Abstract
In both the upper and lower limbs, evidence suggests that short-latency electromyographic (EMG) responses to mechanical perturbations are modulated based on muscle stretch or joint motion, whereas long-latency responses are modulated based on attainment of task-level goals, e.g., desired direction of limb movement. We hypothesized that long-latency responses are modulated continuously by task-level error feedback. Previously, we identified an error-based sensorimotor feedback transformation that describes the time course of EMG responses to ramp-and-hold perturbations during standing balance (Safavynia and Ting 2013; Welch and Ting 2008, 2009). Here, our goals were 1) to test the robustness of the sensorimotor transformation over a richer set of perturbation conditions and postural states; and 2) to explicitly test whether the sensorimotor transformation is based on task-level vs. joint-level error. We developed novel perturbation trains of acceleration pulses such that perturbations were applied when the body deviated from the desired, upright state while recovering from preceding perturbations. The entire time course of EMG responses (∼4 s) in an antagonistic muscle pair was reconstructed using a weighted sum of center of mass (CoM) kinematics preceding EMGs at long-latency delays (∼100 ms). Furthermore, CoM and joint kinematic trajectories became decorrelated during perturbation trains, allowing us to explicitly compare task-level vs. joint feedback in the same experimental condition. Reconstruction of EMGs was poorer using joint kinematics compared with CoM kinematics and required unphysiologically short (∼10 ms) delays. Thus continuous, long-latency feedback of task-level variables may be a common mechanism regulating long-latency responses in the upper and lower limbs.
Keywords: balance, electromyography, postural control, reflex, sensorimotor response
long-latency electromyographic (EMG) responses to perturbations are generated by complex neural mechanisms that can integrate sophisticated information to rapidly produce functionally appropriate motor behaviors (Horak and Macpherson 1996; Matthews 1991; Pruszynski and Scott 2012; Shemmell et al. 2010). These long-latency responses typically occur from 45 to 100 ms following perturbation onset in the upper limb (Kurtzer et al. 2008; Marsden et al. 1981; Matthews 1991) and 70 to 100 ms in the lower limb (Horak et al. 1989; Horak and Macpherson 1996; Nashner 1976). In contrast, short-latency EMG responses arise from stretch reflexes and act to return individual joints to the original limb posture (Liddell and Sherrington 1924; Nichols and Houk 1976; Prochazka 1996; Sinkjaer et al. 1996), regardless of whether they contribute to the desired task-level motor goal (Carpenter et al. 2001; Gollhofer et al. 1989; Kurtzer et al. 2008; Nashner 1977; Pruszynski and Scott 2012; Shemmell et al. 2010). Here we define task-level goals to be motor variables that cannot be directly encoded or controlled by any unique sensory input or motor output. Long-latency responses in the upper and lower limb can be modified as a function of motor intention, motor planning, and obstacle avoidance, reflecting task-level goals (Kurtzer et al. 2008; Pruszynski and Scott 2012; Shemmell et al. 2010). However, most of the conclusions about the functional differences between short- and long-latency responses have been inferred using discrete perturbations across various experimental conditions, where restoring joint configurations could not produce functionally appropriate motor behaviors (Carpenter et al. 2001; Dimitriou et al. 2012; Evarts and Tanji 1976; Gollhofer et al. 1989; Kurtzer et al. 2008; Nashner 1977; Pruszynski et al. 2011a). In these conditions, analysis of EMG responses has been limited to comparisons of the sign and amplitude of responses.
Recently, we demonstrated in standing balance control that an error-based sensorimotor transformation could account for the variations in the amplitude of long-latency responses during discrete ramp-and-hold perturbations, as well as describe the entire time course of the responses (Safavynia and Ting 2013; Welch and Ting 2008, 2009). In these studies, we assumed that center of mass (CoM) kinematics could be used as task-level variables to generate long-latency postural responses. However, it is unclear whether our findings generalize to more dynamic conditions because of the limited duration and static initial conditions of our prior experimental paradigms. Moreover, because the initial joint and CoM kinematics in prior unidirectional perturbations were highly correlated, we could not definitively rule out individual joint feedback as a contributing factor to long-latency responses. Therefore, our goals were 1) to test the robustness of the error-based sensorimotor feedback transformation over a richer set of perturbation conditions and initial postural states; and 2) to explicitly test whether the sensorimotor transformation is based on task-level vs. joint-level error.
While a number of studies suggest that long-latency responses are continuously modulated, during movement, the time course of such responses is not well understood. Evidence suggests that a continuous feedback mechanism governs the long-latency response. For example, the duration of long-latency responses varies as function of perturbation duration (Lee and Tatton 1982), velocity (Lewis et al. 2005), and torque (Finley et al. 2012; Soechting and Lacquaniti 1988). The initiation of long-latency responses to the onset of torque perturbations has similar latency compared with the termination of long-latency responses at the end of torque perturbations, suggesting that a delayed feedback mechanism modulates the time course of the response (Kurtzer et al. 2010). Moreover, the amplitude of long-latency responses varies with perturbation amplitude (Crevecoeur et al. 2012; Diener et al. 1988; Welch and Ting 2009), including those on the order of the natural variability of movement (Crevecoeur et al. 2012). However, a unified description of the sensorimotor processes for generating long-latency responses to perturbations has not been proposed.
Our error-based sensorimotor transformation could reproduce small variations in temporal patterns of long-latency muscle activity evoked during responses to perturbations of varying acceleration and velocity characteristics during standing balance. The entire time course of muscle activity evoked in response to discrete ramp-and-hold perturbations was reconstructed based on a weighted sum of CoM kinematics at a delay of 100 ms in humans (Welch and Ting 2008). Moreover, when the initial acceleration and velocity characteristics were varied, the variations in the shape, timing, and amplitude of muscle were predicted by the sensorimotor transformation (Welch and Ting 2009). We, therefore, demonstrated that the initial components of the long-latency response, which had previously been attributed to feedforward mechanisms (Crago et al. 1976; Diener et al. 1988), could in fact be described by a delayed feedback response that mirrored perturbation acceleration characteristics. In cats, we used the same sensorimotor transformation to predict muscle activity in antagonistic muscle pairs during perturbations that reversed direction after 10–100 ms (Lockhart and Ting 2007); this demonstrated that parameters derived from unidirectional perturbations could predict antagonistic muscle activity in a more complex, reversing perturbation. Here, our goal was to demonstrate robustness of a continuous sensorimotor feedback transformation in human balance control over a wider range of stimulus characteristics and initial body states.
Although long-latency responses are modulated by task-level variables, this has only been shown in specific conditions where task and joint-level demands would predict opposing responses. In the upper extremity, the long-latency response has been shown to be highly adaptive to task demands and vary substantially more across conditions compared with the short-latency response (Calancie and Bawa 1985; Crago et al. 1976; Hammond 1956; Kurtzer et al. 2008; Mutha et al. 2008; Rothwell et al. 1980; Shemmell et al. 2010). Similarly in balance control, the long-latency response has been shown to reflect the direction of CoM displacement and can be independent of the actual joint displacements induced by the perturbation that affect short-latency responses (Carpenter et al. 2001; Nashner 1977). These differences have been identified in specific experimental conditions, where the short- and long-latency responses are in opposition to each other across a pair of antagonists (e.g., where one muscle is excited and the other inhibited). Thus, across a pair of antagonists, one would exhibit a short-latency response with an inhibited long-latency response in one muscle and exhibit a reciprocal response in the other muscle. However, in most cases, the short- and long-latency responses are evoked in the same muscles, although only the long-latency response can be modulated by factors such as voluntary intent (Horak and Macpherson 1996; Shemmell et al. 2010), mechanical interactions across joints (Gielen et al. 1988; Koshland et al. 1991; Kurtzer et al. 2008; Latash 2000; Pruszynski et al. 2009; Soechting and Lacquaniti 1988), limb stiffness (Doemges and Rack 1992; Kimura et al. 2006; Perreault et al. 2008), and desired movement direction (Pruszynski et al. 2009). Here, our goal was to explicitly compare the ability of task-level and joint-level variables in explaining modulation of long-latency responses in the same experimental condition.
We hypothesized that the nervous system uses task-level error feedback to robustly and continuously modulate muscle activity in dynamic balance control. To test our hypothesis, we developed novel perturbation trains applied over a relatively long duration (∼4 s) that allowed us to impart 11 acceleration pulses in succession, prior to the full recovery of balance. In contrast to prior studies where perturbations were imparted during quiet standing, our perturbations allowed us to impose identical accelerations during different CoM displacement and velocity states, which created a richer variation in combinations of CoM acceleration, velocity, and displacement induced by the perturbation. We predicted that feedback of CoM kinematics at long-latency delays (∼100 ms) could reconstruct muscle activity in both discrete perturbations and perturbation trains, regardless of the ongoing movement of the body at the time of perturbation. The successive acceleration pulses imposed when the body was in different joint configurations also caused CoM kinematics to deviate more from joint kinematics than in ramp-and-hold perturbations. We thus predicted that feedback based on task-level error would better reconstruct long-latency responses compared with feedback based on joint-level error. We demonstrate in a single experimental condition that task-level and not joint-level variables continuously modulate long-latency responses in a pair of antagonistic muscles.
MATERIALS AND METHODS
Experimental design.
Twenty-three healthy subjects (14 men, 9 women; mean age ± SD: 22 ± 3 yr) participated in an experimental protocol approved by Institutional Review Boards of Emory University and the Georgia Institute of Technology. All subjects provided written, informed consent.
