Abstract
Cutaneous leishmaniasis (CL) and its associated complications, including mucocutaneous leishmaniasis (MCL) and diffuse CL (DCL) have emerged as important neglected tropical diseases in Latin America, especially in areas associated with human migration, conflict, and recent deforestation. Because of the limitations of current chemotherapeutic approaches to CL, MCL, and DCL, several prototype vaccines are in different states of product and clinical development. We constructed and utilized a Markov decision analytic computer model to evaluate the potential economic value of a preventative CL vaccine in seven countries in Latin America: Bolivia, Brazil, Colombia, Ecuador, Mexico, Peru, and Venezuela. The results indicated that even a vaccine with a relative short duration of protection and modest efficacy could be recommended for use in targeted locations, as it could prevent a substantial number of cases at low-cost and potentially even result in cost savings. If the population in the seven countries were vaccinated using a vaccine that provides at least 10 years of protection, an estimated 41,000-144,784 CL cases could be averted, each at a cost less than the cost of current recommended treatments. Further, even a vaccine providing as little as five years duration of protection with as little as 50% efficacy remains cost-effective compared with chemotherapy; additional scenarios resembling epidemic settings such as the one that occurred in Chaparral, Colombia in 2004 demonstrates important economic benefits.
Keywords: cutaneous leishmaniasis, vaccine, economics, Latin America
1. Introduction
An estimated 1-1.5 million new cases of cutaneous leishmaniasis (CL), a parasitic infection transmitted to humans by the phlebotomine sand fly, occur each year worldwide[1]. Approximately 62,000 of these cases occur in South and Central America and the Caribbean[2]. In recent decades, the incidence of human infection has increased, largely due to human migration, deforestation, urbanization, and adaptation of the Leishmania parasite to additional vectors and mammalian hosts[3]. Movement of populations due conflict and narcotics trafficking has also emerged as risk factors for CL in Latin America[4]. CL infection usually leads to the appearance of skin lesions within several weeks, which typically self heal in months to years[5]. Approximately a dozen Leishmania species causing cutaneous disease are present in South and Central America. CL is sometimes associated with severe chronic outcomes[6]. While cutaneous lesions may heal spontaneously without treatment, infection with some Leishmania species may spread to the nasal mucosa or disseminate to multiple locations on the body many years after initial infection. Mucocutaneous leishmaniasis (MCL) rarely heals if untreated, often resulting in severe scarring and death[7]. Ninety percent of MCL cases globally occur in Bolivia, Brazil, and Peru[8], making the disease of significant public health importance regionally. Selected Leishmania species can also cause diffuse CL (DCL), where non-ulcerative parasite-positive nodules disseminate throughout the body[5]. DCL is usually resistant to treatment and does not self-cure.
Although several drugs are available for CL treatment, many have limitations. The World Health Organization (WHO) recommends pentavalent antimonials as the first line treatment for CL[9]. More recently, liposomal amphotericin B, miltefosine, and other agents have emerged as attractive alternative chemotherapies[10-11]. Despite the high efficacy against CL associated with these drugs, lengthy treatment regimens and toxic drug side-effects may prevent completion of a full treatment regimen. Furthermore, the effectiveness of these treatments against MCL and DCL is less clear.
As an alternative to new chemotherapies, several preventative and therapeutic vaccines for CL are now in different stages of product and clinical development[12-14]. For CL, this alternative appears feasible, as most recovered CL cases are resistant or do not present clinical manifestations of CL to subsequent infections[13]. Subunit vaccines encoding Leishmania antigens and epitopes[14] are of particular interest; several vaccines comprised of sand fly salivary proteins alone or in combination with Leishmania antigens are under development[15]. For leishmaniasis, there is strong evidence that immunity to a salivary protein from the vector adversely impacts parasite survival and growth contributing to control of the disease [16-17]. Our group is pursuing a prototype vaccine against CL caused by L. mexicana infections in Mesoamerica (i.e., southern Mexico and Central America), which is comprised of a recombinant parasite-derived nucleoside hydrolase (NH 36, Chale Balboa et al, 2009[18]), together with one or more antigens from the sand fly of the genus Lutzomyia [19].
Evaluating the potential economic value of a vaccine early in development can help shape the vaccine's characteristics and prepare for successful and timely market release. While some living in CL-endemic countries could undoubtedly benefit from a vaccine, the most appropriate price and target population for the vaccine is less clear. We constructed an economic model of CL infection to delineate the vaccine cost and infection risk required for a preventative vaccine to be beneficial under a range of possible vaccine profiles (i.e., vaccine efficacy, duration of protection, etc).
2. Methods
2.1. Model Structure
We constructed a Markov decision analytic computer simulation model in TreeAge Pro 2009 (TreeAge Software, Williamstown, MA) to evaluate the potential economic value of a cutaneous leishmaniasis vaccine from the societal perspective in seven endemic countries in Latin America: Bolivia, Brazil, Colombia, Ecuador, Mexico, Peru, and Venezuela. Individuals entered the model before they reach one year of age; those vaccinated were offered all doses of the vaccine within the first year, with no attenuated CL risk until completion of the full vaccine regimen. All individuals continued to cycle in the model until death unrelated to leishmaniasis. Each year, individuals had probabilities of transitioning away from or remaining in one of five mutually exclusive Markov states:
-
! !
Uninfected: Individual was healthy and uninfected.
-
! !
Cutaneous Leishmaniasis (CL): Individual was currently infected with CL, develops one or more skin lesions, and only stayed in this state for one year.
-
! !
Mucocutaneous Leishmaniasis (MCL): Individual contracted a more severe disease manifestation usually secondary to CL infection, affecting the oral and nasal mucosa. Scarring is common and disease rarely resolves without treatment. Those affected remain in this state until cured with no relapse or death.
-
! !
Diffuse Cutaneous Leishmaniasis (DCL): Individual contracted a more severe disease manifestation usually secondary to the CL infection, where skin lesions are widely distributed a cross the body. Those affected remain in this state until cured with no relapse or death.
