Abstract
To produce fruiting bodies of Oudemansiella mucida, porcelain fungus, on the oak sawdust medium, additives suitable for the mycelial growth and fruiting body formation were screened. In general, the mycelial growth of the three strains of O. mucida used in this study have been good on oak sawdust mixed rice bran of 20~30%. The mycelia incubated in potato dextrose broth for 7 days were inoculated on oak sawdust medium supplemented with various ratios of rice bran and incubated for 30 days at 25℃ in the dark condition until the mycelia of O. mucida fully colonized the media from top to bottom. Then, top surface of the media in the bottles were horizontally scratched with a spatula and filled with tap water for 3 hours. To induce the primordial formation of O. mucida, the bottles were transferred to the mushroom cultivating room under 12 hrs of light (350 lux) and dark condition with relative humidity of 95% at 17℃. The primordia of O. mucida were formed on the surface of oak sawdust media after 7 days of incubation. The mature fruiting bodies were observed 5 days after primordial formation. The fruiting bodies O. mucida were formed on oak sawdust medium mixed with 5 to 30% rice bran. However, abundant fruiting-bodies of O. mucida were produced in oak sawdust medium supplemented with 20% rice bran. This is the first report associated with an artificial fruiting body production of O. mucida in Korea.
Keywords: Additives, Artificial cultivation, Fruiting bodies, Oudemansiella mucida, Sawdust medium
In nature, the fruiting body of Oudemansiella mucida, one of the edible and medicinal mushrooms belonging to Trichlomataceae, Agaricales, develops on the rotten wood of the broadleaf trees from summer to early autumn (Lee, 1988). O. mucida is collected occasionally from mountains and national parks in Korea (Park and Lee, 1999). Fruiting bodies of O. mucida have been known to possess medically important antifungal substances such as mucidin (strobilurin A) and oudemansin. In the USA, derivatives of mucidin are synthesized for controlling various plant fungal diseases. The derivatives of strobilurin known to kresoxim methyl and azoxystrobin were developed and globally sold in agricultural fungicide markets (Deacon, 2006). Mucidin is also isolated from fruiting bodies of Oudemansiella radicata (Anke et al., 1979, 1983, 1990). The oudemansin from O. mucida contained not only an antifungal activity but also an outstanding inhibitory effect on the sarcoma 180 and Erhrlich carcinoma of mice (Ying et al., 1987).
Although O. mucida has been considered as one of the promising edible and medicinal mushrooms (Ying et al., 1987), there is no published study on the artificial cultivation of the fungus in Korea. As part of preliminary experiment for producing fruiting bodies of O. mucida, the optimal culture conditions of mycelia such as temperature, pH, nutrients and culture media were studied (Jaysinghe et al., 2007). Therefore, the aims of this study are to determine suitable additive contents of rice bran or wheat bran for the mycelial growth and developing fruiting of O. mucida in the sawdust substrate.
Materials and Methods
Culture
Three strains of O. mucida such as IUM 688, IUM 929 and IUM 2345 were obtained from the Culture Collection of Wild Mushroom species (CCWM) in the Department of Biology, University of Incheon (Table 1). To facilitate the experiments in oak sawdust media, three strains of O. mucida were transferred to PDA plates and incubated at 25℃ in the dark condition until they showed a full growth and then kept at 4℃ for further use. Unless otherwise stated, the tests were performed at least 5 times. To prepare each liquid culture of three strains of O. mucida, 100 ml of potato dextrose broth (PDB) were poured into an Erlenmeyer flask (250 ml) and autoclaved for 15 minutes at 121℃. Then, 5 pieces of 5mm mycelial plugs were removed with cork borer from 7 days old PDA plates, inoculated PDB within the Erlenmeyer flask (250 ml) and incubated for 10 days at 25℃ in shaking incubator (150 rpm).
Table 1.
Strains of Oudemansiella mucida used in this study
Screening suitable additive contents of rice bran or wheat bran for the mycelial growth of O. mucida
Oak (Quercus variabilis) sawdust, rice bran and wheat bran were purchased from a Yongsan sawdust company located in Namwon city, Jeonbuk. To determine suitable additives for mycelial growth in oak sawdust medium, each additive was added in the ratios of 5%, 10%, 15%, 20%, 25% and 30% (v/v) in oak sawdust media, adjusted moisture content of 65~70%, put into the round glass columns (2 × 22 cm) and steam-sterilized for 90 minutes at 121℃.
