Summary
Arsenic is one of the most widespread environmental carcinogens and has created devastating human health problems worldwide, yet little is known about mechanisms of biotransformation in contaminated regions. Methylarsonic acid [MAs(V)], extensively utilized as an herbicide, is largely demethylated to more toxic inorganic arsenite, which causes environmental problems. To understand the process of demethylation of methylarsenicals, soil samples commonly used on Florida golf courses were studied. Several soil extracts were found to demethylate MAs(V) to inorganic arsenite [As(III)]. From these extracts, a bacterial isolate was capable of reducing MAs(V) to MAs(III) but not of demethylating to As(III). A second bacterial isolate was capable of demethylating MAs(III) to As(III) but not of reducing MAs(V). A mixed culture could carry out the complete process of reduction and demethylation, demonstrating that demethylation of MAs(V) to As(III) is a two-step process. Analysis of the 16S ribosomal DNA sequences of the two organisms identified the MAs(V)-reducing and the MAs(III)-demethylating isolates as belong to Burkholderia and Streptomyces species respectively. This is the first report of a novel pathway of degradation of a methylarsenical herbicide by sequential reduction and demethylation in a microbial soil community, which we propose plays a significant role in the arsenic biogeocycle.
Introduction
Arsenic is the most prevalent toxic environmental toxin, entering the biosphere primarily from geochemical sources but also from anthropogenic activities such as the use of arsenical herbicides. The metalloid produces a wide range of health effects in humans, including cardiovascular and peripheral vascular diseases, diabetes millitus and various forms of cancer (Abernathy et al., 1999). As a consequence of its ubiquity and carcinogenicity, arsenic ranks first on the US Environmental Protection Agency’s (EPA) Superfund List of Hazardous Substances (http://www.atsdr.cdc.gov/cercla/07list.html).
Geothermal introduction of arsenic into the environment predates the origin of life, and its environmental ubiquity has provided a strong and persistent pressure for the evolution of detoxifying mechanisms (Bhattacharjee and Rosen, 2007; Rensing and Rosen, 2009). Most common are efflux systems that lower the concentration of intracellular arsenic (Rosen and Tamas, 2010). Alternate pathways for biotransformation of arsenic include oxidation of arsenite to arsenate, which can be coupled to energy production (Paez-Espino et al., 2009) or methylation to produce a variety of organic species (Bentley and Chasteen, 2002). Both prokaryotic and eukaryotic microbes methylate arsenic by ArsM As(III) S-adenosylmethionine methyltransferases in an established detoxification process (Qin et al., 2006; 2009). At the latest count, nearly 4000 ArsM genes have been deposited in the NCBI database. Most are of bacterial origin, but there are also a considerable number of fungal, algal and vertebrate homologues. The products of the methylation pathway include the less toxic pentavalent methylated arsenicals methylarsonic acid [MAs(V)], dimethylarsinic acid [DMAs(V) or cacodylate], trimethylarsine oxide [TMAsO(V)] (Challenger, 1951) and the non-toxic trivalent volatile trimethylarsine [TMAs(III)] (Cullen, 2005). Arsenic methylation has been proposed to play a significant role in the arsenic biogeocycle among terrestrial, aquatic and atmospheric surroundings (Rensing and Rosen, 2009).
In addition to the widespread biological genesis of methylated arsenicals, salts of MAs(V) and DMAs(V) have been used as herbicides and insect pesticides. During the Vietnam War, DMAs(V), called Agent Blue, was used as a defoliant. At present 154 commercial products with these compounds are used as herbicides. Many of these are for weed control, especially for cotton, ornamental plants, lawns and golf course turf. Approximately 3 000 000 pounds of MAs(V) and 100 000 pounds of DMAs(V) are in commercial use in the USA. In 2006 the EPA denied eligibility for re-registration of MAs(V) and DMAs(V) as herbicides because the agency concluded that the benefits gained from their use did not outweigh the risk to humans (http://www.epa.gov/oppsrrd1/REDs/organic_arsenicals_red.pdf). In a follow-up in 2009, the EPA banned the use of organic arsenical herbicides after 31 December 2013 (EPA, 2009). The sole exception is for treatment of cotton because the EPA does not anticipate that arsenic in cotton will end up in the food supply.
As the EPA noted, however, arsenic, as an element, does not degrade and remains in soil for extended periods, and that the average aerobic half-life of MAs(V) in soil is 240 days. Its chemical form may change, influencing mobility and bioavailability. Soil organisms further methylate these organic arsenicals, including volatilization of the end product of the ArsM pathway, TMAs(III) (Qin et al., 2006; 2009). A major concern of the EPA is that biotransformation between inorganic arsenicals and methylarsenicals is bidirectional, and that noncarcinogenic MAs(V) is demethylated to more toxic and carcinogenic inorganic arsenic through microbial transformations. Demethylation of methylarsenicals has been studied extensively in soil (Von Endt et al., 1968; Woolson et al., 1982; Akkari et al., 1986; Gao and Buran, 1997; Feng et al., 2005; Maki et al., 2006a; Huang et al., 2007), sludge (Sierra-Alvarez et al., 2006), sediment (Hanaoka et al., 1990), seawater (Sanders, 1979) and fresh water (Maki et al., 2006b; 2009). Demethylating microbes have been found in soil (Von Endt et al., 1968; Maki et al., 2006a,b) and lake water (Maki et al., 2004; 2006a). Only in a few cases have specific microorganisms been associated with demethylation such as Mycobacterium neoaurum (Lehr et al., 2003), Montrachet wine yeast (Crecelius, 1977) and Candida humicola (Cullen et al., 1979), and no molecular description of the demethylation pathway has been reported.
Even though the EPA has ordered its use to cease after 31 December 2013, MAs(V) is still commonly applied as an herbicide on golf courses in Florida. The run-off from such golf courses has contaminated the water supplies of Florida municipalities with arsenic (Pichler et al., 2008; Whitmore et al., 2008) and will remain a problem for decades to come. Water percolating through the soil of simulated golf course greens on which MAs(V) was applied is demethylated to As(V) (Feng et al., 2005). In this study we show that demethylation of MAs(V) in these simulated golf course soils is the result of biological activity. No single microorganism isolated from those soils was capable of demethylating MAs(V). We identified a Burkhoderia species capable of reducing MAs(V) to MAs(III) and a Streptomyces species that could demethylate MAs(III) to As(III). In mixed culture the two bacteria carried out the demethylation process from MAs(V) to As(III). These results illustrate a novel pathway for demethylation of an organic arsenical utilizing a communal sequential reduction and demethylation reactions catalysed by different soil micoorganisms.
