Abstract
Efforts to control life-threatening infections, such as with methicillin-resistant Staphylococcus aureus (MRSA), can be complicated when patients are transferred from one hospital to another. Using a detailed computer simulation model of all hospitals in Orange County, California, we explored the effects when combinations of hospitals tested all patients at admission for MRSA and adopted procedures to limit transmission among patients who tested positive. Called “contact isolation,” these procedures specify precautions for health care workers interacting with an infected patient, such as wearing gloves and gowns. Our simulation demonstrated that each hospital’s decision to test for MRSA and implement contact isolation procedures could affect the MRSA prevalence in all other hospitals. Thus, our study makes the case that further cooperation among hospitals—which is already reflected in a few limited collaborative infection control efforts under way—could help individual hospitals achieve better infection control than they could achieve on their own.
A hospital often has its own infection control personnel, programs, and initiatives that are not coordinated with other hospitals unless they are associated legally or financially. Hospitals frequently invest heavily to control their health care–associated infection rates and often undertake measures that are unrelated to control measures at other hospitals.
One example of the infectious agents that hospitals seek to control is methicillin-resistant Staphylococcus aureus, or MRSA. MRSA is widespread in US hospitals, with an estimated 278,203 hospitalizations (95% confidence interval: 252,788, 303,619) and 6,639 MRSA-related deaths in 20051 and a reported prevalence of 66.4 colonizations or infections per 1,000 inpatients in 2010.2
Indeed, it may be highly cost-effective for an individual hospital to implement MRSA control measures, such as actively screening patients for the bacteria and requiring health care workers to practice contact isolation procedures when interacting with patients who test positive.3 Contact isolation procedures require hospital staff to wear gloves and gowns upon entering a room occupied by an infected patient and whenever interacting with an infected patient or the patient’s environment.
Previously, we showed that hospitals in Orange County, California, which has a population of three million people, were interconnected through direct patient transfers and indirect sharing by the readmission of a patient to a different hospital after an intervening stay at home or elsewhere.4,5 Depending on hospital infection control practices or lapses, such patient sharing was shown to either quell or facilitate the spread of infectious diseases such as MRSA, which patients can carry for months or years, to other regional hospitals, even those miles away from the originating hospital.6
The question then arises whether cooperation among hospitals can lead to gains in infection control, which an individual hospital could not otherwise achieve.
Using a computational model developed by our National Institutes of Health Models of Infectious Disease Agent Study MRSA Working Group, we sought to determine the impact of a two-part intervention: testing all patients at admission for MRSA colonization and employing contact isolation procedures for all staff interacting with positive patients. We assessed the impact of this intervention in Orange County hospitals, individually and in combination, and compared it to the impact when the intervention was implemented in all Orange County hospitals or in a subset of those hospitals.
Study Data And Methods
ORANGE COUNTY PATIENT FLOW AND MRSA TRANSMISSION MODEL
Building on previous work, we further developed our Regional Health-care Ecosystem Analyst model of all twenty-nine acute care hospitals serving adult patients in Orange County. Five hospitals were long-term acute care facilities that served patients with prolonged, high-level medical needs. The model simulated an average of 3,740 virtual patients daily, each represented by a computational agent, moving among these hospitals and the community. The model also simulated the transmission of MRSA within these hospitals.6
In the model, virtual patients testing positive or negative for MRSA were admitted to Orange County hospitals at rates obtained from actual hospital records. These virtual patients were then assigned to a hospital intensive care unit or general ward for lengths-of-stay that corresponded to actual lengths-of-stay for patients admitted to that hospital.
Upon discharge, the model returned some virtual patients to the community and others to a different hospital. Some patients were readmitted to the same or a different hospital at a later date, all according to probabilities derived from empirical data, as previously described.5
Our model contained detailed representations of each adult hospital in the county, including representations of each facility’s intensive care units, where applicable, and general wards and hospital beds in each unit and ward.6 Hospital-specific data were obtained from several sources. The hospital-specific data included annual admissions and hospital lengths-of-stay for adult patients from the California Office of Statewide Health Planning and Development.7
Comprehensive matrices on how Orange County hospitals share patients, through direct interfacility transfers as well as discharges and readmissions, came from detailed analyses of this data set.5 Information about the number of hospital intensive care units, bed capacity, and average length-of-stay within those units came from surveys of each hospital.8 Online Appendix Exhibit 1 provides key model input parameters.9
ACTIVE SURVEILLANCE CULTURES AND CONTACT ISOLATION
The two-part intervention of interest was the testing for MRSA of all patients who were admitted and the subsequent adopting of contact isolation procedures for all patients who tested positive. Appendix Exhibit 2 shows how this intervention was implemented.9
Our experiments assumed that the MRSA screen had a sensitivity (the ability or probability of the test to correctly identify patients who have MRSA) of 75 percent10–13 and specificity (the ability or probability of the test to correctly identify patients who do not have MRSA) of 97 percent. We also assumed a turnaround time, from test start to obtaining results, of two days.14
Contact isolation of a patient began only after a test result returned positive, including both true and false positives—regardless of whether the patient was truly colonized, meaning that the bacteria were present on the body. Contact isolation was modeled as a percentage reduction in MRSA transmission, based upon the degree of compliance that was applied.