Discrete perturbations and perturbation trains of the support surface in the horizontal plane were delivered to all subjects (Fig. 1). Discrete ramp-and-hold translations lasted 570 ms and featured two acceleration bursts of equal magnitude (0.5 g) but opposite direction, spaced 400 ms apart. These accelerations yielded a peak velocity of 30 cm/s and a total perturbation displacement of 12 cm (Fig. 1, left). Perturbation trains featured sequential acceleration bursts applied at 400-ms intervals in the forward, and then in the backward directions, resulting in complex perturbations of stepped velocities in forward followed by backward directions (Fig. 1, right). This resulted in a position trajectory that initially moved forward and reversed direction halfway through the perturbation, returning to the initial position. To ensure that the platform continued to move throughout the perturbation (i.e., did not have zero velocity), the first and last acceleration bursts were set at one-half the magnitude (0.1 g) of the other acceleration bursts (0.2 g). Perturbation trains lasted 4 s, with a total perturbation excursion of 15 cm, and had stepped velocities with a maximum velocity of 15 cm/s (Fig. 1, right). In contrast to previous perturbation trains reported in the literature (Soechting and Lacquaniti 1988), the acceleration pulses we designed were imposed in shorter intervals, which perturbed the body before it had reached equilibrium in response to the prior perturbation. Our perturbation train was similar in concept to pseudorandom perturbations used by Peterka (2002), except that we did not allow the platform to have zero velocity during the perturbation train. All perturbations were administered using a custom two-axis perturbation platform commanded with a Baldor NextMove ESB controller (Fort Smith, AR) through a custom MATLAB interface.
Fig. 1.
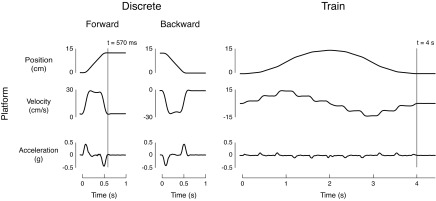
Support-surface perturbation characteristics for discrete perturbations vs. perturbation trains. Discrete ramp-and-hold perturbations (two left panels) exhibited one acceleration burst and a single velocity level. Perturbation trains (right panel) featured multiple acceleration bursts of equal magnitude in forward and backward directions, resulting in stepped velocities in forward and backward directions. Note that the first and last acceleration bursts were at one-half the magnitude of the other acceleration bursts to ensure that the platform had a nonzero velocity level at the midpoint of the perturbation.
Subjects were exposed to three sets of perturbations during the same 3-h experimental session; experimental data were used for both this study and for a previous study (Safavynia and Ting 2013). First a set of 60 discrete translations was randomly presented over 12 directions in the horizontal plane (5 trials per direction) as in previous studies (Chvatal et al. 2011; Safavynia and Ting 2012; Torres-Oviedo and Ting 2007). Next, a set of 120 biphasic, horizontal-plane perturbations which changed direction were randomly presented for use in our previous study (Safavynia and Ting 2013) and is not part of the present study; a full description of these perturbations can be found in Safavynia and Ting (2013). Lastly, 10 identical perturbation trains were administered, which were the main focus of the present study. The order of the perturbation sets was not randomized; however, it has been shown that subjects rapidly adapt postural responses within the first few trials (Keshner et al. 1987). Thus we considered order effects of perturbation sets to be negligible. During all perturbations, subjects were instructed to maintain their balance with their arms folded and their eyes open throughout the perturbations. If subjects took a step during the perturbation, this trial was repeated, and the stepping trial was excluded from analysis.
A subset of administered trials was examined in the present study. We only examined sagittal plane perturbations; this corresponded to forward and backward discrete perturbations as well as perturbation trains (Fig. 1). Multidirectional discrete and biphasic perturbations were not included in this analysis; these results were reported elsewhere (Safavynia and Ting 2013).
Data collection.
Surface EMG activity was recorded at 1,080 Hz from 16 muscles over the right leg and trunk using bipolar electrodes placed ∼2.5 cm apart over the belly of each muscle and oriented in the direction of the muscle fibers (Basmajian et al. 1980). Because the experiment was conducted over one 3-h session, the same electrode placement was used for all perturbations. Despite the large number of muscles recorded, relatively few muscles were activated in perturbation trains due to the small perturbation magnitudes administered. We thus restricted our EMG analysis to a pair of antagonistic muscles active during perturbation trains: tibialis anterior (TA), a monoarticular muscle that acts in ankle dorsiflexion, and medial gastrocnemius (MG), a biarticular muscle that acts in ankle plantarflexion and knee flexion. EMG data were wirelessly transmitted to computer using a Konigsberg telemetry system (Pasadena, CA). Kinematic and kinetic data were also collected in all trials to estimate kinematics of joint angles and CoM. Kinematic data were collected at 120 Hz using an eight-camera Vicon motion capture system (Centennial, CO) and a custom 25-marker set that included head-arms-trunk, thigh, shank, and foot segments. Kinetic data were collected at 1,080 Hz from force plates under the feet (AMTI, Watertown, MA).
Data processing.
Raw EMG data were processed using custom MATLAB routines. EMG data were high-pass filtered at 35 Hz, demeaned, rectified, and then low-pass filtered at 40 Hz, as previously reported (Torres-Oviedo and Ting 2007). For each subject, EMG data were subsequently normalized to peak activity over all analyzed trials, yielding values between 0 and 1.
CoM and joint angle kinematics were calculated from marker and force data. Data from motion-capture markers were low-pass filtered at 50 Hz using a zero-lag third-order Butterworth filter (filtfilt.m). CoM displacement was calculated from kinematic marker data as a weighted sum of segmental masses (Winter 2005). CoM velocity was taken as the derivative of the CoM displacement after smoothing using a third-order Savtizky-Golay filter with a filter size of 48 samples. To reduce error associated with second-derivatives of kinematic data (Risher et al. 1997), we computed CoM acceleration with respect to the feet based on ground reaction forces (F = ma), as previously reported (Welch and Ting 2009; 2008). Integrated CoM acceleration traces were typically similar to CoM velocity based on kinematic data, but were not used because they required that appropriate integration constants be identified. Sagittal-plane joint angles qn for the ankle, knee, and hip were calculated from the dot product of vectors from the joint center to adjacent body segments. Resting joint angles qn-rest were calculated as the average angle from 50 to 500 ms preceding perturbations. Angular displacements θn were defined as (qn − qn-rest). Angular velocities θ̇n and angular accelerations θ̈n were calculated by taking first- and second-order derivatives of the smoothed (third-order Savtizky-Golay filter) angular displacement, respectively.
EMG reconstruction.
To test our hypothesis that the nervous system uses error-based feedback of CoM kinematics to modulate EMG in human standing balance, we reconstructed TA and MG activity in both discrete perturbations and perturbation trains by using linear combinations of CoM kinematics at a delay (Welch and Ting 2009) (Fig. 2A). Our goal was to identify the parameters for a neural feedback controller, where the input to the controller is the kinematic error, and the output is the motor command to muscles. Such a controller can be identified independent of the body dynamics, if the sensor noise is small compared with the effect of a disturbance (see van der Kooij et al. 2005, Eq. 26). As the CoM sway displacement and velocity induced by our perturbation trains was about an order of magnitude greater than that found during quiet standing (Abrahamova and Hlavacka 2008), we were able to use this approach for all of the data collected.
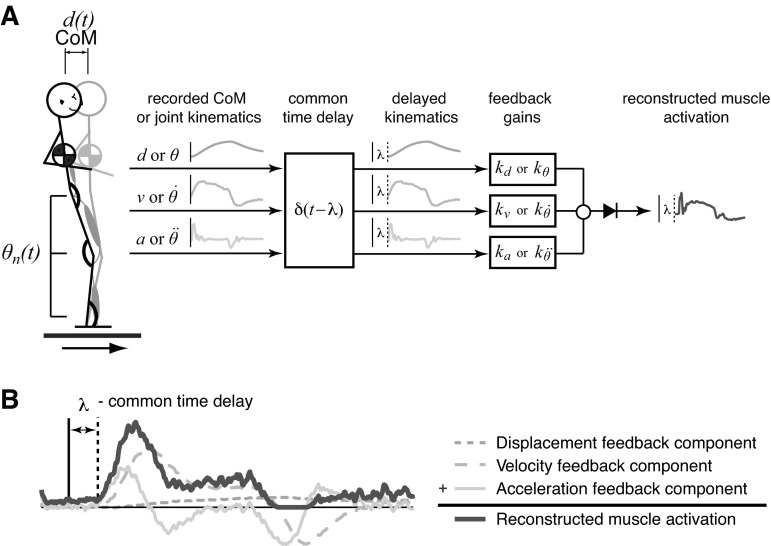
Fig. 2.
Reconstruction of electromyographic (EMG) activity based on center of mass (CoM) or joint kinematics. A: schematic diagram of EMG illustrating that delayed CoM or joint angle kinematics (ankle, knee, hip) were multiplied by feedback gains, added, and half-wave rectified to reconstruct muscle activity throughout discrete perturbations and perturbation trains. Tibialis anterior (TA) and medial gastrocnemius (MG) were reconstructed using both individual joint kinematic signals, as well as combinations of joint kinematics (ankle/knee, knee/hip, ankle/hip), resulting in six input signals. B: the time course of reconstructed EMG signals were a linear sum of delayed displacement, velocity, and acceleration. θn, Angular displacements; d, displacement; v, velocity; a, acceleration; θ, θ̇, and θ̈: joint angular displacement, velocity, and acceleration, respectively; λ, time delay; kd, kv, and ka: feedback gains on CoM displacement, velocity, and acceleration, respectively; kθ, kθ̇, and kθ̈: feedback gains on θ, θ̇, and θ̈, respectively. [Adapted with permission from Welch and Ting (2009)].
