Abstract
Objectives
To develop 3 computer simulation models to determine the potential economic effect of using intravenous (IV) antiviral agents to treat hospitalized patients with influenza-like illness, as well as different testing and treatment strategies.
Study Design
Stochastic decision analytic computer simulation model.
Methods
During the 2009 influenza A(H1N1) pandemic, the Food and Drug Administration granted emergency use authorization of IV neuraminidase inhibitors for hospitalized patients with influenza, creating a need for rapid decision analyses to help guide use. We compared the economic value from the societal and third-party payer perspectives of the following 4 strategies for a patient hospitalized with influenza-like illness and unable to take oral antiviral agents: Strategy 1: Administration of IV antiviral agents without polymerase chain reaction influenza testing. Strategy 2: Initiation of IV antiviral treatment, followed by polymerase chain reaction testing to determine whether the treatment should be continued. Strategy 3: Performance of polymerase chain reaction testing, followed by initiation of IV antiviral treatment if the test results are positive. Strategy 4: Administration of no IV antiviral agents. Sensitivity analyses varied the probability of having influenza (baseline, 10%; range, 10%–30%), IV antiviral efficacy (baseline, oral oseltamivir phosphate; range, 25%–75%), IV antiviral daily cost (range, $20–$1000), IV antiviral reduction of illness duration (baseline, 1 day; range, 1–2 days), and ventilated vs nonventilated status of the patient.
Results
When the cost of IV antiviral agents was no more than $500 per day, the incremental cost-effectiveness ratio for most of the IV antiviral treatment strategies was less than $10,000 per quality-adjusted life-year compared with no treatment. When the cost was no more than $100 per day, all 3 IV antiviral strategies were even more cost-effective. The order of cost-effectiveness from most to least was strategies 3, 1, and 2. The findings were robust to changing risk of influenza, influenza mortality, IV antiviral efficacy, IV antiviral daily cost, IV antiviral reduction of illness duration, and ventilated vs nonventilated status of the patient for both societal and third-party payer perspectives.
Conclusion
Our study supports the use of IV antiviral treatment for hospitalized patients with influenza-like illness.
The 2009 influenza A(H1N1) pandemic raised questions about the role of intravenous (IV) antiviral medications in treating hospitalized (ventilated and nonventilated) patients with influenza-like illness (ILI). Intravenous neuraminidase inhibitors had been under rapid development, with IV peramivir receiving emergency use authorization (EUA) from the Food and Drug Administration (FDA) in October 2009, after the US Secretary of Health and Human Services declared a public health emergency.1–3 The primary motivation for EUA was a lack of alternative drugs for hospitalized patients with influenza A(H1N1) who were unable to take oral or inhaled antiviral agents. Standard FDA approval was not a viable option because efficacy and safety data were limited and the pandemic was already in full bloom. The EUA allowed healthcare providers to administer IV peramivir to patients hospitalized with confirmed or suspected influenza A(H1N1) only if they were unresponsive to or unable to take oral or inhaled antiviral agents.1,3 Contraindications included a history of severe allergic reaction to neuraminidase inhibitors.3
Antiviral medications are the only medications available to reduce the morbidity and mortality of individuals infected with influenza. Neuraminidase inhibitors are an important and widely used class of antiviral agents, the most commonly used being oral oseltamivir phosphate and inhaled zanamivir (both approved by the FDA in 1999).4 In influenza A and influenza B, an enzyme cleaves links between the infected host cell and the influenza virus envelope. This, in turn, allows the viruses that replicated in the host cell to be released to the rest of the body.5 By inhibiting viral replication and thereby limiting the number of viruses in the body, neuraminidase inhibitors could reduce the duration of illness and risk of mortality.6
Because IV peramivir was a novel drug, there were no available clinical trials among higher-risk groups such as pregnant women, pediatric patients, and older adults. It was also unclear how viral resistance to other neuraminidase inhibitors may translate to resistance to peramivir.6
Intravenous antiviral agents such as peramivir have several potential advantages. First, they offer an alternative route of administration, which is especially important for patients who cannot take medication by mouth (such as ventilated patients). Second, when heavy demand may deplete inventories of other antiviral agents such as oseltamivir and zanamivir, IV antiviral agents can serve as another available option. Third, there remains the possibility that strains resistant to other antiviral agents may not be completely resistant to newer antiviral agents such as peramivir, although evidence suggests that oseltamivir-resistant strains may also be resistant to peramivir.7
Intravenous antiviral agents have only recently emerged as potential treatment options, and questions remain about their economic value. Should they be reserved for intensive care unit patients or administered to all hospitalized patients with influenza who cannot take oral antiviral agents? What is a reasonable price for IV antiviral medications? How would the value of IV antiviral agents change with emerging resistance? Should patients be tested for influenza before the initiation of IV antiviral agents, or should IV antiviral treatment be initiated first, followed by confirmatory testing to determine whether treatment should be continued? Will the value be different for seasonal vs pandemic influenza?