Each day the resulting number of new MRSA cases (colonization) in a hospital ward or intensive care unit depended on, first, the number of susceptible and infectious patients on contact isolation; second, the number of those not on contact isolation; and third, the compliance of health care workers with the contact isolation procedures. A more detailed description can be found in the online Appendix Methodology.9
Our baseline scenario assumed that patients who were MRSA-positive lost MRSA carriage over time, with MRSA-carriage duration drawn from a distribution based on published estimates.15–18 One-third of patients who were MRSA-positive remained positive,19 and the remaining two-thirds of MRSA-positive patients gradually became MRSA-negative during the subsequent year. Additional sensitivity analysis explored the effects of indefinite MRSA carriage.
EXPERIMENTS
Experiments explored the effects of implementing active surveillance cultures and contact isolation for MRSA in the following groupings of hospitals. First was single hospitals: implementing surveillance and isolation in only one hospital in the county to assess direct and indirect benefits. Experiments varied according to the hospital implementing the intervention. Second was high-volume hospitals: those with 10,000 or more annual admissions (n = 11). Third was high-capacity hospitals: the largest hospitals in Orange County by bed capacity—three largest (average: 307 beds), five largest (average: 281 beds), and ten largest (average: 232 beds). Fourth was all hospitals in Orange County (N = 29).
Sensitivity analyses varied contact isolation compliance—that is, how well health care workers and other staff members adhered to contact isolation procedures (25 percent, 50 percent, and 75 percent). Outcomes included assessment of the attributable changes in MRSA prevalence in each hospital over time after screening was implemented. In other words, we assessed the relative decrease in MRSA prevalence from pre-intervention levels and the number of averted cases per year after surveillance and isolation took full effect, which was approximately six months after implementation.
LIMITATIONS
Models, by definition, are simplifications of real life and, therefore, do not incorporate all possible factors and outcomes.20,21 For example, our model did not include factors such as comorbidities that could affect MRSA transmission.
Although our model is heavily based on real-world data, all data sources and collection methods have their limitations. For instance, the baseline MRSA prevalence may change from year to year. Our model did not account for hospitals outside Orange County that might exchange patients with Orange County hospitals, although we noted that 86 percent of patients stayed within the county for care.
Orange County is a large metropolitan area with a rather diverse population, including a wide range of racial, ethnic, and socioeconomic groups. However, its hospitals might not be representative of other counties because the number, size, and types of hospitals may differ. In addition, our study focused only on adult patients and did not include pediatric hospitals or long-term care facilities.
Study Results
IMPACT OF INTERVENTION AT ONE HOSPITAL ON OTHER HOSPITALS
Our simulation found that the implementation of surveillance and isolation at one hospital decreased MRSA prevalence not only in that hospital, but also in many other hospitals in the county that had not implemented the intervention. The magnitude of the effect depended on which hospital implemented the intervention.
A hospital employing surveillance and isolation with 75 percent compliance realized a median relative MRSA prevalence decrease of 11.27 percent (range: 6.73–19.08 percent) and averted a median thirty-seven MRSA cases (range: 2–261) during the course of a year.
Indirectly, many of the remaining hospitals—that is, those not employing the intervention—also experienced a reduction in MRSA prevalence (median: 0.27 percent; range: 0.00–12.61 percent) and number of MRSA cases averted per year (median: 1; range: 0–15). For example, when hospital F implemented surveillance and isolation, it experienced a 6.73 percent relative MRSA decrease (averting seven cases a year) and caused a 0.14 percent median (range: 0.0–9.2 percent) relative MRSA decrease in all other hospitals, averting thirty cases per year. Hospital H showed a 19.08 percent median relative MRSA decrease after the intervention was initiated, averting thirty-four cases per year and achieving a 0.28 percent MRSA reduction (range: 0.00–10.22 percent; forty-four cases a year) in all other hospitals.
Surveillance and isolation implemented in hospital I had the largest effect on the network, a median 0.69 percent relative MRSA decrease in all other hospitals (range: 0.06–10.59 percent), averting eighty-six cases per year, and a 16.95 percent median relative decrease in its own MRSA prevalence, averting 261 cases per year.
EFFECTS FOR ALL HOSPITALS ADOPTING SURVEILLANCE AND CONTACT ISOLATION
Exhibit 1 depicts the benefits to acute care hospitals when other hospitals also implement surveillance and isolation. Appendix Exhibit 3 shows the benefits to each facility, both the relative reduction in MRSA prevalence and number of cases averted.9
EXHIBIT 1.
