Abstract
In order to breed a Cordyceps bassiana isolate that stably forms fruiting body in artificial cultivation, isolates derived from subculturing and single spores were tested through mating. From C. bassiana EFCC 783, three subcultured isolates EFCC 2830, EFCC 2831 and EFCC 2832 were obtained and fourteen single conidial isolates were obtained from these three subcultured isolates. Two different morphological types were found in the fourteen single conidial isolates. One type was able to form synnemata and another type was not able to form synnemata. Since switch of morphological type was not observed despite their continuous subculturing, cross was performed between the two types and the formation of fruiting body was examined. Ascospores were obtained from a selected fruiting body formed by hybrid of the cross. Self-cross and combinational cross of the ascospore-derived isolates generated hybrids that stably produce high quality fruiting body in artificial media.
Keywords: Cordyceps bassiana, Mating type, Perithecial fruiting bodies, Single conidial isolates, Synnemata
Cordyceps bassiana was first reported by Li et al. (2001). This species grows on dead Lepidopteran larva under soil or in decayed wood, and it is known to be rare in Korea. Beauveria (Bals.) Vuill. (Hyphomycetes), an imperfect fungus, is presumed to be related to the genus Cordyceps (Clavicipitaceae, Hypocreales, Ascomycota) with respect to its morphological and physiological characters (Humber, 2000). Teleomorph of Beauveria brongniartii (Sacc.) Petch was described as Cordyceps brongniartii by Shimazu et al. (1988). Similarly, teleomorphic stage of another ubiquitous Beauveria sp., B. bassiana (Bals.) Vuill., was described as Cordyceps bassiana from China on a carpenterworm larva (Li et al., 2001). Beauveria bassiana is a nearly ubiquitous fungal pathogen of insects (Humber, 2000). The production of fruiting body from each Beauveria bassiana isolate is rare in natural condition and thus it is very difficult to obtain fruiting bodies through artificial cultivation.
For sustainable fruiting body formation, multispore isolates have a lot of problems. Shrestha et al. (2002) has reported that multi-spore isolates of C. militaris have a lot of different sectors so they do not produce regular yields. Also in vitro fruiting body from multi-spore isolates of C. militaris tend to vary among isolates and their subcultures under the same environmental conditions, showing the clear effects of genetic factors on fruiting (Sung, 1996). These facts are also present in C. bassiana. In C. bassiana, perithecial and non-perithecial as well as normal and abnormal shaped stromata are regularly formed in vitro condition.
Generally, fungal mating system in Ascomycota has been well studied. Edgerton (1914) first showed plus and minus strains in an Ascomycetous genus, Glomerella. Later, Dodge (1920) showed bipolar mating type of heterothallism in Ascobolus magnificus, another ascomycete fungus and expressed the view that ascogonia and antheridia arise from different hyphal branches of the same strain. Many species of Clavicipitaceous genera have been found to be bipolar heterothallic. In entomopathogenic fungus C. militaris, mating type of single isolates to produce artificial fruiting body have been well reported (Shrestha, 2003; Shrestha et al., 2004).
In this study, sequential selection of single conidial isolates and ascospore isolates were performed through self-cross and combinational-cross between the spore isolates to generate good fruiting body forming strain and to determine mating type of Cordyceps bassiana.
Materials and Methods
Fungal isolates and cultures
Subcultures EFCC 2830, EFCC 2831, EFCC 2832 were generated from EFCC 783 preserved in Entomopathogenic Fungal Culture Collection (EFCC), Kangwon National University, Korea. The subcultures were precultured on SDAY agar plate and transferred in SDAY broth. After growing at 25℃ for 3 days, they were diluted in sterilized 1.8 ml Eppendorf tubes from 10-1 to 10-10. The diluted suspension of each subculture were streaked on water agar plates and grown for two days. Fourteen single conidial isolates were obtained from the water agar plate-grown cultures using a dissecting microscope. They were labeled as EFCC 2830-1, EFCC 2830-2, EFCC 2830-3, EFCC 2830-4 (from EFCC 2830), EFCC 2831-1, EFCC 2831-2, EFCC 2831-3, EFCC 2831-4, EFCC 2831-5 (from EFCC 2831), EFCC 2832-1, EFCC 2832-2, EFCC 2832-3, EFCC 2832-4, EFCC 2832-5 (from EFCC 2832).