Three strains of O. mucida were inoculated on the sawdust media in glass columns. A 5 mm agar plug was removed with cork borer from 7 days old PDA plate culture of O. mucida, and placed on the top surface of sawdust media in the glass column. The columns were incubated for 30 days at 25℃ under dark condition. The mycelial growth of O. mucida in oak sawdust media supplemented with rice bran or wheat bran was measured after 30 days of incubation. After 30 days of incubation, a suitable additive was selected and then used for supplements to produce fruiting bodies of O. mucida in the oak sawdust medium.
Cultivation condition of O. mucida on oak sawdust media
After rice bran was selected as a suitable additive for the mycelial growth of O. mucida, oak sawdust medium was prepared to produce fruiting body of O. mucida. The oak sawdust was mixed thoroughly with 5%, 10%, 15%, 20%, 25% and 30% of rice bran (v/v), adjusted the moisture content of 65~70%, put into polypropylene bottles (850 ml), making a hole with glass bar (diameter 1.5 × depth 8 cm) in the center of the media and autoclaved for 90 minutes at 121℃.
About 10 ml of each inoculum was removed from 10 days old liquid culture, inoculated on the top surface of oak sawdust media within polypropylene bottles (850 ml), and incubated for 30 days at 25℃ under 70% relative humidity in the dark condition.
When the mycelia of O. mucida completely colonized the sawdust media from top to bottom, the top surface of the media was horizontally scratched with spatula, filled with tap water for 3 hours and transferred to another room to induce the primordial formation. The bottles were cultured for 6 days at 17℃ under the 12 hours of light (350 lux) and darkness at 95% relative humidity.
After 6 days of incubation at 17℃, primordia of O. mucida were started to form on the top surfaces of the saw dust media. Then, the bottles were transferred to cultivating room and cultured at 20℃ under the 12 hours of illumination (350 lux) and darkness with relative humidity of 95%. Fruiting body formations were examined once a day.
Result and Discussion
Screening of suitable additive contents of rice bran for the mycelial growth of O. mucida
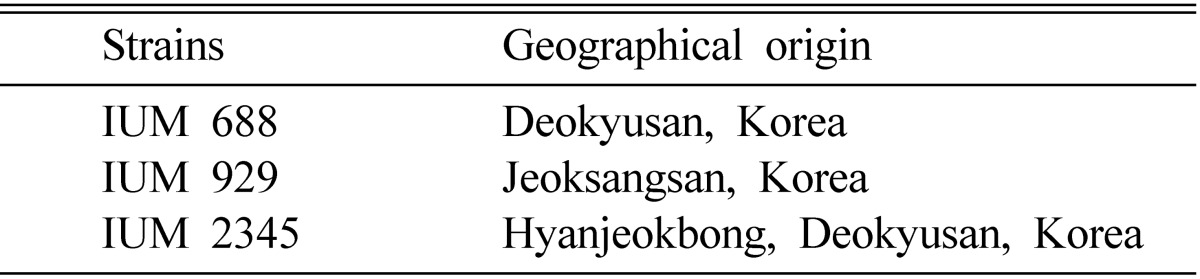
After 30 days of incubation in the glass column at 25℃, the mycelial growth of O. mucida was measured. Of two additives for screening mycelial growth of three strains of O. mucida, rice bran showed a little better mycelial growth than that of wheat bran. The oak sawdust which was supplemented with rice brain of 5~30% showed a good mycelial growth (Table 2). In general, the mycelial growth and density of O. mucida on oak saw dust mixed with rice bran were better than that of wheat bran. All three strains of O. mucida showed good mycelial growth on oak sawdust mixed with 20% rice bran. Even though mycelial growth of oak sawdust supplemented with rice bran and wheat bran was not significantly different, rice bran is cheaper and easier to purchase in the market. Therefore, rice bran was used for supplements of oak sawdust to produce fruiting body of O. mucida. Rew et al. (2004) showed that the mycelial growth of Phellinus baumii was best on oak sawdust mixed with 20% of rice bran (v/v). Shim et al. (2006a) reported that mycelial growth and density of O. radicata were also good on oak sawdust mixed with 10% rice bran. Therefore, rice bran might contain some ingredients to facilitate the good mycelial growth of O. mucida and the other mushrooms.
