar 1999). VAF provides a measure of how well the magnitudes of the measured EMG and the reconstructed EMG match, whereas r2 quantifies how well the shapes of the curves match. Additionally, Wintact were used to reconstruct postspinalization data and Wspinal were used to reconstruct prespinalization data. A two-factor ANOVA (cat, reconstruction condition) followed by Scheffé post hoc test was used to determine significant differences in reconstruction fit values.
Muscle synergies were also extracted from combined pre- and postspinalization data and compared to the muscle synergies identified from the individual extractions. Additionally, it was previously reported that postural muscle latencies were delayed after SCI, so here we also extracted muscle synergies from spinal data during a postural response time window delayed by 50 ms (110–190 ms after perturbation onset) and by 100 ms (160–240 ms after perturbation onset). For one cat, Ni, muscle synergies were also extracted from a data set generated using a subset of the trials collected after spinalization in which the cat remained balanced throughout the entire trial (no experimenter stabilization). Similarity between muscle synergies identified from the various conditions was quantified by calculating the correlation coefficient (r) between the muscle synergy vectors. A pair of muscle synergies having r > 0.834 (cats Go and Ru), r > 0.764 (Re), or r > 0.734 (Ni) were considered similar, which corresponds to the critical value for 8, 10, and 11 muscles, respectively (P = 0.01). To compare the amount of muscle (and muscle synergy) tuning across conditions, we first identified the four perturbation directions with highest muscle (or muscle synergy) activity in each tuning curve and then quantified the number of directions spanned by the four maximum points.
Finally, to determine whether muscle synergy recruitment during balance in the spinal cat was related to a particular biomechanical function or behavioral goal, we extracted functional muscle synergies from a data matrix containing muscle activity as well as subsequent forces under the left hindlimb (Chvatal et al. 2011; Torres-Oviedo et al. 2006). To use NNMF, the positive and negative components of the forces were separated, resulting in six additional data rows to be included in the matrix (Fx+, Fx−, Fy+, Fy−, Fz+, and Fz−). As with the EMG data, each row was normalized to have unit variance before functional muscle synergies were extracted to ensure a uniform representation of variance across the data pool.
RESULTS
Postural responses to perturbation in intact animals were observed in all EMGs from the left hindlimb, as previously reported (Macpherson and Fung 1999). For example, extensors vastus medialis and anterior semimembranosus were active following forward right perturbations that caused the animal to sway backward and leftward, loading the hindlimb (Fig. 1A), and flexors medial sartorius and iliopsoas were active following backward left perturbations caused the animal to sway forward and rightward, unloading the hindlimb (Fig. 1B). After spinalization, postural responses were only observed in muscles that were tonically active in the background period and were not observed in flexors (Fig. 1, A and B). However, flexor muscles could be highly activated by pinching the toes to elicit a flexor reflex or placing tape on the toes to elicit a paw shake (Macpherson and Fung 1999). Therefore the absence of muscle activity in these muscles during postural responses could not be attributed to electrode degradation or a loss of ability to activate flexor muscles. Muscle tuning curves generated from the magnitude of the initial postural response illustrate the directional tuning of each muscle during postural responses (Fig. 1C). In the intact condition, muscles were activated in preferred perturbation directions and forces produced under the left hindlimb were directionally tuned. After spinalization, muscle tuning curves illustrate greatly reduced muscle activity across both flexor and extensor muscles, with degraded directional tuning compared with the intact condition, and force tuning curves were reduced in magnitude but had a shape similar to the intact condition.
Muscle synergy organization is disrupted after spinalization.
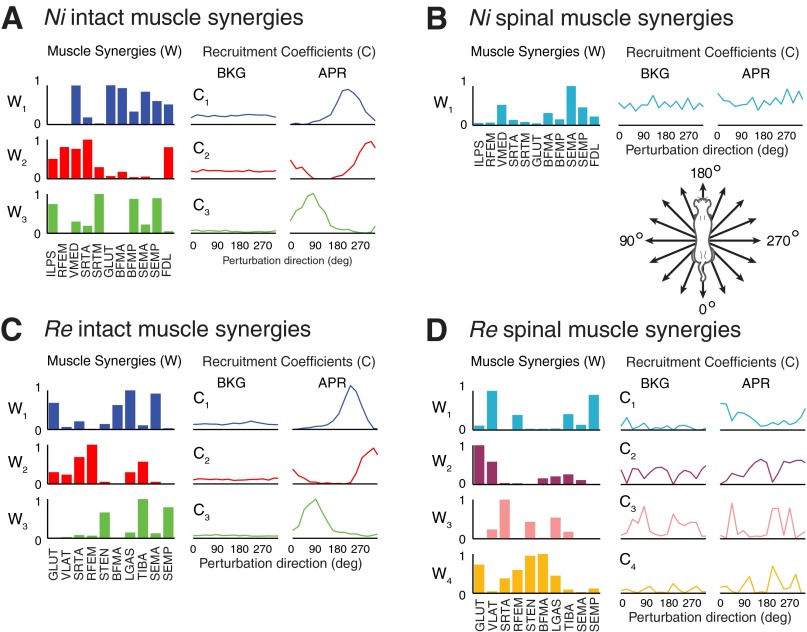
Muscle synergy number and structure were different in the intact and spinal conditions. In the intact conditions, three muscle synergies were required to account for >90% overall VAF and >75% of the variability (VAF) across each muscle and perturbation direction in all four animals (Fig. 2). In contrast, after spinalization, only one muscle synergy was required in three animals (cats Ni, Go, and Ru) whereas four muscle synergies were required in the fourth animal (Re). In the intact condition, muscle synergies had contributions from all muscles recorded, such that each cat generally had one muscle synergy comprised of flexors, one extensor muscle synergy, and a third muscle synergy that varied across cats (Fig. 2, A and C). Spinal muscle synergies had large contributions from extensors, with minimal flexor contributions (Fig. 2, B and D). The postspinalization muscle synergies used by cats Ni, Ru, and Re did not match those used in the intact condition (highest r = 0.554, 0.633, and 0.266 for Ni, Ru, and Re, respectively). However, the extensor muscle synergy used by cat Go after spinalization was similar to a prespinalization muscle synergy (r = 0.897). The same muscle synergies were found when extracting from a slightly later time bin in the spinal condition where EMG amplitudes were somewhat higher.
