Abstract
The rat spinal cord isolated from supraspinal control via a complete low- to midthoracic spinal cord transection produces locomotor-like patterns in the hindlimbs when facilitated pharmacologically and/or by epidural electrical stimulation. To evaluate the role of epidural electrical stimulation in enabling motor control (eEmc) for locomotion and posture, we recorded potentials evoked by epidural spinal cord stimulation in selected hindlimb muscles during stepping and standing in adult spinal rats. We hypothesized that the temporal details of the phase-dependent modulation of these evoked potentials in selected hindlimb muscles while performing a motor task in the unanesthetized state would be predictive of the potential of the spinal circuitries to generate stepping. To test this hypothesis, we characterized soleus and tibialis anterior (TA) muscle responses as middle response (MR; 4–6 ms) or late responses (LRs; >7 ms) during stepping with eEmc. We then compared these responses to the stepping parameters with and without a serotoninergic agonist (quipazine) or a glycinergic blocker (strychnine). Quipazine inhibited the MRs induced by eEmc during nonweight-bearing standing but facilitated locomotion and increased the amplitude and number of LRs induced by eEmc during stepping. Strychnine facilitated stepping and reorganized the LRs pattern in the soleus. The LRs in the TA remained relatively stable at varying loads and speeds during locomotion, whereas the LRs in the soleus were strongly modulated by both of these variables. These data suggest that LRs facilitated electrically and/or pharmacologically are not time-locked to the stimulation pulse but are highly correlated to the stepping patterns of spinal rats.
Keywords: spinal cord stimulation, evoked potentials, EMG, quipazine, strychnine
the lumbosacral spinal circuitry can generate partial weight-bearing stepping of the hindlimbs in rats spinalized as adults when facilitated pharmacologically and/or with epidural electrical stimulation (Courtine et al. 2009; Gerasimenko et al. 2007; Musienko et al. 2011). The mechanisms of pharmacological and/or epidural electrical stimulation that enable motor control (eEmc; Gad et al. 2012) in the spinal circuitry for locomotion are still not clearly understood. During standing, a single bipolar epidural stimulus between L2 and S1 produces three types of evoked responses, i.e., early (ER; latency 1–3 ms), middle (MR; latency 4–6 ms), and late (LRs; latency >7 ms) in the hindlimb muscles in both intact (Gerasimenko et al. 2006) and spinal (Lavrov et al. 2006) rats. Similar responses were observed during rhythmic locomotor-like electromyographic (EMG) activity in the hindlimb muscles of spinal rats while stepping on a motorized treadmill in the presence of epidural stimulation (40 Hz) between L2 and S1 (Lavrov et al. 2008). In addition, the time course of the reemergence of the LRs was similar to that for the recovery of stepping after a complete spinal cord injury (SCI), indicating that LRs are a potential biomarker of functional recovery (Lavrov et al. 2006).
We hypothesized that the pattern of pharmacological neuromodulation of evoked potentials as generated in vivo when performing a specific motor task can be used as a biomarker for the prediction of locomotor characteristics. Presently, we know few details as to which components of the spinal sensorimotor networks are dynamically facilitated or depressed in a phase-dependent pattern during a well-defined postural and/or locomotor task when it is being modulated pharmacologically. Understanding the nature of the electrically and/or pharmacologically evoked responses and their modulation in vivo may provide the insight needed to design optimal rehabilitation paradigms to enable postural control and stepping after a SCI.
Thus the purpose of the present study was to define the epidurally motor-evoked responses in hindlimb muscles of spinal rats when the spinal circuitries are modulated by eEmc during stepping with and without strychnine (Strych) and quipazine (Quip) under different treadmill speed and loading conditions. We hypothesized that 1) the improvement in weight-bearing stepping by modulating the excitability of glycinergic and serotoninergic receptors will be related to the facilitation of MR and LR neuronal circuitries associated with extensor muscles, and 2) the manner in which the MR and LRs are facilitated or depressed during locomotion will be dependent on the patterns of afferent input to the spinal cord.
MATERIALS AND METHODS
Animals and animal care.
Data were obtained from five adult female Sprague-Dawley rats (270–300 g body wt). Pre- and postsurgical animal care procedures have been described in detail previously (Roy et al. 1992). The rats were housed individually with food and water provided ad libitum. All survival surgical procedures were conducted under aseptic conditions with the rats deeply anesthetized with isoflurane gas administered via facemask as needed. All procedures described below are in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Research Committee at University of California, Los Angeles.
Head connector and intramuscular EMG electrode implantation.
A small incision was made at the midline of the skull. The muscles and fascia were retracted laterally, small grooves were made in the skull with a scalpel, and the skull was dried thoroughly. Two Amphenol head connectors with Teflon-coated stainless steel wires (AS 632; Cooner Wire, Chatsworth, CA) were securely attached to the skull with screws and dental cement as described previously (Ichiyama et al. 2005; Roy et al. 1992). Selected hindlimb muscles, i.e., the tibialis anterior (TA) and soleus, were implanted bilaterally with intramuscular EMG recording electrodes as described by Roy et al. (1991). Skin and fascial incisions were made to expose the belly of each muscle. Two wires extending from the skull-mounted connector were routed subcutaneously to each muscle. The wires were inserted into the muscle belly using a 23-gauge needle, and a small notch (approximately 0.5–1.0 mm) was removed from the insulation of each wire to expose the conductor and form the electrodes. The wires were secured in the belly of the muscle via a suture on the wire at its entrance into and exit from the muscle belly. The proper placement of the electrodes was verified during the surgery by stimulating through the head connector and postmortem via dissection.
Spinal cord transection, epidural electrode implantation, and postsurgical animal care procedures.
A partial laminectomy was performed at the T8–T9 vertebral level, and a longitudinal cut was made in the dura to expose the spinal cord. A complete spinal cord transection to include the dura was performed at approximately the T8 spinal level using microscissors. Two surgeons verified the completeness of the transection by lifting the cut ends of the spinal cord and passing a glass probe through the lesion site. Gel foam was inserted into the gap created by the transection as a coagulant and to separate the cut ends of the spinal cord.
For epidural electrode implantation, partial laminectomies were performed to expose the spinal cord at spinal levels L2 and S1. Two Teflon-coated stainless steel wires from the head connector were passed under the spinous processes and above the dura mater of the remaining vertebrae between the partial laminectomy sites. After removing a small portion (∼1-mm notch) of the Teflon coating and exposing the conductor on the surface facing the spinal cord, the electrodes were sutured to the dura mater at the midline of the spinal cord above and below the electrode sites using 8.0 Ethilon suture (Ethicon, New Brunswick, NJ). Two common ground (indifferent EMG and stimulation grounds) wires (∼1 cm of the Teflon removed distally) were inserted subcutaneously in the midback region. All wires (for both EMG and epidural stimulation) were coiled in the back region to provide stress relief.