Table 2.
Effect of two additives on myclial growth of Oudemansiella mucidaa
aEach of 2 additives was mixed with oak (Quercus variabilis) sawdust at the ratio of 5%, 10%, 15%, 20% and 25%, respectively and then, put into the glass column (2 × 22 cm) and autoclaved at 121℃ for 15 min.
bThree strains were inoculated on the sawdust media and cultured for 30 days at 25℃ under dark condition to measure the mycelial growth.
cValue is an average of 5 replications.
Fruiting body production of O. mucida
Conditions for primordial formation
After scratching of mycelia on oak sawdust media, the polypropylene bottles were filled with tap water for 3 hours and then, kept for 7 days at 17℃ under 12 hours of light (350 lux) and dark conditions with relative humidity of 95%. Then, white-milky colored primordia have been started to form on the top surface of the media. The primordia have observed firstly from O. mucida IUM 929, IUM 688 and IUM 2345, respectively.
Conditions for fruiting body formation
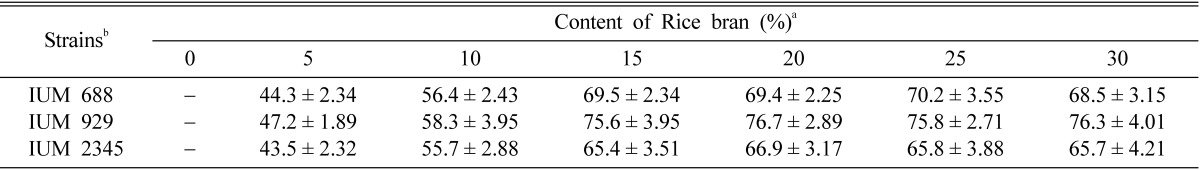
After formation of the primordia, the bottles were transferred to cultivation room, cultured at 20℃ under 12 hours of illumination (350 lux) and darkness with relative humidity of 95%. The fruiting bodies of O. mucida were formed in the media supplemented with rice bran of 5~30% (Table 3). Of three strains, mature fruiting bodies of IUM 929 were developed 5 days after occurrence of primordia (Fig. 1).
Table 3.
The fruiting body production of Oudemansiella mucida on oak sawdust media
aThe sawdust of oak (Quercus variabilis) was supplemented with of 5%, 10%, 15%, 20%, 25% and 30% rice bran (v/v), respectively.
bEach of 3 strains was treated by 10 replications.
Fig. 1.
The fruiting body of Oudemansiella mucida in nature.
In general, fruiting bodies of three strains of O. mucida have been produced in oak sawdust media mixed with rice bran of 5~30%. But, large numbers of fruiting bodies were produced in oak sawdust media mixed with rice bran of 20~30% (Table 3). Even though good fruiting bodies of O. mucida were also produced in the media supplemented with 25~30% rice bran, the contamination rate in this media was relatively higher than those of the media mixed with rice bran of 5~20%. Shim et al. (2006b) reported that fruiting bodies of Armillaria mellea were produced abundantly in the oak saw dust media mixed with 10% rice bran and contamination rate in the saw dust media seemed to be increased in proportion to the higher contents of rice bran. Shim et al. (2006a) and Semerdzieva et al. (1988) also reported that fruiting bodies of O. radicata were formed very well in the oak sawdust media mixed with 10% rice bran.
Now, we are trying to improve the yield and quality of O. mucida through a new innovation experimental method by changing substrates of the media. Then, we are able to produce commercially feasible basidiocarps of O. mucida in a few years. After all, this is the first report associated with fruiting body production of O. mucida in Korea.
