Abstract
Objective
To estimate the economic value of preoperative methicillin-resistant Staphylococcus aureus (MRSA) screening and decolonization for cardiac surgery patients.
Study Design
Monte Carlo decision-analytic computer simulation model.
Methods
We developed a computer simulation model representing the decision of whether to perform preoperative MRSA screening and decolonizing those patients with a positive MRSA culture. Sensitivity analyses varied key input parameters including MRSA colonization prevalence, decolonization success rates, the number of surveillance sites, and screening/decolonization costs. Separate analyses estimated the incremental cost-effectiveness ratio (ICER) of the screening and decolonization strategy from the third-party payer and hospital perspectives.
Results
Even when MRSA colonization prevalence and decolonization success rate were as low as 1% and 25%, respectively, the ICER of implementing routine surveillance was well under $15,000 per quality-adjusted life-year from both the third-party payer and hospital perspectives. The surveillance strategy was economically dominant (less costly and more effective than no testing) for most scenarios explored.
Conclusions
Our results suggest that routine preoperative MRSA screening of cardiac surgery patients could provide substantial economic value to third-party payers and hospitals over a wide range of MRSA colonization prevalence levels, decolonization success rates, and surveillance costs. Healthcare administrators, infection control specialists, and surgeons can compare their local conditions with our study’s benchmarks to make decisions about whether to implement preoperative MRSA testing. Third-party payers may want to consider covering such a strategy.
Infection due to Staphylococcus aureus is a common complication of cardiac surgical procedures. Postoperative S aureus infections have been associated with significant morbidity, prolonged hospitalization, higher medical costs, an increased likelihood of readmission within 90 days postprocedure, and death.1–4 S aureus is the most common cause of sternal wound infections and the leading cause of infective endocarditis in the developed world.1,5 An increasing number of these infections are due to methicillin-resistant S aureus (MRSA), resulting in greater morbidity and mortality than infections from methicillin- sensitive S aureus (MSSA) strains.
Patients about to undergo cardiac surgery tend to have a number of factors predisposing them to postoperative MRSA infections, including older age, comorbid conditions (eg, congestive heart failure, chronic kidney disease, diabetes), and recent hospitalization.6,7 An estimated 30% of Americans are chronic or intermittent carriers of S aureus and nasal MRSA colonization rates from 0.4% to 20.6% have been reported.8–11 Cardiac surgery procedures often are invasive, involving prolonged use of mechanical support devices and indwelling intravenous lines, and introduce foreign materials and tissues (eg, a pacemaker, a prosthetic valve) into the body.1,12
Preoperative screening and decolonization of cardiac surgery patients who are MRSA positive could help prevent postoperative MRSA infections and their sequelae.13–15 Studies have suggested that nasal MRSA colonization is associated with increased rates of postsurgical MRSA infections and poor outcomes, and that decolonization prior to surgery could lower rates of MRSA surgical-site infections and bacteremia.4,11,16,17 However, implementing routine screening and decolonization can be costly, and to date the economic value of such a strategy has not been fully ascertained. Moreover, the appropriateness of such a strategy may differ by rate of MRSA colonization prevalence, which varies geographically and temporally, as well as by individual patient risk factors.
To estimate the economic value of preoperative MRSA screening and decolonization for cardiac surgery patients, we developed a stochastic decision-analytic computer simulation model. Sensitivity analyses varied key model parameters and allowed us to delineate how the cost-effectiveness of this strategy may vary by MRSA colonization prevalence, decolonization success rate, and surveillance/decolonization cost. The results of our model may help clinicians, hospital administrators, third-party payers, and other decision makers determine when and where to use this strategy.
METHODS
Model Structure
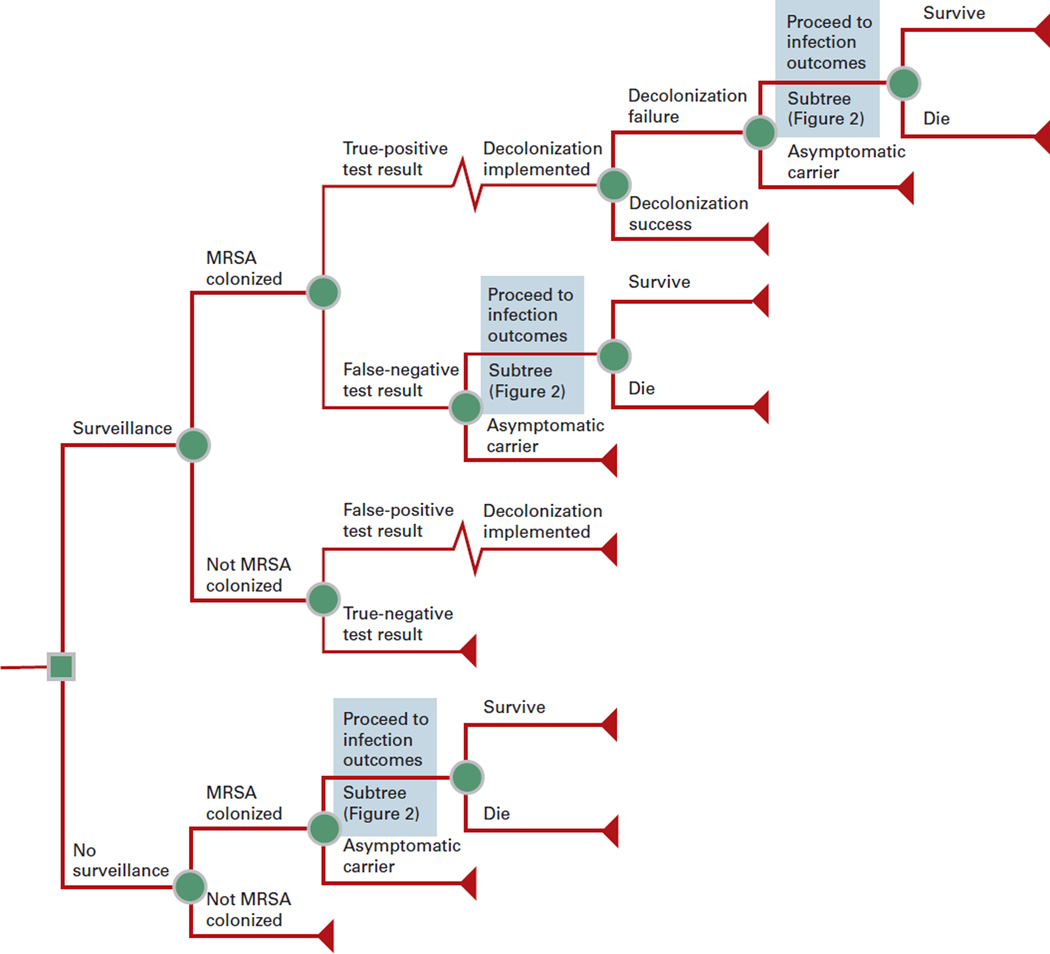
Figure 1 depicts the general structure of our Monte Carlo decision-analytic computer simulation model. This model, constructed using TreeAge Pro 2009 (TreeAge Software, Williamstown, MA), represents the decision of whether to perform preoperative MRSA screening (by means of a single anterior nares culture) and decolonization for those patients with a positive MRSA culture. The time course of each simulation run is the perioperative period for that patient’s cardiac surgical procedure.