Fig. 2.
Muscle synergy composition and recruitment coefficients in the intact and spinalized conditions for 2 representative cats, Ni and Re. A and C: 3 muscle synergy vectors W1–W3 extracted from EMG data during postural responses in the intact condition. Intact muscle synergies had contributions from flexors and extensors. Muscle synergy recruitment coefficients (C1–C3) were consistent across directions during background (BKG) but highly modulated across directions during the posture response (APR). B and D: after spinalization, only 1 muscle synergy was identified for cat Ni (as well as cats Go and Ru, not shown) (B), whereas 4 muscle synergies were required to explain the variability of responses in cat Re (D). Spinal muscle synergies across animals had minimal flexor contributions and exhibited no clear directional tuning. FDL, flexor digitorum longus; VLAT, vastus lateralis; STEN, semitendinosus; LGAS, lateral gastrocnemius; TIBA, tibialis anterior.
For cats Ni, Go, and Ru, all of the muscle synergies identified individually in the intact and spinal conditions were distinctly identified when muscle synergies were extracted from a pooled data set containing both intact and spinal conditions (r = 0.96 ± 0.05 when comparing pooled muscle synergies to those identified from the individual extractions). For cat Re, five muscle synergies were identified in the combined extraction, three of which were similar to the intact muscle synergies (r = 0.98 ± 0.01) and two of which were similar to spinal muscle synergies (r = 0.88 ± 0.05).
Muscle synergy recruitment was directionally tuned in the intact condition, but directional tuning was not as evident in the spinal condition. In the intact condition, after perturbation, each muscle synergy was highly modulated across direction, with the four maximal tuning directions being in consecutive perturbation directions for all muscle synergy tuning curves in all cats, except for one (maximal tuning directions span 4.5 ± 1.7 directions, or 101 ± 38°), and low recruitment for the remaining directions (Fig. 2, A and C). In contrast, postspinalization muscle synergies exhibited less clear directional tuning (Fig. 2, B and D). Across all cats and all muscle synergy tuning curves, the four perturbation directions with maximal muscle synergy recruitment spanned 9.0 ± 3.3 directions, or 203 ± 74°.
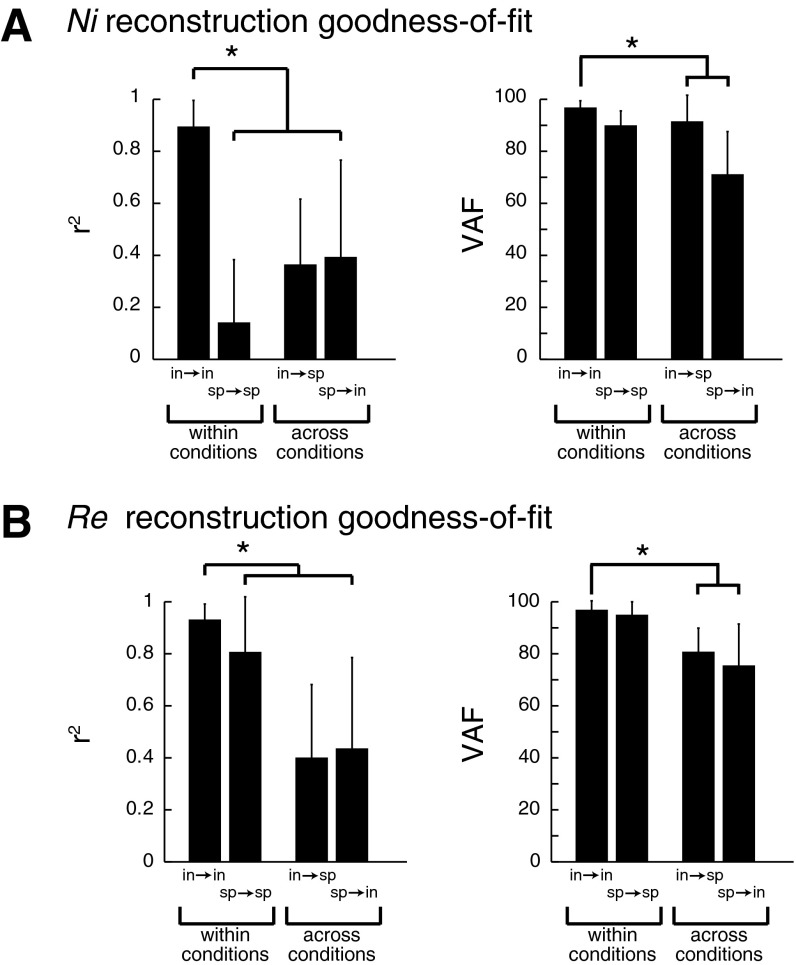
Intact muscle synergies were able to explain variations in intact postural responses (overall VAF = 96.9 ± 1.4 across cats), and spinal muscle synergies were able to explain variations in spinal data (overall VAF = 95.1 ± 1.1 across cats). However, we were unable to adequately reconstruct muscle activity from the intact condition using spinal muscle synergies (overall VAF = 52.9 ± 12.3) or from the spinal condition using intact muscle synergies (overall VAF = 71.6 ± 12.9). Individual muscle tuning curve magnitude and shape were both well-reconstructed when using intact muscle synergies on intact EMG data (Fig. 3; r2 = 0.92 ± 0.07, VAF = 97.1 ± 2.6%). After spinalization, when using spinal muscle synergies to reconstruct spinal EMG data, the muscle tuning curve magnitudes were well-reconstructed (VAF = 92.0 ± 6.4%) but not the tuning curve shapes (r2 = 0.41 ± 0.35). Within each cat, r2 and VAF values describing individual muscle tuning curve reconstructions decreased when using muscle synergies extracted from a different condition (spinal/intact) to reconstruct data (intact/spinal) (Fig. 3). Across all four cats, when intact muscle synergies were used to reconstruct intact data (in→in), r2 were significantly higher compared with any of the other reconstructions (P < 10−14, F3,141 = 30.3, ANOVA followed by Scheffé post hoc, α = 0.01). Likewise, in→in VAF were significantly higher than in→sp and sp→in (P < 10−13, F3,141 = 27.6, ANOVA followed by Scheffé post hoc, α = 0.01) but not significantly different from sp→sp.
Fig. 3.
Goodness of fit for reconstructed muscle tuning curves in the intact and spinal conditions: goodness of fit for cats Ni (A) and Re (B) using muscle synergies within and across conditions. Means ± SD of r2 and variability accounted for (VAF) across muscles for each cat are shown for reconstructions within conditions, i.e., intact muscle synergies used to reconstruct intact data (in→in) and spinal muscle synergies used to reconstruct spinal data (sp→sp), as well as reconstructions across conditions, i.e., intact muscle synergies used to reconstruct spinal data (in→sp) and spinal muscle synergies used to reconstruct intact data (sp→in). In all animals, in→in reconstructions were highest, and fits were degraded when muscle synergies were applied across conditions. *Significant differences by ANOVA followed by Scheffé post hoc test, α = 0.01.
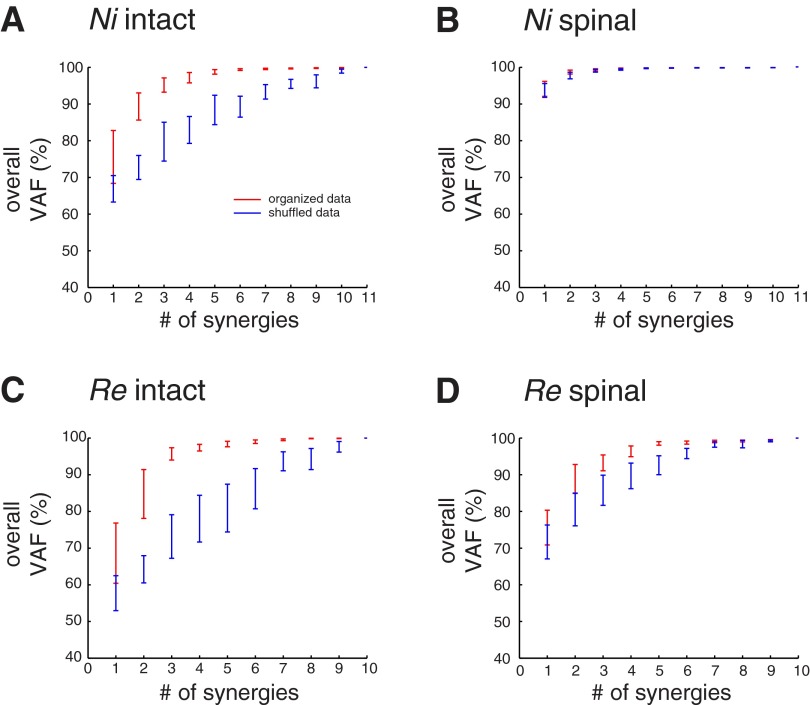
There was little structure in the postspinal data, as CIs for the VAF were reduced compared with the intact condition. In the intact condition there was a rapid rise in the VAF as the number of muscle synergies increased, whereas in a shuffled data set, the VAF increased roughly linearly and the CI did not overlap (Fig. 4, A and C). For all four cats, in the intact condition the VAF CI for the selected number of muscle synergies did not overlap the VAF CI for shuffled data. This demonstrates that the muscle synergies reflect structure in the original data from intact animals. In contrast, after spinalization, VAF increased rapidly in both the original and shuffled data sets, and the CIs were small and often overlapping for cats Ni, Go, and Ru (Fig. 4B). Only cat Re had several nonoverlapping CIs, yet even in this case the postspinalization CIs were much closer together for the original and shuffled data (Fig. 4D). Furthermore, the composition of intact muscle synergies were different from those extracted from shuffled intact data (0 similar synergies were identified for all 4 cats), whereas at least one spinal muscle synergy was similar to the muscle synergies extracted from shuffled spinal data for each cat at the selected number of synergies. For the three cats requiring only one muscle synergy in the spinal condition (Ni, Go, and Ru), the one muscle synergy extracted from shuffled spinal data was nearly identical to the muscle synergy extracted from spinal data (r > 0.99), whereas for cat Re, one spinal muscle synergy was similar to one synergy extracted from shuffled data (r = 0.824). This demonstrates that there was little structure in the spinal data set.