Our model of the neural controller is based on the assumption that kinematic signals are linearly combined to activate muscles (Fig. 2B). Using measured sagittal displacement (d), velocity (v), and acceleration (a) of the CoM, we reconstructed temporal patterns of TA and MG activity [EMGn(t)] according to the equation
where kd, kv, and ka designate feedback gains on CoM displacement, velocity, and acceleration, respectively, λ designates a time delay representing delays in neural transmission and processing, and floor brackets designate half-wave rectification of reconstructed muscle activation patterns.
To test the hypothesis that the nervous system continuously modulates EMG in response to task-level as opposed to local-level feedback, we also reconstructed TA and MG activity in discrete perturbations and perturbation trains based on delayed feedback of joint kinematics (θn, θ̇n, θ̈n) (Fig. 2A). In this formulation, kθ, kθ̇, and kθ̈ designate feedback gains on joint angular displacement, velocity, and acceleration, respectively. TA and MG activity were thus reconstructed according to the equation
All analyses were performed on muscle activity beginning 50 ms before perturbation onset until 500 ms after perturbation offset. This corresponded to a 1.07-s time interval for discrete perturbations, and a 4.55-s time interval for perturbation trains. For each muscle, we first identified the three feedback gains (ki) and common time delay (λ) that best reconstructed EMG activity using either CoM or joint kinematics. All reconstructions were performed according to the cost function
The first term penalized the squared error (em) between averaged and simulated muscle activity with weight μs. The second term penalized the maximum error between simulated and recorded muscle activity at any point in time with weight μk. The ratio of weights (μs and μk) was 10:1 based on prior work (Welch and Ting 2009); this ratio more heavily weighted the integrated error throughout the perturbation as opposed to the maximum error at any given time point. Although this ratio was set at 10:1 for the present study, in general the ratio used does not have a strong effect on the results of the optimization (Welch and Ting 2009). Similarly, the results of the optimization are minimally affected by using a common time delay vs. independent time delays for each kinematic signal (Welch and Ting 2009).
For each subject, averaged TA and MG activity across trials were independently reconstructed using delayed feedback of averaged CoM kinematics, yielding a unique set of feedback gains and time delay for each muscle in each perturbation type. Averaged data across trials were resampled to 1,000 Hz. Because TA was only active in forward discrete perturbations, corresponding to posterior CoM acceleration, TA was analyzed in forward discrete perturbations only. Conversely, MG was only active for backward discrete perturbations, corresponding to anterior CoM acceleration; thus MG was analyzed in backward discrete perturbations. Both muscles were analyzed in perturbation trains because the platform moved in both forward and backward directions. For all reconstructions, the feedback gains ki were constrained to be between −5 and 5, which was an order of magnitude larger than the range of typical feedback gain values; the gains were thus practically unconstrained, as none of the solutions approached the bounds. We used a single lumped time delay, as our previous studies have shown that reconstructions do not improve significantly when delays are allowed to vary independently (Lockhart and Ting 2007). Based on our prior human studies, the time delay λ was restricted to 60–130 ms, with an initial delay λ0 of 100 ms (Welch and Ting 2008, 2009). This range is consistent with long-latency delays observed previously of 70–120 ms (Horak et al. 1989; Horak and Macpherson 1996; Nashner 1976); beyond these limits the goodness of fit for reconstructions drops substantially.
We then evaluated whether averaged TA and MG activity could be reconstructed using delayed feedback of averaged joint angle kinematics. We reconstructed EMG activity of TA and MG independently from feedback of individual joint angles of the lower limb (ankle, knee, hip) as well as from combinations of joints (ankle/knee, knee/hip, ankle/hip). Thus, for the joint angle combinations, six input signals (three kinematic inputs per joint) were used, requiring six feedback gains to be identified (cf. Fig. 2B). Because our model did not converge with more than six inputs, the combination of ankle/knee/hip kinematics was not evaluated. Feedback gains were restricted to values between −5 and 5. For EMG reconstructions based on joint angles, we initially restricted the time delay λ to be between 10 and 130 ms, with an initial delay λ0 of 70 ms, which was in the middle of the allowable range. In addition to long-latency responses (70–100 ms) (Horak et al. 1989; Horak and Macpherson 1996; Nashner 1976), this range also encompassed previously recorded short-latency muscle responses following nerve stimulation (25–40 ms) (Misiaszek 2003), lower leg muscle stretch (∼40 ms) (Sinkjaer et al. 1996), and support-surface perturbations via monosynaptic connections (40–65 ms) (Carpenter et al. 1999; Diener and Dichgans 1988; Diener et al. 1984; Nardone et al. 1990; Sinkjaer et al. 1996).
To examine variability across individual trials within subjects (see Fig. 4B), we also performed trial-by-trial reconstructions of TA and MG using EMG and CoM kinematics. Because of the high-frequency content of the individual EMGs compared with reconstructions based on kinematic data, the goodness-of-fit values were necessarily much lower than that found from averaged data. However, we validated (see Statistical analyses) that the mean feedback gain values for each conditions were not different from that found from the averaged data.
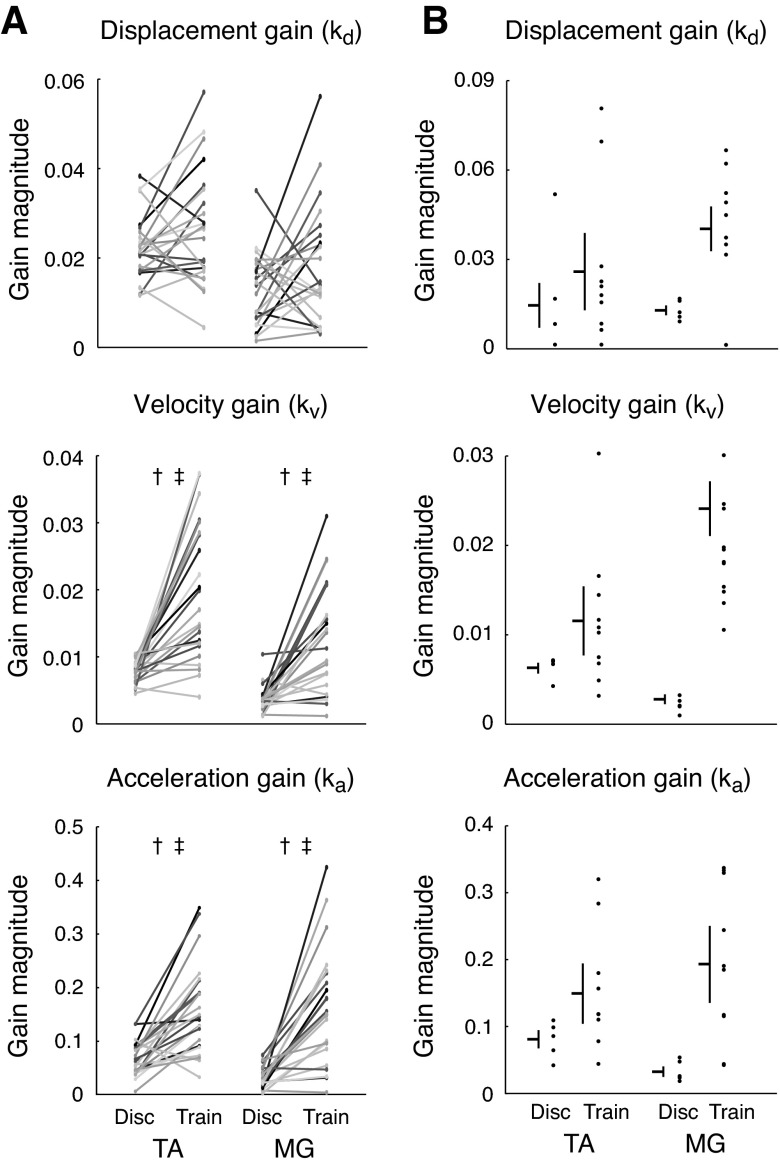
Fig. 4.
Comparison of CoM feedback gains in discrete perturbations (Disc) and perturbation trains (Train). A: across subjects, kv and ka had significantly higher magnitude in perturbation trains vs. discrete perturbations. For both TA (left column) and MG (right column), each pair of connected dots represents the magnitude of feedback gains that best reconstructed averaged muscle activity for one subject in discrete perturbations and perturbation trains (n = 23). There was larger intersubject variability in average feedback gain values in perturbation trains compared with discrete perturbations. †P < 10−4 for mean comparisons using Student's t-test. ‡P < 10−4 for variance comparisons using F-test of equality of variance across all subjects. B: in a representative subject, average gains kv and ka (horizontal lines) were also lower in discrete perturbations. Feedback gains were also more variable from trial to trial in perturbation trains compared with discrete perturbations; vertical lines indicate standard deviation of the mean.
Statistical analyses.