We developed 3 computer simulation models to estimate the potential economic effect of using IV antiviral agents to treat hospitalized patients with ILI, as well as different testing and treatment strategies. Various simulation runs explored seasonal and pandemic influenza scenarios and evaluated the effects of varying patient age, probability of having influenza, ventilated vs nonventilated status of the patient, and the probability of different influenza outcomes such as mortality.
METHODS
Structure of the Model
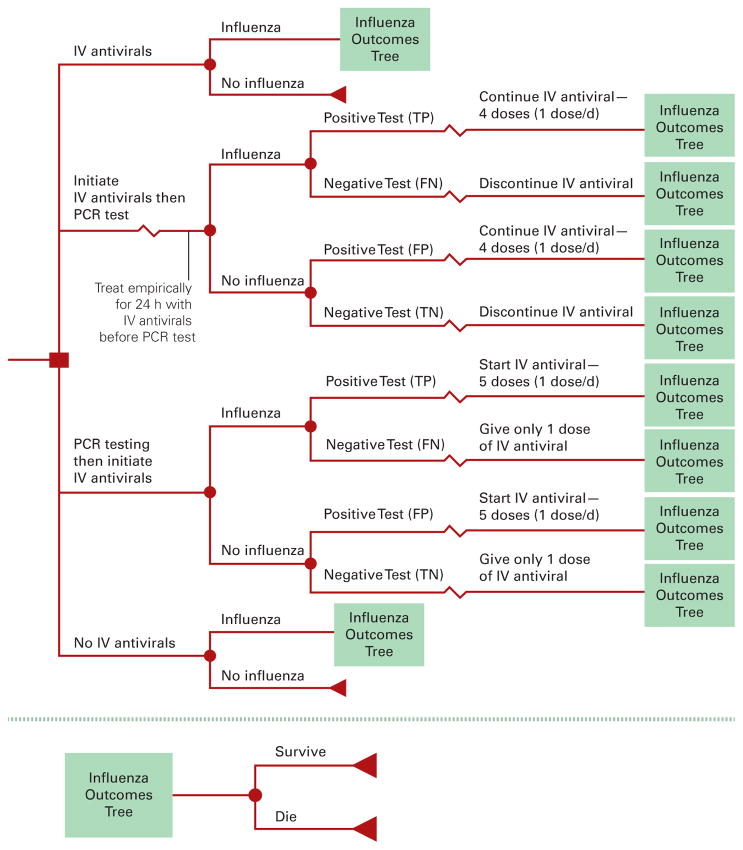
The Figure shows the general structure of our Monte Carlo decision analytic computer simulation model, constructed using commercially available software (TreeAge Pro 2009; TreeAge Software, Williamstown, Massachusetts). Our model represented the economic value from the societal and third-party payer perspectives of the following 4 alternative strategies for a patient hospitalized with ILI and unable to take oral antiviral agents, as per the EUA1–3:
Figure. Decision Tree Model.
IV indicates intravenous; PCR, polymerase chain reaction; TP, true positive; FN, false negative; FP, false positive; TN, true negative.
Strategy 1: Administration of IV Antiviral Agents Without Polymerase Chain Reaction (PCR) Influenza Testing
In this strategy, the patient received a full 5-day course of IV antiviral agents regardless of whether the patient actually had influenza. No PCR influenza testing was performed.
Strategy 2: Initiation of IV Antiviral Treatment, Followed by PCR Testing to Determine Whether the Treatment Should Be Continued
This strategy involved initiation of IV antiviral treatment for hospitalized patients with ILI and then performance of PCR testing for influenza, which required a 24-hour turnaround time for results. A negative test result prompted discontinuation of IV antiviral treatment after a single dose.
Strategy 3: Performance of PCR Testing, Followed by Initiation of IV Antiviral Treatment If the Test Results Were Positive
For this strategy, IV antiviral agents would not be initiated until PCR testing was performed and results were available, delaying treatment for 24 hours. A positive test result prompted initiation of IV antiviral treatment.
Strategy 4: Administration of No IV Antiviral Agents
This strategy involved giving no medical interventions as the treatment.