Fig. 4.
Comparison of reconstruction VAFs across number of muscle synergies extracted from original data vs. shuffled data in both the intact and spinalized conditions in 2 representative cats. Ninety-nine percent confidence intervals (CIs) of the reconstruction VAFs were estimated by bootstrapping methods. A and C: in the intact condition, there was a rapid rise in the VAF as the number of muscle synergies increased for the original data set (red) but a linear rise in VAF from the shuffled data set (blue), indicating that the muscle synergies extracted reflect structure in the data that was lost after shuffling. In all cats, the VAF CI for the selected number of muscle synergies did not overlap the VAF CI for shuffled data in the intact condition. B and D: after spinalization, VAF increased rapidly in both the original and shuffled data sets and the CIs were overlapping (Ni, B, as well as Go and Ru, not shown) or nearly overlapping (Re, D), suggesting that the structure in the original data set was no different from a shuffled version of the data set.
Functional muscle synergies reveal differences in force production after spinalization.
In the intact condition, functional muscle synergies extracted from both muscle activity and forces under the hindlimb demonstrated that EMGs and subsequent forces were highly correlated and a wide variety of force directions were produced, as shown previously (Ting and Macpherson 2005; Torres-Oviedo et al. 2006). In intact animals, four to five functional muscle synergies were required to account for >90% total variability and >75% of the variability across muscles, forces, and perturbation directions (Fig. 5A). The number of muscle synergies required to explain muscles and forces was greater than the number required to explain muscle activity alone and is consistent with previous results in which muscle synergies were extracted from intact perturbation response data across a greater number of muscles and conditions from some of the same cats analyzed here (Torres-Oviedo et al. 2006). For each cat, the muscle synergies were similar (r = 0.93 + 0.05) when the same number of muscle synergies were extracted from combined EMG and force data or EMG data alone. This demonstrates that the addition of forces to the data matrix did not alter the composition of extracted muscle synergies, indicating that muscle synergy activity and force generation were correlated and not independent. When muscle synergies were extracted from EMG activity alone, two muscle synergies with similar directional tuning were identified as a single synergy. With the addition of forces, muscle synergies responsible for different functions are more easily distinguished, further explaining why a greater number of functional muscle synergies were identified compared with muscle synergies extracted from EMG only. For example, a single extensor synergy W1 identified from EMG alone was identified as two functional muscle synergies, one responsible for antigravity function during background across all perturbation directions that turned off during the postural response and the other quiet during background but strongly recruited during the postural response (see also Torres-Oviedo 2006). In general, each functional muscle synergy had contributions from both muscles and forces, suggesting that force production resulted from recruitment of the identified muscle synergies. There were two exceptions observed in cat Go in which functional muscle synergies having large force contributions but no muscle activity were identified. This demonstrates that there was no EMG measurement correlated to force production; for example, no flexor EMG recordings were available from cat Go in this data set, and the generation of flexion forces was found to be independent of the available EMG recordings. Furthermore, across cats, the synergy force vectors spanned several directions in the horizontal plane, demonstrating that the intact cat produces a range of forces during postural responses (Fig. 5, A and B).
Fig. 5.
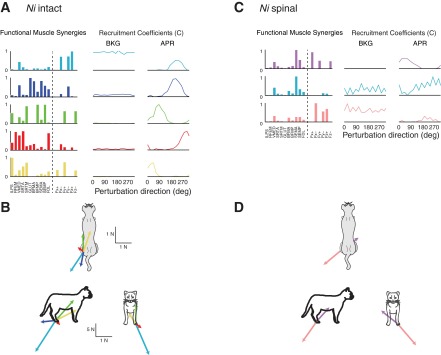
Functional muscle synergies and synergy force vectors in the intact and spinalized conditions for cat Ni. A and B: in the intact condition, EMGs and subsequent forces were highly correlated (A) and a wide variety of force directions were produced (B). C: in contrast, after spinalization, EMGs and forces were not correlated, as synergy 2 has large contributions from muscles but no force contribution, whereas synergy 3 has large force contributions but minimal muscle contributions. D: furthermore, only a restricted set of synergy forces were produced, directed along a single diagonal. Similar trends were observed in cats Go, Re, and Ru, not shown.