Results
MAs(V) demethylation by golf course soils
Previously different types of soil samples of varying porosities and organic content from simulated Florida golf course were shown to demethylate MAs(V) (Feng et al., 2005). The ability of extracts from two of these soils, an uncoated sandy soil (S) and a soil consisting of uncoated sand and peat (S+P), to demethylate MAs(V) was examined. Extracts from both types of soil were prepared with ST 10−1 medium (Maki et al., 2004) and incubated with 1 µM MAs(V) at room temperature for 1 week, following which the arsenic species in each culture were determined (Fig. 1A). Neither soil contained arsenic prior to addition of MAs(V), and no transformation of MAs(V) occurred following incubation in medium alone or in extracts of autoclaved soils. In contrast, nearly complete demethylation of MAs(V) to As(III) was observed in either S or S+P extracts. These results strongly indicate that MAs(V) demethylation is a biological process.
Fig. 1.
Demethylation of MAs(V) to As(III) by soil extracts.
A. Transformation of MAs(V) by soil extracts was analysed after 1 week incubation at room temperature by reverse-phase HPLC-ICP-MS, as described under Experimental procedures, where the x-axis represents retention time, and the y-axis represents relative amounts of arsenic expressed as counts per second (cps). Curve 1: 1 µM MAs(V) in ST 10−1 medium. Curve 2: S+P extracted in ST 10−1 medium. Curve 3: autoclaved S+P extracted in ST 10−1 medium containing 1 µM MAs(V). Curve 4: S extracted in ST 10−1 medium containing 1 µM MAs(V). Curve 5: S+P extracted in ST 10−1 medium containing 1 µM MAs(V). B. The transformation of 1 µM MAs(V) by S+P soil extracted in ST 10−1 medium was assayed after incubation for 3 (curve 2), 5 (curve 3) and 7 (curve 4) days at room temperature. Curve 1: 1 µM MAs(V) control incubated in ST 10−1 medium for 7 days.
Although all of the MAs(V) was converted to As(III) by soil extracts, no single organism capable of demethylating MAs(V) was isolated. The possibility that MAs(V) reduction is an obligatory precursor to demethylation was examined. First, the fate of MAs(V) was examined after shorter incubations with the S+P soil extract (Fig. 1B). After 3 days of incubation, MAs(V) was reduced to MAs(III), with some As(III) production. After an additional 2 days, MAs(III) decreased with a corresponding increase in As(III) and, after a week, there was complete conversion to As(III). These results suggest a two-step pathway, with sequential reduction of MAs(V) to MAs(III), followed by demethylation of MAs(III) to As(III).
Isolation and identification of MAs(V)-reducing bacterium
Extracts of both S and S+P were screened for MAs(V)-demethylating organisms. Serial dilutions of the two soil extracts were applied to ST 10−1 agar plates. Ten colonies from S extracts and 22 colonies from S+P extracts were screened for MAs(V) demethylating activity, but none exhibited ability to demethylate MAs(V).
In contrast, of 22 colonies from S+P that were unable to demethylate, eight exhibited ability to reduce MAs(V) to MAs(III) (a representative isolate is shown in Fig. 2). None of the isolates from extracts of S soil was able to reduce MAs(V). Analysis of 16S ribosomal DNA sequences from the eight colonies revealed that they all belong to the Gram-negative bacterium Burkholderia genus (accession number HM641907) (Fig. S1). Because of the selection conditions, the eight were probably derived from the same single isolate, so only one, Burkholderia sp. MR1, was used for further analysis. Microscopic analysis revealed a rod shaped morphology consistent with Burkholderia.
Fig. 2.
Reduction of MAs(V) by Burkholderia sp. MR1. Reduction of MAs(V) by a bacterial isolate identified as Burkholderia sp. MR1 was assayed by reverse-phase HPLC-ICP-MS, as described under Experimental procedures. Curve 1: 1 µM MAs(V) in ST 10−1 medium. Curve 2: cells of Burkholderia sp. MR1 in ST 10−1 medium containing 1 µM MAs(V).
Burkholderia are related to Pseudomonas, so we considered the possibility that the latter could also reduce MAs(V). Pseudomonas pudita strain KT2440 (Herrero et al., 1990) was found to reduce MAs(V) to MAs(III). The ability of P. pudita strain KT2440 and Burkholderia sp. MR1 to reduce MAs(V) was compared in cells grown in Luria–Bertani (LB) broth, ST 10−1 and M9 minimal medium supplemented with glucose as carbon source (Fig. 3). Neither organism exhibited reductase activity when grown in LB medium (Fig. 3B), and both could reduce when grown in either ST 10−1 (Fig. 3A) or M9 basal salts media (Fig. 3C). ST 10−1 is a nutrient-poor medium that better reflects environmental growth conditions than LB but limits total growth of the culture. For that reason, both organisms were grown overnight in LB, washed free of medium, suspended at equivalent cell densities in either ST 10−1 or M9 and incubated at room temperature for the indicated times. Although reduction was better with Burkholderia sp. MR1, both organisms reduced MAs(V) in either ST 10−1 (Fig. 3D) or M9 (Fig. 3F) but not LB (Fig. 3E), suggesting that the effect of growth in LB medium is reversible. A possibility for the lack of reduction in cells grown in LB is that uptake of MAs(V) is lower in those cells. However, there was no significant difference in uptake in P. pudita strain KT2440 and Burkholderia sp. MR1, whether grown in LB or ST 10−1 media (Fig. 4). This suggests that an unidentified component of LB medium may repress or inhibit reduction.
Fig. 3.
Effect of growth media on MAs(V) reductase activity of Burkholderia sp. MR1 and P. putida KT2440.
A–C. Each bacterial strain was inoculated into ST 10−1 medium (A), LB medium (B) or M9 medium supplemented with 0.2% glucose as carbon source (C), and incubated with 1 µM MAs(V) for 3 days at room temperature, following which reduction was analysed by reverse-phase HPCL-ICP-MS.