Each simulated adult traveled through the decision tree pictured in the Figure and faced a probability draw (first-order trial) at each chance node. This draw was then compared with a value pulled from the probability parameter distribution to determine down which branch he or she traveled (second-order trials). The costs, utilities, and durations of each resulting outcome also drew from their respective probability distributions (second-order trials). Each patient had a probability of having influenza (baseline, 10%).8,9 Test results depended on whether the patient actually had influenza, and the sensitivity and specificity of the test. Intravenous antiviral treatment had a probability (IV antiviral efficacy) of reducing the duration of illness by 1 day (ie, if the dice roll is less than the efficacy number, then the illness duration is reduced; if it is higher, then there is no effect) and the risk of mortality (ie, mortality is reduced by 1 minus IV antiviral efficacy).
Scenarios from the third-party payer perspective considered only the direct costs of illness, while scenarios from the societal perspective included both direct and indirect costs of illness (ie, productivity losses from caregiver time determined by lost wages from time spent with the patient). Each simulation run sent 1000 simulated adults 1000 times through each model, for a total of 1,000,000 trials per scenario. During each run, each parameter drew from the relevant triangular or beta distributions. Table 1 lists the study sources of data inputs for the model.8,10–24
Table 1.
Data Inputs for Model Variables
| Description | Mean | 95% Range | Source | |
|---|---|---|---|---|
| Lower Limit | Upper Limit | |||
| Costs, US $ | ||||
| IV antiviral peramivira | 20 | — | — | PDR Red Book10 |
| Hospitalization per day | ||||
| 18–44 y | 1822 | 1037 | 2607 | AHRQ8 |
| 45–64 y | 1465 | 110 | 2820 | AHRQ8 |
| 65–84 y | 1333 | 805 | 1861 | AHRQ8 |
| Ventilation per day | ||||
| 18–44 y | 2397 | 1605 | 3189 | AHRQ8 |
| 45–64 y | 2194 | 1611 | 2777 | AHRQ8 |
| 65–84 y | 2066 | 1545 | 2587 | AHRQ8 |
| Death in hospital | 6921 | 5191 | 9025 | AHRQ8; Gould et al11 |
| Median hourly wageb | 15.57 | 8.59 | 42.15 | Bureau of Labor Statistics12 |
| PCR test | 10 | 7.37 | 29 | Syrmis et al13; Chidlow et al14 |
| Durations | ||||
| Hospitalized, dc | ||||
| 18–44 y | 2 | 1.8 | 2.2 | AHRQ8 |
| 45–64 y | 3 | 2.7 | 3.3 | AHRQ8 |
| 65–84 y | 4 | 3.8 | 4.2 | AHRQ8 |
| Ventilated, dd | 12 | 6 | 20 | Kumar et al15 |
| Hourly wage, US $ | 8 | — | Assumption | |
| Utilities | ||||
| 1 y Of adult life, QALY | ||||
| 20–64 y | 0.92 | — | — | Gold et al16 |
| 65–70 y | 0.84 | — | — | Gold et al16 |
| Utility | ||||
| Influenza with hospitalization, QALY | 0.5 | 0.38 | 0.63 | Sackett and Torrance17; Tengs and Wallace18 |
| Probabilities | ||||
| Clinical outcomes without vaccination | ||||
| Mortality given influenza with hospitalization | ||||
| 18–44 y | 0.0099 | 0.0086 | 0.0112 | AHRQ8 |
| 45–64 y | 0.0099 | 0.0086 | 0.0112 | AHRQ8 |
| 65–84 y | 0.0144 | 0.0117 | 0.0171 | AHRQ8 |
| Mortality given influenza with ventilation | 0.45 | 0.33 | 0.52 | Jain et al19; Rello et al20; Li et al21 |
| Parents’ income with children <18 y old | Bureau of Labor Statistics22,23 | |||
| Single income | 0.1370 | 0.0374 | 0.2366 | |
| Dual income | 0.4816 | 0.4803 | 0.4832 | |
| PCR test | ||||
| Sensitivity | 0.963 | 0.815 | 0.988 | Chidlow et al14 |
| Specificity | 0.996 | 0.989 | 1 | Chidlow et al14 |
| IV antiviral efficacye | 0.78 | 0 | 0.98 | Jefferson et al24 |
AHRQ indicates Agency for Healthcare Research and Quality; IV, intravenous; PCR, polymerase chain reaction; QALY, quality-adjusted life-year.
Sensitivity analyses on daily cost of IV antiviral agents (baseline oseltamivir phosphate, $20, $100, $500, and $1000).
Eightieth percentile wages from the Bureau of Labor Statistics.12
Nonventilated population.
Ventilated population.
Baseline IV antiviral based on oral oseltamivir, symptom reduction, and hospital stay for 1 d.
The following equation calculated the incremental cost-effectiveness ratio (ICER) of each strategy vs the comparator strategy: Coststrategy x − Coststrategy y = Effectivenessstrategy x − Effectivenessstrategy y, where x represents the strategy and y represents the comparator strategy.