Figure 1.
General Decision Model Structure.
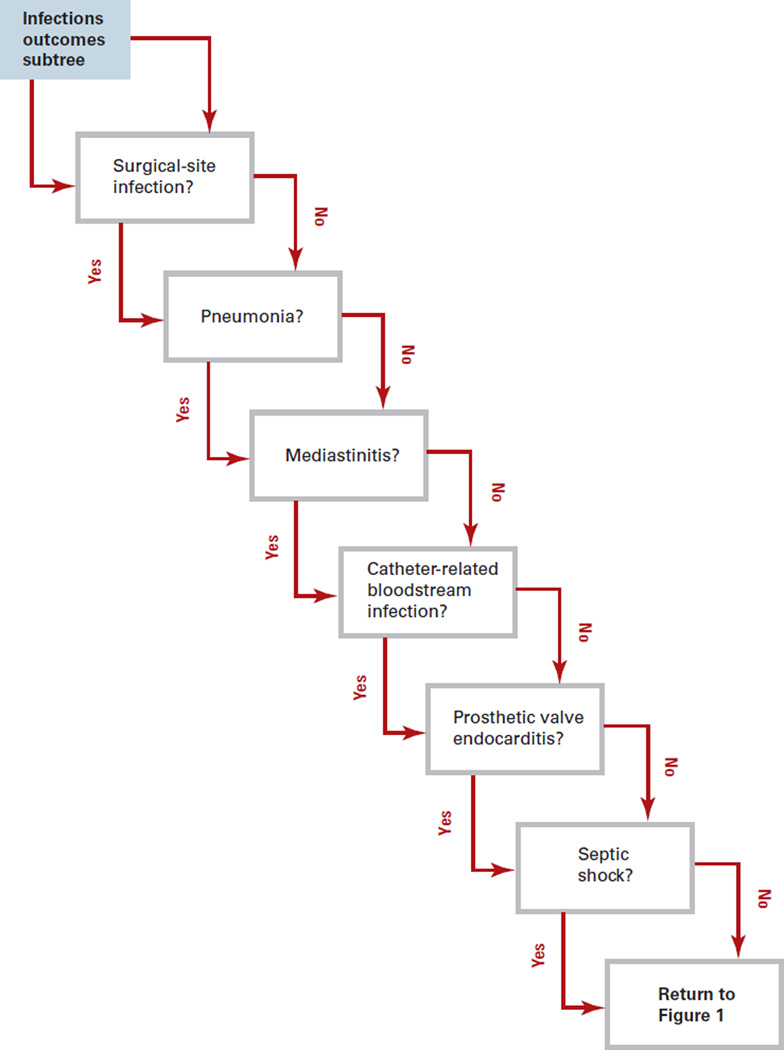
Each patient entering the model had a probability of being MRSA colonized based on the local prevalence of MRSA, but only those who were screened and tested positive underwent decolonization. All positive test results led to decolonization, regardless of true colonization status. Decolonization had a probability of success based on the chosen regimen’s efficacy. Successful decolonization eliminated a patient’s risk of MRSA infection throughout the perioperative period; unsuccessfully decolonized patients retained a probability of developing MRSA infection. Patients who developed a MRSA infection proceeded through the MRSA infection outcomes subtree (Figure 2). Patients entering this subtree had an independent probability of developing each of the following sequelae alone or in combination: mediastinitis, prosthetic valve endocarditis, septic shock, surgical-site infection, catheter- related bloodstream infection, and pneumonia. Returning to Figure 1, each individual with an invasive infection has a probability of death.
Figure 2.
MRSA Infection Outcomes Subtree
In our model the probability of an outcome was not a point estimate, but rather the multiplicative product of the probabilities of all relevant events. For example, the probability of a postoperative MRSA infection was the product of a value drawn from the MRSA colonization probability distribution (range: 0.01 to 0.20) and a value drawn from the postoperative MRSA infection probability distribution (range: 0.0 to 0.4024). Therefore, the probability of a postoperative MRSA infection ranged from the product of the minimum values that can be drawn from each probability distribution (0%) to the product of the maximum possible values drawn from each probability distribution (8.048%).
Each complication necessitates a distinct set of treatment procedures, outcomes, and costs. Surgical-site infections alone do not require additional procedures. Pneumonia requires a chest x-ray. Patients who develop mediastinitis have a probability of undergoing mediastinal exploration. Patients who develop prosthetic valve endocarditis may need a transesophageal or transthoracic echocardiogram, or require a valve replacement procedure. Septic shock necessitates central and arterial line insertion, as well as transesophageal and transthoracic echocardiograms.
MRSA Outcomes
To estimate the probability of each clinical outcome, we conducted a PubMed search using the following key terms: [cardiac], [cardiac surgery], [methicillin-resistant Staphylococcus aureus], and [MRSA]. We limited the search to English-language articles published since 1999 and reviewed all abstracts returned by the search to determine their appropriateness. Case reports, case series, and studies that did not clearly report their population denominators were excluded. We included only studies that clearly characterized study populations and reported on the full set of clinical outcomes from that population (ie, the numerators were properly defined). Parameter distributions in the model reflect the range of values identified by the literature search.
Data Inputs
The median age of simulated patients was 65 years, consistent with the population undergoing cardiac procedures.18 All cost, probability, and utility values and their respective distribution parameters are shown in Table 1. Gamma distributions were used for all cost variables. Hospitalization cost and length of stay (LOS) gamma distributions for each condition were approximated using mean and standard deviation values from the Healthcare Cost and Utilization Project Nationwide Inpatient Sample.19 All probability values drew from beta distributions, with the exceptions of diagnostic test sensitivity and specificity for MRSA, and the probability of developing pneumonia given a MRSA infection, which drew from a triangular distribution. Using a triangular distribution is appropriate when a parameter’s distribution is asymmetric and only the lower limit, mode, and upper limit are known. Such a distribution is shaped like a triangle with the lower limit at the far left of the base, the upper limit at the far right, and the mode at the point of the triangle. Model assumptions were made based on the convention for economic analyses of MRSA and previously published literature. A 3% discount rate was used to standardize all cost and utility measures into 2009 values.
Table 1.