All incision areas were irrigated liberally with warm, sterile saline. All surgical sites were closed in layers using 5.0 Vicryl (Ethicon) for all muscle and connective tissue layers and for the skin incisions in the hindlimbs and 5.0 Ethilon for the back skin incision. All closed incision sites were cleansed thoroughly with saline solution. Analgesia was provided by Buprenex (0.5–1.0 mg/kg subcutaneously 3 times/day). The analgesics were initiated before completion of the surgery and continued for a minimum of 2 days. The rats were allowed to recover fully from anesthesia in an incubator. The rats were housed individually in cages that had ample CareFresh bedding, and the bladders of the spinal rats were expressed manually 3 times daily for the 1st 2 wk after surgery and 2 times daily thereafter. The hindlimbs of the spinal rats were moved passively through a full range of motion once per day to maintain joint mobility. All of these procedures have been described in detail previously (Courtine et al. 2009).
Stimulation and testing procedures.
Bipolar epidural stimulation between L2 and S1 was used to evoke potentials in the hindlimb muscles during standing (1 and 40 Hz) and stepping (40 Hz; Courtine et al. 2009; Gerasimenko et al. 2008; Ichiyama et al. 2008; Lavrov et al. 2006). The rats were stepped bipedally on a specially designed motor-driven rodent treadmill using a body weight support system (de Leon et al. 2002). The rats were stepped under three conditions: 1) weight-bearing stepping during which there was complete foot contact while stepping; 2) toe stepping when only the toes were in contact with the treadmill; and 3) air stepping when there was no foot contact with the treadmill. The evoked potentials and locomotor performance induced by epidural stimulation were determined before and after administration of Quip (0.3 mg/kg; Ichiyama et al. 2008) or Strych (1 mg/kg; de Leon et al. 1999) intraperitoneally.
Kinematics recording parameters.
A four-camera system was calibrated and then used to track reflective markers placed on bony landmarks on the iliac crest, greater trochanter, lateral condyle, lateral malleolus, and the distal end of the fifth metatarsal of both hindlimbs. The video footage was processed using SIMI Motion analysis software (SIMI Reality Motion Systems, Unterschleissheim, Germany) to produce the three-dimensional (3-D) reconstruction of the hindlimb and forelimb movements as well as the 2-D ball-and-stick diagrams and hindlimb trajectory plots (Courtine et al. 2009). The 3-D coordinates for a given marker were calculated using a triangulation procedure that partially accounts for the movement of the skin. The 3-D coordinates then were used to estimate the joint angles. This is a technique that has been used successfully and implemented in many laboratories and has the precision necessary for the present study.
Data analysis.
EMG recordings from the hindlimb muscles were band-pass filtered (1 Hz to 5 kHz), amplified using an A-M Systems Model 1700 Differential AC Amplifier (Carlsborg, WA), and sampled at a frequency of 10 kHz using a custom data acquisition program written in the LabVIEW development environment (National Instruments, Austin, TX) as described previously (Courtine et al. 2009). Evoked potentials were identified using a custom script written in MATLAB (The MathWorks). Peaks were detected using a moving window differentiation technique (Vivó-Truyols et al. 2005) by locating the point at which the slope was 0 (identified as either a peak or a valley) for peaks above a set threshold, i.e., three times the standard deviation of the individual trace (Vivó-Truyols et al. 2005). Figure 3B shows an example of a single trace for MRs and LRs (circles) that were detected along with smaller responses (crosses) that did not qualify as peaks. With a single pulse, the ER was estimated in the window of 1–3 ms, the MR in the window of 4–6 ms, and LRs in the window of >7 ms. At the higher frequency of stimulation (40 Hz), the LRs were estimated as responses with 7- to 25-ms delay. The response latencies were determined from the onset of the stimulation pulse to the identified peak. These criteria scales are based on previously published data (Gerasimenko et al. 2003, 2006; Lavrov et al. 2006, 2008). The integral in the EMG signal was calculated by estimating the area under the curve after rectification of the raw EMG to account for any oscillations in baseline activity as previously described (Roy et al. 1991; Whiting et al. 1984). The LR/MR ratio was calculated as the integral of the LR region to the integral of the MR region. The cumulative integral was calculated by summing the integral from each stimulation pulse across the entire step cycle. The step cycle was determined by analyzing kinematics data using VirtualDubMod (open source video processing, GPL). The step cycle was initiated at the start of the swing phase, i.e., when the toe lost contact with the treadmill, and ended with the start of the next swing phase.
Fig. 3.
Modulation of eEmc evoked potentials during stepping under different loading conditions. A: the effect of load on the modulation of evoked potentials generated for each stimulation pulse in the TA and Sol muscles for a single cycle during weight bearing, toe, and air stepping. The start of each trace is synchronized with the initiation of eEmc. Each trace is 25 ms, i.e., the time between successive eEmc pulses. The red vertical dashed lines denote the MR window. The vertical dashed blue line denotes the beginning of the window for the LRs. The areas outlined by solid red lines denote the LR duration during the swing phase for the TA and during the stance phase for the Sol. B: sample 25-ms trace demonstrating peaks that were detected (circles) and peaks that were not detected (crosses).
Statistical analyses.
All data are reported as means ± SE. Statistically significant differences were determined using a one-way repeated-measures ANOVA. The criterion level for the determination of a statistical difference was set at P < 0.05 for all computations.
RESULTS
Characteristics of motor-evoked responses to epidural stimulation at different frequencies during bipedal standing.