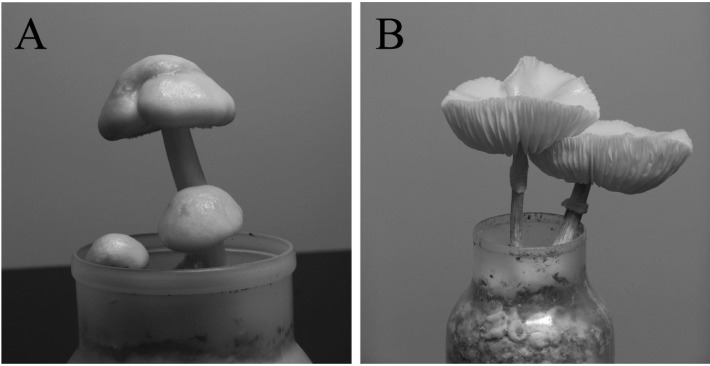
Fig. 2.
The artificial fruiting bodies of Oudemansiella mucida (IUM 929) produced in the oak sawdust medium supplemented with rice bran (A, young; B, old).
Acknowledgement
This work was supported by research grant (NO. 2040393) from Agriculture R & D Promotion Center (ARPC) in the Ministry of Agriculture and Forestry.
References
- 1.Anke T, Hecht HJ, Schramm G, Steglieh W. Antibiotics from basidiomycetes IX. Oudemansin an antifungal antibiotic from Oudemansiella mucida (Schrader ex Fr.) Hochnel (Agaricales) J Antibiot (Tokyo) 1979;32:1112–1117. doi: 10.7164/antibiotics.32.1112. [DOI] [PubMed] [Google Scholar]
- 2.Anke T, Besl H, Mocek U, Steglich W. Antibiotics from basidiomycetes XVIII. Strobilurin C and oudemansin B, two new antifungal metabolites from Xerula species (Agaricales) J Antibiot (Tokyo) 1983;36:661–666. doi: 10.7164/antibiotics.36.661. [DOI] [PubMed] [Google Scholar]
- 3.Anke T, Werle A, Bros M, Steglich W. Antibiotics from basidiomycetes XXXIII. Oudemansin X. A new antifungal F-β-methoxyacrylate from Oudemansiella radicata (Relhan ex Fr.) Sing. J Antibiot (Tokyo) 1990;8:1010–1011. doi: 10.7164/antibiotics.43.1010. [DOI] [PubMed] [Google Scholar]
- 4.Deacon J. Fungal Biology. New York, USA: Black Well Publishing Company; 2006. [Google Scholar]
- 5.Jaysinghe C, Lee GW, Imtiaz OO, Lee MW, Shim MJ, Lee UY, Lee TS. The optimal culture conditions for the mycelial growth of Oudemansiella mucida. Bulletin of Bioresource and Environment. 2007;1:10–14. [Google Scholar]
- 6.Lee JY. Coloured Korean mushrooms. Seoul, Korea: Academy Press; 1988. [Google Scholar]
- 7.Park WH, Lee HD. Illustrated Book of Korean Medicinal Mushrooms. Seoul, Korea: Kyo-Hak Publishing Co.; 1999. [Google Scholar]
- 8.Rew YH, Cho WS, Lee JM, Kim JK. Cultural characteristics of Phellinus baumii grown in bottle. Korean J Mycol. 2004;32:101–104. [Google Scholar]
- 9.Semerdzieva M, Buchalo AS, Hobsch P, Zakordonec OA, Wasser SP, Musilek V. Comparative study of cultures of four species of the genus Oudemansiella. Folia Microbiol. 1988;33:115–120. [Google Scholar]
- 10.Shim JO, Chang KJ, Kim TH, Lee YS, Lee UY, Lee TS, Lee MW. The fruiting body formation of Oudemansiella radicata in the saw dust oak (Quercus variabilis) mixed with rice bran. Mycobiology. 2006;34:30–33. doi: 10.4489/MYCO.2006.34.1.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shim JO, Chang KJ, Lee YS, Park CH, Kim HY, Lee UY, Lee TS, Lee MW. The fruiting body formation of Armillaria mellea on oak dust medium covered with raw carrots. Mycobiology. 2006;34:206–208. doi: 10.4489/MYCO.2006.34.4.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ying JZ, Mao XL, Ma QM, Zong YC, Wen HA. Icons of medicinal fungi from China. Bejing, China: Science Press; 1987. [Google Scholar]