D–F. Each strain was cultured overnight in LB medium, washed and suspended at a density of A600 of 3.0 in ST 10−1 medium (A), LB medium (B) or M9 medium supplemented with 0.2% glucose as carbon source (C), and then incubated with 1 µM MAs(V) at room temperature for 6 h. In each panel curve 1 shows medium with no cells; curve 2 shows P. putida KT2440 and curve 3 shows Burkholderia sp. MR1.
Fig. 4.
Uptake of MAs(V). The ability of Burkholideria sp. MR1 and P. putida KT2440 to take up MAs(V) was assayed as described under Experimental procedures. (●), Burkholderia sp. MR1 in ST 10−1 medium; (○), Burkholderia sp. MR1 in LB medium; (∆), P. pudita KT2440 in ST 10−1 medium; (▼), P. pudita KT2440 in LB medium. The error bars represent the standard deviation of three assays.
Pseudomonas putida strain KT2440 could only reduce MAs(V) to MAs(III) but could not demethylate MAs(III), unlike the environmental P. putida isolates reported to be able to demethylate MAs(V) (Maki et al., 2006a), which might have acquired additional genes. The P. putida genome has two arsRBCH operons (Canovas et al., 2003). In addition, three additional genes following the ars1 operon putatively encode a phosphatase (accession number NP_744079.1), a monooxygenase (accession number NP_744078.1) and a phosphinothricin N-acetyltransferase (accession number NP_744077.1). The monooxygenase and the phosphinothricin N-acetyltransferase possess around 45% similarity to the NADH : flavin oxidoreductase and the GCN5-related N-acetyltransferase, respectively, in ars operons of B. ambifaria AMMD. The functions of these putative proteins in arsenic metabolism are unknown, so it is possible that they contribute to MAs(V) reductase activity. Strains P. putida TEC1 (Galvao and de Lorenzo, 2005), a derivative of KT2440, and a ∆ars1&2 strain derived from TEC1 by deletion of the two ars operons (gift from V. de Lorenzo) were compared with Burkholderia sp. MR1 and Escherichia coli W3110 (Fig. 5). Each P. pudita strain exhibited MAs(V) reductase activity, demonstrating that the activity is not catalysed by a product of either of the ars operons. In contrast, E. coli did not reduce the pentavalent arsenical. No significant difference was observed, which indicates that MAs(V) metabolism is not catalysed by ars operon genes, at least in P. putida KT2440. These results show that both Burkholderia and Pseudomonas but not E. coli have enzymatic mechanisms for MAs(V) reduction, and that this activity is most likely not encoded by traditional ars operons.
Fig. 5.
The P. putida ars operon does not encode a MAs(V) reductase. MAs(V) reduction was assayed in the indicated bacterial cultures following growth in ST 10−1 medium containing 1 µM MAs(V) for 3 days at room temperature. Curve 1: 1 µM MAs(V) control in ST 10−1 medium; curve 2: E. coli strain W3110; curve 3: Burkholderia sp. MR1; curve 4: P. pudita strain Tec1; curve 5: P. putida strain ∆Ars1&2.
Isolation and identification of MAs(III)-demethylating bacterium
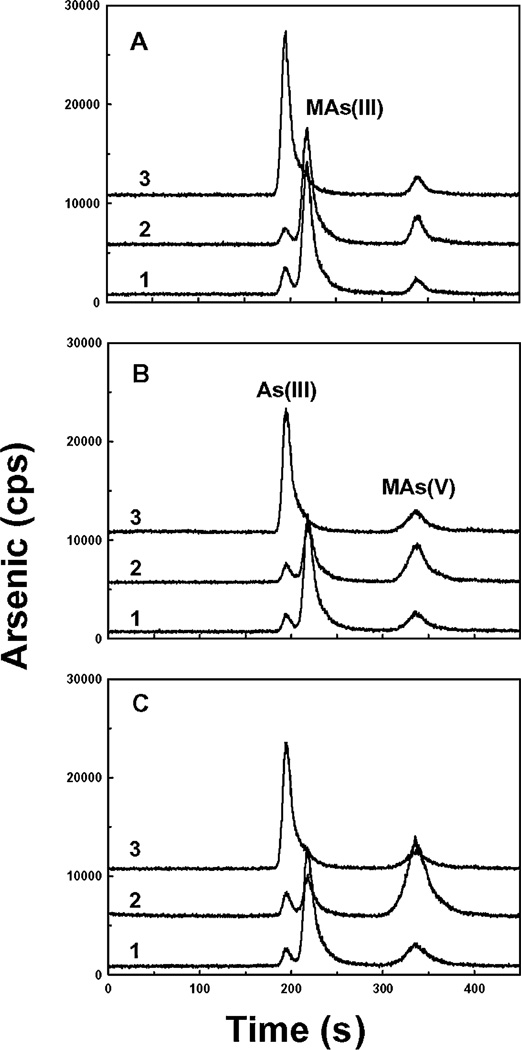
Isolates were screened for the ability to demethylate MAs(III). Individual isolates were incubated with MAs(V) in filter-sterilized S+P extract, either alone or in co-culture with Burkholderia sp. MR1. One isolate capable of demethylation, shown to be a Streptomyces species, was identified (Fig. 6A). By itself, this isolate, Streptomyces sp. MD1, produced only small amounts of As(III) (Fig. 6A, curve 3), but, when co-cultured with Burkholderia sp. MR1, achieved complete transformation of MAs(V) to As(III) (Fig. 6A, curve 4). Since the Burkholderia species only reduces MAs(V) to MAs(III), these results suggest that Streptomyces sp. MD1 catalyses demethylation of MAs(III) to As(III). This was confirmed by demonstrating that Streptomyces sp. MD1, incubated with chemically prepared MAs(III) in the absence of Burkholderia sp. MR1, produced As(III) (Fig. 6B, curve 3). Interestingly, in this experiment much of the MAs(III) spontaneously oxidized to MAs(V) (Fig. 6B, curves 2 and 3), but in co-culture no oxidation was observed (Fig. 6A, curve 4). Analysis of the 16S rDNA sequence (accession number HM641908) from this isolate identified it as a Gram-positive streptomyces (Fig. S2). The cells had a filamentous morphology consistent with that of Streptomyces. Unlike MAs(V) reduction, MAs(III) demethylation was observed with Streptomyces sp. MD1 grown in LB medium, as well as M9 or ST 10−1 medium (Fig. 7), indicating that this activity is constitutive.