In all cases, we quantified the similarity between actual and reconstructed muscle patterns using r2 (squared centered Pearson's correlation coefficient) and variability accounted for (VAF), defined as 100× the square of Pearson's uncentered correlation coefficient (Zar 1999). Both r2 and VAF comparisons were necessary to evaluate goodness of fit (Chvatal et al. 2011; Welch and Ting 2009). r2 is high when the contours of EMG traces are well matched, but is less sensitive to magnitude. Conversely, VAF is high when the magnitudes of EMG traces are well matched, but is less sensitive to the contour of traces. Muscle traces were considered well reconstructed when r2 ≥ 0.5 or VAF ≥ 75%; one-tailed Student's t-tests were performed for the mean of muscle reconstructions against threshold with Bonferroni correction for multiple comparisons (α = 0.0125).
We also compared the differences in magnitude and variance of CoM feedback gains in discrete perturbations vs. perturbation trains. Based on previous modeling studies examining feedback gain variations across perturbation amplitudes (Bingham et al. 2011), we expected the feedback gains to be larger in magnitude and have more variance in perturbation trains, which have smaller accelerations relative to discrete perturbations. Using CoM reconstructions based on averaged EMG, we first performed one-tailed paired t-tests on the mean magnitude of feedback gains across subjects with Bonferroni correction for multiple comparisons (α = 0.0083). We also used a one-tailed F-test of equality of variance to compare the variance of feedback gains across subjects with Bonferroni correction for multiple comparisons (α = 0.0083) (Zar 1999).
We also examined variability of feedback gains within conditions obtained from individual trials. We first validated the feedback gains obtained from individual trial reconstructions by subtracting the value of feedback gains obtained from averaged EMG reconstructions in the same condition for each subject. We then used a two-tailed t-test to test that the mean difference was not different from zero (α = 0.05). We compared the magnitude of feedback gains across perturbation types using two-way ANOVAs (perturbation type × subject) for each feedback gain and muscle with Bonferroni correction for multiple comparisons (α = 0.0083), validating the results using averaged data. We then compared the variance of feedback gains across conditions using Bartlett's test for equal variances.
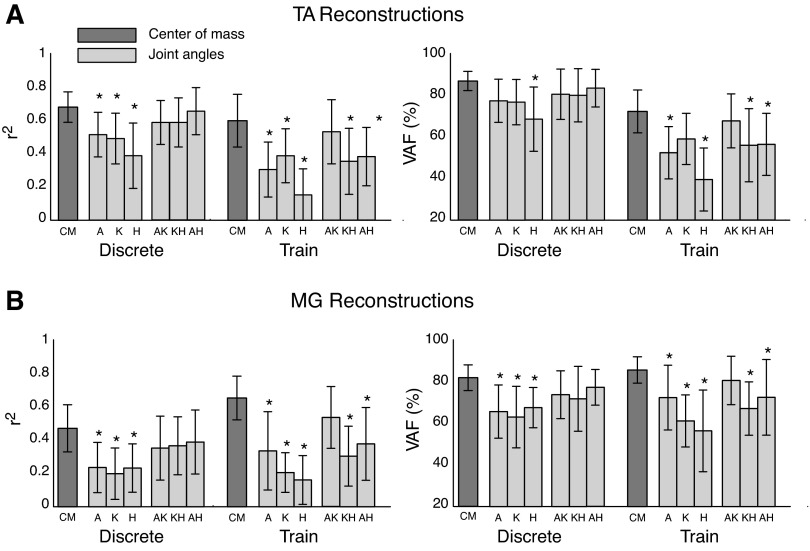
Using r2 and VAF as metrics, we compared the degree to which the different kinematic inputs (CoM, individual joint angles, combinations of joint angles) could reconstruct muscle activity in discrete perturbations and perturbation trains. We performed a three-way ANOVA (subject × kinematic input × perturbation type) on r2 and VAF for both TA and MG reconstructions. We then compared r2 and VAF of CoM constructions to joint kinematic reconstructions using Dunnett's post hoc comparisons (α = 0.05) (Zar 1999).
RESULTS
Summary.
EMG responses in TA and MG during discrete perturbations and perturbation trains were well-reconstructed using feedback gains on CoM kinematics that preceded EMG activity by ∼100 ms, consistent with previously observed delays in postural responses. Mean feedback gain values were of smaller magnitude and less variable in the larger discrete perturbations compared with smaller perturbation trains, both within and across subjects. When EMG responses were reconstructed using joint kinematics, delays were unphysiologically short (median λ = 11 ms), but optimization results were unreliable, as the chosen delays were frequently (340/552 trials, 62%) pinned near the lowest allowable value of 10 ms. Moreover, reconstructions were always better using CoM vs. individual joint kinematic feedback. EMG reconstructions were improved when using multiple-joint kinematics, but were still less accurate than reconstructions based on CoM kinematics. Therefore, error-based feedback of CoM kinematics robustly reconstructs long-latency responses across a variety of kinematic combinations in dynamic states. Moreover, error-based feedback of CoM kinematics was more reliable than joint-level feedback in reconstructing muscle activation patterns throughout human postural responses.
Delayed CoM kinematics robustly reconstructs EMGs across perturbation conditions.
We were able to achieve a wider combination of instantaneous combinations of CoM displacement, velocity, and acceleration in perturbation trains (Fig. 3A, right panel) vs. discrete perturbations (Fig. 3A, two left panels). Notably, changes in CoM acceleration, velocity, and position in discrete perturbations were all in the same direction, but not in perturbation trains. Additionally, perturbation trains had lower magnitudes of CoM velocity and acceleration, and subjects anecdotally reported that these were “gentler” perturbations.
Fig. 3.
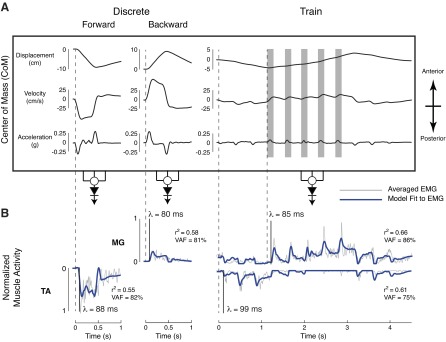
Postural responses to discrete perturbations and perturbation trains. A: average CoM kinematics in discrete perturbations (two left panels) and perturbation trains (right panel). Discrete perturbations started at rest, such that CoM acceleration and velocity were always in the same direction. In perturbation trains, acceleration bursts occurred during different magnitudes and directions of CoM displacement and velocity (gray shaded boxes). Positive values of CoM kinematics indicate anterior motion, and negative values indicate posterior motion. B: in discrete perturbations, TA was activated in forward perturbations, and MG was activated in backward perturbations. Muscle activity was well-reconstructed using delayed CoM feedback. In perturbation trains, both TA and MG were active, and their activity was also reconstructed by delayed (80–100 ms) feedback on CoM kinematics (blue traces). This demonstrates that the activity of each muscle depended on a combination of CoM acceleration, velocity, and displacement. Muscle traces were considered well-reconstructed when r2 ≥ 0.5 or variability accounted for (VAF) ≥ 75%.
In both discrete perturbations and perturbation trains, muscle activity resembled CoM kinematic traces (Fig. 3B) with a delay of ∼100 ms (Fig. 3B, vertical lines), consistent with previously reported delays in long-latency postural responses (Horak et al. 1989; Horak and Macpherson 1996; Nashner 1976). Muscle activity in discrete perturbations was dominated by either TA in forward perturbations or MG in backward perturbations. Both muscles exhibited a burst followed by a plateau of activity that resembled the superposition of CoM acceleration and velocity. In perturbation trains, TA and MG acted as an antagonistic pair. TA bursts were found at the beginning and end of the perturbation, resembling posterior CoM acceleration. MG was active in backward perturbations and more closely resembled anterior CoM velocity traces in both discrete perturbations and perturbation trains.
Across subjects, average TA and MG activity were well-reconstructed in both discrete perturbations and perturbation trains (r2 ≥ 0.5 or VAF ≥ 75%, Table 1) using long-latency delayed feedback of CoM kinematics with a fixed set of CoM feedback gains (kd, kv, ka) for each condition (Fig. 3B). Identified delays λ were highly consistent for both TA and MG across subjects (Table 1), with a grand mean ± SD of 88 ± 10 ms. TA was dominated by posterior CoM velocity and acceleration feedback, while MG was dominated by anterior CoM velocity feedback. Although CoM displacement feedback provided only a minor contribution to muscle reconstructions in the perturbations administered in this experiment, incorporating displacement feedback, nonetheless, improved reconstructions. Thus the limited CoM displacement contribution may likely be related to the particular stimulus characteristics.
Table 1.
Muscle reconstruction parameters based on center of mass kinematics
| Tibialis Anterior |
Medial Gastrocnemius |
|||
|---|---|---|---|---|
| Discrete | Train | Discrete | Train | |
| r2 | 0.68 ± 0.09‡ | 0.60 ± 0.16† | 0.47 ± 0.14 | 0.65 ± 0.13‡ |
| VAF, % | 87 ± 5‡ | 72 ± 10 | 82 ± 6‡ | 86 ± 6‡ |
| n | 23/23 | 17/23 | 21/23 | 23/23 |
| λ, ms | 88 ± 7 | 92 ± 14 | 88 ± 8 | 86 ± 7 |
Values are means ± SD; n/23, no. of individual subjects out of 23 meeting criterion for well-reconstructed muscles. Bold values indicate well-reconstructed muscles. VAF, variability accounted for; λ, time delay.
Mean values significantly above well-reconstructed threshold at P < 0.0125 for n = 4 comparisons.
Mean values significantly above well-reconstructed threshold at P < 10−4.