Data Inputs.
| Variable | Type of Distribution |
Mean or Median |
Standard Deviation |
Rangea | Source |
|---|---|---|---|---|---|
| Costs, $ | |||||
| Surveillance | γ | 9.52 | 1.62 | — | Expert consultation |
| Decolonization | γ | 103.95 | 35.34 | — | Expert consultation |
| Vancomycin (per day) | γ | 9.01 | 5.00 | — | 39 |
| Hospitalization | |||||
| Catheter-related bloodstream infection | γ | 9152.40 | 453.00 | — | 19 |
| Mediastinitis | γ | 13,993.30 | 6886.30 | — | 19 |
| Pneumonia | γ | 6033.34 | 184.60 | — | 19 |
| Prosthetic valve endocarditis | γ | 8635.70 | 2098.50 | — | 19 |
| Septic shock | γ | 11,417.41 | 363.89 | — | 19 |
| Surgical-site infection | γ | 7526.00 | 264.20 | — | 19 |
| Procedure | |||||
| Line insertion | γ | 281.66 | 95.77 | — | 19 |
| Chest x-ray | γ | 25.75 | 8.75 | — | 19 |
| Echocardiogram (transesophageal) | γ | 163.72 | 55.66 | — | 19 |
| Echocardiogram (transthoracic) | γ | 164.71 | 56.00 | — | 19 |
| Mediastinotomy exploration | γ | 762.97 | 259.41 | — | 40 |
| Valve replacement | γ | 2437.18 | 828.64 | — | 40 |
| Utilities (QALYs) | |||||
| Catheter-related bloodstream infection | — | 0.530 | — | — | 41 |
| Mediastinitis | — | 0.530 | — | — | 41 |
| Pneumonia | — | 0.580 | — | — | 20 |
| Prosthetic valve endocarditis | — | 0.530 | — | — | 41 |
| Septic shock | — | 0.530 | — | — | 41 |
| Surgical-site infection | — | 0.642 | — | — | 22 |
| Probability, % | |||||
| Surveillance sensitivity | Δ | 92.6 | — | 63.0–97.0 | 42 |
| Surveillance specificity | Δ | 97.1 | — | 92.2–99.5 | 42 |
| Given MRSA colonization | |||||
| MRSA infection | β | 16.67 | 23.57 | — | 10, 15, 43–51 |
| Given MRSA infection | |||||
| Catheter-related bloodstream infection | β | 22.8 | 21.6 | — | 48, 52 |
| Mediastinitis | β | 23.36 | 14.10 | — | 12, 14 |
| Prosthetic valve endocarditis | β | 12.50 | 1.29 | — | 7, 53–54 |
| Pneumonia | Δ | 33.33 | — | 22.22–44.66 | 14 |
| Septic shock | β | 33.29 | 18.79 | — | 18, 55 |
| Surgical-site infection | β | 28.94 | 15.13 | — | 12, 14, 17, 56 |
| Mortality rate given MRSA infection | |||||
| Catheter-related bloodstream infection | β | 31.50 | 12.80 | — | 52, 57–58 |
| Pneumonia | β | 31.37 | 15.20 | — | 59–67 |
| Prosthetic valve endocarditis | β | 17.91 | 8.86 | — | 68–69 |
| Septic shock | β | 48.44 | 2.21 | — | 12, 14 |
| Length of Stay, Days | |||||
| Catheter-related bloodstream infection | γ | 7.5 | 0.2 | — | 19 |
| Mediastinitis | γ | 9.3 | 1.4 | — | 19 |
| Pneumonia | γ | 5.4 | 0.1 | — | 19 |
| Prosthetic valve endocarditis | γ | 14.2 | 0.3 | — | 19 |
| Septic shock | γ | 8.0 | 1.4 | — | 19 |
| Surgical-site infection | γ | 4.9 | 0.1 | — | 19 |
MRSA indicates methicillin-resistant Staphylococcus aureus; QALYs, quality-adjusted life-years.
Upper and lower limits.
Our model measured effectiveness in quality-adjusted life-years (QALYs). An otherwise healthy 65-year-old had a baseline of 0.84 QALY per year of life.20–22 Patients who did not survive lost QALYs based on their projected life expectancy estimates from the Human Mortality Database.23
Simulation Runs
Each simulation run consisted of 1000 hypothetical patients (each with a distinct set of characteristics) about to undergo cardiac surgery. These patients traveled through the model 1000 times each, accruing a distinct set of costs, utilities, and outcomes each time because of the probabilistic nature of the input parameters. This process generated a total of 1 million patient outcomes for each simulation run.
Economic Outcomes
For each simulation run, we estimated the incremental cost-effectiveness ratio (ICER), expressed in US dollars per QALY, of implementing the screening and decolonization strategy using the following formula:
ICER = (CostMRSA Screening ± Decolonization – CostNo MRSA Screening or Decolonization) ÷ (EffectivenessMRSA Screening ± Decolonization – EffectivenessNo MRSA Screening or Decolonization)
Although some debate exists about the ICER threshold below which an intervention is considered cost-effective, $50,000 per QALY is a commonly used cut point, and ICER values less than $20,000 per QALY offer strong economic support for the adoption of an intervention.24 Strategies that are both less costly and more effective are deemed economically dominant.
Separate analyses evaluated the economic value of screening and decolonization from the following perspectives:
Third-party payer. This perspective accounted for the direct medical costs associated with each outcome.
Hospital. This perspective accounted for the additional hospital LOS associated with each outcome. Added LOS resulted in lost hospital bed days for the hospital.12,25,26 A method described by Graves et al converted lost hospital bed days into costs.27,28
Sensitivity Analyses
Sensitivity analyses varied the values of key input parameters and established the model’s robustness. Specific analyses systematically varied MRSA colonization prevalence from 1% to 40%, and decolonization success rates from 25% to 100%. To simulate the effect of testing sites in addition to the anterior nares (eg, throat, axilla, perianal swabs), the cost of testing was varied up to $200. In addition, for each simulation run, probabilistic sensitivity analyses simultaneously varied all input parameters over the ranges listed in Table 1.
RESULTS
Table 2 shows how the ICER for MRSA surveillance varied with MRSA colonization prevalence and decolonization success rate at single-location ($100) and 2-location ($200) surveillance price points from both the third-party payer and hospital perspectives. Cells labeled “surveillance” indicate scenarios in which testing dominates (ie, is less costly and more effective than) no testing. Intermediate decolonization success rate simulations (data not shown) yielded results consistent with those presented.
Table 2.