Fig. 6.
Demethylation of MAs(V) by Streptomyces sp. MD1. Demethylation of MAs(III) by a bacterial isolate identified as Streptomyces sp. MD1 was assayed by reverse-phase HPLC-ICP-MS, as described under Experimental procedures.
A. Demethylation of MAs(V) in mixed culture. Strains were incubated separately or together in filter-sterilized S+P extract containing 1 µM MAs(V) for 1 week at room temperature. Curve 1: 1 µM MAs(V) in filter-sterilized S+P. Curve 2: Burkholderia sp. MR1. Curve 3: Streptomyces sp. MD1. Curve 4: co-culture of Burkholderia sp. MR1 and Streptomyces sp. MD1.
B. Demethylation of MAs(III) by Streptomyces sp. MD1. MAs(III) was prepared by chemical reduction of MAs(V), as described under Experimental procedures. Line 1: MAs(III) in filter sterilized S+P extract without incubation. Line 2: MAs(III) in filter-sterilized S+P extract after three days of incubation at room temperature. Line 3: Streptomyces sp. MD1 incubated with MAs(III) for 3 days at room temperature.
Fig. 7.
Effect of growth media on MAs(III) demethylating activity of Streptomyces sp. MD1. Streptomyces sp. MD1 was grown overnight in LB medium at 37°C, washed and suspended the same volume in ST 10−1 medium (A), LB medium (B) or M9 medium supplemented with 0.2% glucose as carbon source (C), and incubated with 1 µM MAs(III) for 3 h at room temperature, following which demethylation was analysed by reverse-phase HPCL-ICP-MS. In each panel curve 1 shows 1 µM MAs(III) in medium with no cells and no incubation; curve 2 shows 1 µM MAs(III) in medium with no cells after 3 h; and curve 3 shows Streptomyces sp. MD1 incubated for 3 h in medium containing 1 µM MAs(III).
Discussion
Given the widespread occurrence of arsenic in water and soil, microbial transformation into methylated species takes place continuously worldwide. Although difficult to quantify, we consider it likely that global microbial production of methylarsenicals far exceeds anthropogenic introduction. The major product of the ArsM reaction is DMAs(V), and the final product, which is formed rather slowly, is non-toxic TMAs(III) gas (Cullen, 2005). If this is indeed a pathway for arsenic detoxification, why do microorganisms reduce MAs(V) to the more toxic MAs(III) and then demethylate it to As(III), which is also more toxic than MAs(V)? The most likely explanation is that aerobically As(III) is oxidized to less toxic As(V). Of course, other organisms constantly reduce and methylate As(V) again, which continues the global arsenic cycle. Other possibilities are that MAs(V) reduction produces energy analogous to the role of respiratory arsenate reductases in anaerobes and that demethylation allows for utilization of the methyl groups for biomass. These are unlikely to be assimilatory pathways because the amount of MAs(V) even in highly contaminated soils would not be sufficient for utilization as a primary energy or carbon source, although it could supplement other supplies. In addition, Burkhoderia and Pseudomonas are aerobes that do not have arrAB genes that encode the subunits of the respiratory arsenate reductase.
Regardless of the driving force for evolution of demethylation pathways for MAs(V), the ability has apparently been acquired multiple times by multiple organisms, perhaps as adventitious reactions by pathways with other functions. In some studies single organisms have been reported to carry out conversion of MAs(V) to As(III). For example, Lehr et al. (2003) isolated a strain of Mycobacterium neoaurum that demethylates both MAs(V) and MAs(III). Maki et al. (2006a) identified several strains of P. pudita closely related to strain KT2440 from contaminated sites that could demethylate MAs(V). Although the P. pudita strains examined in this study do not demethylate, environmental isolates may have acquired the requisite genes through horizontal transfer.
In this study we observed that cultures from golf course soil treated with MAs(V) exhibited MAs(V) demethylation as the result of biological activity but were unable to isolate single organisms that could carry out the entire process. Instead, the entire demethylation process required communal action of at least two microbes, reduction by Burkholderia sp. MR1 (or by Pseudomonas sp.) and demethylation by Streptomyces sp. MR1. Many Burkholderia are soil organisms, plant pathogens and/or agriculturally important species. The genome of the related Burkholderia ambifaria AMMD (Fig. 3) has two ars operons and additional non-operon ars genes in Chromosome 1 that include arsR genes for As(III)-responsive transcriptional repressors, arsB genes for arsenite efflux carriers, arsC genes for arsenate reductases, an arsH gene for an NADPH-FMN oxidoreductase and several genes of unknown function, including a putative NADH: flavin oxidoreductase (accession number ABI89703.1) and a putative GCN5-related acetyltransferase genes (accession number ABI89704.1). It is not known whether any of the gene products of the Burkholderia ars operons can reduce MAs(V). The genome contains neither genes for respiratory arsenate reductases or arsenite oxidases nor an arsM gene for an As(III)-S-adenosylmethionione methyltransferase.
Why would Burkholderia sp. MR1 (or P. pudita KT2440) reduce MAs(V) to the more toxic MAs(III)? One explanation is that known Burkholderia and Pseudomonas genomes do not have arsM genes, so cannot further methylate MAs(V) to TMAs(III) gas. These soil organisms take up MAs(V), reduce it to MAs(III), which is then extruded into the medium (Fig. 8). By removing MAs(III) from the cytosol, the cells reduce toxicity to themselves (but not to other organism). Even so, this is probably an adventitious process, especially considering that a P. pudita strain lacking ars operons can still reduce MAs(III). How does MAs(V) get into these bacteria? Perhaps MAs(V) is taken up by phosphate permeases, the route of As(V) uptake into most cells (Rosen and Tamas, 2010). MAs(V) can also be taken up by aquaglyceroporins (AQPs) at low pH as the fully protonated species (Li et al., 2009; McDermott et al., 2010), and both Burkholderia and Pseudomonas have genes encoding AQPs. Or perhaps there are other unidentified transporters that adventitiously take up the pentavalent species. The process of MAs(V) reduction to MAs(III) is also unknown, and identification of the responsible gene(s) is essential. Both organisms have arsC genes for As(V) reduction, but ArsC does not reduce MAs(V). In addition, P. pudita derivatives lacking ars operons reduce MAs(V), demonstrating that MAs(III) is reduced, perhaps adventitiously, by the product of other genes. Once produced, how does the intracellular MAs(III) get out of cells? Both organisms have genes for Acr3 and/or ArsB, the major arsenite extrusion proteins in bacteria (Rosen and Tamas, 2010). It is not known whether these proteins can also catalyse extrusion of MAs(III). AQPs, however, facilitate MAs(III) translocation (Liu et al., 2004). As long as the MAs(III) is produced internally, efflux will always be downhill, allowing a channel such as an AQP to lower the intracellular concentration. In an analogous situation, Sinorhizobium meliloti detoxifies As(V) by internal reduction to As(III) followed by downhill efflux via AqpS (Yang et al., 2005).