Across subjects, velocity and acceleration feedback gains were smaller and less variable in discrete perturbations compared with perturbation trains. Although the magnitudes of CoM kinematics were smaller in perturbation trains (Fig. 3B), the changes in TA and MG activity during perturbation trains could not be explained by simply applying the feedback gains identified from discrete perturbations (Fig. 3B). Across all subjects, mean magnitudes of kv and ka were significantly larger in perturbation trains vs. discrete perturbations for both TA and MG (P < 10−4) (Fig. 4A). Although TA activity was reduced in perturbation trains, it decreased less than would be predicted by the decrease in the magnitude of CoM kinematic deviations in perturbation trains compared with discrete perturbations. MG activity was actually larger in perturbation trains compared with discrete perturbations, resulting in higher identified feedback gains. Moreover, the range, or variance of kv and ka was significantly less constrained in perturbation trains across subjects (P < 10−4) (Fig. 4A). There were no significant differences in magnitude or variance of kd across subjects.
Velocity and acceleration feedback gains were also less variable in discrete perturbations compared with perturbation trains. Although reconstructions of individual trials had poorer goodness-of-fit values (TA Disc, r2: 0.48 ± 0.12; VAF: 73 ± 8%; TA Train, r2: 0.30 ± 0.16; VAF: 43 ± 13%; MG Disc, r2: 0.27 ± 0.13; VAF: 63 ± 10%; MG Train, r2: 0.33 ± 0.13; VAF: 62 ± 10%), the feedback gains obtained from reconstructions of individual trials were not significantly different from feedback gains obtained from reconstructions of averaged EMG (P = 0.81). Feedback gains from individual trials were larger in magnitude in perturbation trains and also more variable (data for a representative subject shown in Fig. 4B), reflecting trial-by-trial variability in feedback gains. There was a significant effect of perturbation type on kv and ka magnitude [TA, kv: F(1,334) = 97.33; ka: F(1,334) = 82.28; MG, kv: F(1,335) = 252.87; ka: F(1,335) = 195.51; P < 10−16 for all ANOVAs]. The variance of kv and ka was greater in perturbation trains as determined by Bartlett's test for equal variances [TA, kv: χ2(1) = 101.12; ka: χ2(1) = 89.86; MG, kv: χ2(1) = 197.37; ka: χ2(1) = 195.21; P < 10−16 for all comparisons].
Delayed CoM kinematics reconstructs muscle activity better than delayed joint kinematics.
A majority of EMG reconstructions using joint kinematics yielded nonphysiological delays (median λ = 11 ms). The identified delays λ were selected near the lower bound of delays (≤12 ms) in 62% (340/552) of reconstructions (cf. Fig. 5B), suggesting that the optimization used to reconstruct EMG data did not converge using short-latency delays for joint kinematic signals in a majority of cases. Moreover, the identified delays were shorter than any physiological delays previously reported for short-latency responses in the lower or upper limb (Brooke et al. 1999; Carpenter et al. 1999; Nardone et al. 1990; Pruszynski et al. 2011b; Sinkjaer et al. 1996).
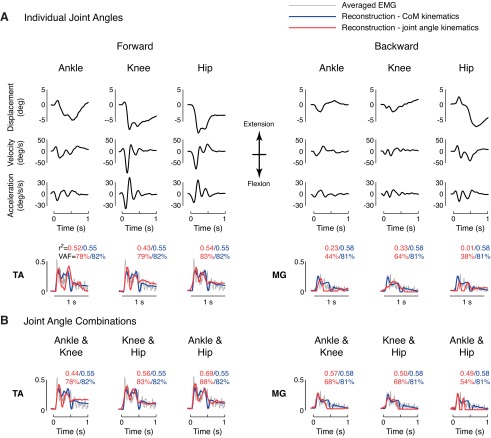
Fig. 5.
Reconstructions of muscle activity in discrete perturbations using CoM vs. joint angle kinematics. Average muscle activity and joint angle kinematics for ankle, knee, and hip are shown for a representative subject. A: reconstructions of EMGs based on individual joint kinematics (red traces) were not as high as reconstructions using CoM kinematics (blue traces). Moreover, delays using joint kinematics (red traces) were unphysiologically short. B: EMG reconstructions based on combinations of joint kinematics improved fits, even in TA, which only crosses the ankle. Nevertheless, muscle reconstructions using CoM kinematics (blue traces) better matched EMG than reconstructions using joint angles.
EMG reconstructions using joint kinematics were not as good as reconstructions using CoM kinematics (example: Figs. 5 and 6, red vs. blue traces). EMG reconstructions based on combinations of joints were better than those using individual joints, but still not as good as reconstructions using CoM kinematics (example: Figs. 5B and 6B, red vs. blue traces).
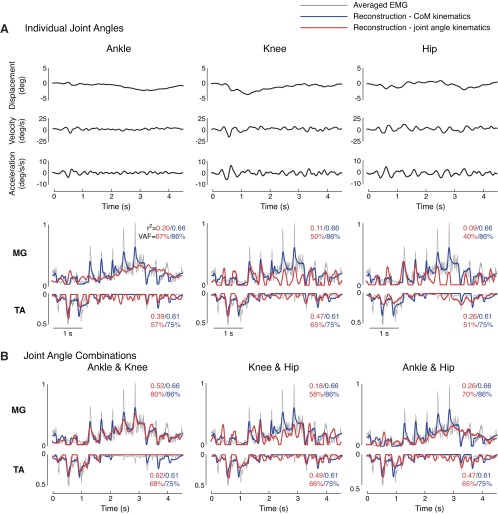
Fig. 6.
Reconstructions of muscle activity in perturbation trains using CoM vs. joint angle kinematics. Muscle reconstructions are shown for the same subject as in Fig. 5. Muscle reconstructions using CoM kinematics (blue traces) had higher goodness-of-fit values than reconstructions using joint angle kinematics (red traces) in both A, individual joint reconstructions, and B, joint combination reconstructions.
EMG reconstructions using CoM kinematics were significantly more similar to actual EMG data than reconstructions using joint kinematics (Fig. 7). There was a significant effect of kinematic inputs on EMG reconstructions for TA and MG [TA, r2: F(12,597) = 20.15; VAF: F(12,597) = 15.63; MG, r2: F(12,597) = 17.47; VAF: F(12,597) = 12.81; P < 10−16 for all ANOVAs]. Across subjects, reconstructions were significantly higher when using CoM kinematics compared with individual joint kinematics (P < 0.05 for 9 of 12 post hoc comparisons using Dunnett's test; Fig. 7) in both discrete perturbations and perturbation trains. Adding multiple joints improved reconstructions based on joint kinematics; however, in perturbation trains, muscle reconstructions using CoM kinematics were still significantly higher than combinations of knee/hip or ankle/hip kinematics (P < 0.05 for all VAF comparisons using Dunnett's post hoc analysis).
Fig. 7.
Goodness-of-fit comparisons for muscle reconstructions using CoM vs. individual and integrated combinations of joint kinematics. A: TA reconstructions. B: MG reconstructions. Goodness-of-fit measures (r2 and VAF) were significantly higher using CoM kinematics (dark gray bars) vs. individual or integrated combinations of joint kinematics (knee/hip, ankle/hip: light gray bars). Reconstructions improved when using combinations of joint kinematics. *P < 0.05 for mean comparisons using Dunnett's post hoc comparisons. A, ankle; K, knee; H, hip; AK, ankle/knee; KH, knee/hip; AH, ankle/hip.
DISCUSSION
A robust, continuous, task-level sensorimotor feedback transformation for long-latency responses.
Here we have shown that muscle activity evoked in long-latency perturbation responses can be robustly described by a continuous, task-level sensorimotor feedback transformation. The novel perturbation trains we used evoked a wider range of CoM kinematic combinations than ramp-and-hold perturbations imposed when subjects were standing quietly (Carpenter et al. 2005; Chvatal et al. 2011; Diener et al. 1988; Henry et al. 1998; McIlroy and Maki 1994; Nashner 1976; Torres-Oviedo and Ting 2007). The perturbations were thus more similar to naturalistic perturbations that can vary over time and can occur when the body is in motion. We demonstrated that the time course of muscle activity reflects the deviations of the CoM acceleration, velocity, and displacement from the desired upright state, even when perturbations are imposed when the body is already deviated from that state. The findings were robust even over a relatively long perturbation train (∼4 s), during which time subjects may be able to alter their motor strategies voluntarily (Jacobs and Horak 2007). As long-latency responses have been observed to reflect perturbation characteristics (Finley et al. 2012; Kurtzer et al. 2010; Lee and Tatton 1982; Lewis et al. 2005), and appear to operate even for very small perturbation amplitudes (Crevecoeur et al. 2012; Peterka 2000, 2002), a common, continuous, and robust sensorimotor feedback process likely regulates long-latency muscle activity. Moreover, we show that the precise pattern of response can be described by summed and rectified CoM kinematic signals consistent with the summation and rectification properties of neural firing mechanisms.