Each simulation run generated a mean ICER and a 95% confidence interval. A strategy was considered cost-effective if the ICER was less than $50,000 per quality-adjusted life-year (QALY).16 However, because there is some debate about the exact threshold for cost-effectiveness, we report the resulting ICER values so that individual readers may make their own determination of what constitutes a cost-effective intervention.
Data Inputs
The study used various inputs and their corresponding distributions for the probabilities, costs, durations, and utilities in the model. Hospital mortality from influenza drew from a beta distribution. All other variables drew from triangular distributions. A 3% discount rate converted all past and future costs to 2009 values.25
Because PCR testing is unavailable and is not part of standard care in many inpatient settings, our model did not include PCR test costs for the IV antiviral and no IV antiviral strategies.26 The hospitalization costs for these arms did not include PCR testing. The PCR test costs were added to the 2 strategies that included PCR testing.
Effectiveness was measured in QALYs. Influenza caused QALY decrements for the duration of illness.17,18 All QALY accruals were age adjusted based on the quality-of-life utility obtained by Gold et al.16 Patients who survived accrued age-adjusted and discounted (3% discount rate) QALYs based on life-expectancy estimates from the Human Mortality Database; patients who did not survive did not accrue these QALYs.16,27
Sensitivity Analysis
Probabilistic (Monte Carlo) sensitivity analyses explored all parameters simultaneously using all distributions examined. Sensitivity analysis varied these following key variables: the probability of having influenza (baseline, 10%; range, 10%–30%),9 IV antiviral efficacy (baseline, oral oseltamivir; range, 25%–75%), IV antiviral daily cost (baseline, $20 [oral oseltamivir]; range, $20–$1000), IV antiviral reduction of illness duration (baseline, 1 day; range, 1–2 days), patient age (baseline, 20 years; range, 20–60 years), and influenza mortality (baseline, seasonal influenza mortality; range, up to twice the seasonal influenza mortality for a pandemic scenario). These sensitivity analyses involved fixing the variable of interest and then allowing the rest to pull from the distributions examined. We evaluated seasonal and pandemic influenza scenarios, as well as IV antiviral treatment in ventilated and nonventilated patients.
RESULTS
Table 2 and Table 3 give the results at baseline, while varying IV antiviral efficacy (25%–75%) (ie, the proportion by which IV antiviral agents will reduce mortality) and IV antiviral daily cost from the third-party payer perspective for the seasonal and pandemic scenarios. Each table gives the ICER (compared with the most economically favorable option) for each strategy. Calculated ICER 95% confidence intervals demonstrated no overlaps, suggesting that the differences were significant.
Table 2.
ICERs of Different Intravenous Antiviral Strategies in the Ventilated Population From the Third-Party Payer Perspectivea
| Variable | IV Antiviral Efficacy in Reducing Hospital Stay by 1 d, ICER | ||
|---|---|---|---|
| 25% | 50% | 75% | |
| Seasonal Influenza Scenario | |||
| IV antiviral cost of $100 per day | |||
| PCR testing, then start IV antiviral agents | — | — | — |
| Start IV antiviral agents, then PCR testing | 39,674 | 39,674 | 39,674 |
| Give no IV antiviral agent | Dominated by start IV antiviral agents, then PCR testing | Dominated by start IV antiviral agents, then PCR testing | Dominated by start IV antiviral agents, then PCR testing |
| Give IV antiviral agents | 69 | 67 | 67 |
| IV antiviral cost of $500 per day | |||
| PCR testing, then start IV antiviral agents | 41 | — | — |
| Give no IV antiviral agent | — | Dominated by PCR testing, then start IV antiviral agents | Dominated by PCR testing, then start IV antiviral agents |
| Start IV antiviral agents, then PCR testing | 198,370 | 198,370 | 198,370 |
| Give IV antiviral agents | 360 | 366 | 369 |
| IV antiviral cost of $1000 per day | |||
| Give no IV antiviral agent | — | — | — |
| PCR testing, then start IV antiviral agents | 331 | 154 | 53 |
| Start IV antiviral agents, then PCR testing | 396,739 | 396,739 | 396,739 |
| Give IV antiviral agents | 738 | 740 | 740 |
| Pandemic Influenza Scenario With Twice the Seasonal Influenza Mortality | |||
| IV antiviral cost of $100 per day | |||
| PCR testing, then start IV antiviral agents | — | — | — |
| Start IV antiviral agents, then PCR testing | 39,674 | 39,674 | 39,674 |
| Give no IV antiviral agent | Dominated by start IV antiviral agents, then PCR testing | Dominated by start IV antiviral agents, then PCR testing | Dominated by start IV antiviral agents, then PCR testing |
| Give IV antiviral agents | 68 | 68 | 68 |
| IV antiviral cost of $500 per day | |||
| PCR testing, then start IV antiviral agents | 32 | — | — |
| Give no IV antiviral agent | — | Dominated by PCR testing, then start IV antiviral agents | Dominated by PCR testing, then start IV antiviral agents |
| Start IV antiviral agents, then PCR testing | 198,370 | 198,370 | 198,370 |
| Give IV antiviral agents | 363 | 366 | 363 |
| IV antiviral cost of $1000 per day | |||
| Give no IV antiviral agent | — | — | Dominated by PCR testing, then start IV antiviral agents |
| PCR testing, then start IV antiviral agents | 283 | 90 | — |
| Start IV antiviral agents, then PCR testing | 396,739 | 396,739 | 396,739 |
| Give IV antiviral agents | 739 | 736 | 736 |
ICER indicates incremental cost-effectiveness ratio (in US $ per QALY); IV, intravenous; PCR, polymerase chain reaction; QALY, quality-adjusted life-year.