Relative Reduction In MRSA Prevalence For Various Active Surveillance And Contact Isolation Campaigns Implemented At 75 Percent Contact Isolation Compliance Over 10 Simulated Years
| This hospital only (%) | 5 highest- capacity hospitals (%) | 10 highest- capacity hospitals (%) | All hospitals (%) | |
|---|---|---|---|---|
| ACUTE CARE HOSPITAL AFFECTED | ||||
|
| ||||
| A | 14.9 | 2.2 | 2.8 | 20.2 |
| B | 13.6 | 14.4 | 14.8 | 15.6 |
| C | 13.5 | 2.3 | 3.5 | 19.6 |
| D | 10.3 | 11.4 | 12.0 | 13.6 |
| E | 8.3 | 1.0 | 2.7 | 11.9 |
| F | 6.7 | 2.3 | 4.7 | 12.2 |
| G | 9.9 | 1.6 | 2.9 | 14.9 |
| H | 19.1 | 1.9 | 2.6 | 22.8 |
| I | 17.0 | 17.2 | 17.7 | 18.5 |
| J | 12.5 | 1.2 | 14.2 | 16.8 |
| K | 10.6 | 1.2 | 12.6 | 14.6 |
| L | 16.9 | 17.6 | 19.3 | 20.2 |
| M | 12.5 | 2.3 | 3.6 | 17.2 |
| N | 9.4 | 1.7 | 3.1 | 15.5 |
| O | 11.2 | 1.6 | 13.2 | 15.6 |
| P | 13.1 | 1.3 | 2.1 | 15.9 |
| Q | 8.7 | 0.7 | 9.7 | 10.6 |
| R | 15.6 | 0.36 | 0.7 | 16.4 |
| S | 9.8 | 1.2 | 2.4 | 13.5 |
| T | 11.3 | 1.7 | 2.8 | 20.2 |
| U | 8.6 | 0.9 | 9.2 | 9.4 |
| V | 12.2 | 12.7 | 13.1 | 13.9 |
| W | 10.5 | 2.9 | 4.7 | 17.8 |
| X | 9.3 | 1.5 | 3.0 | 16.5 |
|
| ||||
| COUNTYWIDE ACUTE CARE REDUCTION | ||||
|
| ||||
| Mean | — | 4.3 | 7.4 | 16.0 |
| Median | — | 1.7 | 4.2 | 15.7 |
SOURCE Author-generated data. NOTE MRSA is methicillin-resistant Staphylococcus aureus.
When all Orange County hospitals implemented the intervention simultaneously at a 75 percent contact isolation compliance, acute care hospitals experienced a further proportional decrease in MRSA prevalence beyond what they could achieve alone (median: 3.85 percent additional decrease; range: 0.81–8.97 percent). Long-term acute care facilities experienced an even higher proportional decrease in MRSA prevalence (median: 12.13 percent additional decrease; range: 1.29–15.35 percent).
Notably, only twelve hospitals, including two long-term acute care facilities, had more gains—that is, a reduction in MRSA prevalence—when the ten highest-capacity hospitals implemented the intervention compared to when they implemented surveillance and isolation individually (relative change differences of 2.85 percent or less). Fourteen facilities had larger reductions when those with 10,000 admissions or more implemented the intervention, versus when individual hospitals did so (Appendix Exhibit 3).9
Exhibit 2 illustrates the increasing benefits—relative decreases in MRSA prevalence and number of averted cases per year—to all Orange County hospitals when more hospitals adopted the intervention (see Appendix Exhibit 4 for full version).9 The more hospitals that implemented surveillance and isolation, the greater was the reduction in MRSA prevalence and the more MRSA cases were averted in each hospital.
EXHIBIT 2.
Benefits Of Implementing Active Surveillance And Contact Isolation Campaigns In Selected Subsets Of Hospitals Over 10 Simulated Years
| Percent contact isolation compliance | Highest- capacity hospital | 3 highest- capacity hospitals | 5 highest- capacity hospitals | 10 highest- capacity hospitals | 11 highest- volume hospitalsa | All hospitals |
|---|---|---|---|---|---|---|
| MEDIAN REDUCTION IN EACH HOSPITAL’S MRSA PREVALENCE (%) | ||||||
|
| ||||||
| 25 | 0.2 | 0.5 | 0.7 | 1.5 | 1.6 | 5.7 |
| 50 | 0.3 | 0.9 | 1.3 | 3.1 | 3.1 | 10.9 |
| 75 | 0.4 | 1.3 | 1.7 | 4.7 | 4.8 | 15.9 |
|
| ||||||
| MEDIAN NUMBER OF MRSA CASES AVERTED IN EACH HOSPITALb | ||||||
|
| ||||||
| 25 | 1.0 | 2.1 | 2.2 | 4.1 | 4.2 | 18.8 |
| 50 | 1.1 | 2.9 | 4.1 | 7.3 | 7.8 | 36.2 |
| 75 | 1.4 | 4.0 | 5.7 | 10.1 | 10.2 | 52.6 |
SOURCE Author-generated data. NOTE MRSA is methicillin-resistant Staphylococcus aureus.
Those with 10,000 or more admissions (11 in Orange County).
Per year, after the implementation of surveillance and isolation has taken full effect (approximately six months).