Fig. 8.
Demethylation of MAs(V) is a two-step process carried out by a microbial community. Demethylation of MAs(V) in soil takes place by sequential reduction to MAs(III) by Burkholderia sp. MR1 followed by demethylation of MAs(III) to As(III) by Streptomyces sp. MD1. Burkholderia sp. MR1 takes up MAs(V) by unknown transporters (a), possibly phosphate transporters or AQPs, and extrudes MAs(III) by efflux systems (b), possibly ArsB, Acr3 or AQPs. Streptomyces sp. MD1 takes up MAs(III), possibly by AQPs or glucose permeases (c), and extrudes As(III) by ArsB or Acr3 (d).
As MAs(III) appears, other organism are challenged to detoxify it. Note that, as MAs(III) is re-oxidized to MAs(V), Burkholderia continuously reduces it back to MAs(III) (Fig. 6A, curve 4), so other microbes must have evolved MAs(III) detoxification pathways to deal with the build-up of MAs(III). Considering that As(III) is less toxic than MAs(III), demethylation of MAs(III) to As(III) by Streptomyces might be a detoxification process. It is interesting to note that groundwater from a former herbicide production plant on the Menominee River in Wisconsin was shown to contain high concentrations of MAs(III) (McKnight-Whitford et al., 2010), which suggests that first microbial reduction of MAs(V) may be widespread and second that MAs(III) is indeed a problem even with an oxidizing atmosphere. Following demethylation, some microorganisms can re-methylate As(III), but, again, the genome of the closely related Streptomyces ceolicolor does not have an arsM gene. Streptomyces sp. MR1 can take up MAs(III), perhaps via AQPs or by glucose permeases, which also transport MAs(III) (Liu et al., 2006; Jiang et al., 2010). Inside the cells, MAs(III) is demethylated to As(III), again by unknown mechanisms. It is not known whether the methyl group is incorporated into biomass or simply evolves it as CO2. Characterization of this pathway is also essential to more completely understand environmental arsenic cycling. Finally, Streptomyces has both arsB and acr3 genes, so demethylation to As(III) allows for extrusion via one or more of the arsenite efflux systems, where it can be re-oxidized to As(V) and remethylated, continuing the arsenic biogeocycle.
Experimental procedures
Chemicals
The commercial herbicide Ortho Weed-B-Gone Crabgrass Killer Formula II, which contains calcium methanearsonate as its sole active ingredient, was used as the source of monomethylarsonic acid [MAs(V)]. The concentration of stock solutions of MAs(V) was determined by ICP-MS (ELAN 9000, PerkinElmer, Waltham, MA, USA). Unless otherwise mentioned, all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Strains
The P. putida strains used in this study KT2440 (Franklin et al., 1981), Tec1 (Galvao and de Lorenzo, 2005) and a double ars operon strain ∆Ars1&2 derived from Tec1 were kindly supplied by Víctor de Lorenzo, Centro Nacional de Biotecnología–CSIC, Madrid, Spain. Escherichia coli strain W3110 (Bachmann, 1987) was also used.
Demethylation of MAs(V) by soil extracts
Two types of soil samples from simulated golf course greens, including uncoated sand (S) and uncoated sand and peat (S+P), were examined (Feng et al., 2005). To prepare soil extracts, native or autoclaved soils were mixed with ST 10−1 culture medium (0.5 g l−1 Difco Bacto Peptone and 0.05 g l−1 yeast extract (Becton, Dickinson and Company) (Maki et al., 2006b) in a 1:1 ratio (g:ml) and incubated for 2 h with moderate shaking. After centrifugation at 500 r.p.m. for 1 min to remove the soil, each of the resulting supernatant solutions was incubated with 1 µM MAs(V) with shaking at 200 r.p.m. at room temperature for the indicated lengths of time. The cultures were then centrifuged at 13 000 r.p.m. for 10 min to remove cells, and the resulting supernatants were filtered with a Microcon Ultracel YM-3 centrifugal filter (Millipore Corp, Billerica, MA, USA). Arsenic species in the filtered samples were analysed with a Series 2000 HPLC connected to an ELAN 9000 ICP-MS (Perkin Elmer, Waltham, MA, USA) using a Jupiter 5µ C18 300A reverse-phase C18 column (Chromservis s.r.o., Brno, Czech Republic), as described previously (Qin et al., 2006).
Isolation of MAs(V) reducing and MAs(III) demethylating microorganisms
S+P or S soil extracts were diluted and spread on ST 10−1 plates containing 1 µM MAs(V). The plates were incubated for 3 days at room temperature. Single colonies were purified by re-streaking, isolated and incubated in ST 10−1 medium containing 1 µM MAs(V) at room temperature with shaking at 200 r.p.m. To examine the morphology of the isolated strains, the cells were fixed, stained with 1% methylene blue solution and observed by visible microscopy. After 3 days, transformation of MAs(V) was analysed by HPLC-ICP-MS, as described above. Although no single isolate was capable of demethylating MAs(V), a Burkholderia sp. isolate designated Burkholderia sp. MR1 [MAs(V) reducing] (vide infra) reduced MAs(V) to MAs(III). To further screen for MAs(III)-demethylating organisms, S+P soil extracts were filter-sterilized using a 0.2 µm Corning syringe filter and inoculated with the MAs(V)-reducing bacterium Burkholderia sp. or other isolates to reduce MAs(V) to MAs(III).