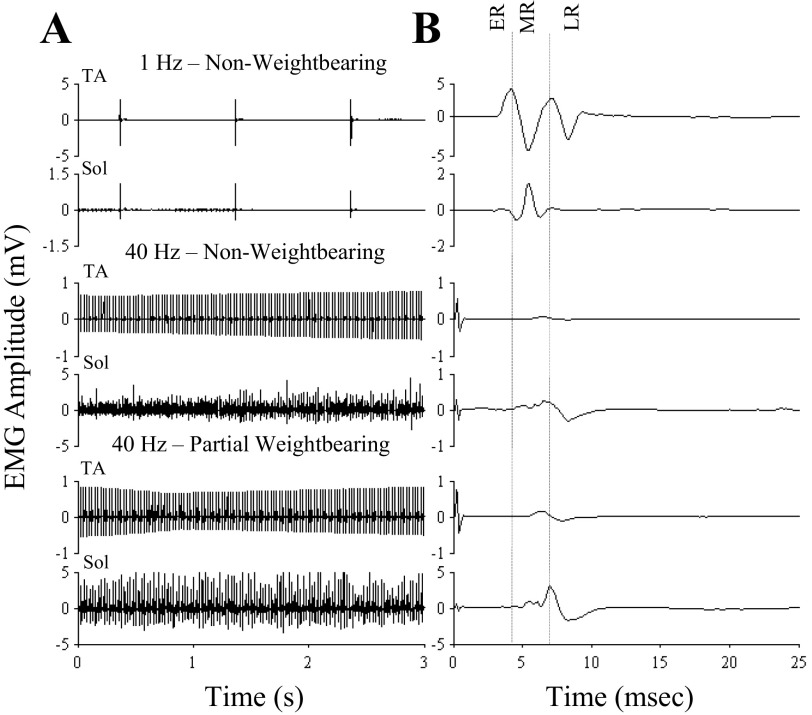
Stimulation at a low frequency evoked responses within three general time frames in the TA and soleus when the rat was not bearing weight, i.e., an ER (1–3 ms latency), MR (4–6 ms latency), and LR (>7 ms latency; Fig. 1, top) as demonstrated previously (Gerasimenko et al. 2003, 2006; Lavrov et al. 2006, 2008). Epidural stimulation at 40 Hz facilitated tonic EMG activity in both the TA and soleus muscles during bipedal standing while the rat was partially weight bearing (Fig. 1, bottom traces). The average latency for these responses in both muscles was between 4 and 6 ms, i.e., a latency corresponding to a MR (Gerasimenko et al. 2006; Lavrov et al. 2006). Note the higher amplitudes at the lower frequency reflecting a depression in the evoked potentials at 40 Hz. There was a large MR in the soleus during partial weight bearing (Fig. 1, bottom traces), whereas there was no or minimal ER, MR, and LR in the absence of weight bearing in either muscle (Fig. 1, middle traces).
Fig. 1.
Evoked potentials during standing under different loading and stimulation conditions. A: tibialis anterior (TA) and soleus (Sol) electromyographic (EMG) amplitudes with the rat supported in a harness in a bipedal standing position while nonweight bearing and electrical enabling motor control (eEmc) between L2 and S1 at 1 Hz (top traces) and during nonweight bearing (middle traces) and partial weight bearing (bottom) and eEmc between L2 and S1 at 40 Hz. B: average of the motor-evoked potentials during the 3 s of recordings shown in A identifying the early (ER; latency 1–3 ms), middle (MR; latency 4–6 ms), and late (LRs; latency >7 ms) responses. The range of latencies for each response is based on previous observations (Gerasimenko et al. 2006; Lavrov et al. 2008). Note the different scales for the EMG amplitudes.
Characteristics of motor-evoked responses to epidural stimulation during bipedal stepping.
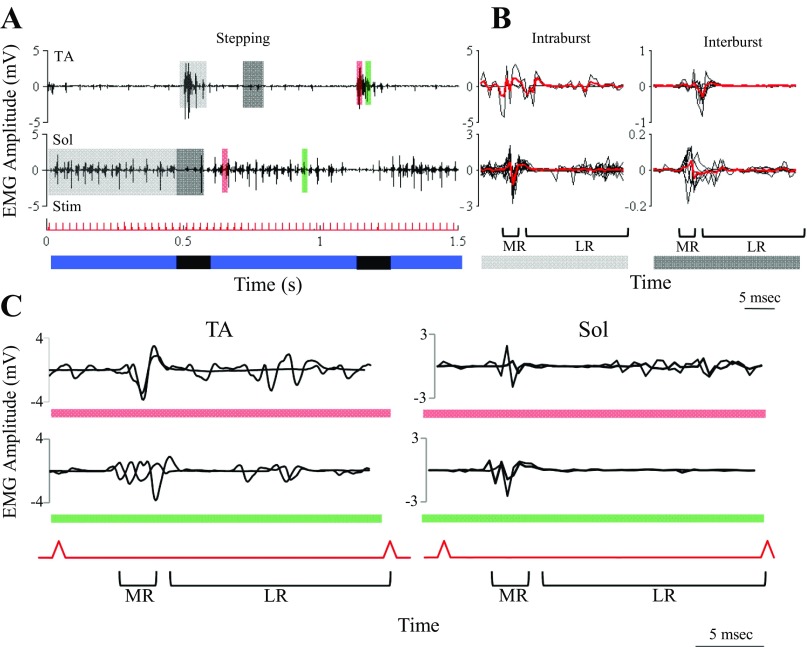
The latencies and amplitudes of the evoked potentials to epidural stimulation were modulated in a phase-dependent manner during bipedal stepping on a treadmill. These patterns were modulated to a large extent based on the presence or absence of an EMG burst (see light and dark gray shaded areas in Fig. 2A). Averages of all responses during (intraburst) and between (interburst) EMG bursts of the TA and soleus are shown in Fig. 2B. The amplitudes of the responses are 5- to 10-fold higher during than between bursts. In addition, the number of LRs is greater during compared with between bursts. The evoked responses, however, are further modulated as a function of whether they are induced in the early vs. late phase of the flexor (TA) or extensor (soleus) EMG (see orange and green in Fig. 2C). The latency of the MR is slightly longer in the TA than the soleus throughout the burst, and in the later phase of the soleus burst there are little or no LRs.
Fig. 2.
Modulation of eEmc evoked potentials during stepping. A: TA and Sol EMG during the stance (blue) and swing (black) phases of stepping on a treadmill at 13.5 cm/s with partial weight bearing under the influence of eEmc (40 Hz between L2 and S1). Light gray highlight, during intraburst interval; dark gray highlight, during interburst interval; red and green highlights, early and late phases of the EMG burst, respectively; Stim, eEmc pulse. B: the MR and LRs for all motor-evoked potentials during the intraburst interval (left plots for the areas highlighted in light gray in A) and during the interburst interval (right plots for the areas highlighted in dark gray in A) are shown as black traces, and the red bold line shows the average of all potentials. C: zoomed-in view of the early (top traces) and late (bottom traces) phases of the TA and Sol EMG bursts highlighted in A. Note the presence of both an MR and LRs during both phases of the EMG burst in the TA and for the early phase in the Sol but only an MR for the late phase in the Sol.