In contrast, functional muscle synergies identified after spinalization demonstrated that EMGs and forces were not correlated and that only a restricted set of forces was produced. In the spinal condition, three to six functional muscle synergies were required to account for >75% of the variability across muscles, forces, and directions (Fig. 5C). Each muscle synergy was generally composed of either muscles or forces, such that few had large contributions from both. This indicates a low correlation between muscle synergy recruitment and force production. Furthermore, the synergy force vectors after spinalization were directed along a single diagonal, suggesting a passive loading/unloading rather than an active force production in response to the perturbations (Fig. 5, C and D). This decoupling of muscle synergy activation and force production and restriction in force vector direction persisted even when the number of functional muscle synergies extracted was high. For example, cat Re required six functional muscle synergies in the spinal condition, yet forces were nonetheless aligned along the diagonal.
DISCUSSION
Our results suggest that the ability to stand and resist small perturbations to balance after spinal injury is due to neuromuscular mechanisms different from those used for directional balance control in intact animals. In previous analyses of these data, the small amplitude of muscle activity evoked from spinalized animals required analysis of later time bins, suggesting a delayed response. However, the similarity of muscle coordination in background, APR, and delayed-APR time bins after spinalization suggests that there is no coordinated response evoked by perturbation as in intact animals. We demonstrated that the modular structure of postural responses for balance control was greatly altered after spinalization, confirming that the balance capabilities after spinalization may be quite limited. Furthermore, the functional relationships between muscle synergy recruitment and forces to restore balance found in intact animals were not present in spinalized animals. Taken together, these results suggest that the muscle synergies necessary for directional balance control are not accessible after spinalization, likely because they require supraspinal connectivity for their recruitment but possibly because of disruption of spinal circuitry necessary for balance control.
Muscle synergy analysis lends insight into explicit differences in muscle coordination of balance that were previously difficult to quantify because of the low amplitude and variable timing of muscle responses to perturbation. Spinalized cats can be trained to stand independently (De Leon et al. 1998) and withstand small perturbations to balance (Macpherson and Fung 1999); however, their postural response muscle activity has low amplitude. Previous analysis showed that postural responses were both smaller and delayed in spinalized compared with intact animals when comparing individual muscles. Although flexors could be appropriately activated by flexor and paw shake reflexes after spinalization, flexors were not activated during postural responses. Muscle synergy analysis allowed us to examine the coordination across muscles irrespective of amplitude and timing. In spinalized animals, the similarity of muscle coordination in background, postural response, and delayed-postural response time bins suggests that no coordinated balance response was evoked by perturbations in spinalized animals. In contrast, modular organization in intact animals differed significantly in the postural response compared with the background period because of recruitment of specific balance control circuitry. Thus, in spinalized animals, mechanisms of weight support and balance may be due to local scaling up or down of background EMG patterns (Heckman et al. 2003; Hyngstrom et al. 2007) or due to spinal circuits mediating muscle responses to stretch (Nichols 1994). Therefore, the identified muscle response to balance perturbations in spinalized animals most likely cannot be scaled up or reduced in latency to achieve directional balance control.
The differences in motor modules identified in the intact and spinalized conditions suggest that the structure and organization of postural responses are greatly altered in SCI. In the three animals requiring a single muscle synergy to describe postural responses after spinalization, CIs on the VAF in EMG reconstructions using the identified muscle synergies overlapped with those from shuffled data (in contrast to intact data), suggesting that the structure of the spinalized data set could not be clearly differentiated from randomized data. In the spinalized conditions the overlapping CIs were found even as the number of muscle synergies extracted was increased (cf. Fig. 4, B and D). These results corroborate the idea that no organized postural response is evoked by the perturbation in these animals. Some of these differences may be attributed to the fact that muscle activity was compiled from multiple sessions because only eight muscles could be sampled in a single session and the entire set of directions was often not obtained within a single recording session because of fatigue. However, the same conditions applied to the control data in which highly structured responses were obtained. Furthermore, using the same approach, we identified more muscle synergies in the spinalized condition than the intact condition in one animal (cat Re). In contrast to the other three animals, these muscle synergies did reveal structure in the data, as the VAF due to the same number of muscle synergies decreased considerably when data were shuffled. However, these muscle synergies were different from those identified in the intact condition. Because of the large extensor contributions and lack of directional tuning, these muscle synergies could reflect other neural mechanisms causing coordinated muscle activity such as spasticity or heterogenic stretch responses. This animal was also the most greatly impaired and required the most assistance in standing. Similarly, in human SCI preliminary analyses show increased variability and an increased number of “abnormal” muscle synergies during locomotion in more impaired individuals (Hayes et al. 2011). While muscle synergy analysis may ultimately lead to improved measures of motor coordination across neuromotor impairments, we still lack fundamental knowledge to interpret how muscle synergy composition, recruitment, and number relate to functionally relevant changes due to motor experience, impairment, or rehabilitation.