In a patient aged 20 years (influenza prevalence, 10%) given 1 dose per day for a 5-day treatment course. Dash mark (—) indicates the best strategy compared with all other strategies.
Table 3.
ICERs of Different Intravenous Antiviral Strategies in the Nonventilated Population From the Third-Party Payer Perspectivea
| Variable | IV Antiviral Efficacy in Reducing Hospital Stay by 1 d, ICER | ||
|---|---|---|---|
| 25% | 50% | 75% | |
| Seasonal Influenza Scenario | |||
| IV antiviral cost of $100 per day | |||
| PCR testing, then start IV antiviral agents | — | — | — |
| Start IV antiviral agents, then PCR testing | 39,674 | 39,674 | 39,674 |
| Give no IV antiviral agent | Dominated by start IV antiviral agents, then PCR testing | Dominated by start IV antiviral agents, then PCR testing | Dominated by start IV antiviral agents, then PCR testing |
| Give IV antiviral agents | 802 | 800 | 790 |
| IV antiviral cost of $500 per day | |||
| Give no IV antiviral agent | — | — | — |
| PCR testing, then start IV antiviral agents | 1733 | 1484 | 1301 |
| Start IV antiviral agents, then PCR testing | 198,370 | 198,370 | 198,370 |
| Give IV antiviral agents | 4266 | 4201 | 4236 |
| IV antiviral cost of $1000 per day | |||
| Give no IV antiviral agent | — | — | — |
| PCR testing, then start IV antiviral agents | 6438 | 5473 | 5176 |
| Start IV antiviral agents, then PCR testing | 396,739 | 396,739 | 396,739 |
| Give IV antiviral agents | 8666 | 8596 | 8536 |
| Pandemic Influenza Scenario With Twice the Seasonal Influenza Mortality | |||
| IV antiviral cost of $100 per day | |||
| PCR testing, then start IV antiviral agents | — | — | — |
| Start IV antiviral agents, then PCR testing | 39,674 | 39,674 | 39,674 |
| Give no IV antiviral agent | Dominated by start IV antiviral agents, then PCR testing | Dominated by start IV antiviral agents, then PCR testing | Dominated by start IV antiviral agents, then PCR testing |
| Give IV antiviral agents | 789 | 794 | 797 |
| IV antiviral cost of $500 per day | |||
| Give no IV antiviral agent | — | — | — |
| PCR testing, then start IV antiviral agents | 1499 | 1171 | 972 |
| Start IV antiviral agents, then PCR testing | 198,370 | 198,370 | 198,370 |
| Give IV antiviral agents | 4310 | 4202 | 4290 |
| IV antiviral cost of $1000 per day | |||
| Give no IV antiviral agent | — | — | — |
| PCR testing, then start IV antiviral agents | 5554 | 4732 | 3876 |
| Start IV antiviral agents, then PCR testing | 396,739 | 396,739 | 396,739 |
| Give IV antiviral agents | 8506 | 8553 | 8571 |
ICER indicates incremental cost-effectiveness ratio (in US $ per QALY); IV, intravenous; PCR, polymerase chain reaction; QALY, quality-adjusted life-year.
In a patient aged 20 years (influenza prevalence, 10%) given 1 dose per day for a 5-day treatment course. Dash mark (—) indicates the best strategy compared with all other strategies.