-
! !
Prior Infection: Individual had recently recovered from CL, MCL, or DCL and is at risk for MCL and DCL[20-21]. Individuals remained in this state for 10 years (or until they died or developed CL, DCL, or MCL), before returning to be uninfected.
-
! !
Death: Individual died of causes unrelated to leishmaniasis and ceased to cycle through the model.
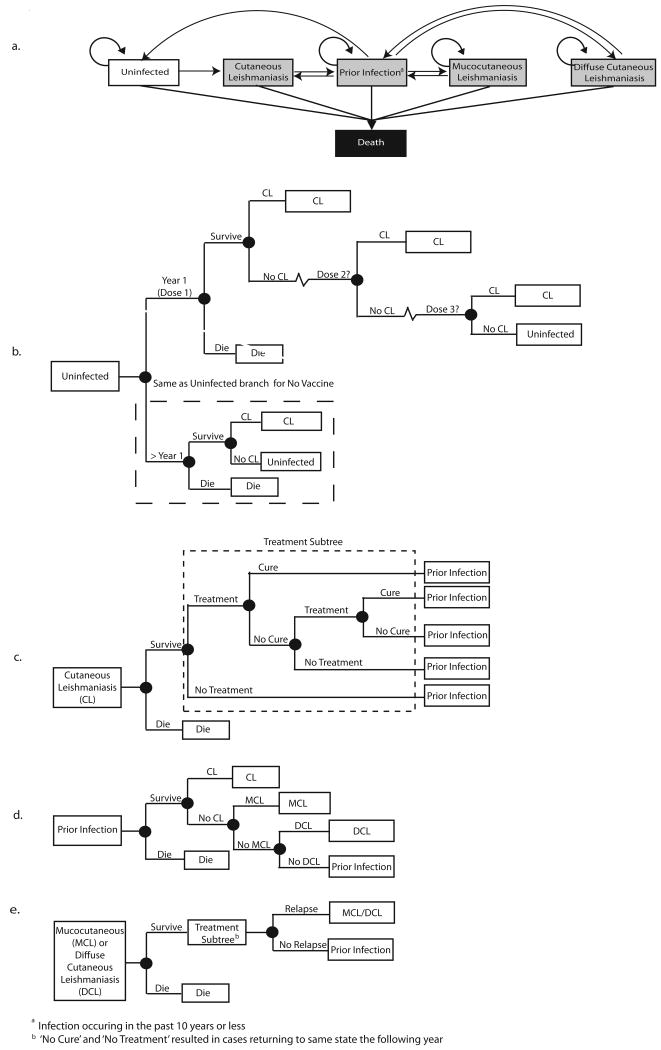
Figures 1a-e show all transition possibilities between states as well as state-specific events, such as treatment, treatment cure, and reinfection. For instance, a CL case could seek treatment and be cured, in which case the duration of their disease was shorter than those not cured by treatment; CL episodes were assumed to last one year or less[22]. Those recovering from CL had a risk of developing Leishmania strain-specific outcomes such as MCL (approx. 2%) or DCL (approx. 5%) shown in Table 1. Individuals had a possibility of multiple CL episodes, assuming any subsequent infection was a different Leishmania strain.
Figure 1.
Cutaneous Leishmaniasis Model Structure, a. Health States, b. Uninfected Health State, c. Cutaneous Leishmaniasis Health State, d. Prior Infection Health State, e. Mucocutaneous or Diffuse Leishmaniasis Health State.
Table 1.
Model Inputs
| Parameter | Value | Ref |
|---|---|---|
| Probabilities | ||
|
| ||
| Strain Distribution | ||
| Andean Countries a | ||
| L. braziliensis | 49% | [24] |
| L. panamensis | 27% | [24] |
| L. guyanensis | 4% | [24] |
| L. amazonensis | 5% | [24] |
| Other | 15% | [24] |
| Brazil | ||
| L. amazonensis | 20% | assumption b |
| L. braziliensis | 60% | [29] |
| L. guyanensis | 20% | assumption b |
| Mexico | ||
| L. mexicana | 100% | assumption b |
| Lifetime Risk of MCL given infection by L. braziliensis, L. panamensis, or L. guyanensis | 2% | [55-57] |
| Lifetime Risk of DCL given infection by L. amazonensis | 30% c | [58] |
|
| ||
| Cure Rates | ||
|
| ||
| Penatvalent antimonials (CL) | 77% d | [59] |
| (40-100%) | ||
| Pentavalent antimonials (MCL) | 67% d | [10] |
| (28-94%) | ||
| Pentamidine (MCL) | 93% | [10] |
| Amphotericin B (MCL) | 89% | [10] |
| Any Treatment (DCL) | (0-10%) e | [60] |
|
| ||
| Costs f | ||
|
| ||
| Pentavalent antimonials, CL (intralesional) g | $56.46 | [61] |
| Pentavalent antimonials, CL (systemic)e | $169.37 | [61] |
| Pentavalent antimonials, MCL and DCL (systemic) g | $254.06 | [61] |
| Pentamidine | $0 | [61] |
| Amphotericin B (MCL and DCL) | $150 | [61] |
| Lab Materials (per visit) | $0.50 | [62] |
Bolivia, Colombia, Ecuador, Peru, Venezuela
Based on expert opinion from Brazil
Overall risk of DCL following CL was 5%
Triangular distribution
Uniform distribution
All costs calculated using a 50kg person
Calculated using the average of both generic and non-generic versions of sodium stibogluconate and meglumine antimoniate
Vaccination occurred at month 0 and 1 for the 2-dose presentation (baseline scenarios) and month 0, 1, and 6 for the 3-dose presentation in the model, where everyone received the first dose of vaccine. Receipt of subsequent doses depended on compliance; no protection was granted until completion of full vaccine regimen. CL risk in the model depended upon a person's vaccination status. A 3% discount rate[23] converted all costs to 2012 US$ and future costs to their 2012 value.