In contrast to recent studies examining muscle synergies in poststroke hemiplegia, we found that an increased number of muscle synergies did not necessarily correspond to better motor function and that for SCI muscle synergy structure and recruitment patterns may be more important in characterizing motor deficits. Although we found a reduced number of muscle synergies in three animals, these were not merged versions of the intact muscle synergies, as found in poststroke locomotion (Clark et al. 2010). Moreover, we found an increased number of muscle synergies in the most impaired animal, and these had dissimilar muscle synergy composition compared with the intact condition. This is consistent with preliminary reports in humans with SCI, who may exhibit a greater number of muscle synergies than healthy individuals but with very different muscle synergy composition. Moreover, even in the stroke literature, the number of muscle synergies cannot completely account for differences in motor performance in individuals with poststroke hemiplegia who have the same number of muscle synergies as control subjects. Furthermore, it has been shown that the structure of muscle synergies can change based on the acuteness of the neural insult (Cheung et al. 2012; Gizzi et al. 2011). Therefore, the composition and recruitment of muscle synergies may be equally important as the number of muscle synergies in understanding motor impairments.
Here functional muscle synergy analysis revealed that spinal muscle synergies were not correlated to unique functional outputs as they were in the intact condition, suggesting that directional balance responses were absent after spinalization. Our functional muscle synergy analysis reveals correlations between muscle synergy recruitment and components of ground-reaction forces or accelerations that are subsequently generated (Chvatal et al. 2011; Torres-Oviedo et al. 2006). Previous such analyses suggest that in the intact nervous system muscle synergies produce a broad set of force vector directions for balance control, allowing robust control of center of mass (CoM) direction following a perturbation. In cat and human balance control the addition of forces to the muscle synergy analysis does not alter the muscle synergies identified by using EMG alone, indicating that forces during the postural response are highly correlated to evoked EMGs. However, in spinalized animals such correlations between muscle synergy recruitment and force production are disrupted, as forces and EMGs were identified in separate muscle synergies, even when animals were able to maintain balance. The forces that were produced were limited to two force directions (even when 4 muscle synergies were identified in 1 animal) and could have been generated based on the intrinsic biomechanical properties of the limb rather than a coordinated muscular response due to sensorimotor feedback. Thus spinal cats may use muscle stiffness or passive forces to maintain balance, as muscle synergy recruitment changed little in the response period compared with quiet standing. These findings corroborate studies in rabbits with spinal lesions indicating that evoked EMG signals do not functionally contribute to postural control on a tilting platform (Lyalka et al. 2009). Therefore, prior studies demonstrating that spinalized animals can be trained to stand (De Leon et al. 1998) likely relied on mechanisms other than the brain stem-mediated automatic postural response (Deliagina et al. 2008; Horak and Macpherson 1996) to maintain stability.
Our results suggest that the muscle synergies used for postural control are not accessible via spinal circuits alone. Studies in the frog have demonstrated that locomotor and reflexive behaviors may be constructed by recruiting muscle synergies organized in both the brain stem and spinal cord (Roh et al. 2011). Basic locomotor patterns may be produced by spinal CPGs (McCrea and Rybak 2008; Rossignol et al. 2006), which are modulated by sensory feedback pathways within the spinal cord (Forssberg et al. 1980; Rossignol et al. 2008), even in the absence of descending input. However, balance control requires multisensory integration and interlimb coordination that is not evident in spinalized animals (Macpherson and Fung 1999) or animals lacking connectivity to the brain stem (Deliagina et al. 2008; Lyalka et al. 2009) and likely requires brain stem processing (Stapley and Drew 2009). However, we do not know whether supraspinal input is responsible for recruiting muscle synergies encoded in the spinal cord or is involved in the patterning of muscle synergies. Recent evidence suggests that muscle synergies may be encoded in the spinal cord but recruited via different pathways for locomotion and reactive balance control (Chvatal and Ting 2012, 2013). We also cannot rule out the possibility that aspects of directional balance control are modulated by spinal circuits but disrupted by spinalization. It is likely that the muscle synergies identified in the postspinalization condition resulted from loading or stretch responses mediated by the spinal cord (Dietz et al. 1992; Nichols 1994). Therefore our work has demonstrated dissociation between mechanisms of weight support, locomotion, and balance control, suggesting that different interventions may be necessary to restore those functions after SCI.
GRANTS
This work is supported by National Institute of Neurological Disorders and Stroke Grant NS-058322 to L. H. Ting.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.A.C. and L.H.T. conception and design of research; S.A.C. and G.T.-O. analyzed data; S.A.C., J.M.M., G.T.-O., and L.H.T. interpreted results of experiments; S.A.C. and L.H.T. prepared figures; S.A.C. and L.H.T. drafted manuscript; S.A.C., J.M.M., G.T.-O., and L.H.T. edited and revised manuscript; S.A.C., J.M.M., G.T.-O., and L.H.T. approved final version of manuscript; J.M.M. performed experiments.
ACKNOWLEDGMENTS
We thank Claire Honeycutt for her contributions to some of the initial analysis of data.