While the influences of task-level vs. joint-level variables on long-latency responses have been previously reported, we explicitly demonstrate that task-level variables better describe evoked muscle activity than joint-level variables. In prior studies, the influences of joint-level vs. task-level variables on the amplitude of long-latency responses were dissociated by examining modulation of the response across specially designed experimental conditions. For example, in two-dimensional arm movements, task-level (i.e., hand location) and local-level (i.e., joint angle) variables cannot be distinguished for a specific hand location, so the differential modulation of short- and long-latency EMG amplitudes was inferred by examining perturbation responses across disparate conditions (Dimitriou et al. 2012; Evarts and Tanji 1976; Kurtzer et al. 2006, 2008; Pruszynski et al. 2011a; Scott 1999). In balance control studies, task-level and joint-level responses are similar in perturbations that translate the support surface; thus the differential modulation of short- and long-latency responses has been identified by comparing responses of translational perturbations to rotational perturbations (Carpenter et al. 1999; Gollhofer et al. 1989; Nashner 1976; Ting and Macpherson 2004). Here, we used a train of translational perturbations during which the task-level CoM kinematic deviations were initially highly correlated to the joint deviations, but became decorrelated over time. Thus in a single condition we were able to show that the entire time course of EMG activity at long-latency delays was better described by task-level vs. individual joint information. Moreover, the delays identified for reconstructions based on joint angles were unphysiologically short (median λ = 11 ms). Reconstructions based on joint kinematics improved when information from two joints were used, even in the case of TA, a monoarticular muscle that acts solely at the ankle joint. Such improvements highlight the fact that individual muscular responses reflect motion of joints distant to the muscle (Kurtzer et al. 2008; Welch and Ting 2009). Muscle reconstructions would likely continue to improve if additional joint information were used, as incorporating multiple joint kinematics allows for a partial estimation of CoM kinematics. However, in our experiment, we were still unable to identify physiologically plausible delays using kinematics from two joints. Ultimately, incorporating all joints would be equivalent to using CoM kinematics, as body CoM is derived from joint angles of multiple body segments as well as segment masses and length. Our data support the idea that long-latency postural responses are due to multisensory integration mediated by brain stem pathways (Allum and Honegger 1998; Horak and Macpherson 1996; Stapley and Drew 2009).
While the form of the sensorimotor feedback transformation was consistent across perturbation conditions, the sensitivity of the response increased in smaller perturbations. Although a fixed set of feedback gains could be used to describe each perturbation condition, different feedback gains were identified across conditions. Our perturbation train used small translational accelerations (<0.2 g, 6 cm/s steps) compared with the discrete, ramp-and-hold perturbations (0.5 g, 30 cm/s) typically used to elicit long-latency postural responses in this and other studies (0.1–0.6 g, 15–40 cm/s) (Chvatal et al. 2011; Horak et al. 1989; Torres-Oviedo and Ting 2007; Welch and Ting 2009). Feedback gains were typically higher for perturbation trains, consistent with prior work demonstrating that the scaling of muscle activity with perturbation magnitude is not linear (Welch and Ting 2009), and that smaller gain values are required in larger perturbations to maintain stability in both the sagittal (Park et al. 2004) and frontal (Bingham et al. 2011) plane. Furthermore, feedback gains for the smaller magnitude perturbation trains were more variable across subjects and trials and spanned a greater range. This is consistent with the idea that there is redundancy in the allowable amplitude of feedback gains and the resulting postural strategies in smaller vs. larger perturbations (Bingham et al. 2011; Creath et al. 2005; Horak and Macpherson 1996).
Common task-level mechanisms for motor control.
A common sensorimotor feedback transformation pattern likely coordinates multiple muscles across the body for coordinated movement. Here we demonstrated in sagittal plane balance that antagonistic pairs of muscles are driven by deviations in a common task-level control signal, but with different sensitivities. Our results were particularly surprising, given that the pair of muscles we used were not strictly antagonistic, as TA crosses only the ankle and MG crosses both the knee and ankle. The sensitivity of each muscle to both positive and negative deviations in CoM kinematics is consistent with the idea that each kinematic channel has both excitatory and inhibitory influences that continuously shape the time course of motoneuron and muscle activity. Thus periods of both muscle activity and quiescence can be explained in a manner that reflects neuronal summation of synaptic inputs. Although responses in proximal muscles were very small in our perturbation trains, in larger sagittal ramp-and-hold perturbations, proximal muscle activity can also be reconstructed by CoM feedback, even in responses that involve large hip joint motion (Welch and Ting 2008, 2009). Therefore the fact that CoM kinematics are reflected in EMG responses likely reflects a task-level sensorimotor feedback transformation rather than the direct feedback of joint kinematics to the muscles spanning that joint. Furthermore, driving muscles across the body using a common task-level feedback transformation may facilitate the coordination of the proximal and distal joints during movement (Gribble and Ostry 1999; Zajac and Gordon 1989) and power transfer (Fregly and Zajac 1996; van Antwerp et al. 2007; Zajac 1993), as net joint torques depend on muscle activity and torque generation at distant joints (Park et al. 2004; van Antwerp et al. 2007). Likewise, we recently demonstrated that the recruitment of muscle synergies that specify patterns of muscle coordination could be predicted by task-level error feedback of CoM kinematics in multidirectional perturbation responses (Safavynia and Ting 2013). Recruitment of multiple muscles by common command signals based on task-level variables may thus provide a mechanism by which task-level motor goals are implemented to coordinate multiple body segments (Ting 2007; Ting and McKay 2007).
Task-level sensorimotor feedback may, therefore, be a common neural mechanism underlying long-latency responses in both the upper and lower limbs. Task-level variables representing abstract motor goals are represented throughout the nervous system, including in spinal (Nichols 1989), cortical (Kalaska et al. 1997; Taube et al. 2006), and subcortical circuits (Jacobs and Horak 2007). For example, in reaching, task-level variables, including hand movement direction, velocity (Georgopoulos et al. 1986), end-point force (Georgopoulos et al. 1992), hand location (Sergio and Kalaska 1997), and arm orientation (Scott and Kalaska 1997), are represented in pyramidal neurons of motor cortex. Long-latency responses in the arm have been shown to involve motor cortex pathways and vary according to task-level goals (Cheney and Fetz 1985; Evarts and Tanji 1976; Kurtzer et al. 2008; Pruszynski et al. 2011a). In the lower limb, information about orientation and limb length can be assembled from ascending afferent signals in the dorsal root ganglia (Weber et al. 2007) and dorsal spinocerebellar tract (Poppele et al. 2002). Reliable estimation of the CoM requires integration of proprioceptive, vestibular, and/or visual information (Peterka 2002), likely in the brain stem. Indeed, during balance control, reticular formation neurons respond to task-level variables such as limb orientation (Deliagina et al. 2008) or direction of center of pressure shifts, independent of the limbs involved (Stapley and Drew 2009). Moreover, corticospinal pathways also modulate long-latency responses in perturbations to balance (Taube et al. 2006). Therefore, task-level goals for a wide variety of tasks are represented in neuromotor circuits throughout the nervous system and likely share mechanisms for transforming these motor goals into functional muscle activation patterns.
We further propose that long-latency perturbation responses provide rapid, goal-directed feedback responses to perturbations that can be superimposed upon an ongoing movement pattern, whether reactive or voluntary. We have shown that the task-level feedback response reflects deviations of both the initial and perturbed state of the body from the desired, upright state in both muscles and muscle synergies (Safavynia and Ting 2013). Although it has been previously suggested that postural sway is a feedforward mechanism because peak EMG precedes peak CoM displacement (Fitzpatrick et al. 1992), we have shown here and in other studies that EMG activity in balance is evoked in a feedback manner based on CoM motion (Lockhart and Ting 2007; Welch and Ting 2008, 2009). It is likely that such a continuous sensorimotor feedback response to perturbations works in parallel with feedforward motor commands that may alter the desired posture (van der Kooij and de Vlugt 2007) or shift the center of pressure location during standing (Latash et al. 2003; Loram and Lakie 2002). Furthermore, long-latency postural responses to perturbations during voluntary movements such as walking or whole body reaching appear to be superimposed upon the ongoing motor pattern (Chvatal and Ting 2012; Oliveira et al. 2012; Trivedi et al. 2010) at similar latencies to standing, and modulated according to desired motion of the CoM, e.g., task-level goals. Similarly, in reaching, long-latency EMG following arm perturbations has also been explained by the superposition of reactive and voluntary neural commands (Pruszynski et al. 2011b). Moreover, long-latency responses are modified to support voluntary movement goals (Pruszynski et al. 2009), as subjects differentially correct for identical arm perturbations depending on the desired endpoint hand location (Schlerf and Ivry 2011), movement goals (Cheney and Fetz 1985; Evarts and Tanji 1976), visumotor feedback (Pruszynski et al. 2008), or bimanual coordination constraints (Dimitriou et al. 2012). Thus, while short-latency responses may regulate the stiffness of individual joints to reduce the mechanical effects of perturbation, long-latency responses serve to restore the limb to the desired motor goal based on continuous, task-level sensorimotor feedback.
GRANTS
This work is supported by National Institutes of Health (NIH) Grant R01 NS058322 to L. H. Ting. S. A. Safavynia is supported by a Medical Scientist Training Program Fellowship (NIH 5 T32 GM08169-24).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.A.S. and L.H.T. conception and design of research; S.A.S. performed experiments; S.A.S. analyzed data; S.A.S. and L.H.T. interpreted results of experiments; S.A.S. prepared figures; S.A.S. drafted manuscript; S.A.S. and L.H.T. edited and revised manuscript; S.A.S. and L.H.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank D. Joseph Jilk for implementing the perturbations and assisting with data collection. We thank Jeffrey T. Bingham for technical assistance and scientific discussions regarding data analysis.