One isolate of Streptomyces sp. called Streptomyces sp. MD1 (MAs(III) demethylating) (vide infra) was identified through this second round of screening. This isolate was incubated in filter sterilized S+P extract containing with 1 µM MAs(V) in the presence or absence of the Burkholderia isolate for 4 days at room temperature, following which MAs(V) transformation products were analysed by HPLC-ICP-MS. The MAs(III)-demethylating activity was confirmed by incubating the Streptomyces isolate with MAs(III) prepared from MAs(V) by reduction with metabisulfite/thiosulfate (Reay and Asher, 1977). This chemically prepared MAs(III) reagent was adjusted to pH 6.5 and analysed by HPLC-ICP-MS. MAs(III) was added to filter sterilized S+P extract at 1 µM, final concentration, and incubated with or without the Streptomyces sp. isolate for the indicated times, following which transformation of MAs(III) was analysed by HPLC-ICP-MS.
MAs(V) reduction and MAs(III) demethylation in selected growth media
To analyse the effect of growth medium on MAs(V) reductase activity of Burkholderia sp. MR1 and P. putida KT2440, each was separately cultured with 1 µM MAs(V) in ST 10−1, LB (Sambrook et al., 1989) or M9 medium (Sambrook et al., 1989) supplemented with 0.2% D-glucose as carbon source, at room temperature with shaking (200 r.p.m.) for 3 days. To examine the reversibility of inhibition of MAs(V) reduction by LB, Burkholderia sp. MR1 and P. putida KT2440 were cultured in LB overnight. After washing the cells once with either ST 10−1, LB or M9 medium supplemented with 0.2% D-glucose, the cell density was adjusted to A600 = 3.0, transferred to the same medium and incubated with 1 µM MAs(V) at room temperature with shaking (200 r.p.m.) for 6 h. To examine the effect of growth media on MAs(III)-demethylating activity, Streptomyces sp. MD1 was cultured in LB medium overnight at room temperature, washed once with ST 10−1, LB or M9 medium supplemented with 0.2% D-glucose, transferred into the same volume of the respective media and incubated with 1 µM MAs(III) at room temperature with shaking (200 r.p.m.) for 3 h. MAs(V) transformation in each culture was analysed by HPLC-ICP-MS.
MAs(V) reduction by Pseudomonas putida KT2440 derivatives
MAs(V) reductase activity of P. putida strains was analysed and compared with that of Burkholderia sp. MR1 and E. coli strain W3110. Each strain was separately incubated with fresh 1 µM MAs(V) in ST 10−1 medium at RT with shaking (200 r.p.m.). Cultures of P. putida Tec1 and ∆Ars1&2 were supplemented with 20 µg ml−1 uracil. After 3 days, MAs(V) transformation in each culture was analysed by HPLC-ICP-MS.
MAs(V) uptake by Burkholderia sp. MR1 and P. putida KT2440
Cultures (100 ml) of Burkholderia sp. MR1 and P. putida strains of KT2440 were grown to A600 = 1 in LB medium. The cells were collected, washed once and suspended in either 10−1 or LB medium at a density of A600 = 10. MAs(V) was added to 1 ml of the prepared cell solutions at a final concentration of 1 mM to initiate the transport reaction. Portions (0.1 ml) were withdrawn at the indicated times, filtered through 0.45 mm pore size nitrocellulose filters (Millipore), washed twice with 4 ml of the same medium and air dried. The filters were digested with 0.3 ml of 70% HNO3 at 70°C for 30 min, allowed to cool to room temperature and diluted with 5.7 ml of deionized water to produce a final concentration of HNO3 of 3.5%. Arsenic in the prepared samples was quantified by ICP-MS. Arsenic solutions in the range of 1–50 p.p.b. were prepared in 3.5% HNO3 using an arsenic standard (Ricca Chemical Company, Arlington, TX, USA).
16S rDNA sequence analysis
Total DNA from isolates was purified following cetyltrimethylammonium bromide extraction (Wilson, 2001). For the MAs(III)-demethylating isolate it was first necessary to treat with lysozyme before CTAB extraction (Zehner et al., 2005). Primers were designed from the highly conserved regions of the bacterial 16S ribosomal DNA(Lane et al., 1985; Weisburg et al., 1991). The sequences of two sense oligonucleotides, f27 and f2L, were, respectively, 5′-AGAGTTTGATCCTG GCTCAG-3′ and 5′-CCAGCAGCCGCGGTAATAC-3′ and those of the two antisense oligonucleotides, 1492r and r3L, were, respectively, 5′-GGCTACCTTGTTACGACTT-3′ and 5′-TTGCGCTCGTTGCGGGACT-3′. To amplify 16S rDNA, polymerase chain reaction (PCR) was performed for each of the extracted total DNA with an annealing temperature of 61°C using Pfu Turbo DNA polymerase (STRATAGENE) and primer set of 27f and 1492r. Each PCR product was gel-purified using a QIAquick Gel Extraction Kit (QIAGEN Sciences) and then incubated with dATP, MgCl2 and Taq polymerase (Invitrogen) at 75°C for 1 h to attach dATP to the 3′ end. Each dATP-attached fragment was TA-cloned into pGEM-T easy vector (Promega). T7 (sense) and SP6 (antisense) primers 5′-TAATACGACTCACTATAGGG-3′ and 5′-ATTTAGGTGACACTATAGAA-3′, respectively, were designed from the pGEM-T easy vector. Sequencing reactions for each cloned 16S rDNA were performed with f27, f2L, 1492r, r3L, T7 or SP6 primers, and each sequence was analysed using a CEQ 2000XL DNA Analysis System (Beckman Coulter). The partial sequence obtained for each 16S rDNA was searched with NCBI BLAST to determine species of each isolate. The GenBank accession numbers for the 16S rDNA sequences of Burkholderia sp. MR1 and Streptomyces sp. MD1 are HM641907 and HM641908 respectively. For phylogenetic analyses, each 16S rDNA sequence was aligned with those of 10 randomly selected strains from the same genus available in the DDBJ/EMBL/ GenBank databases using CLUSTAL W version 1.83 (Thompson et al., 1994) at the DDBJ web site (http://clustalw.ddbj.nig.ac.jp/top-j.html). Phylogenetic tree for each genus was constructed by neighbour-joining (Saitou and Nei, 1987), with distance calculation by a two-parameter method (Kimura, 1980) and drawn with TreeView (Page, 1996).