Ascospore isolates
For the isolation of single ascospores, ascospore discharge method was used. When fruiting body was attached to inside part of Petri dish lid, its ascospores dropped on water agar plate. Single ascospores on the surface of water agar plate were picked up using a dissecting microscope and transferred to SDAY agar plates (Table 1). The medium for synnemata and fruiting formation was prepared by mixing 60 g of brown rice, 10 g of silkworm pupae and 65 ml of water in 1000 ml polypropylene bottle and by autoclaving for 20 min at 121℃ under 15 psi. Mycelial suspensions derived from single spore isolate was prepared by growing in SDAY broth for 3 days at 25℃ and used as an inoculum. After inoculation, the medium for fruiting formation was incubated at 20℃, under 1000 lux light and 70% humidity for 55 days.
Table 1.
Fruiting body formation by self- and combinational-mating of single spore isolates of Cordyceps bassiana EFCC2830 (top), EFCC2831 (middle), and EFCC2832 (bottom)

○, fruiting body formation; ×, no fruiting body formation; ▵, moderate fruiting body formation.
Mating experiment
Single ascospore isolates derived from combination of EFCC isolates were used to observe the nature of fruiting body formed. For genetic crosses, mycelial suspensions of two isolates were inoculated together on the fruiting body formation medium and incubated in favorable conditions. For the determination of mating type, in vitro fruiting body from single isolates as well as from all combinations of crosses were observed for perithecium formation on stromata.
Results and Discussion
Mating characteristics of single conidial isolates
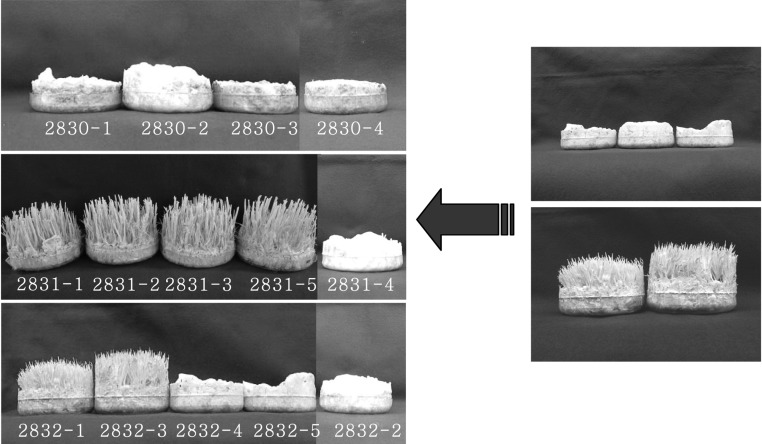
The 14 single conidial isolates from subcultures EFCC 2830, EFCC 2831, EFCC 2832 showed two different morphological types. One type showed only white mycelial growth and another types showed yellow synnemata formation (Fig. 1). Mating through self and combinations of single conidial isolates showed both characteristics in brown rice medium (Table 1). Mating between EFCC2830-derived single spore isolates were not able to form fruiting body. While mating between EFCC 2831-derived single spore isolates and between EFCC 2832-derived single spore isolates resulted in fruiting body formation (Table 1).
Fig. 1.
Two morphological features shown by 14 single conidial isolates from Cordyceps bassiana subcultures EFCC2830, EFCC 2831 and EFCC 2832. Right figure shows fruiting body formation type (bottom) and no fruiting body formation type (top).