Incremental Cost-Effectiveness Ratios of Performing Surveillance and Decolonization for Different Cost, MRSA Prevalence, and Decolonization Success Rate Scenariosa
| Decolonization Success Rate | MRSA Colonization Prevalence | ||||
|---|---|---|---|---|---|
| 1% | 2.5% | 5% | 10% | 15% | |
| Third-Party Payer Perspective: Single-Location Surveillance ($100) | |||||
| 25% | 5851 | 2677 | 35 | Surveillance | Surveillance |
| 50% | 2007 | Surveillance | Surveillance | Surveillance | Surveillance |
| 75% | 1210 | Surveillance | Surveillance | Surveillance | Surveillance |
| 100% | 122 | Surveillance | Surveillance | Surveillance | Surveillance |
| Third-Party Payer Perspective: 2-Location Surveillance ($200) | |||||
| 25% | 12,887 | 3161 | 879 | Surveillance | Surveillance |
| 50% | 4551 | 585 | Surveillance | Surveillance | Surveillance |
| 75% | 1751 | Surveillance | Surveillance | Surveillance | Surveillance |
| 100% | 1037 | Surveillance | Surveillance | Surveillance | Surveillance |
| Hospital Perspective: Single-Location Surveillance ($100) | |||||
| 25% | 2224 | Surveillance | Surveillance | Surveillance | Surveillance |
| 50% | Surveillance | Surveillance | Surveillance | Surveillance | Surveillance |
| 75% | Surveillance | Surveillance | Surveillance | Surveillance | Surveillance |
| 100% | Surveillance | Surveillance | Surveillance | Surveillance | Surveillance |
| Hospital Perspective: 2-Location Surveillance ($200) | |||||
| 25% | 4411 | Surveillance | Surveillance | Surveillance | Surveillance |
| 50% | Surveillance | Surveillance | Surveillance | Surveillance | Surveillance |
| 75% | Surveillance | Surveillance | Surveillance | Surveillance | Surveillance |
| 100% | Surveillance | Surveillance | Surveillance | Surveillance | Surveillance |
MRSA indicates methicillin-resistant Staphylococcus aureus.
The incremental cost-effectiveness ratio is expressed in US dollars per quality-adjusted life-year. Cells labeled “surveillance” indicate scenarios in which testing dominated (ie, was less costly and more effective than) no testing.
Third-Party Payer Perspective
Universal MRSA surveillance was either a strongly cost-effective (ICER <$13,000 per QALY) or dominant strategy for a wide variety of decolonization success and MRSA colonization prevalence rates. For scenarios that used a single anterior nares swab ($100 cost of surveillance and decolonization), MRSA testing was the dominant strategy when MRSA colonization prevalence was ≥2.5% and decolonization was ≥50% successful, MRSA prevalence was >5% and decolonization was ≥25% successful, and for all decolonization success rates when MRSA colonization prevalence was ≥10%. Universal MRSA surveillance remained cost-effective (ie, ICER ≤$50,000 per QALY) for all other scenarios when the MRSA colonization prevalence was ≥1%. In scenarios where 2-location surveillance ($200 price point) was utilized, MRSA testing was the dominant strategy when MRSA colonization prevalence was ≥2.5% and decolonization was ≥75% successful, MRSA prevalence was ≥5% and decolonization was ≥50% successful, and when decolonization success rates were ≥25% and MRSA colonization prevalence was ≥10%. Universal MRSA surveillance was cost-effective for all other scenarios where MRSA colonization prevalence was ≥1%.
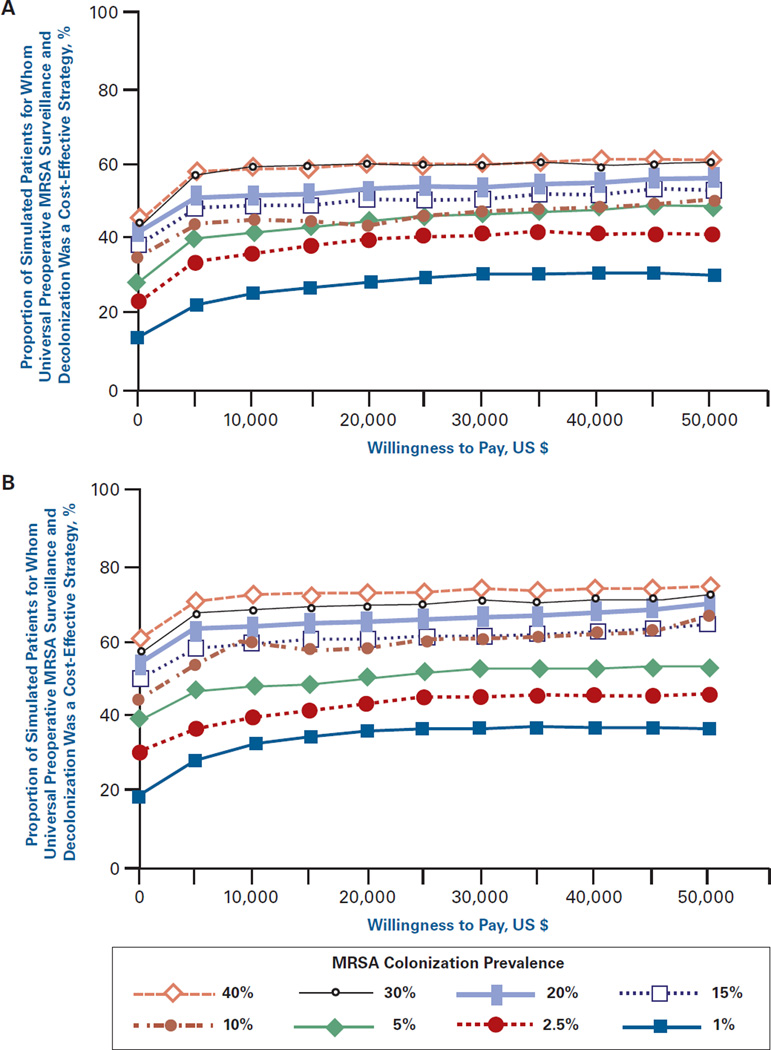
Figure 3A and Figure 3B show the acceptability curves for different MRSA colonization prevalence levels when the cost of decolonization was $200 and the probability of decolonization success was 25% (Figure 3A) and 50% (Figure 3B). These show the proportion of simulated patients for whom preoperative MRSA testing was a cost-effective intervention (compared with no testing) at different willingness-to-pay levels. The curves in Figure 3A indicate, for example, that when MRSA prevalence was 10% and the maximum willingness- to-pay was $50,000, universal surveillance was cost-effective approximately 50% of the time. As the prevalence of MRSA increased, surveillance became the optimal choice the majority of the time at lower willingness-to-pay thresh olds (~$10,000 at 20% prevalence, and ~$2500 for both 30% and 40% MRSA colonization prevalence). In Figure 3B, the rate of decolonization success increases to 50% and all of the curves shift upward.
Figure 3.
Acceptability Curves for Different MRSA Prevalence Rates When (A) Cost of Screening and Decolonization Is $200 and the Probability of Decolonization Success Is 25% and (B) Cost of Screening and Decolonization Is $200 and the Probability of Decolonization Success Is 50%
Hospital Perspective
Simulations using the opportunity cost of lost bed days showed surveillance to be economically dominant at even lower MRSA colonization prevalence and decolonization success rates. For surveillance at a single site (cost $100), when MRSA prevalence was ≥1% and decolonization success probability was ≥50%, or MRSA prevalence was ≥2.5% and decolonization success was ≥25%, surveillance dominated no surveillance. Even when colonization prevalence was <2.5% and decolonization was <50% successful, universal surveillance was still a strongly cost-effective intervention (ICER of $2224 per QALY). In scenarios using a 2-location surveillance strategy ($200 price point), testing was strongly cost-effective (ICER of $4411 per QALY) when MRSA colonization prevalence was 1% and the probability of decolonization success was 25%. Preoperative screening and decolonization was the dominant strategy when MRSA colonization prevalence was ≥2.5% and decolonization success was ≥25%, or MRSA prevalence was ≥1% and the decolonization success rate was ≥50%.