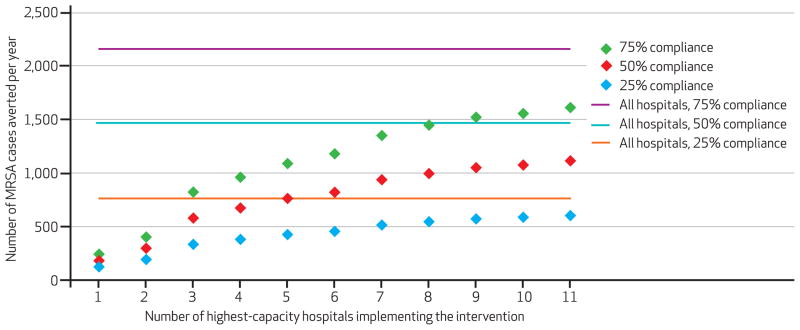
However, note that this relationship was not linear. Doubling the number of hospitals that employed surveillance and isolation from the five to the ten highest-capacity hospitals—that is, increasing the average number of people screened per year from 93,280 to 160,678—more than doubled the benefits.
BENEFITS OF IMPROVING COMPLIANCE WITH CONTACT PRECAUTIONS
Exhibit 2 also delineates how MRSA prevalence decreased, and the number of cases averted increased, when compliance with contact precautions was improved. The effects were somewhat linear.
Increasing isolation compliance from 25 percent to 50 percent tended to double the effects, while improving compliance from 50 percent to 75 percent further doubled the effects. These results emphasize the importance of not only increasing the number of hospitals implementing surveillance and isolation, but also of increasing isolation compliance in all participating hospitals.
These trends raise the question of whether greater reductions in MRSA prevalence are attained by improving compliance in hospitals already implementing the intervention or by focusing on convincing more hospitals to adopt the intervention. The answer depends on the size and connectedness of the hospitals.
Exhibit 3 shows the number of MRSA cases averted per year countywide when surveillance and isolation procedures were implemented in increasing numbers of county hospitals, progressively adding the next-largest hospital by bed capacity. The results highlight the benefits of improving compliance, which increases as more hospitals participate, and they enable comparison of the incremental gains from increasing the number of hospitals performing surveillance and isolation procedures with the gains from increasing isolation compliance in the currently participating hospitals.
EXHIBIT 3. Number Of MRSA Cases Averted Per Year Countywide By Implementing Surveillance And Contact Isolation In The Highest-Capacity Hospitals At Varying Contact Isolation Compliance Rates Over 10 Simulated Years.
SOURCE Author-generated data. NOTES For number of highest-capacity hospitals, starting with the largest and progressively adding the next-largest by capacity. MRSA is methicillin-resistant Staphylococcus aureus.
EFFECTS OF MRSA CARRIAGE
Additional scenarios exploring the effects of making all MRSA colonization indefinite underscored the benefits of active surveillance cultures and isolation. When all hospitals implemented surveillance and isolation with 75 percent compliance, the median relative decrease in MRSA prevalence for Orange County was 15.8 percent with MRSA carriage loss and 19.8 percent with indefinite carriage.
Discussion
Our study showed that concerted infection control campaigns among a regional group of hospitals can yield substantial indirect benefits for hospitals involved and for hospitals not involved in the campaign. What’s more, the synergistic effects of persuading other hospitals in the same county to implement control mechanisms can help individual hospitals achieve better MRSA control than they can on their own. The more hospitals that work together, the more benefits accrue; doubling the number of hospitals that adopt the intervention can more than double the improvement in infection control.
These synergistic effects arise from the fact that hospitals often share patients throughout a county. Even when one hospital enforces strict infection control policies, its patients can be infected by MRSA-colonized patients from other hospitals that have less stringent infection control policies.
In fact, for some hospitals, having other hospitals implement some degree of MRSA control may be as effective or even more effective than improving procedures in a single hospital. Although our study explored the possibility of all hospitals’ using surveillance and contact isolation, our general finding would hold even if hospitals used different infection control procedures, as long as the procedures reduced the prevalence of MRSA colonization.
There has been some effort to stimulate cooperation among hospitals in controlling MRSA and other hospital-acquired infections. Statewide approaches and interventions to reduce hospital-acquired infections have been successful in Iowa; Pennsylvania; Michigan; New York; Wisconsin; and the Siouxland region of Iowa, Nebraska, and South Dakota.22–27
In the Siouxland region, the implementation of surveillance cultures and isolation led to a reduction in infections from another bacterium, vancomycin-resistant Enterococcus.23 Other collaborative efforts, such as the Iowa Healthcare Collaborative, have had success from several initiatives. A majority (93 percent) of Iowa’s hospitals implemented the MRSA control measures recommended by the 5 Million Lives Campaign of the Institute for Healthcare Improvement to some degree.27
Although it encountered implementation problems, the Pittsburgh Regional Health Initiative has created a culture of change to improve overall patient safety by providing opportunities to learn, increasing general awareness, and identifying common problems and solutions.28
These initiatives show the willingness of multiple hospitals to work together toward a common goal and to achieve an overall reduction in disease burden. However, to our knowledge, there has not been an evaluation of potential synergistic effects of facility interventions in a region.
In recent years, a number of collaborative initiatives have evolved.29 For example, in California the Safety Net Initiative, with a grant from the Blue Shield of California Foundation, is building a learning collaborative among public hospitals to reduce hospital-acquired infections—specifically, central-line infections and sepsis.