Stepping under different levels of body weight support modulates the sensory information received by the spinal cord. To begin to understand better the unique changing “footprints” reflected in the responses to different load-bearing conditions, we examined the latencies of the responses throughout the step cycle to each evoked stimulus administered tonically at 40 Hz. In this way, we could observe the response to each stimulus during consecutive 25-ms epochs throughout the flexor and extensor phases of the step cycle (Fig. 3A). The start of each 25-ms epoch was synchronized with each stimulation pulse with the initial epoch plotted at the bottom of the figure and beginning with the initiation of the swing phase of the step cycle. The LRs in the TA tended to persist for more consecutive 25-ms epochs during air stepping compared with weight-bearing stepping. Clear variations in the MR and LRs related to the early and late phases of soleus activation were observed (similar to Fig. 2). The MR in the soleus is prominent and relatively consistent in amplitude and latency throughout the extension phase of the step cycle during air stepping and toe stepping. During weight-bearing stepping, however, there was a progressive decrease in the amplitude of the MR during the stance phase. This finding is consistent with a concomitant decrease in proprioception related to load bearing during the stance phase of the step. A reduction in the duration of the LRs in the soleus (i.e., in the number of 25-ms epochs and the duration of responses within a given epoch) occurred with load bearing.
Varying the speed of stepping largely affects the EMG burst duration of ankle extensors but not flexors in intact (Roy et al. 1991) and spinal (Courtine et al. 2009) rats. We examined the behavior of motor-evoked potentials in flexor and extensor muscles during stepping at different treadmill speeds. Plotting the evoked responses to consecutive 25-ms epochs between stimuli for a single step cycle at different speeds demonstrates different patterns of changes in the amplitudes and durations for the LRs compared with the MR (Fig. 4A). Increased treadmill speed resulted in a decrease in the number of epochs during the stance phase but no change in the number of epochs during the swing phase. The amplitude of the MR in the TA increased with speed of stepping, whereas the LRs were small and occurred randomly during the swing phase at all speeds tested. In the soleus, a prominent MR was present and occurred only during the stance phase. The largest MR amplitudes generally occurred at the beginning of stance and progressively decreased throughout the remainder of the stance phase. The LRs in the soleus also were present only during the stance phase. At the initiation of stance, LRs were sustained throughout each 25-ms epoch but with succeeding epochs the initiation of the LRs had a progressively longer delay following each stimulus (Fig. 4A). The amplitudes of the individual LR did not seem to vary consistently across the speeds tested. Another feature of the LRs in the soleus was the inverse relationship between the speed of stepping and the number of 25-ms epochs in which LRs occurred (Fig. 4, A and B).
Fig. 4.
eEmc evoked potentials during stepping at different treadmill speeds. A: the effect of treadmill speed on the modulation of the evoked potentials generated for each stimulation pulse in the TA and Sol muscles for a single step cycle. The layout is similar to that in Fig. 3. B: the average (n = 5 rats, 10 steps per rat) LR/MR ratio of the cumulative integral in the TA and Sol when stepping at 6, 13.5, and 21 cm/s. C: the cumulative levels of activation over consecutive 25-ms epochs during the swing and stance phases are shown for each of the 3 speeds. * And †: significantly different from 6 and 13.5 cm/s, respectively, at P > 0.05.
The ratio of LR to MR integral in the TA remained constant as a function of speed (Fig. 4B). In contrast, the ratio of the LR to MR integral progressively decreased with increasing speed in the soleus. The relative rate of cumulative integral for the TA or the soleus was not remarkably different across the three speeds studied (Fig. 4C). The absolute rate of accumulation, however, was ∼5-fold greater in the soleus than TA. The total cumulative integral for the soleus, but not the TA, decreased with increasing treadmill speed.
Pharmacological modulation of motor-evoked potentials during nonweight-bearing standing and stepping.
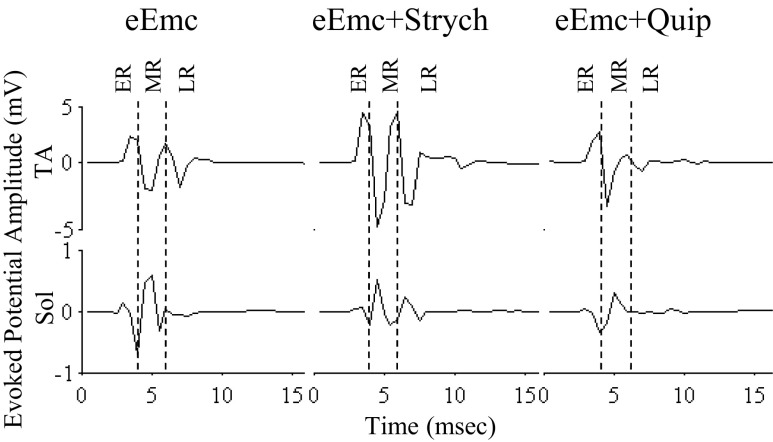
eEmc (1 Hz) at a suprathreshold intensity (>4 V) when the limbs were nonweight bearing elicited an ER, MR, and LR in the TA and an ER and MR in the soleus (Fig. 5, similar to Fig. 1, top traces). In the presence of eEmc, Strych (a glycinergic antagonist) facilitated the MR in the TA, whereas Quip (a serotoninergic agonist) reduced the MR in both muscles. The addition of Strych or Quip in concert with eEmc modulated the stepping pattern of the spinal rats (Fig. 6 and Supplemental Video S1, available in the data supplement online at the Journal of Neurophysiology web site). eEmc+Strych modestly increased the prominence of the MR in the TA while markedly increasing the LRs in the soleus during the stance phase. The number and duration of LRs in the soleus were higher with eEmc+Strych than with eEmc alone (Fig. 6), suggesting that blocking glycinergic receptors resulted in disinhibition of the neural networks and facilitated stepping.
Fig. 5.
Evoked potentials during standing under the influence of eEmc with and without strychnine (Strych) or quipazine (Quip). Average amplitude (10 responses) of the evoked potentials induced by eEmc (1 Hz) from the Sol and TA with the rat under nonweight-bearing conditions with and without Quip or Strych administration.
Fig. 6.
Evoked potentials during stepping under the influence of eEmc with or without Strych or Quip. A: modulation of the evoked potentials generated by each stimulation pulse with eEmc alone and after Quip or Strych administration with eEmc. The layout is similar to that in Fig. 3. B: angle-angle plots (knee vs. ankle) for a single step cycle with eEmc alone and after Quip or Strych administration with eEmc. deg, Degrees. C: x-y plots representing the trajectory of the foot marker (metatarsophalangeal, MTP) during a single step cycle with eEmc alone and after Quip or Strych administration with eEmc.
The MR and LRs were more prominent under eEmc+Quip relative to eEmc alone corresponding to a more prolonged stance phase. The effects of Quip on the pattern of the evoked potentials throughout the step cycle differed in several ways from that of Strych. For example, there was significantly longer duration of LRs and a more prominent MR occurring throughout the stance phase with eEmc+Quip compared with eEmc+Strych in the soleus. The differences in the pattern of stepping are demonstrated in Fig. 6B. The angle-angle plots with eEmc alone demonstrate the smaller excursions in the ankle and knee angles during a single step cycle compared with eEmc+Quip and eEmc+Strych. Administration of Strych or Quip increased the excursion at both the knee and ankle joints, more so with Quip than Strych. The foot (metatarsophalangeal) trajectory in the x-y plane also demonstrates the differences in the pattern of stepping with eEmc alone compared with eEmc+Quip and eEmc+Strych (Fig. 6C). The administration of Quip with eEmc resulted in a higher foot position during the swing phase than with eEmc+Strych and eEmc alone. Similar patterns of evoked potentials were observed across several animals (Fig. 7).