Ventilated Population
For the ventilated population under seasonal influenza conditions, the ICER of the IV antiviral treatment vs no treatment was less than $10,000 per QALY in most scenarios, as long as the IV antiviral cost was no more than $500 per day, except for strategy 2 (initiation of IV antiviral treatment, followed by PCR testing to determine whether the treatment should be continued). In fact, when IV antiviral cost was no more than $100 per day, all 3 IV antiviral strategies were even more cost-effective (ie, provided cost and health benefits) than no treatment, with the order from most to least cost-effective being strategies 3 (performance of PCR testing, followed by initiation of IV antiviral treatment if the test results are positive), 1 (administration of IV antiviral agents without PCR influenza testing), and 2 (initiation of IV antiviral treatment, followed by PCR testing to determine whether the treatment should be continued). As the risk of influenza increased from 10%, the antiviral strategies grew even more economically favorable. Similarly, increasing the IV antiviral illness reduction effect to 2 days further enhanced the cost-effectiveness of all IV antiviral strategies.
These findings were robust to various sensitivity analyses. Pandemic conditions increased the economic value of only the IV antiviral strategies. The order of cost-effectiveness of the strategies did not change as long as IV antiviral treatment cost no more than $100 per day. Switching from the third-party payer perspective to the societal perspective captured additional cost savings for the IV antiviral strategies.
Nonventilated Population
Findings for the nonventilated population were similar to those for the ventilated population. Although the IV antiviral strategies were slightly less cost-effective for the comparatively healthier nonventilated population, the ICERs for most of the IV antiviral strategies fell far below $10,000 per QALY when the IV antiviral cost was no more than $100 per day. The results of sensitivity analyses for the nonventilated population were not substantially different from those for the ventilated population.
DISCUSSION
With the 2009 pandemic and concerns that the circulating influenza A(H1N1) virus might be more virulent than typical circulating seasonal influenza viruses, the US Secretary of Health and Human Services indicated that certain influenza A(H1N1) interventions might be approved for emergency use. On October 23, 2009, the FDA granted EUA for IV peramivir with the following restrictions1–3: (1) it was to be used only to treat patients hospitalized with the 2009 influenza A(H1N1) virus and (2) patients treated had to have laboratory-confirmed 2009 influenza A(H1N1) virus infection or an unidentifiable subtype of influenza A virus suspected to be influenza A(H1N1) virus and be (a) someone who was unresponsive to oral or inhaled antiviral therapy or (b) someone in whom drug delivery through enteral (eg, oseltamivir) or inhaled (eg, zanamivir) routes was not feasible.
Our study strongly supports the use of IV antiviral agents for hospitalized patients with severe influenza complications during influenza A(H1N1) pandemic and other influenza seasons. The results also suggest that initial use of PCR testing to guide IV antiviral treatment continuation may be more cost-effective. Our finding that this strategy is economically dominant is especially compelling because interventions that not only save lives, but also decrease costs are uncommon. Our study supports IV antiviral treatment in ventilated and nonventilated patients.
Determining the epidemiological and clinical characteristics of the influenza A(H1N1) pandemic was challenging. As the pandemic progressed, attack rate and mortality estimates changed on a weekly basis. Therefore, our goal was to use conservative initial values for our model to establish thresholds above which IV antiviral treatment would no longer be cost-effective. Because we determined that the IV antiviral agents and PCR strategy would be cost-effective and in most cases economically dominant, even at seasonal influenza parameters as long as the influenza A(H1N1) strain was at least as virulent as typical seasonal influenza strains, such a strategy is justified during a pandemic.
Peramivir has several advantages over other neuraminidase inhibitors. It was the only available IV neuraminidase inhibitor during the 2009 influenza A(H1N1) pandemic. With a reported efficacy similar to that of oral antiviral agents, peramivir provides an alternative drug route for patients who have an inability to take oral medications, poor gastrointestinal absorption, or any other respiratory illness that requires mechanical ventilation. Intravenous administration can also help ensure patient compliance with receiving the full course of antiviral agents (many patients may not complete the full course of oral or inhaled antiviral agents). Conversely, because IV peramivir has not undergone nearly as much testing as oseltamivir and zanamivir, efficacy and safety data are limited, rendering extensive cross-comparisons between peramivir and other antiviral agents difficult. Resistance to other antiviral medications may lead to resistance to peramivir and vice versa. For example, the FDA reported that some influenza viruses that are highly resistant to oseltamivir were also cross-resistant to peramivir via the H275 resistance gene.7 In another study,28 the resistance profile of zanamivir was better than that of peramivir.
Price is also an important issue when a new therapy reaches the market. Although these decisions involve several factors, our study helps benchmark what levels of pricing may be appropriate. The benefits of IV antiviral agents support a higher price point than that of oral antiviral agents. However, it is unclear how different pricing levels may affect adoption of use.