DISCUSSION
Our results suggest that routine preoperative screening of cardiac surgery patients may be a cost-effective strategy for a wide range of MRSA colonization prevalence levels, decolonization success rates, and screening/decolonization costs. In fact, this strategy could even save money for both hospitals and third-party payers. The value of MRSA testing strategy lies in its ability to prevent the substantial morbidity and mortality associated with MRSA infections in cardiac surgery patients.14 Patients undergoing major heart surgery, particularly MRSA carriers, are at risk of experiencing severe postoperative infections, including surgical-site infection, mediastinitis, catheter-related bloodstream infection, septic shock, and infective endocarditis, which in turn increase the length of hospital stays, the cost of care, and the likelihood of mortality.17,29–31
Our study explored a wide range of decolonization success rates because the precise effectiveness of different decolonization regimens for cardiac surgery patients remains unclear. Additionally, antimicrobial resistance, as well as patient compliance, influence decolonization success. Similar sensitivity analyses were used to estimate the impact of doubling the price of screening and decolonization. This was an important consideration because culturing more than 1 body site increases cost, and because local pricing varies for medical and laboratory services as well as pharmaceuticals.
Studies have identified nasal carriage of S aureus as a major risk factor for MRSA infection after cardiac surgery and have shown that intranasal mupirocin treatment reduces sternal wound infection after cardiac surgery.29,32 Segers et al found that a regimen consisting of perioperative naso- and oropharynx application of 0.12% chlorhexidine gluconate decreased cultures positive for S aureus by 57.5% and reduced the rate of lower respiratory tract infections and deep surgical-site infections after cardiac surgery.33 Kluytmans et al reported that a preoperative regimen of nasal mupirocin significantly reduced the rate of surgical-site infections in patients undergoing cardiac surgery, warranting a more structured clinical trial to assess the efficacy of such a decolonization strategy in the cardiac surgical population.29 van Rijen et al also found that the number of surgical-site infections attributable to S aureus can be decreased by screening and decolonizing S aureus carriers on admission to the hospital.34
Although long-term decolonization is hard to achieve as a chronic or transient carrier state is common and many patients become recolonized when they return to home or to other healthcare environments, decolonization only needs to achieve short-term success to prevent perioperative infections.35 Future studies may aim to clarify the short-term success rates of different decolonization regimens. However, based on our model, decolonization needs to be successful in only 25% of attempts to remain a cost-effective strategy for the prevention of perioperative MRSA infection in cardiac surgery patients.
Computer simulation models can assist—but should not replace—human decision making; models can identify and clarify key relationships and the factors that individual surgeons, surgical unit administrators, hospital infection control personnel, and policy makers should consider when determining policies and courses of action. Because our simulations demonstrated cost savings (surveillance dominating no surveillance) from the third-party payer perspective, they provide economic support for insurer-covered preoperative screening and decolonization among patients preparing to undergo cardiac surgery. Models also can raise key questions, guide the design of future epidemiologic and clinical studies, and complement retrospective and prospective clinical and epidemiologic studies. Although clinical and epidemiologic studies can provide important insights, their results may not be applicable to different settings. Computer simulation models can extend and extrapolate findings from retrospective and prospective studies, and they also can help delineate the relationships among various parameters and outcomes.
In many ways, our model may actually underestimate the economic value of MRSA screening. First, while constructing the model, we endeavored to remain conservative about the benefits of MRSA surveillance, deliberately choosing high surveillance and decolonization costs ($100 and $200), including only the most common potential MRSA complications, and using the least expensive procedures to diagnose and treat each MRSA clinical condition. Second, our model did not account for the possible transmission of MRSA from a carrier to other patients; screening and decolonization could limit the spread of MRSA in healthcare settings. Third, our model only accounted for MRSA infections and not MSSA infections. Routine screening also can be used to detect MSSA colonization. Incorporating MSSA screening and decolonization (for those who test positive) into our model would provide additional economic value beyond that for MRSA screening alone, suggesting that preoperative screening and decolonization is an even more cost-effective intervention than estimated herein. Fourth, decreasing the incidence of S aureus infections could spare patients antibiotic courses and in turn reduce the selection pressure for the development of antibiotic resistance.36–38 Finally, additional comorbidities could make patients even more susceptible to poor MRSA infection outcomes.
Limitations
All computer simulation models are simplifications of real life and cannot fully represent the myriad complexities of real life; no model can fully represent every possible MRSA outcome. Model inputs drew from a variety of sources, including studies of varying (and often small) sample size and differing quality of design. The findings of this model may not be applicable to patients undergoing emergency cardiac surgery, as the urgency of surgery may not allow enough time for preoperative screening and decolonization.
CONCLUSIONS
Our results suggest that universal preoperative MRSA screening of cardiac surgery patients is a strongly cost-effective or cost-saving intervention from both third-party payer and hospital perspectives over a wide range of MRSA colonization prevalence, decolonization success rates, and screening/ decolonization costs. Clinical practitioners and hospital administrators stand to benefit from a decrease in healthcare-associated MRSA infections, improved patient outcomes, and the release of valuable monetary, personnel, and physical resources for alternative use. These stakeholders can compare their local conditions with our study’s benchmarks and make decisions about whether to implement preoperative MRSA testing. Third-party payers may want to consider covering such a strategy, as structuring reimbursements to provide coverage for preoperative MRSA screening and decolonization could generate cost savings.
Take-Away Points.
Routine preoperative methicillin-resistant Staphylococcus aureus (MRSA) screening of cardiac surgery patients could provide substantial economic value to third-party payers and hospitals over a wide range of MRSA colonization prevalence levels (≥1%), decolonization success rates (≥25%), and decolonization costs (up to $200 per patient)
-
▪
Healthcare administrators, infection control specialists, and surgeons can compare their local conditions with our study’s benchmarks to make decisions about whether to implement preoperative MRSA testing.
-
▪
Third-party payers may want to consider covering such a strategy.
Acknowledgments
Funding Source: This research was supported by National Institute of General Medical Sciences Models of Infectious Agent Study (MIDAS) Grant 1U54GM088491-0109. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Author Disclosures: The authors (BYL, AEW, RRB, VG, GJL, BYKT, KJS, RRM) report no relationship or financial interest with any entity that would pose a conflict of interest with the subject matter of this article.