Acknowledgements
This work was supported by NIH grants GM55425 to BPR. We thank Victor de Lorenzo for the gift of strains and for valuable discussions.
Footnotes
Supporting information
Additional Supporting Information may be found in the online version of this article:
Fig. S1. Phylogenetic relatedness of Burkholderia sp. MR1. A phylogenetic tree was constructed based on 16S rDNA sequences of the MAs(V) reducing strain Burkholderia sp. MR1 and 10 selected species from the genus Burkholderia. Bootstrap values calculated for 1000 subsets are indicated on each branch. The numbers in parentheses indicate accession numbers for each species, and the scale bar represents 1% sequence dissimilarity.
Fig. S2. Phylogenetic relatedness of Streptomyces sp. MD1. A phylogenetic tree was constructed based on 16S rDNA sequences of the MAs(III) demethylating strain Streptomyces sp. MD1 and 10 selected species from the genus Streptomyces. Bootstrap values calculated for 1000 subsets are indicated on each branch. The numbers in parentheses indicate accession numbers for each species, and the scale bar represents 1% sequence dissimilarity.
Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Abernathy CO, Liu YP, Longfellow D, Aposhian HV, Beck B, Fowler B, et al. Arsenic: health effects, mechanisms of actions, and research issues. Environ Health Perspect. 1999;107:593–597. doi: 10.1289/ehp.99107593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkari KH, Frans RE, Lavy TL. Factors affecting degradation of MSMA in soil. Weed Sci. 1986;34:781–788. [Google Scholar]
- Bachmann BJ. In: Derivations and genotypes of some mutant derivatives of Escherichia coli K12. In Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. Neidhardt FC, Ingraham JL, Low KB, Magasanik B, Schaechter M, Umbarger HE, editors. Washington, DC, USA: American Society for Microbiology; 1987. pp. 1190–1219. [Google Scholar]
- Bentley R, Chasteen TG. Microbial methylation of metalloids: arsenic, antimony, and bismuth. Microbiol Mol Biol Rev. 2002;66:250–271. doi: 10.1128/MMBR.66.2.250-271.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee H, Rosen BP. Arsenic metabolism in prokaryotic and eukaryotic microbes. In: Nies DHS, Simon S, editors. Molecular Microbiology of Heavy Metals. Heidelberg, Germany: Springer-Verlag; 2007. pp. 371–406. [Google Scholar]
- Canovas D, Cases I, de Lorenzo V. Heavy metal tolerance and metal homeostasis in Pseudomonas putida as revealed by complete genome analysis. Environ Microbiol. 2003;5:1242–1256. doi: 10.1111/j.1462-2920.2003.00463.x. [DOI] [PubMed] [Google Scholar]
- Challenger F. Biological methylation. Adv Enzymol Relat Subj Biochem. 1951;12:429–491. doi: 10.1002/9780470122570.ch8. [DOI] [PubMed] [Google Scholar]
- Crecelius EA. Changes in the chemical speciation of arsenic following ingestion by man. Environ Health Perspect. 1977;19:147–150. doi: 10.1289/ehp.7719147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen WR. The toxicity of trimethylarsine: an urban myth. J Environ Monit. 2005;7:11–15. doi: 10.1039/b413752n. [DOI] [PubMed] [Google Scholar]
- Cullen WR, McBride BC, Pickett AW. The transformation of arsenicals by Candida humicola. Can J Microbiol. 1979;25:1201–1205. doi: 10.1139/m79-187. [DOI] [PubMed] [Google Scholar]
- EPA (Environmental Protection Agency) Organic arsenicals: product cancellation order and amendments to terminate uses. Fed Regist. 2009;74:50187–50194. [Google Scholar]
- Feng M, Schrlau JE, Snyder R, Snyder GH, Chen M, Cisar JL, Cai Y. Arsenic transport and transformation associated with MSMA application on a golf course green. J Agric Food Chem. 2005;53:3556–3562. doi: 10.1021/jf047908j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin FC, Bagdasarian M, Bagdasarian MM, Timmis KN. Molecular and functional analysis of the TOL plasmid pWWO from Pseudomonas putida and cloning of genes for the entire regulated aromatic ring meta cleavage pathway. Proc Natl Acad Sci USA. 1981;78:7458–7462. doi: 10.1073/pnas.78.12.7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvao TC, de Lorenzo V. Adaptation of the yeast URA3 selection system to gram-negative bacteria and generation of a {delta}betCDE Pseudomonas putida strain. Appl Environ Microbiol. 2005;71:883–892. doi: 10.1128/AEM.71.2.883-892.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Buran RG. Environmental factors affecting rates of arsine evolution from mineralization of arsenicals in soil. J Environ Qual. 1997;26:753–763. [Google Scholar]
- Hanaoka K, Hasegawa S, Kawabe N, Tagawa S, Kaise T. Aerobic and anaerobic degradation of several arsenicals by sedimentary micro-organisms. Appl Organomet Chem. 1990;4:239–243. [Google Scholar]
- Herrero M, de Lorenzo V, Timmis KN. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J Bacteriol. 1990;172:6557–6567. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JH, Scherr F, Matzner E. Demethylation of dimethylarsinic acid and arsenobetaine in different organic soils. Water Air Soil Pollut. 2007;182:31–41. [Google Scholar]
- Jiang X, McDermott JR, Abdul Ajees A, Rosen BP, Liu Z. Trivalent arsenicals and glucose use different translocation pathways in mammalian GLUT1. Metallomics. 2010;2:211–219. doi: 10.1039/b920471g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Lane DJ. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic Acid Techniques in Bacterial Systematics. Chichester, UK: John Wiley & Sons; 1991. pp. 115–175. [Google Scholar]
- Lehr CR, Polishchuk E, Delisle MC, Franz C, Cullen WR. Arsenic methylation by microorganisms isolated from sheepskin bedding materials. Hum Exp Toxicol. 2003;22:325–334. doi: 10.1191/0960327103ht353oa. [DOI] [PubMed] [Google Scholar]