2.2. Model Parameters
We conducted a literature review on cutaneous leishmaniasis in the Americas using MEDLINE and the following terms: [cutaneous leishmaniasis], [tegumentary leishmaniasis], [leishmaniasis treatment], [mucocutaneous leishmaniasis], [diffuse cutaneous leishmaniasis], and [cutaneous leishmaniasis vaccine]. Countries selected were chosen based on the completeness of available data. Data from the year 2000 on was used if available. Table 1 contains a list of model parameters and their sources. Country-specific breakdowns of Leishmania by species[24] were used to estimate the likelihood of clinical outcomes such as MCL, DCL, spontaneous cure, and illness duration. Although recent reports suggest little change in Leishmania species distribution has occurred over the past 20 years [25-26] in some regions, changes in species distribution over time may cause the risk of species-dependent outcomes (i.e., MCL and DCL) to deviate from our estimates.
CL cases had a 20-60% likelihood of seeking treatment; those with MCL or DCL had a 40-100% (∼2 times greater) chance of receiving treatment. CL cases were initially given 20mg/kg of pentavalent antimonials for 20 days, as recommended by the WHO[9], and were retreated with either pentamidine or pentavalent antimonials upon treatment failure[27]. Cases were not retreated after experiencing two treatment failures. Pentavalent antimonials (30 day regimen) were used to treat those with DCL and MCL initially, while pentamidine and amphotericin B were administered if disease persisted[10] (i.e., relapse); individuals sought a maximum of four rounds of treatment per DCL or MCL episode.
2.3. Cost-Effectiveness Analysis
Each simulation run sent 1,000 individuals through the model 1,000 times for a total of 1,000,000 outcomes. The cost per CL case averted is defined as:
| Equation 1 |
The benefit of vaccination was evaluated based on whether the cost per case averted was <$180, the cost of the recommended CL treatment with pentavalent antimonials. Negative values indicated that vaccination averted CL cases as well as costs (i.e., cost saving).
2.4. Sensitivity Analyses
At baseline, a 2-dose cutaneous leishmaniasis vaccine cost $0.5/dose, with an efficacy of 70%, which lasted for 5 years. CL infection risk was varied from 0.005-5%, as reported in endemic and epidemic settings in the countries of interest [28-31]. Sensitivity analyses were conducted for cost per vaccine dose (range: $0.5-10), vaccine efficacy (range: 50-90%), compliance with subsequent doses in the vaccine regimen (range: 50-100%), vaccine protection duration (range: 5-20 years), and the likelihood of revaccination after protection from prior vaccination has expired (range: 50-100%). These scenarios were also evaluated using a 3-dose vaccine.
3. Results
3.1. Overview
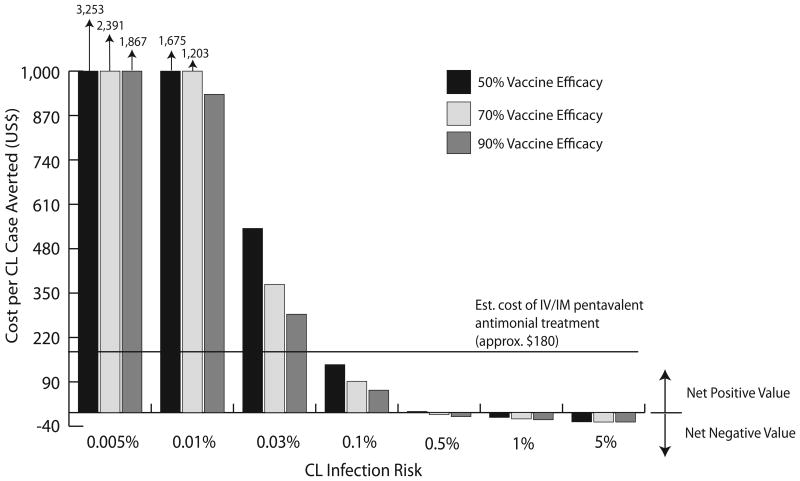
A vaccine with our baseline vaccine profile (2-dose, $0.5/dose, 70% vaccine efficacy, 5 year protection duration) required ≥ 0.1% infection risk to cost less to avert a case than the cost of treatment with pentavalent antimonials ($180), shown in Figure 2. At this risk level and vaccine profile, vaccination could avert 14-52 CL cases per 1,000 individuals vaccinated, and 50-93% of MCL cases and 40-91% of DCL cases, depending on revaccination and compliance (Table 3). Infection risk needed to be 0.5% for a vaccine with these characteristics to save costs compared to if no vaccine was used, and if vaccination provided protection for 10 years or longer, CL risk was able to be 0.03% in order for averting a case to cost less than treatment (Figure 2).
Figure 2.
Cost per Cutaneous Leishmaniasis (CL) Case Averted. (doses=2, vaccine cost= $0.5/dose, duration of protection = 5 years, compliance with full vaccine regimen = 100%, revaccination = 100%).
Table 3.