REFERENCES
- Abrahamova D, Hlavacka F. Age-related changes of human balance during quiet stance. Physiol Res 57: 957–964, 2008 [DOI] [PubMed] [Google Scholar]
- Allum JH, Honegger F. Interactions between vestibular and proprioceptive inputs triggering and modulating human balance-correcting responses differ across muscles. Exp Brain Res 121: 478–494, 1998 [DOI] [PubMed] [Google Scholar]
- Basmajian JV, Blumenstein R, Dismatsek M. Electrode Placement in EMG Biofeedback. Baltimore, MD: Williams and Wilkins, 1980 [Google Scholar]
- Bingham JT, Choi JT, Ting LH. Stability in a frontal plane model of balance requires coupled changes to postural configuration and neural feedback control. J Neurophysiol 106: 437–448, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke JD, McIlroy WE, Staines WR, Angerilli PA, Peritore GF. Cutaneous reflexes of the human leg during passive movement. J Physiol 518: 619–628, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calancie B, Bawa P. Firing patterns of human flexor carpi radialis motor units during the stretch reflex. J Neurophysiol 53: 1179–1193, 1985 [DOI] [PubMed] [Google Scholar]
- Carpenter MG, Allum JH, Honegger F. Vestibular influences on human postural control in combinations of pitch and roll planes reveal differences in spatiotemporal processing. Exp Brain Res 140: 95–111, 2001 [DOI] [PubMed] [Google Scholar]
- Carpenter MG, Allum JHJ, Honegger F. Directional sensitivity of stretch reflexes and balance corrections for normal subjects in the roll and pitch planes. Exp Brain Res 129: 93–113, 1999 [DOI] [PubMed] [Google Scholar]
- Carpenter MG, Thorstensson A, Cresswell AG. Deceleration affects anticipatory and reactive components of triggered postural responses. Exp Brain Res 167: 433–445, 2005 [DOI] [PubMed] [Google Scholar]
- Cheney PD, Fetz EE. Comparable patterns of muscle facilitation evoked by individual corticomotoneuronal (CM) cells and by single intracortical microstimuli in primates: evidence for functional groups of CM cells. J Neurophysiol 53: 786–804, 1985 [DOI] [PubMed] [Google Scholar]
- Chvatal SA, Ting LH. Voluntary and reactive recruitment of locomotor muscle synergies during perturbed walking. J Neurosci 32: 12237–12250, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chvatal SA, Torres-Oviedo G, Safavynia SA, Ting LH. Common muscle synergies for control of center of mass and force in non-stepping and stepping postural behaviors. J Neurophysiol 106: 999–1015, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crago PE, Houk JC, Hasan Z. Regulatory actions of human stretch reflex. J Neurophysiol 39: 925–935, 1976 [DOI] [PubMed] [Google Scholar]
- Creath R, Kiemel T, Horak F, Peterka R, Jeka J. A unified view of quiet and perturbed stance: simultaneous co-existing excitable modes. Neurosci Lett 377: 75–80, 2005 [DOI] [PubMed] [Google Scholar]
- Crevecoeur F, Kurtzer IL, Scott SH. Fast corrective responses are evoked by perturbations approaching the natural variability of posture and movement tasks. J Neurophysiol 107: 2821–2832, 2012 [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Beloozerova IN, Zelenin PV, Orlovsky GN. Spinal and supraspinal postural networks. Brain Res Rev 57: 212–221, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener HC, Dichgans J. On the role of vestibular, visual and somatosensory information for dynamic postural control in humans. Prog Brain Res 76: 253–262, 1988 [DOI] [PubMed] [Google Scholar]
- Diener HC, Dichgans J, Bootz F, Bacher M. Early stabilization of human posture after a sudden disturbance: influence of rate and amplitude of displacement. Exp Brain Res 56: 126–134, 1984 [DOI] [PubMed] [Google Scholar]
- Diener HC, Horak FB, Nashner LM. Influence of stimulus parameters on human postural responses. J Neurophysiol 59: 1888–1905, 1988 [DOI] [PubMed] [Google Scholar]
- Dimitriou M, Franklin DW, Wolpert DM. Task-dependent coordination of rapid bimanual motor responses. J Neurophysiol 107: 890–901, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doemges F, Rack PM. Changes in the stretch reflex of the human first dorsal interosseous muscle during different tasks. J Physiol 447: 563–573, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evarts EV, Tanji J. Reflex and intended responses in motor cortex pyramidal tract neurons of monkey. J Neurophysiol 39: 1069–1080, 1976 [DOI] [PubMed] [Google Scholar]
- Finley JM, Dhaher YY, Perreault EJ. Contributions of feed-forward and feedback strategies at the human ankle during control of unstable loads. Exp Brain Res 217: 53–66, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick RC, Gorman RB, Burke D, Gandevia SC. Postural proprioceptive reflexes in standing human subjects: bandwidth of response and transmission characteristics. J Physiol 458: 69–83, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregly BJ, Zajac FE. A state-space analysis of mechanical energy generation, absorption, and transfer during pedaling. J Biomech 29: 81–90, 1996 [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, Ashe J, Smyrnis N, Taira M. The motor cortex and the coding of force. Science 256: 1692–1695, 1992 [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, Schwartz AB, Kettner RE. Neuronal population coding of movement direction. Science 233: 1416–1419, 1986 [DOI] [PubMed] [Google Scholar]
- Gielen CC, Ramaekers L, van Zuylen EJ. Long-latency stretch reflexes as co-ordinated functional responses in man. J Physiol 407: 275–292, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollhofer A, Horstmann GA, Berger W, Dietz V. Compensation of translational and rotational perturbations in human posture: stabilization of the centre of gravity. Neurosci Lett 105: 73–78, 1989 [DOI] [PubMed] [Google Scholar]
- Gribble PL, Ostry DJ. Compensation for interaction torques during single- and multijoint limb movement. J Neurophysiol 82: 2310–2326, 1999 [DOI] [PubMed] [Google Scholar]
- Hammond PH. The influence of prior instruction to the subject on an apparently involuntary neuro-muscular response. J Physiol 132: 17P–18P, 1956 [PubMed] [Google Scholar]
- Henry SM, Fung J, Horak FB. EMG responses to maintain stance during multidirectional surface translations. J Neurophysiol 80: 1939–1950, 1998 [DOI] [PubMed] [Google Scholar]
- Horak FB, Diener HC, Nashner LM. Influence of central set on human postural responses. J Neurophysiol 62: 841–853, 1989 [DOI] [PubMed] [Google Scholar]
- Horak FB, Macpherson JM. Postural orientation and equilibrium. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am. Physiol. Soc., 1996, sect. 12, chapt. 7, p. 255–292 [Google Scholar]
- Jacobs JV, Horak FB. Cortical control of postural responses. J Neural Transm 114: 1339–1348, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaska JF, Scott SH, Cisek P, Sergio LE. Cortical control of reaching movements. Curr Opin Neurobiol 7: 849–859, 1997 [DOI] [PubMed] [Google Scholar]
- Keshner EA, Allum JH, Pfaltz CR. Postural coactivation and adaptation in the sway stabilizing responses of normals and patients with bilateral vestibular deficit. Exp Brain Res 69: 77–92, 1987 [DOI] [PubMed] [Google Scholar]
- Kimura T, Haggard P, Gomi H. Transcranial magnetic stimulation over sensorimotor cortex disrupts anticipatory reflex gain modulation for skilled action. J Neurosci 26: 9272–9281, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland GF, Hasan Z, Gerilovsky L. Activity of wrist muscles elicited during imposed of voluntary movements about the elbow joint. J Mot Behav 23: 91–100, 1991 [DOI] [PubMed] [Google Scholar]
- Kurtzer I, Pruszynski JA, Herter TM, Scott SH. Primate upper limb muscles exhibit activity patterns that differ from their anatomical action during a postural task. J Neurophysiol 95: 493–504, 2006 [DOI] [PubMed] [Google Scholar]
- Kurtzer I, Pruszynski JA, Scott SH. Long-latency and voluntary responses to an arm displacement can be rapidly attenuated by perturbation offset. J Neurophysiol 103: 3195–3204, 2010 [DOI] [PubMed] [Google Scholar]
- Kurtzer IL, Pruszynski JA, Scott SH. Long-latency reflexes of the human arm reflect an internal model of limb dynamics. Curr Biol 18: 449–453, 2008 [DOI] [PubMed] [Google Scholar]
- Latash ML. Modulation of simple reaction time an the background of an oscillatory action: implications for synergy organization. Exp Brain Res 131: 85–100, 2000 [DOI] [PubMed] [Google Scholar]
- Latash ML, Ferreira SS, Wieczorek SA, Duarte M. Movement sway: changes in postural sway during voluntary shifts of the center of pressure. Exp Brain Res 150: 314–324, 2003 [DOI] [PubMed] [Google Scholar]
- Lee RG, Tatton WG. Long latency reflexes to imposed displacements of the human wrist: dependence on duration of movement. Exp Brain Res 45: 207–216, 1982 [DOI] [PubMed] [Google Scholar]
- Lewis GN, Perreault EJ, MacKinnon CD. The influence of perturbation duration and velocity on the long-latency response to stretch in the biceps muscle. Exp Brain Res 163: 361–369, 2005 [DOI] [PubMed] [Google Scholar]
- Liddell EGT, Sherrington C. Reflexes in response to stretch (myotatic reflexes). Proc R Soc Lond B Biol Sci 96: 212–242, 1924 [Google Scholar]
- Lockhart DB, Ting LH. Optimal sensorimotor transformations for balance. Nat Neurosci 10: 1329–1336, 2007 [DOI] [PubMed] [Google Scholar]