Present addresses: J. M. Macpherson, jM Scientific Consulting, 1305 Heater Ct., West Linn, OR 97068; G. Torres-Oviedo, University of Pittsburgh, Pittsburgh, PA 15261.
REFERENCES
- Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res 412: 84–95, 1987 [DOI] [PubMed] [Google Scholar]
- Cappellini G, Ivanenko YP, Poppele RE, Lacquaniti F. Motor patterns in human walking and running. J Neurophysiol 95: 3426–3437, 2006 [DOI] [PubMed] [Google Scholar]
- Carter MC, Smith JL. Simultaneous control of two rhythmical behaviors. II. Hindlimb walking with paw-shake response in spinal cat. J Neurophysiol 56: 184–195, 1986 [DOI] [PubMed] [Google Scholar]
- Cheung VC, d'Avella A, Bizzi E. Adjustments of motor pattern for load compensation via modulated activations of muscle synergies during natural behaviors. J Neurophysiol 101: 1235–1257, 2009a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung VC, d'Avella A, Tresch MC, Bizzi E. Central and sensory contributions to the activation and organization of muscle synergies during natural motor behaviors. J Neurosci 25: 6419–6434, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung VC, Piron L, Agostini M, Silvoni S, Turolla A, Bizzi E. Stability of muscle synergies for voluntary actions after cortical stroke in humans. Proc Natl Acad Sci USA 106: 19563–19568, 2009b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung VC, Turolla A, Agostini M, Silvoni S, Bennis C, Kasi P, Paganoni S, Bonato P, Bizzi E. Muscle synergy patterns as physiological markers of motor cortical damage. Proc Natl Acad Sci USA 109: 14652–14656, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chvatal SA, Ting LH. Common muscle synergies for balance and walking. Front Comput Neurosci 7: 48, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chvatal SA, Ting LH. Voluntary and reactive recruitment of locomotor muscle synergies during perturbed walking. J Neurosci 32: 12237–12250, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chvatal SA, Torres-Oviedo G, Safavynia SA, Ting LH. Common muscle synergies for control of center of mass and force in nonstepping and stepping postural behaviors. J Neurophysiol 106: 999–1015, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DJ, Ting LH, Zajac FE, Neptune RR, Kautz SA. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J Neurophysiol 103: 844–857, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leon RD, Hodgson JA, Roy RR, Edgerton VR. Full weight-bearing hindlimb standing following stand training in the adult spinal cat. J Neurophysiol 80: 83–91, 1998 [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Beloozerova IN, Zelenin PV, Orlovsky GN. Spinal and supraspinal postural networks. Brain Res Rev 57: 212–221, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz V, Gollhofer A, Kleiber M, Trippel M. Regulation of bipedal stance: dependency on “load” receptors. Exp Brain Res 89: 229–231, 1992 [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Leon RD, Harkema SJ, Hodgson JA, London N, Reinkensmeyer DJ, Roy RR, Talmadge RJ, Tillakaratne NJ, Timoszyk W, Tobin A. Retraining the injured spinal cord. J Physiol 533: 15–22, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B. An Introduction to the Bootstrap. New York: Chapman and Hall, 1993 [Google Scholar]
- Forssberg H, Grillner S, Halbertsma J, Rossignol S. The locomotion of the low spinal cat. II. Interlimb coordination. Acta Physiol Scand 108: 283–295, 1980 [DOI] [PubMed] [Google Scholar]
- Fung J, Macpherson JM. Attributes of quiet stance in the chronic spinal cat. J Neurophysiol 82: 3056–3065, 1999 [DOI] [PubMed] [Google Scholar]
- Giszter S, Patil V, Hart C. Primitives, premotor drives, and pattern generation: a combined computational and neuroethological perspective. Prog Brain Res 165: 323–346, 2007 [DOI] [PubMed] [Google Scholar]
- Gizzi L, Nielsen JF, Felici F, Ivanenko YP, Farina D. Impulses of activation but not motor modules are preserved in the locomotion of subacute stroke patients. J Neurophysiol 106: 202–210, 2011 [DOI] [PubMed] [Google Scholar]
- Grillner S. Locomotion in vertebrates: central mechanisms and reflex interaction. Physiol Rev 55: 247–304, 1975 [DOI] [PubMed] [Google Scholar]
- Hart CB, Giszter SF. A neural basis for motor primitives in the spinal cord. J Neurosci 30: 1322–1336, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes HB, VanHiel LR, Chvatal SA, Ting LH, Tansey KE, Trumbower RD. Modularity of muscle activity during robot-assisted locomotion in persons with incomplete spinal cord injury (Abstract). Neuroscience Meeting Planner 2011: 808–13, 2011 [Google Scholar]
- Heckman CJ, Lee RH, Brownstone RM. Hyperexcitable dendrites in motoneurons and their neuromodulatory control during motor behavior. Trends Neurosci 26: 688–695, 2003 [DOI] [PubMed] [Google Scholar]