Authorship Information: Concept and design (BYL, AEW, RRB, VG, KJS, RRM); acquisition of data (AEW, RRB, VG, GJL); analysis and interpretation of data (AEW, RRB, VG, GJL, BYKT, KJS, RRM); drafting of the manuscript (BYL, AEW, VG, BYKT); critical revision of the manuscript for important intellectual content (BYL, BYKT, KJS); statistical analysis (AEW, RRB, VG, GJL); obtaining funding (BYL); administrative, technical, or logistic support (VG, BYKT, KJS, RRM); and supervision (BYL).
REFERENCES
- 1.Söderquist B. Surgical site infections in cardiac surgery: microbiology. APMIS. 2007;115(9):1008–1111. doi: 10.1111/j.1600-0463.2007.00833.x. [DOI] [PubMed] [Google Scholar]
- 2.Paule SM, Mehta M, Hacek DM, Gonzalzles TM, Robicsek A, Peterson LR. Chromogenic media vs real-time PCR for nasal surveillance of methicillin-resistant Staphylococcus aureus: impact on detection of MRSA-positive persons. Am J Clin Pathol. 2009;131(4):532–539. doi: 10.1309/AJCP18ONZUTDUGAQ. [DOI] [PubMed] [Google Scholar]
- 3.Anderson DJ, Kaye KS, Chen LF, et al. Clinical and financial outcomes due to methicillin resistant Staphylococcus aureus surgical site infection: a multi-center matched outcomes study. PLoS One. 2009;4(12):e8305. doi: 10.1371/journal.pone.0008305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tom TS, Kruse MW, Reichman RT. Update: methicillin-resistant Staphylococcus aureus screening and decolonization in cardiac surgery. Ann Thorac Surg. 2009;88(2):695–702. doi: 10.1016/j.athoracsur.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Cosgrove SE, Fowler VG., Jr Management of methicillin-resistant Staphylococcus aureus bacteremia [published correction appears in Clin Infect Dis.2008;47(3) 437] Clin Infect Dis. 2008;46(suppl 5):S386–S393. doi: 10.1086/533595. [DOI] [PubMed] [Google Scholar]
- 6.Big C, Malani PN. Staphylococcus aureus bloodstream infections in older adults: clinical outcomes and risk factors for in-hospital mortality. J Am Geriatr Soc. 2010;58(2):300–305. doi: 10.1111/j.1532-5415.2009.02666.x. [DOI] [PubMed] [Google Scholar]
- 7.Hsu RB, Chu SH. Impact of methicillin resistance on clinical features and outcomes of infective endocarditis due to Staphylococcus aureus. Am J Med Sci. 2004;328(3):150–155. doi: 10.1097/00000441-200409000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Carrier M, Marchand R, Auger P, et al. Methicillin-resistant Staphylococcus aureus infection in a cardiac surgical unit. J Thorac Cardiovasc Surg. 2002;123(1):40–44. doi: 10.1067/mtc.2002.118505. [DOI] [PubMed] [Google Scholar]
- 9.Gorwitz RJ, Kruszon-Moran D, McAllister SK, et al. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001–2004. J Infect Dis. 2008;197(9):1226–1234. doi: 10.1086/533494. [DOI] [PubMed] [Google Scholar]
- 10.Davis KA, Stewart JJ, Crouch HK, Florez CE, Hospenthal DR. Methicillin-resistant Staphylococcus aureus (MRSA) nares colonization at hospital admission and its effect on subsequent MRSA infection. Clin Infect Dis. 2004;39(6):776–782. doi: 10.1086/422997. [DOI] [PubMed] [Google Scholar]
- 11.Trautmann M, Stecher J, Hemmer W, Luz K, Panknin HT. Intranasal mupirocin prophylaxis in elective surgery. A review of published studies. Chemotherapy. 2008;54(1):9–16. doi: 10.1159/000112312. [DOI] [PubMed] [Google Scholar]
- 12.Reddy SL, Grayson AD, Smith G, Warwick R, Chalmers JA. Methicillin resistant Staphylococcus aureus infections following cardiac surgery: incidence, impact and identifying adverse outcome traits. Eur J Cardiothorac Surg. 2007;32(1):113–117. doi: 10.1016/j.ejcts.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Graf K, Sohr D, Haverich A, Kuhn C, Gastmeier P, Chaberny IF. Decrease of deep sternal surgical site infection rates after cardiac surgery by a comprehensive infection control program. Interact Cardiovasc Thorac Surg. 2009;9(2):282–286. doi: 10.1510/icvts.2009.205286. [DOI] [PubMed] [Google Scholar]
- 14.Mastoraki A, Kriaras I, Douka E, Mastoraki S, Stravopodis G, Geroulanos S. Methicillin-resistant Staphylococcus aureus preventing strategy in cardiac surgery. Interact Cardiovasc Thorac Surg. 2008;7(3):452–456. doi: 10.1510/icvts.2008.176156. [DOI] [PubMed] [Google Scholar]
- 15.Schelenz S, Tucker D, Georgeu C, et al. Significant reduction of endemic MRSA acquisition and infection in cardiothoracic patients by means of an enhanced targeted infection control programme. J Hosp Infect. 2005;60(2):104–110. doi: 10.1016/j.jhin.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 16.Hacek DM, Paule SM, Thomson RB, Jr, Robicsek A, Peterson LR. Implementation of a universal admission surveillance and decolonization program for methicillin-resistant Staphylococcus aureus (MRSA) reduces the number of MRSA and total number of S aureus isolates reported by the clinical laboratory. J Clin Microbiol. 2009;47(11):3749–3752. doi: 10.1128/JCM.01223-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muñoz P, Hortal J, Giannella M, et al. Nasal carriage of S. aureus increases the risk of surgical site infection after major heart surgery. J Hosp Infect. 2008;68(1):25–31. doi: 10.1016/j.jhin.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Combes A, Trouillet JL, Joly-Guillou ML, Chastre J, Gibert C. The impact of methicillin resistance on the outcome of poststernotomy mediastinitis due to Staphylococcus aureus. Clin Infect Dis. 2004;38(6):822–829. doi: 10.1086/381890. [DOI] [PubMed] [Google Scholar]
- 19.Levit K, Wier L, Stranges E, Ryan K, Elixhauser A. HCUP Facts and Figures: Statistics on Hospital-based Care in the United States, 2007. Rockville, MD: Agency for Healthcare Research and Quality; 2009. [Accessed June 9, 2010]. http://www.hcup-us.ahrq.gov/reports/factsandfigures/2007/TOC_2007.jsp. [Google Scholar]
- 20.Gold MR, Franks P, McCoy KI, Fryback DG. Toward consistency in cost-utility analyses: using national measures to create condition-specific values. Med Care. 1998;36(6):778–792. doi: 10.1097/00005650-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care. 2000;38(6):583–637. doi: 10.1097/00005650-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Selai C, Rosser R. Eliciting EuroQol descriptive data and utility scale values from inpatients. A feasibility study. Pharmacoeconomics. 1995;8(2):147–158. doi: 10.2165/00019053-199508020-00006. [DOI] [PubMed] [Google Scholar]