- Loram ID, Lakie M. Human balancing of an inverted pendulum: position control by small, ballistic-like, throw and catch movements. J Physiol 540: 1111–1124, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden CD, Merton PA, Morton HB. Human postural responses. Brain 104: 513–534, 1981 [DOI] [PubMed] [Google Scholar]
- Matthews PB. The human stretch reflex and the motor cortex. Trends Neurosci 14: 87–91, 1991 [DOI] [PubMed] [Google Scholar]
- McIlroy WE, Maki BE. The “deceleration response” to transient perturbation of upright stance. Neurosci Lett 175: 13–16, 1994 [DOI] [PubMed] [Google Scholar]
- Misiaszek JE. Early activation of arm and leg muscles following pulls to the waist during walking. Exp Brain Res 151: 318–329, 2003 [DOI] [PubMed] [Google Scholar]
- Mutha PK, Boulinguez P, Sainburg RL. Visual modulation of proprioceptive reflexes during movement. Brain Res 1246: 54–69, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone A, Corra T, Schieppati M. Different activations of the soleus and gastrocnemii muscles in response to various types of stance perturbation in man. Exp Brain Res 80: 323–332, 1990 [DOI] [PubMed] [Google Scholar]
- Nashner LM. Adapting reflexes controlling the human posture. Exp Brain Res 26: 59–72, 1976 [DOI] [PubMed] [Google Scholar]
- Nashner LM. Fixed patterns of rapid postural responses among leg muscles during stance. Exp Brain Res 30: 13–24, 1977 [DOI] [PubMed] [Google Scholar]
- Nichols TR. The organization of heterogenic reflexes among muscles crossing the ankle joint in the decerebrate cat. J Physiol 410: 463–477, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TR, Houk JC. Improvement in linearity and regulation of stiffness that results from actions of stretch reflex. J Neurophysiol 39: 119–142, 1976 [DOI] [PubMed] [Google Scholar]
- Oliveira AS, Gizzi L, Kersting UG, Farina D. Modular organization of balance control following perturbations during walking. J Neurophysiol 108: 1895–1906, 2012 [DOI] [PubMed] [Google Scholar]
- Park S, Horak FB, Kuo AD. Postural feedback responses scale with biomechanical constraints in human standing. Exp Brain Res 154: 417–427, 2004 [DOI] [PubMed] [Google Scholar]
- Perreault EJ, Chen K, Trumbower RD, Lewis G. Interactions with compliant loads alter stretch reflex gains but not intermuscular coordination. J Neurophysiol 99: 2101–2113, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterka RJ. Postural control model interpretation of stabilogram diffusion analysis. Biol Cybern 82: 335–343, 2000 [DOI] [PubMed] [Google Scholar]
- Peterka RJ. Sensorimotor integration in human postural control. J Neurophysiol 88: 1097–1118, 2002 [DOI] [PubMed] [Google Scholar]
- Poppele RE, Bosco G, Rankin AM. Independent representations of limb axis length and orientation in spinocerebellar response components. J Neurophysiol 87: 409–422, 2002 [DOI] [PubMed] [Google Scholar]
- Prochazka A. Proprioceptive feedback and movement regulation. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am. Physiol. Soc., 1996, sect. 12, chapt. 3, p. 89–127 [Google Scholar]
- Pruszynski JA, Kurtzer I, Lillicrap TP, Scott SH. Temporal evolution of “automatic gain-scaling”. J Neurophysiol 102: 992–1003, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruszynski JA, Kurtzer I, Nashed JY, Omrani M, Brouwer B, Scott SH. Primary motor cortex underlies multi-joint integration for fast feedback control. Nature 478: 387–390, 2011a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruszynski JA, Kurtzer I, Scott SH. The long-latency reflex is composed of at least two functionally independent processes. J Neurophysiol 106: 449–459, 2011b [DOI] [PubMed] [Google Scholar]
- Pruszynski JA, Kurtzer I, Scott SH. Rapid motor responses are appropriately tuned to the metrics of a visuospatial task. J Neurophysiol 100: 224–238, 2008 [DOI] [PubMed] [Google Scholar]
- Pruszynski JA, Scott SH. Optimal feedback control and the long-latency stretch response. Exp Brain Res 218: 341–359, 2012 [DOI] [PubMed] [Google Scholar]
- Risher DW, Schutte LM, Runge CF. The use of inverse dynamics solutions in direct dynamics simulations. J Biomech Eng 119: 417–422, 1997 [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Traub MM, Marsden CD. Influence of voluntary intent on the human long-latency stretch reflex. Nature 286: 496–498, 1980 [DOI] [PubMed] [Google Scholar]
- Safavynia SA, Ting LH. Sensorimotor feedback based on task-relevant error robustly predicts temporal recruitment and multidirectional tuning of muscle synergies. J Neurophysiol 109: 31–45, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safavynia SA, Ting LH. Task-level feedback can explain temporal recruitment of spatially-fixed muscle synergies throughout postural perturbations. J Neurophysiol 107: 159–177, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlerf JE, Ivry RB. Task goals influence online corrections and adaptation of reaching movements. J Neurophysiol 106: 2622–2631, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SH. Apparatus for measuring and perturbing shoulder and elbow joint positions and torques during reaching. J Neurosci Methods 89: 119–127, 1999 [DOI] [PubMed] [Google Scholar]
- Scott SH, Kalaska JF. Reaching movements with similar hand paths but different arm orientations. I. Activity of individual cells in motor cortex. J Neurophysiol 77: 826–852, 1997 [DOI] [PubMed] [Google Scholar]
- Sergio LE, Kalaska JF. Systematic changes in directional tuning of motor cortex cell activity with hand location in the workspace during generation of static isometric forces in constant spatial directions. J Neurophysiol 78: 1170–1174, 1997 [DOI] [PubMed] [Google Scholar]
- Shemmell J, Krutky MA, Perreault EJ. Stretch sensitive reflexes as an adaptive mechanism for maintaining limb stability. Clin Neurophysiol 121: 1680–1689, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkjaer T, Andersen JB, Larsen B. Soleus stretch reflex modulation during gait in humans. J Neurophysiol 76: 1112–1120, 1996 [DOI] [PubMed] [Google Scholar]
- Soechting JF, Lacquaniti F. Quantitative evaluation of the electromyographic responses to multidirectional load perturbations of the human arm. J Neurophysiol 59: 1296–1313, 1988 [DOI] [PubMed] [Google Scholar]
- Stapley PJ, Drew T. The pontomedullary reticular formation contributes to the compensatory postural responses observed following removal of the support surface in the standing cat. J Neurophysiol 101: 1334–1350, 2009 [DOI] [PubMed] [Google Scholar]
- Taube W, Schubert M, Gruber M, Beck S, Faist M, Gollhofer A. Direct corticospinal pathways contribute to neuromuscular control of perturbed stance. J Appl Physiol 101: 420–429, 2006 [DOI] [PubMed] [Google Scholar]
- Ting LH. Dimensional reduction in sensorimotor systems: a framework for understanding muscle coordination of posture. Prog Brain Res 165: 299–321, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting LH, Macpherson JM. Ratio of shear to load ground-reaction force may underlie the directional tuning of the automatic postural response to rotation and translation. J Neurophysiol 92: 808–823, 2004 [DOI] [PubMed] [Google Scholar]
- Ting LH, McKay JL. Neuromechanics of muscle synergies for posture and movement. Curr Opin Neurobiol 17: 622–628, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Oviedo G, Ting LH. Muscle synergies characterizing human postural responses. J Neurophysiol 98: 2144–2156, 2007 [DOI] [PubMed] [Google Scholar]
- Trivedi H, Leonard JA, Ting LH, Stapley PJ. Postural responses to unexpected perturbations of balance during reaching. Exp Brain Res 202: 485–491, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Antwerp KW, Burkholder TJ, Ting LH. Inter-joint coupling effects on muscle contributions to endpoint force and acceleration in a musculoskeletal model of the cat hindlimb. J Biomech 40: 3570–3579, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooij H, de Vlugt E. Postural Responses Evoked by Platform Pertubations Are Dominated by Continuous Feedback. J Neurophysiol 98: 730–743, 2007 [DOI] [PubMed] [Google Scholar]
- van der Kooij H, van Asseldonk E, van der Helm FC. Comparison of different methods to identify and quantify balance control. J Neurosci Methods 145: 175–203, 2005 [DOI] [PubMed] [Google Scholar]
- Weber DJ, Stein RB, Everaert DG, Prochazka A. Limb-state feedback from ensembles of simultaneously recorded dorsal root ganglion neurons. J Neural Eng 4: S168–S180, 2007 [DOI] [PubMed] [Google Scholar]
- Welch TD, Ting LH. A feedback model explains the differential scaling of human postural responses to perturbation acceleration and velocity. J Neurophysiol 101: 3294–3309, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch TD, Ting LH. A feedback model reproduces muscle activity during human postural responses to support-surface translations. J Neurophysiol 99: 1032–1038, 2008 [DOI] [PubMed] [Google Scholar]
- Winter DA. Biomechanics and Motor Control of Human Movement. Hoboken, NJ: John Wiley & Sons, 2005 [Google Scholar]
- Zajac FE. Muscle coordination of movement: a perspective. J Biomech 26, Suppl 1: 109–124, 1993 [DOI] [PubMed] [Google Scholar]
- Zajac FE, Gordon ME. Determining muscle's force and action in multi-articular movement. Exerc Sport Sci Rev 17: 187–230, 1989 [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. Upper Saddle River, NJ: Prentice-Hall, 1999, p. 663 [Google Scholar]