Mating characteristics of single ascospore isolates
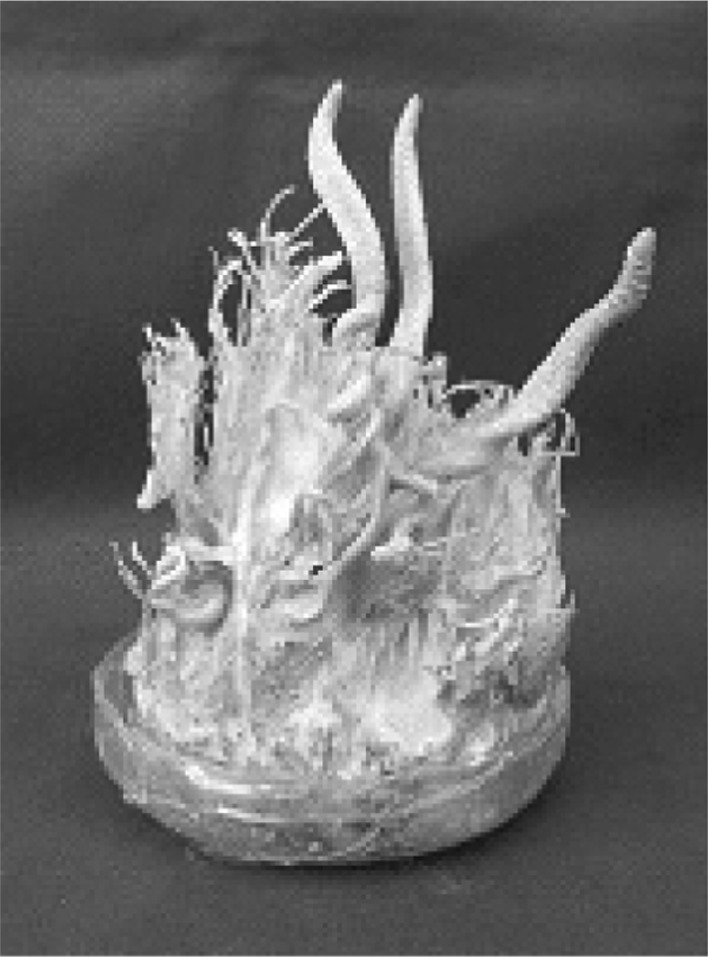
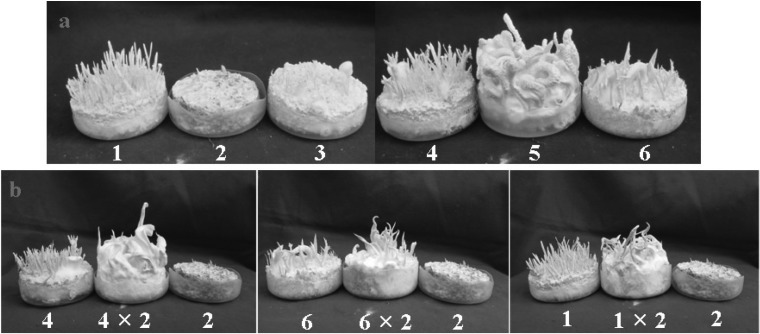
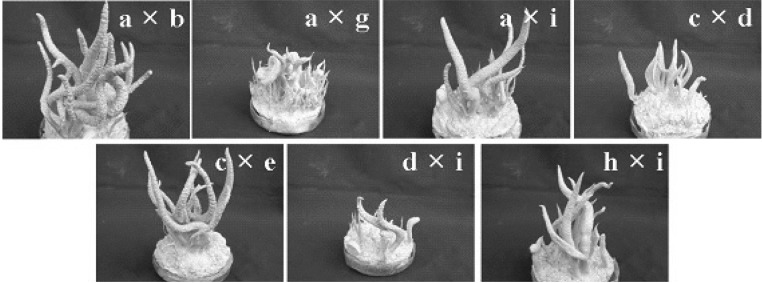
Based on the results of Table 1, the hybrid from cross EFCC2832-3 with EFCC2832-3 (Fig. 2) was chosen for further study. Six ascospores obtained from the fruiting body formed by the hybrid were named as EFCC2832-3-1 to EFCC2832-3-6. Cross of these six ascospore isolates through self- and combinational-mating revealed that fruiting body can be produced only cross between EFCC 2832-3-1 and EFCC 2832-3-2, between EFCC 2832-3-2 and EFCC 2832-3-4, between EFCC 2832-3-2 and EFCC 2832-3-6 (Fig. 3) and between EFCC 2832-3-5 and EFCC 2832-3-5. Among these cross tests, good fruiting body formation and mycelial growth was found in cross between EFCC 2832-3-5 and EFCC 2832-3-5. Thus hybrid from EFCC 2832-3-5xEFCC 2832-3-5 was named as EFCC 12511. Nine ascospores were also obtained from self-cross of EFCC 12511 and named as EFCC 12511-a to EFCC 12511-i. Cross was performed among these 9 ascospore isolates with combination and the results is given at Table 2. Seven different combinations of cross showed the formation of fruiting body. Their formation of fruiting body is shown in Fig. 4. Cross EFCC 12511-a with 12511-b produced better fruiting body than other cross combinations. Thus we further selected this cross-hybrid and from which ten ascospores were obtained and named as EFCC 12511-a × b-1 to EFCC 12511-a × b-10. Table 3 is combinational cross results of these ten ascospore isolates. 13 combinations produced perithecial stromata when inoculated together (Fig. 5). But all the 10 isolates produced various types of non-perithecial stromata, when inoculated alone. It could be observed that two isolates of a combination produced perithecial stromata when they were of opposite mating types and no stromata or nonperithecial stromata formed if two isolates were of same mating type. Although their mating type was not perfectly known, these isolates followed general mating rule of ascomycetes.