The Health Services Advisory Group of California, in collaboration with the Hospital Association of Southern California, has launched a Centers for Medicare and Medicaid Services Learning and Action Network to improve the quality of care. A major component is the Reducing Healthcare-Associated Infections Project. Its emphasis is preventing catheter-associated urinary tract infections, Clostridium difficile infections, and surgical site infections.
The California Healthcare-Associated Infection Prevention Initiative is another similar collaborative. The primary motivation for these collaboratives has been to share knowledge and best practices rather than to closely coordinate infection control measures.
To date, few studies have demonstrated and quantified the synergistic effects of hospitals within a large region coordinating infection control measures to achieve greater gains than they could achieve individually. Understanding these added benefits could provide extra motivation to hospitals to work more closely together. Computational modeling and simulation can be a useful tool for understanding infection control, especially at a regional level. Forecasting the effects of policies before enacting them can save valuable time and resources.
To that end, our study aimed to demonstrate the benefits of cooperation for each hospital, which may motivate hospitals to overcome barriers to cooperation. These obstacles are real. Hospitals have different budgets, resources, leadership, and competing priorities.30 Enforcing the same infection control procedures may be easier in some hospitals and more challenging in others.31 Information systems, reporting measures, and organizational structures may also be quite disparate.
It is also possible that as Medicare and other insurers continue to reduce reimbursements to hospitals with health care–associated infections, a hospital could use the achievement of infection rates that are lower than its competitors as a selling point to patients, insurers, and potential partners. A hospital could then make successful infection control policies and interventions into potential competitive advantages that it would be loath to share with other hospitals.32,33
As in this study, modeling and simulation can help extend the data collected from retrospective and prospective studies and make the case for cooperation. Modeling and simulation can serve as virtual “policy laboratories” for public health officials, policy makers, hospital administrators, and other health care decision makers. Here, modeling enables us to quantify the indirect and added benefits attained from regional efforts of admission screening for MRSA and greater compliance with contact precautions.
Conclusion
Our study shows that coordination of MRSA prevention practices in infection control, even among small groups of hospitals, can benefit all hospitals in a county, even those that do not implement the intervention. The effects of persuading other hospitals in the same county to implement control mechanisms can help hospitals achieve better infection control than they could on their own. The more hospitals that work together, the greater the benefits accrued.
This relationship is also nonlinear; doubling the number of hospitals can more than double the improvement in infection control. Concerted infection-control campaigns reflect the fact that hospitals are rarely isolated islands but instead share patients extensively with other hospitals in a county. All hospitals may also benefit greatly from improved compliance with infection control measures.
Supplementary Material
Acknowledgments
This study was supported by the National Institute of General Medical Sciences Models of Infectious Disease Agent Study (MIDAS) grants No. 5U54GM088491-02 and No. 1U01GM076672, National Institute of Allergy and Infectious Diseases Grant No. 1RC4A1092327-01, and the Pennsylvania Department of Health. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Computational resources were provided by the Center for Simulation and Modeling and Pittsburgh Supercomputing Center.
Biographies
 Bruce Y. Lee is an associate professor of medicine, epidemiology, and biomedical informatics at the University of Pittsburgh.
Bruce Y. Lee is an associate professor of medicine, epidemiology, and biomedical informatics at the University of Pittsburgh.
In this month’s Health Affairs, Bruce Lee and coauthors report on their simulation of efforts to control life-threatening hospital-acquired infections when patients are transferred from one hospital to another. The simulation demonstrated that each hospital’s decision to test for methicillin-resistant Staphylococcus aureus, or MRSA, and contain the bacterium’s spread could affect prevalence in all other hospitals—and thus makes the case that collaborative infection control efforts can help individual hospitals achieve better infection control than they can achieve on their own.
Lee is an associate professor of medicine, epidemiology, and biomedical informatics at the University of Pittsburgh. He founded and directs the Public Health and Infectious Disease Computational and Operations Research (PHICOR) group, which specializes in designing economic and operational computer models, simulations, and tools that help decision makers tackle infectious diseases of global importance.
Lee’s previous positions include serving as senior manager at Quintiles Transnational; working in biotechnology equity research at Montgomery Securities; and cofounding Integrigen, a biotechnology and bioinformatics company. Lee has authored more than 130 scientific publications as well as three books. He received his undergraduate and medical degrees from Harvard University and an MBA from Stanford University. Lee completed his medicine residency training at the University of California, San Diego.
 Sarah M. Bartsch is a research coordinator and senior analyst in the PHICOR group, University of Pittsburgh.
Sarah M. Bartsch is a research coordinator and senior analyst in the PHICOR group, University of Pittsburgh.
Sarah Bartsch is a research coordinator and senior analyst in the PHICOR group, University of Pittsburgh. She earned her master’s degree in public health, with a concentration in epidemiology, from the Ohio State University.
 Kim F. Wong is an assistant research professor in the Center for Simulation and Modeling, University of Pittsburgh.
Kim F. Wong is an assistant research professor in the Center for Simulation and Modeling, University of Pittsburgh.
Kim Wong is an assistant research professor in the Center for Simulation and Modeling and in the Department of Chemistry at the University of Pittsburgh. His expertise lies at the intersection of physics-based modeling and simulation, software development, and high-performance computing. Wong has applied his experience in these areas toward the development of an agent-based model simulating disease dynamics. He received a doctorate in chemical physics from the University of Texas at Austin.