Our model aimed to underestimate the value of IV antiviral treatment. We did not quantify the potential benefit of decreased viral shedding with the use of IV antiviral agents. In addition, our pandemic scenarios represent an influenza pandemic with twice the mortality rate of seasonal influenza. Higher mortality rates would only strengthen the results of our study.
There are several limitations to our study. No computer model can fully represent every possible influenza event and outcome. By definition, models are simplifications of real life. Our study assumed that patients were admitted to the hospital primarily for ILI and did not have other major comorbidities that would contribute to mortality. However, the effect of comorbidities in real life can be variable and unexpected. Moreover, this may increase corresponding resource-use (eg, intubation and mechanical ventilation). Our model did not incorporate the possible adverse effects of IV antiviral medications. Moreover, our study did not consider the potential effects of IV antiviral medications on transmission and communicability (ie, IV antiviral medications may decrease the degree and duration of influenza virus shedding).
CONCLUSIONS
During the 2009 influenza A(H1N1) pandemic, the FDA granted EUA of IV neuraminidase inhibitors for hospitalized patients with influenza, creating a need for rapid economic studies to help guide their use. Our study supports the use of IV antiviral treatment for hospitalized patients (ventilated and nonventilated) with ILI. If available, initial PCR testing to help guide treatment may be an even more cost-effective strategy. The use of IV antiviral agents in this setting may save money and offer health benefits.
Take-Away Points.
During the 2009 influenza A(H1N1) pandemic, the Food and Drug Administration granted emergency use authorization of peramivir because of a dearth of alternative drugs for patients unable to take oral or inhaled antiviral agents and because of limited safety and efficacy data for peramivir. Our economic model suggests the following:
Intravenous antiviral agents are a cost-effective intervention for hospitalized patients with severe influenza-like illness.
The use of PCR influenza testing to guide whether to initiate intravenous antiviral treatment is the most cost-effective strategy.
The use of intravenous antiviral agents in this setting may save money and offer health benefits.
Our findings should help guide policy makers and clinicians in the use of such novel treatments during other influenza seasons.
Acknowledgments
Funding Source: This study was funded by grant 5U54GM088491-02 from the National Institute of General Medical Sciences Models of Infectious Disease Agent Study, 5R01LM009132-03 from the National Library of Medicine, and 5P01HK000086-02 from the Centers for Disease Control and Prevention. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Author Disclosures: The authors (BYL, JHYT, RRB, SMM, AEW, SMZ, KJS, RKZ) report no relationship or financial interest with any entity that would pose a conflict of interest with the subject matter of this article.
Authorship Information: Concept and design (BYL, RRB, AEW, SMZ, KJS); acquisition of data (BYL, AEW, SMZ, RKZ); analysis and interpretation of data (BYL, JHYT, RRB, SMM, SMZ); drafting of the manuscript (BYL, JHYT, RRB, SMM, AEW, RKZ); critical revision of the manuscript for important intellectual content (BYL, JHYT, RRB, SMM, SMZ, KJS, RKZ); statistical analysis (BYL); provision of study materials or patients (BYL); obtaining funding (BYL); administrative, technical, or logistic support (BYL, RRB, KJS); and supervision (BYL).
References
- 1.U.S. Food and Drug Administration. FDA authorizes emergency use of intravenous antiviral peramivir for 2009 H1N1 influenza for certain patients, settings. [Accessed December 17, 2010];2009 Oct 23; http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm187813.htm.
- 2.Birnkrant D, Cox E. The Emergency Use Authorization of peramivir for treatment of 2009 H1N1 influenza. N Engl J Med. 2009;361(23):2204–2207. doi: 10.1056/NEJMp0910479. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. H1N1 Flu: Emergency Use Authorization (EUA) of Medical Products and Devices: Peramivir IV. Atlanta, GA: Dept of Health and Human Services; 2009. [Google Scholar]
- 4.U.S. Food and Drug Administration. Drugs@FDA: FDA approved drug products. [Accessed December 17, 2010]; http://www.accessdata.fda.gov/scripts/cder/drugsatfda/
- 5.Centers for Disease Control and Prevention. Neuraminidase inhibitors for treatment of influenza A and B infections [published correction appears in MMWR Morb Mortal Wkly Rep. 1999;48(49):1139] MMWR Recomm Rep. 1999;48(RR-14):1–9. [PubMed] [Google Scholar]
- 6.Moscona A. Neuraminidase inhibitors for influenza. N Engl J Med. 2005;353(13):1363–1373. doi: 10.1056/NEJMra050740. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Food and Drug Administration. Peramivir IV questions and answers for health care providers. [Accessed December 17, 2010]; http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm187980.htm.