Cases of CL, MCL, and DCL with and without vaccination (per 1,000 people)
| Vaccine Efficacy | CL Risk | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 0.005% | 0.01% | 0.03% | 0.1% | 0.5% | 1% | 5% | |||
| No Vaccine | CL | 4 | 7 | 23 | 75 | 373 | 742 | 3,550-3,551 | |
| MCL | 0.02 | 0.04 | 0.1 | 0.4 | 2 | 3 | 8 | ||
| DCL | 0.06 | 0.1 | 0.4 | 1 | 5 | 9 | 23 | ||
|
| |||||||||
| Vaccine | CL | 50% | 2-3 | 4-7 | 11-20 | 38-65 | 190-324 | 381-647 | 1,895-3,122 |
| 70% | 1-3 | 2-6 | 7-19 | 23-61 | 116-305 | 233-608 | 1,180-2,940 | ||
| 90% | 0.4-3 | 0.8-6 | 3-17 | 8-57 | 42-286 | 85-568 | 435-2,753 | ||
| MCL | 50% | 0.005-0.01 | 0.006-0.02 | 0.02-0.07 | 0.05-0.2 | 0.2-1 | 0.4-2 | 2-5 | |
| 70% | 0.002-0.01 | 0.002-0.02 | 0.007-0.06 | 0.03-0.2 | 0.2-1 | 0.3-2 | 1-5 | ||
| 90% | 0.0003-0.01 | 0.0007-0.02 | 0.002-0.05 | 0.01-0.2 | 0.05-1 | 0.1-1 | 0.5-5 | ||
| DCL | 50% | 0.009-0.03 | 0.02-0.06 | 0.04-0.2 | 0.1-0.6 | 0.7-3 | 1-5 | 5-14 | |
| 70% | 0.004-0.03 | 0.008-0.05 | 0.03-0.15 | 0.08-0.5 | 0.4-2 | 0.8-4 | 3-13 | ||
| 90% | 0.002-0.02 | 0.005-0.05 | 0.009-0.1 | 0.03-0.5 | 0.1-2 | 0.3-4 | 1-12 | ||
3.2. Costs and Cases with and without Vaccination
Table 2 shows the costs accrued per 1,000 people in the model using no vaccine and a two dose vaccine assuming a 70% vaccine efficacy for each vaccine cost and protection duration. Ranges represent the variation that occurred across compliance and revaccination rates. As expected, vaccine efficacy had little to no impact on vaccination costs. A two dose vaccine costing $1/dose costs approximately $5,736-12,879 per 1,000 people vaccinated if the risk was 0.01% and duration of protection was 5 years; this cost was reduced by 40% if the protection duration was 10 years and by 60% if vaccine protected against infection for 20 years. Not vaccinating became more costly than vaccinating at 0.5% infection risk, also shown in Figure 2. At this infection risk, a vaccine could cost up to $0.5/dose, $1/dose, and $2/dose and still save costs if protection duration were 5, 10, and 20 years, respectively.
Table 2.
Cost of vaccination and no vaccination (per 1,000 people)
| CL Risk | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 0.005% | 0.01% | 0.03% | 0.1% | 0.5% | 1% | 5% | ||
|
| ||||||||
| No Vaccine | $111-123 | $227-253 | $689-703 | $2,285-2,407 | $11,369-11,792 | $22,671-23,230 | $105,656-109,327 | |
| Duration of Protection = 5 Years | ||||||||
| Vaccine | $0.5/dose | $2,869-6,439 | $2,955-6,478 | $3,332-6,619 | $4,593-7,128 | $9,978 -11,677 | $13,645-20,592 | $42,662-89,470 |
| $1/dose | $5,643-12,848 | $5,736-12,879 | $6,097-13,033 | $7,364-13,557 | $14,750-16,455 | $19,908-23,495 | $49,061-92,654 | |
| $2/dose | $11,201-25,684 | $11,285-25,747 | $11,615-25,958 | $12,800-26,757 | $19,733-31,300 | $27,908-36,941 | $40,130-103,683 | |
| $5/dose | $27,873-64,113 | $27,958-64,137 | $28,343-64,290 | $29,608-64,756 | $36,834-67,620 | $45,829-71,122 | $99,272-113,453 | |
| $10/dose | $55,644-128,200 | $55,749-128,254 | $56,102-128,467 | $57,271-129,243 | $63,743-133,564 | $72,389-139,007 | $135,160-181,499 | |
| Duration of Protection = 10 Years | ||||||||
| $0.5/dose | $1,774-3,505 | $1,850-3,541 | $2,211-3,696 | $3,530-4,217 | $7,144-10,493 | $10,887-19,690 | $40,215-85,908 | |
| $1/dose | $3,448-6,975 | $3,538-7,011 | $3,896-7,163 | $5,164-7,671 | $10,580-12,235 | $14,336-21,228 | $43,271-88,403 | |
| $2/dose | $4,072-13,945 | $6,879-14,008 | $7,207-14,236 | $8,345-15,014 | $14,937-19,571 | $16,795-25,623 | $28,859-71,637 | |
| $5/dose | $16,872-34,751 | $16,960-34,781 | $17,322-34,925 | $18,582-35,448 | $25,751-38,334 | $34,796-41,883 | $70,225-100,309 | |
| $10/dose | $33,649-69,481 | $33,713-69,530 | $34,046-69,786 | $35,191-79,577 | $41,745-75,071 | $49,711-80,796 | $84,061-125,341 | |
| Duration of Protection = 20 Years | ||||||||
| $0.5/dose | $1,231-2,059 | $1,305-2,097 | $1,640-2,241 | $2,784-2,853 | $5,717-9,893 | $9,373-18,032 | $38,871-81,255 | |
| $1/dose | $2,347-4,087 | $2,438-4,124 | $2,797-4,274 | $3,991-4,806 | $7,756-10,959 | $11,461-19,340 | $40,196-83,837 | |
| $2/dose | $4,610-8,152 | $4,684-8,209 | $5,005-8,433 | $6,111-9,290 | $9,603-13,967 | $11,107-23,324 | $23,501-86,347 | |
| $5/dose | $11,429-20,265 | $11,504-20,296 | $11,865-20,443 | $13,048-20,975 | $19,929-23,987 | $27,643-28,429 | $57,574-92,646 | |
| $10/dose | $22,742-40,497 | $22,825-40,558 | $23,143-40,802 | $24,204-41,623 | $30,591-46,418 | $38,167-52,317 | $55,628-103,071 | |
Table 3 presents the number of resultant CL, MCL, and DCL cases with and without vaccination. Changing vaccine protection duration had little to no effect on resulting CL cases. Lower (50%) revaccination and compliance probabilities frequently caused little to no cases of CL being averted in addition to more costs being accrued. As can be seen in the table, vaccination not only averted CL cases (21-57%), but also led to a 21-58% reduction in MCL and 59-74% decrease in DCL cases regardless of vaccine efficacy.