 S. Levent Yilmaz is a research assistant professor in the Center for Simulation and Modeling, University of Pittsburgh.
S. Levent Yilmaz is a research assistant professor in the Center for Simulation and Modeling, University of Pittsburgh.
S. Levent Yilmaz is a research assistant professor in the Center for Simulation and Modeling at the University of Pittsburgh. He earned his doctorate in mechanical engineering and materials science from the University of Pittsburgh.
 Taliser R. Avery is a research associate at Harvard Medical School.
Taliser R. Avery is a research associate at Harvard Medical School.
Taliser Avery is a research associate at Harvard Medical School and the Harvard Pilgrim Health Care Institute. She earned her master’s degree in epidemiology from Boston University.
 Ashima Singh is a senior analyst in the PHICOR group, University of Pittsburgh.
Ashima Singh is a senior analyst in the PHICOR group, University of Pittsburgh.
Ashima Singh is a senior analyst in the PHICOR group at the University of Pittsburgh, where she is pursuing her doctorate in epidemiology. She earned her master’s degree in biomedical informatics from the University of Medicine and Dentistry of New Jersey.
 Yeohan Song is a former senior analyst in the PHICOR group, University of Pittsburgh.
Yeohan Song is a former senior analyst in the PHICOR group, University of Pittsburgh.
Yeohan Song was a senior analyst at the PHICOR group at the time this article was written. He earned his bachelor’s degree in neuroscience from the University of Pittsburgh and is currently in medical school at the University of Michigan.
 Diane S. Kim is an assistant clinical research coordinator at the School of Medicine, University of California, Irvine.
Diane S. Kim is an assistant clinical research coordinator at the School of Medicine, University of California, Irvine.
Diane Kim is an assistant clinical research coordinator in the Division of Infectious Diseases, School of Medicine, at the University of California, Irvine. She earned a bachelor’s degree in animal physiology and neuroscience from the University of California, San Diego.
 Shawn T. Brown is an assistant professor of biostatistics at the University of Pittsburgh.
Shawn T. Brown is an assistant professor of biostatistics at the University of Pittsburgh.
Shawn Brown is an assistant professor of biostatistics at the University of Pittsburgh and a research fellow at the Pittsburgh Supercomputing Center. He conducts research in agent-based modeling for public health decision making and simulations of vaccine supply chains in many countries throughout the world. Brown earned his doctorate in theoretical chemistry from the University of Georgia.
 Margaret A. Potter is the director of the Center for Public Health Practice, Graduate School of Public Health, University of Pittsburgh.
Margaret A. Potter is the director of the Center for Public Health Practice, Graduate School of Public Health, University of Pittsburgh.
Margaret Potter is the director of the Center for Public Health Practice, Graduate School of Public Health, at the University of Pittsburgh. She holds several other positions at the university, including codirector for Preparedness Policy in the Center for Public Health Preparedness; associate professor of health policy and management; and codirector of the Center for National Preparedness. She earned her law degree from Rutgers University.
 Richard Platt is a professor and chair of the Department of Population Medicine, Harvard Medical School.
Richard Platt is a professor and chair of the Department of Population Medicine, Harvard Medical School.
Richard Platt is a professor and chair of the Department of Population Medicine, Harvard Medical School, and executive director of the Harvard Pilgrim Health Care Institute. He is principal investigator for several major projects, including a Prevention Epicenter program at the Centers for Disease Control and Prevention. Platt is a member of the Institute of Medicine Roundtable on Value and Science-Driven Health Care and the American Association of Medical Colleges Advisory Panel on Research. He earned his medical degree and a master’s degree in epidemiology from Harvard University.
 Susan S. Huang is an associate professor at the School of Medicine, University of California, Irvine.
Susan S. Huang is an associate professor at the School of Medicine, University of California, Irvine.
Susan Huang is an associate professor in the Division of Infectious Diseases, School of Medicine, at the University of California (UC), Irvine, and the medical director of epidemiology and infection prevention at UC Irvine Medical Center. Her research has focused on health care–associated infections, including identifying the burden, risk factors, and strategies for preventing disease and containing its spread. Huang received her medical degree from the Johns Hopkins University and her master’s degree in public health from Harvard University.
Contributor Information
Bruce Y. Lee, Email: BYL1@pitt.edu, Associate professor of medicine, epidemiology, and biomedical informatics and director of the Public Health and Infectious Diseases Computational and Operations Research (PHICOR) group at the University of Pittsburgh, in Pennsylvania
Sarah M. Bartsch, Research coordinator and senior analyst in the PHICOR group at the University of Pittsburgh
Kim F. Wong, Assistant research professor in the Center for Simulation and Modeling and in the Department of Chemistry at the University of Pittsburgh
S. Levent Yilmaz, Research assistant professor in the Center for Simulation and Modeling at the University of Pittsburgh.
Taliser R. Avery, Research associate at Harvard Medical School and the Harvard Pilgrim Health Care Institute, in Boston, Massachusetts
Ashima Singh, Senior analyst in the PHICOR group at the University of Pittsburgh.