- 8.Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project. HCUP facts and figures: statistics on hospital-based care in the United States. [Accessed December 17, 2010]. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Flu activity & surveillance: past weekly surveillance reports. [Accessed December 17, 2010]; http://www.cdc.gov/flu/weekly/fluactivity.htm.
- 10.PDR Red Book: Pharmacy’s Fundamental Reference. Montvale, NJ: Thompson Healthcare Inc; 2009. [Google Scholar]
- 11.Gould MK, Dembitzer AD, Sanders GD, Garber AM. Low-molecular-weight heparins compared with unfractionated heparin for treatment of acute deep venous thrombosis: a cost-effectiveness analysis. Ann Intern Med. 1999;130(10):789–799. doi: 10.7326/0003-4819-130-10-199905180-00002. [DOI] [PubMed] [Google Scholar]
- 12.Bureau of Labor Statistics. Occupational employment statistics: May 2008 national occupational employment and wage estimates: United States. [Accessed December 17, 2010]; http://www.bls.gov/oes/2008/may/oes_nat.htm.
- 13.Syrmis MW, Whiley DM, Thomas M, et al. A sensitive, specific, and cost-effective multiplex reverse transcriptase–PCR assay for the detection of seven common respiratory viruses in respiratory samples. J Mol Diagn. 2004;6(2):125–131. doi: 10.1016/S1525-1578(10)60500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chidlow G, Harnett G, Williams S, Levy A, Speers D, Smith DW. Duplex real-time reverse transcriptase PCR assays for rapid detection and identification of pandemic (H1N1) 2009 and seasonal influenza A/H1, A/H3, and B viruses. J Clin Microbiol. 2010;48(3):862–866. doi: 10.1128/JCM.01435-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar A, Zarychanski R, Pinto R, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302(17):1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 16.Gold MR, Franks P, McCoy KI, Fryback DG. Toward consistency in cost-utility analyses: using national measures to create condition-specific values. Med Care. 1998;36(6):778–792. doi: 10.1097/00005650-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Sackett DL, Torrance GW. The utility of different health states as perceived by the general public. J Chronic Dis. 1978;31(11):697–704. doi: 10.1016/0021-9681(78)90072-3. [DOI] [PubMed] [Google Scholar]
- 18.Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care. 2000;38(6):583–637. doi: 10.1097/00005650-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Jain S, Kamimoto L, Bramley AM, et al. 2009 Pandemic Influenza A (H1N1) Virus Hospitalizations Investigation Team. Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N Engl J Med. 2009;361(20):1935–1944. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 20.Rello J, Rodríguez A, Ibañez P, et al. H1N1 SEMICYUC Working Group. Intensive care adult patients with severe respiratory failure caused by influenza A (H1N1)v in Spain. Crit Care. 2009;13(5):eR148. doi: 10.1186/cc8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li G, Yilmaz M, Kojicic M, et al. Outcome of critically ill patients with influenza virus infection. J Clin Virol. 2009;46(3):275–278. doi: 10.1016/j.jcv.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bureau of Labor Statistics. Employment characteristics of families in 2008. [Accessed December 17, 2010];2009 May 27; http://www.bls.gov/news.release/archives/fa-mee_05272009.pdf.
- 23.Bureau of Labor Statistics. Employment characteristics of families in 2007. [Accessed December 17, 2010]; http://www.bls.gov/news.release/archives/famee_05302008.pdf.
- 24.Jefferson TO, Demicheli V, Di Pietrantonj C, Jones M, Rivetti D. Neuraminidase inhibitors for preventing and treating influenza in healthy adults. Cochrane Database Syst Rev. 2006;3:CD001265. doi: 10.1002/14651858.CD001265.pub2. [DOI] [PubMed] [Google Scholar]
- 25.Shepard DS. In: Cost-effectiveness in Health and Medicine. Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. New York: Oxford University Press; 1996. [Google Scholar]; J Ment Health Policy Econ. 1999;2(2):91–92. [Google Scholar]
- 26.Centers for Disease Control and Prevention. Influenza diagnostic testing during the 2009–2010 flu season. [Accessed December 17, 2010];2009 Sep 29; http://www.cdc.gov/h1n1flu/diagnostic_testing_public_qa.htm.
- 27.Wilmoth JR, Shkolnikov V. Human Mortality Database. [Accessed December 17, 2010];2008 http://www.mortality.org/
- 28.Dulek DE, Williams JV, Creech CB, et al. Use of intravenous zanamivir after development of oseltamivir resistance in a critically Ill immunosuppressed child infected with 2009 pandemic influenza A (H1N1) virus. Clin Infect Dis. 2010;50(11):1493–1496. doi: 10.1086/652655. [DOI] [PubMed] [Google Scholar]