Fig. 7.
Evoked potentials during stepping under the influence of eEmc with or without Strych or Quip from multiple animals. Modulation of evoked potentials generated by each stimulation pulse with eEmc alone and after Strych and Quip administration for 2 steps (red and blue) from each of 3 animals (#1, #4, and #7). The layout is similar to that in Fig. 3.
The total activation of the soleus and TA based on the EMG responses was measured to determine the relative importance of the modulation of the MR and LR components during the flexor and extensor phases of the step cycle. To do this, we determined the area under the rectified EMG burst (integrated area of the rectified EMG burst) during the MR region and the LR region after each stimulation pulse (Fig. 8A). For the total step cycle, the integral of both the MR and LRs and the total integral in the TA were higher with eEmc+Quip and eEmc+Strych than eEmc alone and lower with eEmc+Strych than with eEmc+Quip. Similar increases in the integral for the LRs and the total integral were observed with eEmc+Quip and eEmc+Strych relative to eEmc alone in the soleus. The overall effect of eEmc+Strych or eEmc+Quip relative to eEmc alone was similar, i.e., dominated by the LRs in both the TA and soleus.
Fig. 8.
Average evoked potentials during stepping under the influence of eEmc with or without Strych or Quip. A: average integral levels in the TA (top) and Sol (bottom) EMG in the MR, LR, and total regions and the relative percentage of the LR (LR/Total) during stepping (13.5 cm/s) with eEmc alone (red), eEmc+Strych (blue), and eEmc+Quip (green). * And †: significantly different from eEmc and eEmc+Strych, respectively, at P > 0.05. B: the average MR amplitude and latency for each stimulus pulse are shown for the eEmc alone (red), eEmc+Strych (blue), and eEmc+Quip (green) conditions. C: plots showing the average MR latency (red line) and LR duration (vertical lines; a, 1st LR; b, last LR) for each stimulation pulse within a step cycle. D: average cumulative integral curves for the TA and Sol EMG during the swing and stance phases for all step cycles for the MR, LR, and total regions under the 3 conditions tested. Values are means ± SE for 5 rats, 10 steps per rat.
More unique features of the effects of Strych and Quip became apparent when comparing the time course of the changes in the MR within the flexor and extensor phases of the step cycle (Figs. 8B and 9A). For example, the peak level of activation in the soleus during stance was higher with eEmc alone than with eEmc+Quip or eEmc+Strych (Fig. 8B). The amplitude of the MR in response to eEmc+Strych was more modest but at the same time persisted at a more constant level throughout the stance phase. Note also that eEmc+Quip resulted in a more prolonged stance phase compared with eEmc alone or eEmc+Strych (the results were similar for the LRs). The impact of the enhancement of the swing and stance phases attributable to Strych and Quip was made apparent by the more prolonged activation within each 25-ms epoch (Fig. 8C). The net effect of the stimulation pulse on the LR integral relative to the MR integral was greatest for the eEmc+Strych condition and smallest with eEmc alone during the stance phase (Fig. 9A).
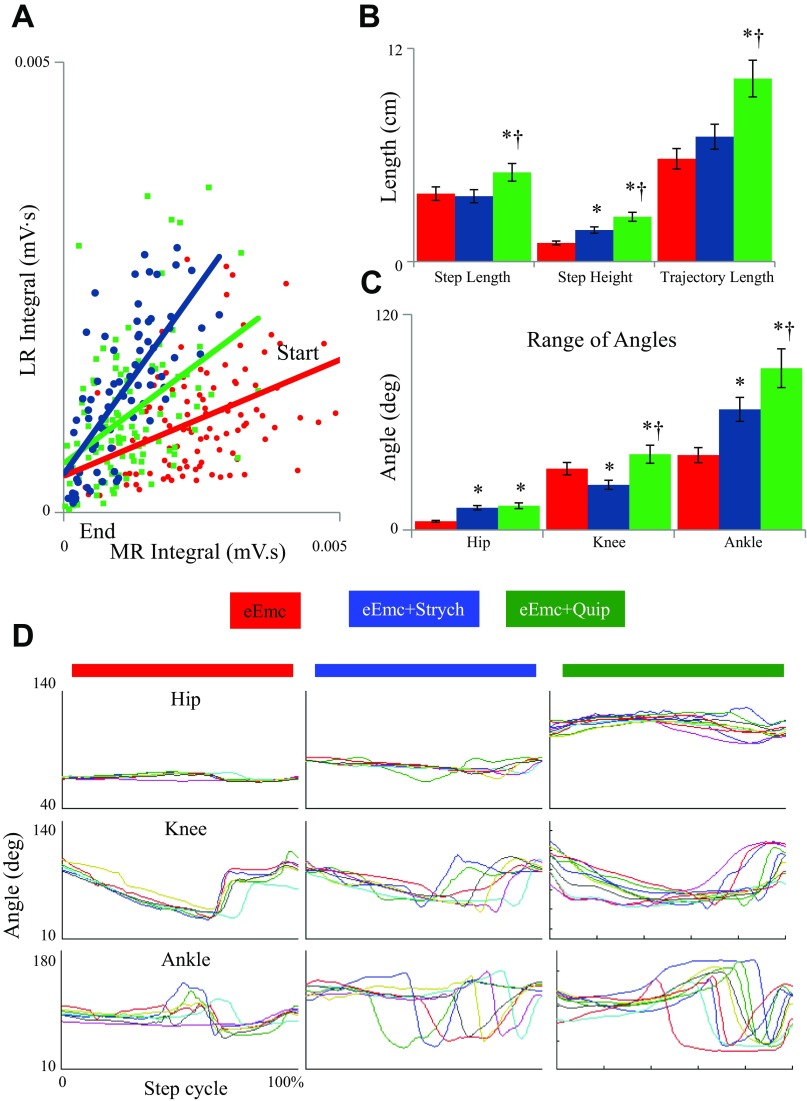
Fig. 9.