- 23.Human Mortality Database. University of California, Berkeley (USA), and Max Planck Institute for Demographic Research (Germany) [Accessed January 21, 2008];2008 http://www.mortality.org. [Google Scholar]
- 24.Braithwaite RS, Meltzer DO, King JT, Jr, Leslie D, Roberts MS. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care. 2008;46(4):349–356. doi: 10.1097/MLR.0b013e31815c31a7. [DOI] [PubMed] [Google Scholar]
- 25.Bhatia JY, Pandey K, Rodrigues C, Mehta A, Joshi VR. Postoperative wound infection in patients undergoing coronary artery bypass graft surgery: a prospective study with evaluation of risk factors. Indian J Med Microbiol. 2003;21(4):246–251. [PubMed] [Google Scholar]
- 26.Shorr AF, Tabak YP, Killian AD, Gupta V, Liu LZ, Kollef MH. Health-care-associated bloodstream infection: a distinct entity? Insights from a large U.S. database. Crit Care Med. 2006;34(10):2588–2595. doi: 10.1097/01.CCM.0000239121.09533.09. [DOI] [PubMed] [Google Scholar]
- 27.Graves N. Economics and preventing hospital-acquired infection. Emerg Infect Dis. 2004;10(4):561–566. doi: 10.3201/eid1004.020754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graves N, Halton K, Lairson D. Economics and preventing hospital-acquired infection: broadening the perspective. Infect Control Hosp Epidemiol. 2007;28(2):178–184. doi: 10.1086/510787. [DOI] [PubMed] [Google Scholar]
- 29.Kluytmans JA, Mouton JW, Ijzerman EP, et al. Nasal carriage of Staphylococcus aureus as a major risk factor for wound infections after cardiac surgery. J Infect Dis. 1995;171(1):216–219. doi: 10.1093/infdis/171.1.216. [DOI] [PubMed] [Google Scholar]
- 30.Jakob HG, Borneff-Lipp M, Bach A, et al. The endogenous pathway is a major route for deep sternal wound infection. Eur J Cardiothorac Surg. 2000;17(2):154–160. doi: 10.1016/s1010-7940(00)00327-4. [DOI] [PubMed] [Google Scholar]
- 31.Toumpoulis IK, Anagnostopoulos CE, Derose JJ, Jr, Swistel DG. The impact of deep sternal wound infection on long-term survival after coronary artery bypass grafting. Chest. 2005;127(2):464–471. doi: 10.1378/chest.127.2.464. [DOI] [PubMed] [Google Scholar]
- 32.Cimochowski GE, Harostock MD, Brown R, Bernardi M, Alonzo N, Coyle K. Intranasal mupirocin reduces sternal wound infection after open heart surgery in diabetics and nondiabetics. Ann Thorac Surg. 2001;71(5):1572–1578. doi: 10.1016/s0003-4975(01)02519-x. [DOI] [PubMed] [Google Scholar]
- 33.Segers P, Speekenbrink RG, Ubbink DT, van Ogtrop ML, de Mol BA. Prevention of nosocomial infection in cardiac surgery by decontamination of the nasopharynx and oropharynx with chlorhexidine gluconate: a randomized controlled trial. JAMA. 2006;296(20):2460–2466. doi: 10.1001/jama.296.20.2460. [DOI] [PubMed] [Google Scholar]
- 34.van Rijen MM, Bonten M, Wenzel RP, Kluytmans JA. Intranasal mupirocin for reduction of Staphylococcus aureus infections in surgical patients with nasal carriage: a systematic review. J Antimicrob Chemother. 2008;61(2):254–261. doi: 10.1093/jac/dkm480. [DOI] [PubMed] [Google Scholar]
- 35.Cookson BD. The emergence of mupirocin resistance: a challenge to infection control and antibiotic prescribing practice. J Antimicrob Chemother. 1998;41(1):11–18. doi: 10.1093/jac/41.1.11. [DOI] [PubMed] [Google Scholar]
- 36.Gaudreau MC, Lacasse P, Talbot BG. Protective immune responses to a multi-gene DNA vaccine against Staphylococcus aureus. Vaccine. 2007;25(5):814–824. doi: 10.1016/j.vaccine.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 37.Bal AM, Gould IM. Antibiotic resistance in Staphylococcus aureus and its relevance in therapy. Expert Opin Pharmacother. 2005;6(13):2257–2269. doi: 10.1517/14656566.6.13.2257. [DOI] [PubMed] [Google Scholar]
- 38.John CC, Schreiber JR. Therapies and vaccines for emerging bacterial infections: learning from methicillin-resistant Staphylococcus aureus. Pediatr Clin North Am. 2006;53(4):699–713. doi: 10.1016/j.pcl.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Bounthavong M, Hsu DI, Okamoto MP. Cost-effectiveness analysis of linezolid vs vancomycin in treating methicillin-resistant Staphylococcus aureus complicated skin and soft tissue infections using a decision analytic model. Int J Clin Pract. 2009;63(3):376–386. doi: 10.1111/j.1742-1241.2008.01958.x. [DOI] [PubMed] [Google Scholar]
- 40.Drug Topics Red Book 2008. Montvale, NJ: Thomson Healthcare; 2008. [Google Scholar]
- 41.Sackett DL, Torrance GW. The utility of different health states as perceived by the general public. J Chronic Dis. 1978;31(11):697–704. doi: 10.1016/0021-9681(78)90072-3. [DOI] [PubMed] [Google Scholar]
- 42.Reyes J, Hidalgo M, Diaz L, et al. Characterization of macrolide resistance in Gram-positive cocci from Colombian hospitals: a countrywide surveillance. Int J Infect Dis. 2007;11(4):329–336. doi: 10.1016/j.ijid.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 43.von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med. 2001;344(1):11–16. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 44.Ellis MW, Hospenthal DR, Dooley DP, Gray PJ, Murray CK. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis. 2004;39(7):971–979. doi: 10.1086/423965. [DOI] [PubMed] [Google Scholar]
- 45.Corbella X, Dominguez MA, Pujol M, et al. Staphylococcus aureus nasal carriage as a marker for subsequent staphylococcal infections in intensive care unit patients. Eur J Clin Microbiol Infect Dis. 1997;16(5):351–357. doi: 10.1007/BF01726362. [DOI] [PubMed] [Google Scholar]
- 46.Lee YL, Cesario T, Gupta G, et al. Surveillance of colonization and infection with Staphylococcus aureus susceptible or resistant to methicillin in a community skilled-nursing facility. Am J Infect Control. 1997;25(4):312–321. doi: 10.1016/s0196-6553(97)90023-7. [DOI] [PubMed] [Google Scholar]