Fig. 2.
Fruiting body of Cordyceps bassiana formed by self-cross of EFCC 2832-3 single conidial isolates.
Fig. 3.
Fruiting body formation by single ascospore isolates from self-cross of Cordyceps bassiana EFCC 2832-3. Numbers (1-6) indicate single ascospore isolates (from 2832-3-1 to 2832-3-6). Top (a), mycelila growth of the six single ascospore isolates on fruiting body formation media. Bottom (b), fruiting body formation by cross of two different single ascospore isolates.
Table 2.
Fruiting body formation by cross among 9 single conidial isolates of Cordyceps bassiana EFCC 12511

+, fruiting body formation; -, no fruiting body formation.
Fig. 4.
Fruiting body formation by single ascospore isolates from self-cross of Cordyceps bassiana EFCC 12511. Alphabet (a, b ,c, d, e, f, g, h, i) indicates single ascospore isolates (from 12511-a to 12511-i). Example of fruiting body formed by cross of two different single ascospore isolates.
Table 3.
Fruiting body formation by cross among 10 single ascospore isolates of Cordyceps bassiana EFCC 12511a × b

+, fruiting body formation; -, no fruiting body formation.
Fig. 5.
Fruiting body formed by cross of single ascospore isolates of Cordyceps bassiana EFCC 12511-a × b. Numbers (1-6) indicate single ascospore isolates (from EFCC 12511-a × b-1 to EFCC 12511-a × b-10).
For sustainable artificial fruiting body formation, mating type is very important (Shrestha et al., 2004). As the multispore isolates have a lot of sectors, it does not bring regular yield (Shrestha et al., 2002). Thus it is need to determine mating type using single spore isolates. In this work we tested the approach using single conidium and ascospore isolates. Through serial selection and repeated cross we could generate isolates that can produce fruiting body stably in artificial media. In this way we potentially determined the two opposite mating types. With the finding of mating type we expect that C. bassiana can be better cultivated by breeding.
Acknowledgement
The authors wish to acknowledge the financial support from Ministry of Agricultural Forestry and ARPC to Entomopathogenic Fungal Culture Collection (EFCC), Kangwon National University. And also to acknowledge to provide Cordyceps Research Institute for facilities to carry out this study.
References
- 1.Dodge BO. The life history of Ascobolus magnificus: Origin of ascocarp from two strains. Mycologia. 1920;12:115–134. [Google Scholar]
- 2.Edgerton CW. Plus and minus strains in the genus Glomerella. Am J Bot. 1914;1:244–254. [Google Scholar]
- 3.Humber RA. Fungal pathogens and parasites of insects. In: Priest FG, Goodfellow M, editors. Applied Microbial Systematics. Dordrecht: Kluwer Acadenic Publishers; 2000. pp. 203–230. [Google Scholar]
- 4.Kobayasi Y. The genus Cordyceps and its allies. Sci Rept Tokyo Bunrika Daigaku Sect B. 1941;5:53–260. [Google Scholar]
- 5.Kobayasi Y, Shimizu D. Cordyceps species from Japan, 6. Bull Nat Sci Mus. 1983;9:15. [Google Scholar]
- 6.Li Z, Li C, Huang B, Fan M. Discovery and demonstration of the teleomorph of Beauveria bassiana (Bals.) Vuill, an important entomogenous fungus. Chiness Science Bulletin. 2001;46(9):751–753. [Google Scholar]
- 7.Shimazu M, Mitsuhasi W, Hashinoto H. Cordyceps brongniarii sp. nov., the teleomorph of Beauveria brongniarii. Trans Mycol Soc Jpn. 1988;29:323–330. [Google Scholar]
- 8.Shrestha B, Nam IS, Kim HK, Sung JM. Effect of sector of isolates on fruiting of Cordyceps militaris. The Korean Society of Mycology News Letter. 2002;14:98. [Google Scholar]
- 9.Shrestha B. Growth characteristics of somatic mycelium and mating system of Cordyceps militaris (L. ex Fr.) Link. Chucheon, Korea: Kangwon National University; 2003. Doctoral Thesis. [Google Scholar]
- 10.Shrestha B, Kim HK, Sung GH, Spatafora JW, Sung JM. Bipolar heterothallism, a principal mating system of Cordyceps militaris in vitro. Biothechnology and Bioprocess Engineering. 2004;9:440–446. [Google Scholar]
- 11.Sung JM. Insect-borne fungi of Korea. Seoul: Kyo-Hak Publishing Co., Ltd; 1996. [Google Scholar]