Evoked potentials and kinematics characteristics during stepping under the influence of eEmc with and without Strych or Quip. A: each dot represents the MR and LR integral from a single eEmc pulse from the start to the end of the stance phase (n = 3 rats, 5 steps per rat). B: the average step length, step height, and trajectory length are shown for the eEmc alone (red), eEmc+Strych (blue), and eEmc+Quip (green) conditions (n = 5 rats, 5 steps per rat). C: average range of angle excursions during a step cycle for the eEmc alone (red), eEmc+Strych (blue), and eEmc+Quip (green) conditions (n = 5 rats, 5 steps per rat). D: hip, knee, and ankle angles from normalized step cycles for each condition (n = 3 rats, 3 steps per rat). * And † in B and C: significantly different from eEmc and eEmc+Strych, respectively, at P > 0.05.
To examine further the dynamics of this activation pattern relative to the phase of the step cycle, we calculated the cumulative integrated EMG attributable to the MR and LRs (Fig. 8D). The importance of this cumulative effect, theoretically, would be related to the speed of movement occurring throughout the step cycle as well as to the duration of the flexor and extensor phases of the step cycle. This rationale is based on the assumption that a higher and longer level of activation (increased MR and LR amplitude) results in higher muscle forces attributable to more muscle fibers being activated or the muscle fibers being activated at a higher frequency. Given this interpretation, the results predict that under the conditions of the present experiment the level of activation attributable to the MR is consistently higher than that attributable to the LRs in response to eEmc alone (Figs. 8B and 9A). The opposite tends to occur in response to eEmc+Strych. eEmc+Quip also enhances the LRs relative to the MR during the stance phase compared with eEmc alone, has a unique effect in prolonging the duration of this enhancement, and probably contributes significantly to a longer stance phase (Fig. 8B).
Compared with eEmc alone, eEmc+Strych increased step height during treadmill locomotion (Fig. 9B). In addition, the excursion at the hip and ankle joints was greater and that at the hip smaller at the knee with eEmc+Strych than eEmc alone (Fig. 9, C and D). eEmc+Quip had a more robust effect on the step kinematics increasing step length, step height, and trajectory length compared with both eEmc alone and eEmc+Strych (Fig. 9B). With eEmc+Strych, the excursion at all joints was greater than with eEmc alone and greater at the knee and ankle than with eEmc+Strych (Fig. 9, C and D).
DISCUSSION
We recently demonstrated progressive postlesion changes in the MR and LRs in the extensor muscles during standing (Lavrov et al. 2006) and phase-dependent modulation during stepping in control rats (Gerasimenko et al. 2006) and humans (Courtine et al. 2007) and in spinal rats (Lavrov et al. 2008) and SCI patients (Dy et al. 2010). As observed previously (de Leon et al. 1999; Gerasimenko et al. 2007), locomotion was facilitated by eEmc and by Quip or Strych in spinal animals. The present data demonstrate in an adult in vivo preparation using an unanesthetized rat that there are unique patterns of evoked potentials having a range of delay times relative to the stimulation pulses and predictable changes in the persistence of poststimulation responses that appear to play a role in defining the kinematics of the stance and swing phases of the step cycle. These unique patterns generate predictable functional footprints that could be used to formulate optimal combinations of electrical and pharmacological neuromodulation of the spinal circuitry in facilitating specific motor tasks even when there is no supraspinal input to the lumbosacral spinal cord.
What are the MR and LRs in the EMG bursts?
Although the MR have some properties consistent with spinal cord monosynaptic circuits (Lavrov et al. 2006, 2008) and their latency is similar to the responses recorded with magnetic spinal cord stimulation in normal and spinal rats (Chiba et al. 2003), there is no compelling reason to assume that this response can be attributed solely to the classic monosynaptic reflex when stimulating Ia afferents. As the MR is a single response with a relatively constant latency after the stimulation pulse, it may represent a simple mechanism involving a few spinal networks that could facilitate the generation of the locomotor pattern. The latency of the first component of the LRs, on the other hand, as recorded during standing probably results from more than one synaptic delay (Lavrov et al. 2006). Some combination of eEmc and afferent input derived from the moving hindlimbs appears to facilitate multiple spikes within the LR region of the EMG burst. We speculate that the multiple peaks of the LRs reflect primarily variable numbers of synaptic events within the constantly changing neural networks involved at any given phase of the step cycle rather than synchronized reflex responses being generated with a similar delay. Among the neural networks that control the extensor muscles, we speculate that there could be multiple parallel, as well as in series, networks that generate the LRs that are not strictly time-linked to individual stimuli.
Multiple LRs of varying amplitudes and latencies reflect the dynamic proprioception associated with the moving hindlimbs and suggest that the origin of these evoked responses is due to activation of a complex interneuronal network modulated pharmacologically, electrically, and through proprioception. These responses cannot be classified as stereotypical reflex responses generated by activation of specific fibers and/or pathways.
The lack of LRs in both the soleus and TA during the interburst period (Fig. 2B) could be attributed to presynaptic inhibition between antagonistic muscles. The mechanism for the systematic and progressive delay of ∼2 ms with successive stimuli within the stance phase of the step cycle is not clear. Intuitively, it seems unlikely to be only attributable to a progressively increasing complexity, i.e., number of synapses, of the network. Given that the evoked potentials were recorded in the muscles, there could be several simpler mechanisms to explain the systematic delay of LRs during stance. There could be a progressive change in the neuronal excitability in response to successive stimuli, a property that theoretically could be at the level of the neuromuscular junction, the motoneurons, or the interneuronal networks driving the motoneurons. Given that this phenomenon is not evident in the MR, the most logical site for this effect would seem to be the interneuronal networks that drive and coordinate the motoneurons.
Step cycle-dependent mechanisms for stepping.
The formation of the EMG bursts in the soleus and TA involved the modulation of both the MR and LRs, but the modulation of the LRs was phase-dependent only in the soleus (Figs. 2 and 3). In contrast, EMG activity in both the flexor and extensor muscles showed only MR during the interburst interval (Fig. 2). Thus it appears that the neural networks responsible for the genesis of the EMG pattern for extensor muscles during stepping are mediated by the modulation of MR, whereas this process is associated with a switching from MR to LR pathways in the flexor muscles (Gerasimenko et al. 2006). LRs were present during the entire TA burst of the swing phase and during the first portion of the soleus burst that is related to foot placement on the treadmill during the initial portion of the stance phase. The later portion of the soleus burst contains only an MR and is related to the supportive and extension reactions during the later portion of the stance phase. Thus the interval of the step cycle where LRs were observed requires precise movement and control.
Based on these findings, LRs probably reflect the activation of spinal networks involved in complex motor programs, such as coordination of precise movements during stepping. These networks could be responsible for planning and executing the next step. During the swing phase, these networks may be planning the position of the foot for the next stance phase based on afferent information received during the previous step and from the contralateral hindlimb. During the beginning of the stance phase when the foot touches the treadmill, it seems likely that the afferent information processed in the spinal cord helps in maintaining balance, posture, coordination of different hindlimb muscles, and the ability to make appropriate adjustments (Musienko et al. 2012; Zhong et al. 2012). During the later portion of the stance phase, these circuits are normally “disconnected” to provide only supportive reactions. We speculate that during this disconnected state, the neural networks also are dedicated to modulating the MR and LRs of the contralateral limb.