3.3. Cost per CL Case Averted
Figure 2 depicts the cost per CL case averted by CL risk and vaccine efficacy, assuming a 2-dose vaccine provided 5 years of protection and cost $0.5/dose. Perfect compliance and revaccination were assumed for these scenarios to determine the CL risk at which revaccination could be recommended, due to the cost of averting a case by vaccination being less than the cost of recommended CL treatment (i.e., $180 for systemic pentavalent antimonials). As the graph indicates, infection risk needed to be ≥0.1% for averting a case with vaccination to cost less than treatment ($66-140/case averted depending on vaccine efficacy) assuming this vaccine profile. At this risk, a 3-dose vaccine with the same cost and protection duration required a 70% efficacy to be below this cost threshold ($155 and $114/case averted for 70% and 90% efficacy, respectively).
Across the vaccine profiles examined, CL risk needed to be at least 0.03% for the vaccine to cost less to avert a case than the cost of treatment. At this risk, a 2-dose vaccine costing $0.5/dose providing 10 years of protection cost $142 to avert a case of CL if vaccine efficacy was 90%. Similarly, a 2-dose vaccine providing 20 years of protection could cost as much as $1/dose if vaccine efficacy was ≥90% and only cost $171/case averted. Averting a case of CL costs ∼1.5-3 times more with a 3-dose vaccine compared to a 2-dose vaccine; costs became less comparable as compliance and revaccination neared 50%.
Vaccinating with a two or three-dose vaccine actually saved costs ($1-34) at infection risks of ≥0.5%, a protection duration as low as 5 years, and a vaccine cost of $0.5/dose. Cost savings continued at vaccination costs of up to $2/dose for a two and three dose vaccine as long as at least 10 and 20 years of protection were provided, respectively. If the revaccination likelihood were 50%, vaccination often remained cost saving; decreasing compliance to 50% frequently caused vaccination to no longer save costs. For example, a 2-dose vaccine costing $0.5/dose with 70% efficacy that provides 5 years' protection saves $6/case averted if an individual is fully revaccinated every 5 years. This savings doubles if protection duration was 10 years and would only cost $19/case averted if the cost per dose increased from $0.5 to $1. Use of a $5/dose vaccine was not below this threshold unless CL risk resembles a highly endemic or epidemic setting (i.e., 1-5% risk) and does not save costs unless risk resembled an epidemic (i.e., 5%).
4. Discussion
Our results indicate that even a vaccine with a relatively short duration of protection and modest efficacy could be recommended for use in targeted locations, as it could prevent a substantial number of cases at little cost and potentially even result in cost savings. In recent years, country-wide incidence estimates from Brazil, Bolivia, Colombia, Ecuador, Peru, and Venezuela have been approximately 0.03% or higher[32-37] equating to a target population of approximately 308 million people within these countries alone[38-43]. If the population in these countries were vaccinated using a vaccine with our baseline profile that provided protection for 10 years or more, 41,000-144,784 CL cases could be averted, each at a cost less than the cost of the recommended treatment.
If countries with similar rates of CL globally were targeted, this target population increases to 703 million. Even a vaccine providing 5 years of protection and requiring a 0.1% infection risk to cost less to avert a case than treatment would have a target population of 191 million. These are likely conservative estimates, as infection risk in certain regions within an endemic country may be higher than the risk in the country overall. In fact, although the estimated overall CL risk in Bolivia is around 0.03%, some regions have reported risks as high as 1%[28]; therefore, vaccinating in these regions may even result in cost savings.
Additionally, vaccination against CL could be highly beneficial in the event of an epidemic. The Leishmania outbreak seen in Chaparral, Colombia in 2004 reported attack rates of up to 8,444 CL cases per 100,000 residents[44]. If this population had been previously vaccinated with a vaccine containing our baseline characteristics, 478-1,827 out of the 2,810 resulting cases and $13,384-58,464 could have been averted, depending on the level of compliance and revaccination achieved.
Although vaccinating before age one would allow for utilization of existing delivery systems (thus saving start-up costs), evaluation of regional CL case demographics may aid in identifying and targeting other high risk groups for vaccination and may be a practical alternative approach in some locations. For example, men (ages 10 – 45 years) working in wooded areas have been identified as a group at high risk for Leishmania infection in areas of Colombia and Mexico[45-47]. In contrast, comparable gender-specific attack rates reported in a Peruvian study, where 79% of CL risk was attributable to factors associated with indoor living quarters (e.g., lamps, fireplaces, etc), suggest domestic transmission was more predominant in this location[24]. Vaccinating certain occupational groups in the former example during the years when they are most at risk for infection may also be a cost-effective strategy.
Other target populations for vaccination could include military personnel and those at risk for visceral leishmaniasis (VL). Genetic analyses have shown that the genomes of disease-causing Leishmania species are highly conserved[48]. This information along with additional studies demonstrating cross-protection between species[49-50] show promise for a CL vaccine to additionally provide partial protection against VL.
This and similar economic analyses of vaccines undergoing development are informative for vaccine developers, funders, and other decision makers, and assist in bringing the market closer to development[51-53]. Our analyses show that in many cases a CL vaccine could have as low as 50% efficacy (0.03% risk if 20 year protection duration and $0.5/dose, 0.1% risk if 5-10 year protection duration and $0.5-2/dose) or provide protection for as little as 5 years (if risk was ≥0.1%) and still cost less than treatment. While efficacy and protection duration will ultimately be determined by the vaccine's formulation, such findings could be helpful when determining vaccine price points and target populations.
5. Limitations
Models are simplistic representations of the real world and therefore include assumptions of real-world events. We evaluated the benefit of vaccine introduction in seven countries; therefore, probabilities of outcomes (MCL and DCL) were calculated accordingly. Focusing on a single country or region would change these probabilities, and thus the benefit of vaccination. We conservatively assume that individuals were at risk of MCL and DCL up to 10 years after CL, although some report this duration to be up to 30 years. Although treatment costs can vary greatly by country, treatment costs that were used are comparable to direct medical costs per patient reported during the cutaneous leishmaniasis outbreak in Colombia[22] and reports from Brazil[54].