- Li RY, Ago Y, Liu WJ, Mitani N, Feldmann J, McGrath SP, et al. The rice aquaporin Lsi1 mediates uptake of methylated arsenic species. Plant Physiol. 2009;150:2071–2080. doi: 10.1104/pp.109.140350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Carbrey JM, Agre P, Rosen BP. Arsenic trioxide uptake by human and rat aquaglyceroporins. Biochem Biophys Res Commun. 2004;316:1178–1185. doi: 10.1016/j.bbrc.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Liu Z, Sanchez MA, Jiang X, Boles E, Landfear SM, Rosen BP. Mammalian glucose permease GLUT1 facilitates transport of arsenic trioxide and methylarsonous acid. Biochem Biophys Res Commun. 2006;351:424–430. doi: 10.1016/j.bbrc.2006.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott JR, Jiang X, Beene LC, Rosen BP, Liu Z. Pentavalent methylated arsenicals are substrates of human AQP9. Biometals. 2010;23:119–127. doi: 10.1007/s10534-009-9273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight-Whitford A, Chen B, Naranmandura H, Zhu C, Le XC. New method and detection of high concentrations of monomethylarsonous acid detected in contaminated groundwater. Environ Sci Technol. 2010;44:5875–5880. doi: 10.1021/es100273b. [DOI] [PubMed] [Google Scholar]
- Maki T, Hasegawa H, Watarai H, Ueda K. Classification for dimethylarsenate-decomposing bacteria using a restrict fragment length polymorphism analysis of 16S rRNA genes. Anal Sci. 2004;20:61–68. doi: 10.2116/analsci.20.61. [DOI] [PubMed] [Google Scholar]
- Maki T, Takeda N, Hasegawa H, Ueda K. Isolation of monomethylarsonic acid-mineralizing bacteria from arsenic contaminated soils of Ohkunoshima Island. Appl Organometal Chem. 2006a;20:538–544. [Google Scholar]
- Maki T, Watarai H, Kakimoto T, Takahashi M, Hasegawa H, Ueda K. Seasonal dynamics of dimethylarsenic acid degrading bacteria dominated in Lake Kibagata. Geomicrobiol J. 2006b;23:311–318. [Google Scholar]
- Maki T, Hirota W, Ueda K, Hasegawa H, Azizur Rahman M. Seasonal dynamics of biodegradation activities for dimethylarsinic acid (DMA) in Lake Kahokugata. Chemosphere. 2009;77:36–42. doi: 10.1016/j.chemosphere.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Paez-Espino D, Tamames J, de Lorenzo V, Canovas D. Microbial responses to environmental arsenic. Biometals. 2009;22:117–130. doi: 10.1007/s10534-008-9195-y. [DOI] [PubMed] [Google Scholar]
- Page RD. TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- Pichler T, Brinkmann R, Scarzella GI. Arsenic abundance and variation in golf course lakes. Sci Total Environ. 2008;394:313–320. doi: 10.1016/j.scitotenv.2008.01.046. [DOI] [PubMed] [Google Scholar]
- Qin J, Rosen BP, Zhang Y, Wang G, Franke S, Rensing C. Arsenic detoxification and evolution of trimethylarsine gas by a microbial arsenite S-adenosylmethionine methyltransferase. Proc Natl Acad Sci USA. 2006;103:2075–2080. doi: 10.1073/pnas.0506836103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Lehr CR, Yuan C, Le XC, McDermott TR, Rosen BP. Biotransformation of arsenic by a Yellowstone thermoacidophilic eukaryotic alga. Proc Natl Acad Sci USA. 2009;106:5213–5217. doi: 10.1073/pnas.0900238106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reay PF, Asher CJ. Preparation and purification of 74As-labeled arsenate and arsenite for use in biological experiments. Anal Biochem. 1977;78:557–560. doi: 10.1016/0003-2697(77)90117-8. [DOI] [PubMed] [Google Scholar]
- Rensing C, Rosen BP. Heavy metals cycles (arsenic, mercury, selenium, others. In: Schaechter M, editor. Encyclopedia of Microbiology. Oxford, UK: Elsevier; 2009. pp. 205–219. [Google Scholar]
- Rosen BP, Tamas MJ. Arsenic transport in prokaryotes and eukaryotic microbes. Adv Exp Med Biol. 2010;679:47–55. doi: 10.1007/978-1-4419-6315-4_4. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sanders JG. Microbial role in the demethylation and oxidation of methylated arsenicals in seawater. Chemosphere. 1979:135–137. [Google Scholar]
- Sierra-Alvarez R, Yenal U, Field JA, Kopplin M, Gandolfi AJ, Garbarino JR. Anaerobic biotransformation of organo-arsenical pesticides monomethylarsonic acid and dimethylarsinic acid. J Agric Food Chem. 2006;54:3959–3966. doi: 10.1021/jf053223n. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Endt DW, Kearney PC, Kafman DD. Degradation of monosodium methanearsonic acid by soil microorganisms. J Agric Food Chem. 1968;16:17–20. [Google Scholar]
- Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmore TJ, Riedinger-Whitmore MA, Smoak JM, Kolasa KV, Goddard EA, Bindler R. Arsenic contamination of lake sediments in Florida: evidence of herbicide mobility from watershed soils. J Paleolimnol. 2008:869–884. [Google Scholar]
- Wilson K. Preparation of genomic DNA from bacteria. Curr Protoc Mol Biol. 2001 doi: 10.1002/0471142727.mb0204s56. Chapter 2: Unit 2.4. [DOI] [PubMed] [Google Scholar]
- Woolson EA, Aharonson N, Ladevaia R. Application of the high-performance liquid chromatography-flameless atomic absorption method ot the study of alkyl arsenical herbicide metabolism in soil. J Agric Food Chem. 1982;30:580–584. [Google Scholar]
- Yang HC, Cheng J, Finan TM, Rosen BP, Bhattacharjee H. Novel pathway for arsenic detoxification in the legume symbiont Sinorhizobium meliloti . J Bacteriol. 2005;187:6991–6997. doi: 10.1128/JB.187.20.6991-6997.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehner S, Kotzsch A, Bister B, Süssmuth RD, Méndez C, Salas JA, van Pée KH. A regioselective tryptophan 5-halogenase is involved in pyrroindomycin biosynthesis in Streptomyces rugosporus LL-42D005. Chem Biol. 2005;12:445–452. doi: 10.1016/j.chembiol.2005.02.005. [DOI] [PubMed] [Google Scholar]