The role of afferent input on the effect of eEmc in facilitating stepping.
In the absence of any supraspinal influence, the sensory input from hindlimb receptors determines the formation of adaptive motor patterns in spinal rats during stepping facilitated by epidural stimulation (Lavrov et al. 2008). In fact, the spinal rats can perform forward, sideward, and backward stepping depending on the direction of the treadmill movement (Courtine et al. 2009; Shah et al. 2012). In addition, during bilateral stepping facilitated by eEmc of spinal rats with a unilateral deafferentation, each limb is dependent on the afferent information from the ipsilateral side (Lavrov et al. 2008). As the animal recovers, there is a reorganization of the spinal circuitry to adapt for the lack of afferent information from the deafferented side.
In this study, we demonstrate a direct relationship between the LRs in the soleus and the afferent information being processed in the spinal cord (Fig. 6). The number of LRs increases significantly during weight-bearing stepping compared with toe stepping or air stepping (Fig. 3). In addition, we show that with increasing treadmill speeds, the number of LRs in the soleus decreases, whereas the MR remains consistent (Fig. 4). Thus the number of LRs is associated with the duration of foot contact and loading with the treadmill. Combined, these findings demonstrate a very close link between stepping and the presence of LRs in spinal animals (present data and Lavrov et al. 2008) and that there is an important role of afferent information in shaping the LRs.
Mechanisms of neuropharmacological modulation and activation of spinal networks.
de Leon et al. 1999 demonstrated that the ability of spinal cats to step can be dramatically and rapidly enhanced by inhibiting the glycinergic pathways. They also reported a prolonged EMG burst duration during step testing in cats that were trained to stand. In contrast to the lengthened stance phase of the step cycle based on the EMG burst duration of the soleus, in the presence of eEmc+Strych the stance duration was reduced compared with eEmc alone (Fig. 8B). Our data suggest that the modulation of the step cycle by Strych could be attributed to enhanced LRs and MR but with a relatively greater effect on the LRs during both the swing and stance phases of the step cycle (Fig. 8, B and C).
A wide range of studies demonstrates the importance of 5-HT-mediated neuromodulation of locomotion (Jacobs and Fornal 1993; Rossignol et al. 1998). These studies have reported 5-HT-mediated increases in cycle period, primarily during the stance phase, in spinal animals. In the present study, we show that the effects of Quip on the step cycle differ in several ways from that of Strych. Relative to eEmc alone, eEmc+Quip increased the MR and LRs in the TA to a greater extent than that observed with eEmc+Strych. The largest qualitative difference in the soleus between eEmc+Quip and eEmc+Strych was the greater prominence of the MR relative to the LR with eEmc+Quip. In addition, the MR and LRs occurred over a more prolonged period during eEmc+Quip compared with eEmc+Strych, resulting in a significantly longer stance phase with eEmc+Quip (Fig. 8, B and C). Based on these comparisons, one might predict that the combined effects of these two drugs could be complementary or even synergistic given that their mechanisms of neuromodulation of the locomotor networks have fundamentally different characteristics. These pharmacological interventions neuromodulate the physiological state of the spinal networks so that they will process the ensemble of sensory information associated with the kinetics and kinematics of stepping. This processing, in turn, generates a predictable efferent pattern from the relevant motor pools. In essence, the combination of the physiological state and the sensory ensemble that it reads generates a unique, predictable footprint.
Can the functionality of the spinal circuitry be embedded in the dynamic patterns of the MR and LRs during stepping in spinal rats?
The present data demonstrate distinct differences in the temporal dynamics of the modulation of the MR and LRs throughout the stance phase of a step. In the presence of Strych, the physiological state of the circuitry seems to be modulated in a way that results in markedly enhanced LRs and MR immediately on placement of the paw during the second phase of extension (Forssberg et al. 1980), resulting in a significantly greater stiffness, i.e., less yield, in the early phase of stance (Fig. 8B). The physiological state generated by Quip, on the other hand, results in a considerably more moderate response to foot placement in the LRs and MR and consequently results in less stiffness and a greater yield. Another feature of Quip is a prolongation of both the LRs and MR resulting in a longer stance phase (Fig. 8, B and C). These two contrasting pharmacological effects on the stance phase demonstrate dramatically different pharmacological footprints on the spinal circuitry and how it responds to proprioception (Figs. 8B and 9B). These results also demonstrate the temporal dynamics of both the LRs and MR and can potentially serve as important biomarkers for understanding the relationship between pharmacological treatments and the kinetics and kinematics of stepping (Fig. 9, C and D).
GRANTS
This research was supported by the National Institute of Biomedical Imaging and Bioengineering Grant R01-EB-007615, the National Institute of Neurological Disorders and Stroke Grant R01-NS-062009, Christopher and Dana Reeve Foundation, Walkabout Foundation, Fundamental Research Program #7 of the Presidium Russian Academy of Sciences, and the Russian Foundation for Basic Research Grants 13-04-01091 and 13-04-12030 ofi-m.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
P.G. and V.R.E. conception and design of research; P.G., I.A.L., P.S., H.Z., and R.R.R. performed experiments; P.G. and V.R.E. analyzed data; P.G., R.R.R., V.R.E., and Y.G. interpreted results of experiments; P.G. and V.R.E. prepared figures; P.G. and V.R.E. drafted manuscript; P.G., P.S., R.R.R., V.R.E., and Y.G. edited and revised manuscript; P.G., P.S., H.Z., R.R.R., V.R.E., and Y.G. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Maynor Herrera for providing excellent animal care and Sharon Zdunowski for technical assistance.