6. Conclusion
Vaccination with a preventive CL vaccine providing 5-10 years of protection from infection caused by New World Leishmania species endemic to the seven most highly endemic Latin American countries is often less costly than treatment and sometimes cost-saving (i.e., economically dominant) across a range of scenarios.
Highlights.
-
! !
We constructed and utilized a computer simulation model to evaluate the economic value of a CL vaccine.
-
! !
Modest protection duration and efficacy are required for CL vaccination to cost less than treatment.
-
! !
A preventative CL vaccine could save costs at moderate levels of infection risk.
Acknowledgments
We acknowledge the support of the Carlos Slim Health Institute.
Funding: Supported by the Vaccine Modeling Initiative (VMI), funded by the Bill and Melinda Gates Foundation and the National Institute of General Medical Sciences Models of Infectious Disease Agent Study (MIDAS) through grant 5U54GM088491-02. Funders had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization. Global Alert and Response (GAR) : WHO Report on Global Surveillance of Epidemic-prone Infectious Diseases - Leishmaniasis 2012 2012. [cited 2012 May 18]; Available from: http://www.who.int/csr/resources/publications/CSR_ISR_2000_1leish/en/index.html.
- 2.Hotez PJ, Bottazzi ME, Franco-Paredes C, Ault SK, Periago MR. The neglected tropical diseases of Latin America and the Caribbean: a review of disease burden and distribution and a roadmap for control and elimination. PLoS Negl Trop Dis. 2008;2(9):e300. doi: 10.1371/journal.pntd.0000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferro C, Marin D, Gongora R, Carrasquilla MC, Trujillo JE, Rueda NK, et al. Phlebotomine vector ecology in the domestic transmission of American cutaneous leishmaniasis in Chaparral, Colombia. Am J Trop Med Hyg. 2011 Nov;85(5):847–56. doi: 10.4269/ajtmh.2011.10-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beyrer C, Villar JC, Suwanvanichkij V, Singh S, Baral SD, Mills EJ. Neglected diseases, civil conflicts, and the right to health. Lancet. 2007 Aug 18;370(9587):619–27. doi: 10.1016/S0140-6736(07)61301-4. [DOI] [PubMed] [Google Scholar]
- 5.Reithinger R, Coleman PG. Treating cutaneous leishmaniasis patients in Kabul, Afghanistan: cost-effectiveness of an operational program in a complex emergency setting. BMC Infect Dis. 2007;7:3. doi: 10.1186/1471-2334-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bern C, Maguire JH, Alvar J. Complexities of assessing the disease burden attributable to leishmaniasis. PLoS Negl Trop Dis. 2008;2(10):e313. doi: 10.1371/journal.pntd.0000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marsden PD. Mucosal leishmaniasis (“espundia” Escomel, 1911) Trans R Soc Trop Med Hyg. 1986;80(6):859–76. doi: 10.1016/0035-9203(86)90243-9. [DOI] [PubMed] [Google Scholar]
- 8.Leishmaniasis: Burden of Disease. [cited 2012 April 11];2012 Available from: http://www.who.int/leishmaniasis/burden/en/
- 9.Control of the leishmaniases. Geneva: World Health Organization; 2010. WHO Expert Committee on the Control of Leishmaniasis. [Google Scholar]
- 10.Amato VS, Tuon FF, Siqueira AM, Nicodemo AC, Neto VA. Treatment of Mucosal Leishmaniasis in Latin America: Systematic Review. American Journal of Tropical Medicine and Hygiene. 2007;77(2):266–74. [PubMed] [Google Scholar]
- 11.Almeida OL, Santos JB. Advances in the treatment of cutaneous leishmaniasis in the new world in the last ten years: a systematic literature review. An Bras Dermatol. 2011 Jun;86(3):497–506. doi: 10.1590/s0365-05962011000300012. [DOI] [PubMed] [Google Scholar]
- 12.Dumonteil E. Vaccine development against Trypanosoma cruzi and Leishmania species in the post-genomic era. Infect Genet Evol. 2009 Dec;9(6):1075–82. doi: 10.1016/j.meegid.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Okwor I, Uzonna J. Vaccines and vaccination strategies against human cutaneous leishmaniasis. Hum Vaccin. 2009 May;5(5):291–301. doi: 10.4161/hv.5.5.7607. [DOI] [PubMed] [Google Scholar]
- 14.Duthie MS, Raman VS, Piazza FM, Reed SG. The development and clinical evaluation of second-generation leishmaniasis vaccines. Vaccine. 2012 Jan 5;30(2):134–41. doi: 10.1016/j.vaccine.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomes R, Teixeira C, Oliveira F, Lawyer PG, Elnaiem DE, Meneses C, et al. KSAC, a defined Leishmania antigen, plus adjuvant protects against the virulence of L. major transmitted by its natural vector Phlebotomus duboscqi. PLoS Negl Trop Dis. 2012 Apr;6(4):e1610. doi: 10.1371/journal.pntd.0001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bethony JM, Cole RN, Guo X, Kamhawi S, Lightowlers MW, Loukas A, et al. Vaccines to combat the neglected tropical diseases. Immunol Rev. 2011 Jan;239(1):237–70. doi: 10.1111/j.1600-065X.2010.00976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomes R, Oliveira F. The immune response to sand fly salivary proteins and its influence on leishmania immunity. Front Immunol. 2012;3:110. doi: 10.3389/fimmu.2012.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chale-Balboa WG, Mut-Martin M, Ramirez-Sierra MJ, Garcia-Miss MR, Dumonteil E. A combination DNA vaccine encoding nucleoside hydrolase 36 and glycoproteine 63 protects female but not male hamsters against Leishmania mexicana. Parasite. 2009 Sep;16(3):227–30. doi: 10.1051/parasite/2009163227. [DOI] [PubMed] [Google Scholar]