- Honeycutt CF, Gottschall JS, Nichols TR. Electromyographic responses from the hindlimb muscles of the decerebrate cat to horizontal support surface perturbations. J Neurophysiol 101: 2751–2761, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak FB, Macpherson JM. Postural orientation and equilibrium. In Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am Physiol Soc, 1996, sect. 12, p. 255–292 [Google Scholar]
- Hyngstrom AS, Johnson MD, Miller JF, Heckman CJ. Intrinsic electrical properties of spinal motoneurons vary with joint angle. Nat Neurosci 10: 363–369, 2007 [DOI] [PubMed] [Google Scholar]
- Jacobs R, Macpherson JM. Two functional muscle groupings during postural equilibrium tasks in standing cats. J Neurophysiol 76: 2402–2411, 1996 [DOI] [PubMed] [Google Scholar]
- Kargo WJ, Ramakrishnan A, Hart CB, Rome LC, Giszter SF. A simple experimentally based model using proprioceptive regulation of motor primitives captures adjusted trajectory formation in spinal frogs. J Neurophysiol 103: 573–590, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DD, Seung HS. Learning the parts of objects by non-negative matrix factorization. Nature 401: 788–791, 1999 [DOI] [PubMed] [Google Scholar]
- Lyalka VF, Orlovsky GN, Deliagina TG. Impairment of postural control in rabbits with extensive spinal lesions. J Neurophysiol 101: 1932–1940, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson JM. Strategies that simplify the control of quadrupedal stance. I. Forces at the ground. J Neurophysiol 60: 204–217, 1988 [DOI] [PubMed] [Google Scholar]
- Macpherson JM, Fung J. Weight support and balance during perturbed stance in the chronic spinal cat. J Neurophysiol 82: 3066–3081, 1999 [DOI] [PubMed] [Google Scholar]
- Macpherson JM, Lywood DW, Van Eyken A. A system for the analysis of posture and stance in quadrupeds. J Neurosci Methods 20: 73–82, 1987 [DOI] [PubMed] [Google Scholar]
- McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev 57: 134–146, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S. Integration of posture and locomotion in acute decerebrate cats and in awake, freely moving cats. Prog Neurobiol 28: 161–195, 1987 [DOI] [PubMed] [Google Scholar]
- Nichols TR. A biomechanical perspective on spinal mechanisms of coordinated muscular action: an architecture principle. Acta Anat (Basel) 151: 1–13, 1994 [DOI] [PubMed] [Google Scholar]
- Nooijen CF, Ter Hoeve N, Field-Fote EC. Gait quality is improved by locomotor training in individuals with SCI regardless of training approach. J Neuroeng Rehabil 6: 36, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira AS, Gizzi L, Kersting UG, Farina D. Modular organization of balance control following perturbations during walking. J Neurophysiol 108: 1895–1906, 2012 [DOI] [PubMed] [Google Scholar]
- Roh J, Cheung VC, Bizzi E. Modules in the brain stem and spinal cord underlying motor behaviors. J Neurophysiol 106: 1363–1378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S, Barriere G, Frigon A, Barthelemy D, Bouyer L, Provencher J, Leblond H, Bernard G. Plasticity of locomotor sensorimotor interactions after peripheral and/or spinal lesions. Brain Res Rev 57: 228–240, 2008 [DOI] [PubMed] [Google Scholar]
- Rossignol S, Chau C, Brustein E, Belanger M, Barbeau H, Drew T. Locomotor capacities after complete and partial lesions of the spinal cord. Acta Neurobiol Exp (Wars) 56: 449–463, 1996 [DOI] [PubMed] [Google Scholar]
- Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol Rev 86: 89–154, 2006 [DOI] [PubMed] [Google Scholar]
- Schepens B, Stapley P, Drew T. Neurons in the pontomedullary reticular formation signal posture and movement both as an integrated behavior and independently. J Neurophysiol 100: 2235–2253, 2008 [DOI] [PubMed] [Google Scholar]
- Stapley PJ, Drew T. The pontomedullary reticular formation contributes to the compensatory postural responses observed following removal of the support surface in the standing cat. J Neurophysiol 101: 1334–1350, 2009 [DOI] [PubMed] [Google Scholar]
- Stein PS, Daniels-McQueen S. Modular organization of turtle spinal interneurons during normal and deletion fictive rostral scratching. J Neurosci 22: 6800–6809, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting LH, Macpherson JM. A limited set of muscle synergies for force control during a postural task. J Neurophysiol 93: 609–613, 2005 [DOI] [PubMed] [Google Scholar]
- Torres-Oviedo G, Macpherson JM, Ting LH. Muscle synergy organization is robust across a variety of postural perturbations. J Neurophysiol 96: 1530–1546, 2006 [DOI] [PubMed] [Google Scholar]
- Torres-Oviedo G, Ting LH. Muscle synergies characterizing human postural responses. J Neurophysiol 98: 2144–2156, 2007 [DOI] [PubMed] [Google Scholar]
- Tresch MC, Saltiel P, Bizzi E. The construction of movement by the spinal cord. Nat Neurosci 2: 162–167, 1999 [DOI] [PubMed] [Google Scholar]
- Zar J. Biostatistical Analysis. Upper Saddle River, NJ: Prentice-Hall, 1999 [Google Scholar]