Yeohan Song, Former senior analyst in the PHICOR group at the University of Pittsburgh, is now a medical student at the University of Michigan, in Ann Arbor.
Diane S. Kim, Assistant clinical research coordinator in the Division of Infectious Diseases, School of Medicine, University of California, Irvine
Shawn T. Brown, Assistant professor of biostatistics at the University of Pittsburgh and a research fellow at the Pittsburgh Supercomputing Center
Margaret A. Potter, Director of the Center for Public Health Practice, Graduate School of Public Health, at the University of Pittsburgh
Richard Platt, Professor and chair of the Department of Population Medicine, Harvard Medical School, and executive director of the Harvard Pilgrim Health Care Institute.
Susan S. Huang, Associate professor in the Division of Infectious Diseases, School of Medicine, University of California, Irvine
NOTES
- 1.Klein E, Smith DL, Laxminarayan R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg Infect Dis. 2007;13(12):1840–6. doi: 10.3201/eid1312.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jarvis WR, Jarvis AA, Chinn RY. National prevalence of methicillin-resistant Staphylococcus aureus in inpatients at United States health care facilities, 2010. Am J Infect Control. 2012;40(3):194–200. doi: 10.1016/j.ajic.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Lee BY, Bailey RR, Smith KJ, Muder RR, Strotmeyer ES, Lewis GJ, et al. Universal methicillin-resistant Staphylococcus aureus (MRSA) surveillance for adults at hospital admission: an economic model and analysis. Infect Control Hosp Epidemiol. 2010;31(6):598–606. doi: 10.1086/652524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee BY, McGlone SM, Song Y, Avery TR, Eubank S, Chang CC, et al. Social network analysis of patient sharing among hospitals in Orange County, California. Am J Public Health. 2011;101(4):707–13. doi: 10.2105/AJPH.2010.202754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang SS, Avery TR, Song Y, Elkins KR, Nguyen CC, Nutter SK, et al. Quantifying interhospital patient sharing as a mechanism for infectious disease spread. Infect Control Hosp Epidemiol. 2010;31(11):1160–9. doi: 10.1086/656747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee BY, McGlone SM, Wong KF, Yilmaz SL, Avery TR, Song Y, et al. Modeling the spread of methicillin-resistant Staphylococcus aureus (MRSA) outbreaks throughout the hospitals in Orange County, California. Infect Control Hosp Epidemiol. 2011;32(6):562–72. doi: 10.1086/660014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.California Health and Human Services Agency. Office of Statewide Health Planning and Development Healthcare Information Resource Center [home page on the Internet] Sacramento (CA): OSHPD; [updated 2010 Oct 4; cited 2012 Sep 6]. Available from: http://www.oshpd.ca.gov/HID/HIRC/ [Google Scholar]
- 8.Elkins KR, Nguyen CC, Kim D, Meyers H, Cheung M, Huang SS. Successful strategies for high participation in three regional health-care surveys: an observational study. BMC Med Res Methodol. 2011;11:176. doi: 10.1186/1471-2288-11-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.To access the Appendix, click on the Appendix link in the box to the right of the article online.
- 10.Yang E, Tan J, Eells S, Rieg G, Tagudar G, Miller L. Body site colonization in patients with community-associated methicillin-resistant Staphylococcus aureus and other types of S. aureus skin infections. Clin Microbiol Infect. 2010;16(5):425–31. doi: 10.1111/j.1469-0691.2009.02836.x. [DOI] [PubMed] [Google Scholar]
- 11.Widmer AF, Mertz D, Frei R. Necessity of screening of both the nose and the throat to detect methicillin-resistant Staphylococcus aureus colonization in patients upon admission to an intensive care unit. J Clin Microbiol. 2008;46(2):835. doi: 10.1128/JCM.02276-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rohr U, Wilhelm M, Muhr G, Gatermann S. Qualitative and semi-quantitative characterization of nasal and skin methicillin-resistant Staphylococcus aureus carriage of hospitalized patients. Int J Hyg Environ Health. 2004;207(1):51–5. doi: 10.1078/1438-4639-00266. [DOI] [PubMed] [Google Scholar]
- 13.Mertz D, Frei R, Jaussi B, Tietz A, Stebler C, Fluckiger U, et al. Throat swabs are necessary to reliably detect carriers of Staphyolcoccus aureus. Clin Infect Dis. 2007;45(4):475–7. doi: 10.1086/520016. [DOI] [PubMed] [Google Scholar]
- 14.Reyes J, Hidalgo M, Diaz L, Rincón S, Moreno J, Vanegas N, et al. Characterization of macrolide resistance in Gram-positive cocci from Colombian hospitals: a countrywide surveillance. Int J Infect Dis. 2007;11(4):329–36. doi: 10.1016/j.ijid.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Huang SS, Rifas-Shiman SL, Warren DK, Fraser VJ, Climo MW, Wong ES, et al. Improving methicillin-resistant Staphylococcus aureus surveillance and reporting in intensive care units. J Infect Dis. 2007;195(3):330–8. doi: 10.1086/510622. [DOI] [PubMed] [Google Scholar]