REFERENCES
- Chiba A, Oshio K, Inase M. Magnetically evoked EMGs in rats. Neurol Res 25: 87–91, 2003. [DOI] [PubMed] [Google Scholar]
- Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, Song B, Ao Y, Ichiyama RM, Lavrov I, Roy RR, Sofroniew MV, Edgerton VR. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci 12: 1333–1342, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Harkema SJ, Dy CJ, Gerasimenko YP, Dyhre-Poulsen P. Modulation of multisegmental monosynaptic responses in a variety of leg muscles during walking and running in humans. J Physiol 582: 1125–1139, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon RD, Reinkensmeyer DJ, Timoszyk WK, London NJ, Roy RR, Edgerton VR. Use of robotics in assessing the adaptive capacity of the rat lumbar spinal cord. Prog Brain Res 137: 141–149, 2002. [DOI] [PubMed] [Google Scholar]
- de Leon RD, Tamaki H, Hodgson JA, Roy RR, Edgerton VR. Hindlimb locomotor and postural training modulates glycinergic inhibition in the spinal cord of the adult spinal cat. J Neurophysiol 82: 359–369, 1999. [DOI] [PubMed] [Google Scholar]
- Dy CJ, Gerasimenko YP, Edgerton VR, Dyhre-Poulsen P, Courtine G, Harkema SJ. Phase-dependent modulation of percutaneously elicited multisegmental muscle responses after spinal cord injury. J Neurophysiol 103: 2808–2820, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton VR, Leon RD, Harkema SJ, Hodgson JA, London N, Reinkensmeyer DJ, Roy RR, Talmadge RJ, Tilakaratne NJ, Timoszyk W, Tobin A. Retraining the injured spinal cord. J Physiol 533: 15–22, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossberg H, Grillner S. The locomotion of the acute spinal cat injected with clonidine i.v. Brain Res 50: 184–186, 1973. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Grillner S, Halbertsma J. The locomotion of the low spinal cat. I. Coordination within a hindlimb. Acta Physiol Scand 108: 269–281, 1980. [DOI] [PubMed] [Google Scholar]
- Gad P, Woodbridge J, Lavrov I, Zhong H, Roy RR, Sarrafzadeh M, Edgerton VR. Forelimb EMG-based trigger to control an electronic spinal bridge to enable hindlimb stepping after a complete spinal cord lesion in rats. J Neuroeng Rehabil 9: 38, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko Y, Roy RR, Edgerton VR. Epidural stimulation: comparison of the spinal circuits that generate and control locomotion in rats, cats and humans. Exp Neurol 209: 417–425, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko YP, Avelev VD, Nikitin OA, Lavrov IA. Initiation of locomotor activity in spinal cats by epidural stimulation of the spinal cord. Neurosci Behav Physiol 33: 247–254, 2003. [DOI] [PubMed] [Google Scholar]
- Gerasimenko YP, Ichiyama RM, Lavrov IA, Courtine G, Cai L, Zhong H, Roy RR, Edgerton VR. Epidural spinal cord stimulation plus quipazine administration enable stepping in complete spinal adult rats. J Neurophysiol 98: 2525–2536, 2007. [DOI] [PubMed] [Google Scholar]
- Gerasimenko YP, Lavrov IA, Courtine G, Ichiyama RM, Dy CJ, Zhong H, Roy RR, Edgerton VR. Spinal cord reflexes induced by epidural spinal cord stimulation in normal awake rats. J Neurosci Methods 157: 253–263, 2006. [DOI] [PubMed] [Google Scholar]
- Harkema SJ, Gerasimenko YP, Hodes J, Burdick J, Angeli C, Chen Y, Ferreira C, Willhite A, Rejc E, Grossman R, Edgerton VR. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing and assisted stepping after motor complete paraplegia: a case study. Lancet 377: 1938–1947, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama RM, Courtine G, Gerasimenko YP, Yang GJ, van den Brand R, Lavrov IA, Zhong H, Roy RR, Edgerton VR. Step training reinforces specific spinal locomotor circuitry in adult spinal rats. J Neurosci 28: 7370–7375, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama RM, Gerasimenko YP, Zhong H, Roy RR, Edgerton VR. Hindlimb stepping movements in complete spinal rats induced by epidural spinal cord stimulation. Neurosci Lett 383: 339–344, 2005. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. 5-HT and motor control: a hypothesis. Trends Neurosci 16: 346–352, 1993. [DOI] [PubMed] [Google Scholar]
- Lavrov I, Dy CJ, Fong AJ, Gerasimenko Y, Courtine G, Zhong H, Roy RR, Edgerton VR. Epidural stimulation induced modulation of spinal locomotor networks in adult spinal rats. J Neurosci 28: 6022–6029, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavrov I, Gerasimenko YP, Ichiyama RM, Courtine G, Zhong H, Roy RR, Edgerton VR. Plasticity of spinal cord reflexes after a complete transection in adult rats: relationship to stepping ability. J Neurophysiol 96: 1699–1710, 2006. [DOI] [PubMed] [Google Scholar]
- Musienko P, Courtine G, Tibbs J, Kilimnik V, Savochin A, Garfinkel A, Roy RR, Edgerton VR, Gerasimenko Y. Somatosensory control of balance during locomotion in decerebrated cat. J Neurophysiol 107: 2072–2082, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musienko P, van den Brand R, Maerzendorfer O, Roy RR, Gerasimenko Y, Edgerton VR, Courtine G. Controlling specific locomotor behaviors through multidimensional monoaminergic modulation of spinal circuitries. J Neurosci 31: 9262–9278, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson GA, Goldberger ME. The development and recovery of motor function in spinal cats. II. Pharmacological enhancement of recovery. Exp Brain Res 62: 387–400, 1986. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Chau C, Brustein E, Giroux N, Bouyer L, Barbeau H, Reader TA. Pharmacological activation and modulation of the central pattern generator for locomotion in the cat. Ann NY Acad Sci 860: 346–359, 1998. [DOI] [PubMed] [Google Scholar]
- Roy RR, Hodgson JA, Lauretz S, Pierotti DJ, Gayek RJ, Edgerton VR. Chronic spinal cord injured cats: surgical procedures and management. Lab Anim Sci 42: 335–343, 1992. [PubMed] [Google Scholar]
- Roy RR, Hutchison DL, Pierotti DJ, Hodgson JA, Edgerton VR. EMG patterns of rat ankle extensors and flexors during treadmill locomotion and swimming. J Appl Physiol 70: 2522–2529, 1991. [DOI] [PubMed] [Google Scholar]
- Shah PK, Gerasimenko Y, Shyu A, Lavrov I, Zhong H, Roy RR, Edgerton VR. Variability in step training enhances locomotor recovery after a spinal cord injury. Eur J Neurosci 36: 2054–2062, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivó-Truyols G, Torres-Lapasió JR, van Nederkassel AM, Vander Heyden Y, Massart D. Automatic program for peak detection and deconvolution of multi-overlapped chromatographic signals part I: peak detection. J Chromatogr A 1096: 133–145, 2005. [DOI] [PubMed] [Google Scholar]
- Whiting WC, Gregor RJ, Roy RR, Edgerton VR. A technique for estimating mechanical work of individual muscles in the cat during treadmill locomotion. J Biomech 17: 685–694, 1984. [DOI] [PubMed] [Google Scholar]
- Zhong H, Roy RR, Nakada K, Zdunowski S, Khalili N, de Leon R, Edgerton VR. Accomodation of the spinal cat to a tripping pertubation. Front Physiol 3: 112, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.