- 47.Morange-Saussier V, Giraudeau B, van der Mee N, Lermusiaux P, Quentin R. Nasal carriage of methicillin-resistant Staphylococcus aureus in vascular surgery. Ann Vasc Surg. 2006;20(6):767–772. doi: 10.1007/s10016-006-9088-x. [DOI] [PubMed] [Google Scholar]
- 48.Coello R, Glynn JR, Gaspar C, Picazo JJ, Fereres J. Risk factors for developing clinical infection with methicillin-resistant Staphylococcus aureus (MRSA) amongst hospital patients initially only colonized with MRSA. J Hosp Infect. 1997;37(1):39–46. doi: 10.1016/s0195-6701(97)90071-2. [DOI] [PubMed] [Google Scholar]
- 49.Fishbain JT, Lee JC, Nguyen HD, et al. Nosocomial transmission of methicillin-resistant Staphylococcus aureus: a blinded study to establish baseline acquisition rates. Infect Control Hosp Epidemiol. 2003;24(6):415–421. doi: 10.1086/502224. [DOI] [PubMed] [Google Scholar]
- 50.Huang SS, Platt R. Risk of methicillin-resistant Staphylococcus aureus infection after previous infection or colonization. Clin Infect Dis. 2003;36(3):281–285. doi: 10.1086/345955. [DOI] [PubMed] [Google Scholar]
- 51.Garrouste-Orgeas M, Timsit JF, Kallel H, et al. Colonization with methicillin-resistant Staphylococcus aureus in ICU patients: morbidity, mortality, and glycopeptide use. Infect Control Hosp Epidemiol. 2001;22(11):687–692. doi: 10.1086/501846. [DOI] [PubMed] [Google Scholar]
- 52.Pujol M, Peña C, Pallares R, et al. Nosocomial Staphylococcus aureus bacteremia among nasal carriers of methicillin-resistant and methicillin-susceptible strains. Am J Med. 1996;100(5):509–516. doi: 10.1016/s0002-9343(96)00014-9. [DOI] [PubMed] [Google Scholar]
- 53.Yoon HJ, Choi JY, Kim CO, Kim JM, Song YG. A comparison of clinical features and mortality among methicillin-resistant and methicillin-sensitive strains of Staphylococcus aureus endocarditis. Yonsei Med J. 2005;46(4):496–502. doi: 10.3349/ymj.2005.46.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuo CB, Lin JC, Peng MY, Wang NC, Chang FY. Endocarditis: impact of methicillin-resistant Staphylococcus aureus in hemodialysis patients and community-acquired infection. J Microbiol Immunol Infect. 2007;40(4):317–324. [PubMed] [Google Scholar]
- 55.Mekontso-Dessap A, Kirsch M, Brun-Buisson C, Loisance D. Post-sternotomy mediastinitis due to Staphylococcus aureus: comparison of methicillin-resistant and methicillin-susceptible cases. Clin Infect Dis. 2001;32(6):877–883. doi: 10.1086/319355. [DOI] [PubMed] [Google Scholar]
- 56.Hill EE, Peetermans WE, Vanderschueren S, Claus P, Herregods MC, Herijgers P. Methicillin-resistant versus methicillin-sensitive Staphylococcus aureus infective endocarditis. Eur J Clin Microbiol Infect Dis. 2008;27(6):445–450. doi: 10.1007/s10096-007-0458-2. [DOI] [PubMed] [Google Scholar]
- 57.Whitby M, McLaws ML, Berry G. Risk of death from methicillin-resistant Staphylococcus aureus bacteraemia: a meta-analysis. Med J Aust. 2001;175(5):264–267. doi: 10.5694/j.1326-5377.2001.tb143562.x. [DOI] [PubMed] [Google Scholar]
- 58.Johannes RS. Epidemiology of early-onset bloodstream infection and implications for treatment. Am J Infect Control. 2008;36(10):S171, e13–e17. doi: 10.1016/j.ajic.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 59.Wenisch C, Laferl H, Szell M, et al. A holistic approach to MRSA eradication in critically ill patients with MRSA pneumonia. Infection. 2006;34(3):148–154. doi: 10.1007/s15010-006-5107-7. [DOI] [PubMed] [Google Scholar]
- 60.Drinka P, Faulks JT, Gauerke C, Goodman B, Stemper M, Reed K. Adverse events associated with methicillin-resistant Staphylococcus aureus in a nursing home. Arch Intern Med. 2001;161(19):2371–2377. doi: 10.1001/archinte.161.19.2371. [DOI] [PubMed] [Google Scholar]
- 61.DeRyke CA, Lodise TP, Jr, Rybak MJ, McKinnon PS. Epidemiology, treatment, and outcomes of nosocomial bacteremic Staphylococcus aureus pneumonia. Chest. 2005;128(3):1414–1422. doi: 10.1378/chest.128.3.1414. [DOI] [PubMed] [Google Scholar]
- 62.Fleisch F, Zbinden R, Vanoli C, Ruef C. Epidemic spread of a single clone of methicillin-resistant Staphylococcus aureus among injection drug users in Zurich, Switzerland. Clin Infect Dis. 2001;32(4):581–586. doi: 10.1086/318716. [DOI] [PubMed] [Google Scholar]
- 63.Athanassa Z, Siempos II, Falagas ME. Impact of methicillin resistance on mortality in Staphylococcus aureus VAP: a systematic review. Eur Respir J. 2008;31(3):625–632. doi: 10.1183/09031936.00081007. [DOI] [PubMed] [Google Scholar]
- 64.Wunderink RG, Rello J, Cammarata SK, Croos-Dabrera RV, Kollef MH. Linezolid vs vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest. 2003;124(5):1789–1797. [PubMed] [Google Scholar]
- 65.Jeffres MN, Isakow W, Doherty JA, et al. Predictors of mortality for methicillin-resistant Staphylococcus aureus health-care-associated pneumonia: specific evaluation of vancomycin pharmacokinetic indices. Chest. 2006;130(4):947–955. doi: 10.1378/chest.130.4.947. [DOI] [PubMed] [Google Scholar]
- 66.Hageman JC, Uyeki TM, Francis JS, et al. Severe community-acquired pneumonia due to Staphylococcus aureus 2003–04 influenza season. Emerg Infect Dis. 2006;12(6):894–899. doi: 10.3201/eid1206.051141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kollef KE, Reichley RM, Micek ST, Kollef MH. The modified APACHE II score outperforms Curb65 pneumonia severity score as a predictor of 30-day mortality in patients with methicillin-resistant Staphylococcus aureus pneumonia. Chest. 2008;133(2):363–369. doi: 10.1378/chest.07-1825. [DOI] [PubMed] [Google Scholar]
- 68.Gammie JS, O’Brien SM, Griffith BP, Peterson ED. Surgical treatment of mitral valve endocarditis in North America. Ann Thorac Surg. 2005;80(6):2199–2204. doi: 10.1016/j.athoracsur.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 69.David TE, Gavra G, Feindel CM, Regesta T, Armstrong S, Maganti MD. Surgical treatment of active infective endocarditis: a continued challenge. J Thorac Cardiovasc Surg. 2007;133(1):144–149. doi: 10.1016/j.jtcvs.2006.08.060. [DOI] [PubMed] [Google Scholar]