- 19.Tavares NM, Silva RA, Costa DJ, Pitombo MA, Fukutani KF, Miranda JC, et al. Lutzomyia longipalpis saliva or salivary protein LJM19 protects against Leishmania braziliensis and the saliva of its vector, Lutzomyia intermedia. PLoS Negl Trop Dis. 2011;5(5):e1169. doi: 10.1371/journal.pntd.0001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weigle K, Gore Saravia N. Natural History, Clinical Evolution, an the Host-Parasite Interaction in New World Cutaneous Leishmaniasis. Clinics in Dermatology. 1996;14:433–50. doi: 10.1016/0738-081x(96)00036-3. [DOI] [PubMed] [Google Scholar]
- 21.Jones TC, Johnsons WD, Jr, Barretto AC, Lago E, Badaro R, Cerf B, Reed SG, Netto EM, Tada MS, Franca F, Wiese K, Golightly L, Fikrig E, Costa JML, Cuba CC, Marsden PD. Epidemiology of American Cutaneous Leishmaniasis Due to Leishmania braziliensis braziliensis. The Journal of Infectious Diseases. 1987;156(1):73–83. doi: 10.1093/infdis/156.1.73. [DOI] [PubMed] [Google Scholar]
- 22.Vega JC, Sanchez BF, Montero LM, Montana R, Del Pilar Mahecha M, Duenes B, et al. Short communication: The cost-effectiveness of cutaneous leishmaniasis patient management during an epidemic in Chaparral, Colombia in 2004. Trop Med Int Health. 2007 Dec;12(12):1540–4. doi: 10.1111/j.1365-3156.2007.01962.x. [DOI] [PubMed] [Google Scholar]
- 23.Shepard DS. Cost-effectiveness in Health and Medicine. In: Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. J Ment Health Policy Econ. 2. Vol. 2. New York: Oxford University Press; 1996. 1999. Jun 1, pp. 91–2. [Google Scholar]
- 24.Davies CR, Reithinger R, Campbell-Lendrum D, Feliciangeli D, Borges R, Rodriguez N. The Epidemiology and Control of Leishmaniasis in Andean Countries. Cadernos de Saúde Pública. 2000;16(4):925–50. doi: 10.1590/s0102-311x2000000400013. [DOI] [PubMed] [Google Scholar]
- 25.Jirmanus L, Glesby MJ, Guimaraes LH, Lago E, Rosa ME, Machado PR, et al. Epidemiological and clinical changes in American tegumentary leishmaniasis in an area of Leishmania (Viannia) braziliensis transmission over a 20-year period. Am J Trop Med Hyg. 2012 Mar;86(3):426–33. doi: 10.4269/ajtmh.2012.11-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urbano J, Sanchez-Moreno ME, Ovalle CE, Rosales MJ, Camargo YC, Gutierrez-Sanchez R, et al. Characterization of cutaneous isolates of Leishmania in Colombia by isoenzyme typing and kDNA restriction analysis. Rev Ibero-Latinoam Parasitol. 2011;70(1):16–24. [Google Scholar]
- 27.David CV, Craft N. Cutaneous and Mucocutaneous Leishmaniasis. Dermatologic Therapy. 2009;22:491–502. doi: 10.1111/j.1529-8019.2009.01272.x. [DOI] [PubMed] [Google Scholar]
- 28.Tedesqui VL, Calleja NCC, Parra R, Pabon JP, Boia MN, Carvalho-Costa FA. Active Surveillance of American tegumentary leishmaniasis in endemic areas in rural. Bolivia: Sociedade Brasileira Medicina Tropical; 2011. [DOI] [PubMed] [Google Scholar]
- 29.Bedoya-Pacheco SJ, Araujo-Melo MH, Valete-Rosalino CM, Pimentel MI, Conceicao-Silva F, Schubach AO, et al. Endemic tegumentary leishmaniasis in Brazil: correlation between level of endemicity and number of cases of mucosal disease. Am J Trop Med Hyg. 2011 Jun;84(6):901–5. doi: 10.4269/ajtmh.2011.10-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valderrama-Ardila C, Alexander N, Ferro C, Cadena H, Marin D, Holford TR, et al. Environmental risk factors for the incidence of American cutaneous leishmaniasis in a sub-Andean zone of Colombia (Chaparral, Tolima) Am J Trop Med Hyg. 2010 Feb;82(2):243–50. doi: 10.4269/ajtmh.2010.09-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.García AL, Parrado R, Rojas E, Delgado R, Dujardin JC, Reithinger R. Leishmaniasis in Bolivia: comprehensive review and current status. Am J Trop Med Hyg. 2009;80(5):704–11. [PubMed] [Google Scholar]
- 32.Brazil: Pan American Health Organization. 2007. [Google Scholar]
- 33.Colombia: Pan American Health Organization. 2007. [Google Scholar]
- 34.Peru: Pan American Health Organization. 2007. [Google Scholar]
- 35.Venezuela: Pan American Health Organization (PAHO) 2007. [Google Scholar]
- 36.Bolivia: Pan American Health Organization. 2007. [Google Scholar]
- 37.Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS One. 2012;7(5):e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Countries: Brazil. May 2012 ed: World Health Organization, 2012.
- 39.Countries: Bolivia (Plurinational State of). May 2012 ed: World Health Organization, 2012.
- 40.Countries: Colombia. May 2012 ed, 2012.
- 41.Countries: Ecuador. May 2012 ed: World Health Organization, 2012.
- 42.Countries: Peru. May 2012 ed: World Health Organization, 2012.
- 43.Countries: Venezuela (Bolivarian Republic of). May 2012 ed: World Health Organization, 2012.