- 16.Robicsek A, Beaumont JL, Peterson LR. Duration of colonization by methicillin-resistant Staphylococcus aureus after hospital discharge and risk. Clin Infect Dis. 2009;48(7):910–3. doi: 10.1086/597296. [DOI] [PubMed] [Google Scholar]
- 17.Sanford MD, Widmer AF, Bale MJ, Jones RN, Wenzel RP. Efficient detection and long-term persistance of the carriage for methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 1994;19(6):1123–8. doi: 10.1093/clinids/19.6.1123. [DOI] [PubMed] [Google Scholar]
- 18.Scanvic A, Denic L, Gaillon S, Giry P, Andremont A, Lucet JC. Duration of colonization by methicillin-resistant Staphylococcus aureus after hospital discharge and risk factors for prolonged carriage. Clin Infect Dis. 2001;32(10):1393–8. doi: 10.1086/320151. [DOI] [PubMed] [Google Scholar]
- 19.Kluytman J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10(3):505–20. doi: 10.1128/cmr.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee BY. Digital decision making: computer models and antibiotic prescribing in the twenty-first century. Clin Infect Dis. 2008;46(8):1139–41. doi: 10.1086/529441. [DOI] [PubMed] [Google Scholar]
- 21.Lee BY, Biggerstaff BJ. Screening the United States blood supply for West Nile Virus: a question of blood, dollars, and sense. PLoS Med. 2006;3(2):e99. doi: 10.1371/journal.pmed.0030099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belongia EA, Knobloch MJ, Kieke BA, Davis JP, Janette C, Besser RE. Impact of statewide program to promote appropriate antimicrobial drug use. Emerg Infect Dis. 2005;11(6):912–20. doi: 10.3201/eid1106.050118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ostrowsky BE, Trick WE, Sohn AH, Quirk SB, Holt S, Carson LA, et al. Control of vancomycin-resistant enterococcus in health care facilities in a region. N Engl J Med. 2001;344(19):1427–33. doi: 10.1056/NEJM200105103441903. [DOI] [PubMed] [Google Scholar]
- 24.Pronovost P. Interventions to decrease catheter-related bloodstream infections in the ICU: the Keystone Intensive Care Unit Project. Am J Infect Control. 2008;36(10):S171.e1–5. doi: 10.1016/j.ajic.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Schulman J, Stricof RL, Stevens TP, Holzman IR, Shields EP, Angert RM, et al. Development of a statewide collaborative to decrease NICU central line–associated bloodstream infections. J Perinatol. 2009;29(9):591–9. doi: 10.1038/jp.2009.18. [DOI] [PubMed] [Google Scholar]
- 26.Sirio CA, Segel KT, Keyser DJ, Harrison EI, Lloyd JC, Weber RJ, et al. Pittsburgh Regional Healthcare Initiative: a systems approach for achieving perfect patient care. Health Aff (Millwood) 2003;22(5):157–65. doi: 10.1377/hlthaff.22.5.157. [DOI] [PubMed] [Google Scholar]
- 27.Ward MM, Clabaugh G, Evans TC, Herwaldt LA. A successful, voluntary, multicomponent statewide effort to reduce health care–associated infections. Am J Med Qual. 2012;27(1):66–73. doi: 10.1177/1062860611405506. [DOI] [PubMed] [Google Scholar]
- 28.Sirio CA, Keyser DJ, Norman H, Weber RJ, Muto CA. Shared learning and the drive to improve patient safety: lessons learned from the Pittsburgh Regional Healthcare Initiative. In: Henriksen K, Battles JB, Marks ES, Lewin DI, editors. Advances in patient safety: from research to implementation, vol. 3, Surveillance. Rockville (MD): Agency for Healthcare Research and Quality; 2005. pp. 153–65. [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Division of Healthcare Quality Promotion. State-based HAI prevention [Internet] Atlanta (GA): CDC; [updated 2012 Mar; cited 2012 Apr 6]. Available from: http://www.cdc.gov/HAI/stateplans/state-hai-plans/ca.html. [Google Scholar]
- 30.Coyne JS, Richards MT, Short R, Shultz K, Singh SG. Hospital cost and efficiency: do hospital size and ownership type really matter? J Healthc Manag. 2009;54(3):163–74. [PubMed] [Google Scholar]
- 31.Peterson A, Marquez P, Terashita D, Burwell L, Mascola L. Hospital methicillin-resistant Staphylococcus aureus active surveillance practices in Los Angeles County: implications of legislation-based infection control, 2008. Am J Infect Control. 2010;38(8):653–6. doi: 10.1016/j.ajic.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Brown J, Doloresco F, III, Mylotte JM. “Never events”: not every hospital-acquired infection is preventable. Clin Infect Dis. 2009;49(5):743–6. doi: 10.1086/604719. [DOI] [PubMed] [Google Scholar]
- 33.Campbell DA, Jr, Englesbe MJ, Kubus JJ, Phillips LRS, Shanley CJ, Velanovich V, et al. Accelerating the pace of surgical quality improvement: the power of hospital collaboration. Arch Surg. 2010;145(10):985–91. doi: 10.1001/archsurg.2010.220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.