- 44.Pardo RH, Cabrera OL, Becerra J, Fuya P, Ferro C. [Lutzomyia longiflocosa as suspected vector of cutaneous leishmaniasis in a focus of cutaneous leishmaniasis on the sub-andean region of Tolima department, Colombia, and the knowledge on sandflies by the inhabitants] Biomedica. 2006 Oct;26(Suppl 1):95–108. [PubMed] [Google Scholar]
- 45.Andrade-Narvaez FJ, Canto Lara SB, Van Wynsberghe NR, Rebollar-Tellez EA, Vargas-Gonzalez A, Albertos-Alpuche NE. Seasonal transmission of Leishmania (Leishmania) mexicana in the state of Campeche, Yucatan Peninsula, Mexico. Mem Inst Oswaldo Cruz. 2003 Dec;98(8):995–8. doi: 10.1590/s0074-02762003000800002. [DOI] [PubMed] [Google Scholar]
- 46.Andrade-Narvaez FJ, Vargas-Gonzalez A, Canto-Lara SB, Damian-Centeno AG. Clinical picture of cutaneous leishmaniases due to Leishmania (Leishmania) mexicana in the Yucatan peninsula, Mexico. Mem Inst Oswaldo Cruz. 2001 Feb;96(2):163–7. doi: 10.1590/s0074-02762001000200005. [DOI] [PubMed] [Google Scholar]
- 47.Weigle KA, Santrich C, Martinez F, Valderrama L, Saravia NG. Epidemiology of cutaneous leishmaniasis in Colombia: environmental and behavioral risk factors for infection, clinical manifestations, and pathogenicity. J Infect Dis. 1993 Sep;168(3):709–14. doi: 10.1093/infdis/168.3.709. [DOI] [PubMed] [Google Scholar]
- 48.Peacock CS, Seeger K, Harris D, Murphy L, Ruiz JC, Quail MA, et al. Comparative genomic analysis of three Leishmania species that cause diverse human disease. Nat Genet. 2007 Jul;39(7):839–47. doi: 10.1038/ng2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tonui WK, Titus RG. Cross-protection against Leishmania donovani but not L. Braziliensis caused by vaccination with L. Major soluble promastigote exogenous antigens in BALB/c mice. Am J Trop Med Hyg. 2007 Mar;76(3):579–84. [PubMed] [Google Scholar]
- 50.Aguilar-Be I, da Silva Zardo R, Paraguai de Souza E, Borja-Cabrera GP, Rosado-Vallado M, Mut-Martin M, et al. Cross-protective efficacy of a prophylactic Leishmania donovani DNA vaccine against visceral and cutaneous murine leishmaniasis. Infect Immun. 2005 Feb;73(2):812–9. doi: 10.1128/IAI.73.2.812-819.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee BY, Burke DS. Constructing target product profiles (TPPs) to help vaccines overcome post-approval obstacles. Vaccine. 2010 Apr 1;28(16):2806–9. doi: 10.1016/j.vaccine.2009.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee BY, McGlone SM. Pricing of new vaccines. Hum Vaccin. 2010 Aug;6(8):619–26. doi: 10.4161/hv.6.8.11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee BY, Wiringa AE. The 2009 H1N1 influenza pandemic: a case study of how modeling can assist all stages of vaccine decision-making. Hum Vaccin. 2011 Jan 1;7(1):115–9. doi: 10.4161/hv.7.1.13740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Modabber F, Buffet PA, Torreele E, Milon G, Croft SL. Consultative meeting to develop a strategy for treatment of cutaneous leishmaniasis. Institute Pasteur, Paris. 13-15 June, 2006. Kinetoplastid Biol Dis. 2007;6:3. doi: 10.1186/1475-9292-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soto J, Valda-Rodriquez L, Toledo J, Vera-Navarro L, Luz M, Monasterios-Torrico H, Vega J, Berman J. Comparison of Generic to Branded Pentavalent Antimony for Treatment of New World Cutaneous Leishmaniasis. The American Journal of Tropical Medicine and Hygiene. 2004;71(5):577–81. [PubMed] [Google Scholar]
- 56.Mitropoulos P, Konidas P, Durkin-Konidas M. New World Cutaneous Leishmaniasis: Updated Review of Current and Future Diagnosis and Treatment. Journal of the American Academy of Dermatology 2010 August. 2010;63(2):309–22. doi: 10.1016/j.jaad.2009.06.088. [DOI] [PubMed] [Google Scholar]
- 57.Herwaldt BL. Leishmaniasis. The Lancet 1999 October 2. 1999;354:1191–9. doi: 10.1016/S0140-6736(98)10178-2. [DOI] [PubMed] [Google Scholar]
- 58.Desjeux P. Information on the Epidemiology and Control of the Leishmaniases by Country or Territory. World Health Organization (WHO) 1991 [Google Scholar]
- 59.Tuon FF, Amato VS, Graf ME, Siqueira AM, Nicodemo AC, Neto VA. Treatment of New World Cutaneouls Leishmaniasis - A Systematic Review with a Meta-Analysis. International Journal of Dermatology. 2008;47:109–24. doi: 10.1111/j.1365-4632.2008.03417.x. [DOI] [PubMed] [Google Scholar]
- 60.Zerpa O, Ulrich M, Blanco B, Polegre M, Avila A, Matos N, Mendoza I, Pratlong F, Ravel C, Convit J. Diffuse Cutaneous Leishmaniasis Responds to Miltefosine but then Relapses. British Journal of Dermatology. 2007;156:1328–35. doi: 10.1111/j.1365-2133.2007.07872.x. [DOI] [PubMed] [Google Scholar]
- 61.Control of the Leishmaniases. Geneva: World Health Organization; 2010. [Google Scholar]
- 62.Vega JC, Sanchez BF, Montero LM, Montana R, del Pilar Mahecha M, Duenes B, Baron AR, Reithinger R. The Cost-Effectiveness of Cutaneous Leishmaniasis Patient Management During an Epidemic in Chaparral, Columbia in 2004. Tropical Medicine and International Health. 2007;12(12):1540–4. doi: 10.1111/j.1365-3156.2007.01962.x. [DOI] [PubMed] [Google Scholar]