Abstract
We provide a systematic review of epidemiological surveys of autistic disorder and pervasive developmental disorders (PDDs) worldwide. A secondary aim was to consider the possible impact of geographic, cultural/ethnic, and socioeconomic factors on prevalence estimates and on clinical presentation of PDD. Based on the evidence reviewed, the median of prevalence estimates of autism spectrum disorders was 62/10 000. While existing estimates are variable, the evidence reviewed does not support differences in PDD prevalence by geographic region nor of a strong impact of ethnic/cultural or socioeconomic factors. However, power to detect such effects is seriously limited in existing data sets, particularly in low-income countries. While it is clear that prevalence estimates have increased over time and these vary in different neighboring and distant regions, these findings most likely represent broadening of the diagnostic concets, diagnostic switching from other developmental disabilities to PDD, service availability, and awareness of autistic spectrum disorders in both the lay and professional public. The lack of evidence from the majority of the world's population suggests a critical need for further research and capacity building in low- and middle-income countries. Autism Res 2012, 5: 160–179. © 2012 International Society for Autism Research, Wiley Periodicals, Inc.
Keywords: epidemiology, prevalence, global health, low- and middle-income countries
Introduction
Autism is a life-long neurodevelopmental condition interfering with the person's ability to communicate and relate to others. Since the earliest epidemiological surveys in the 1960s, a wealth of data has become available, indicating a much higher prevalence of the condition than previously thought [Fombonne, 2003a, 2005; Fombonne, Quirke, & Hagen, 2011]. It is now recognized that some individuals with the condition are able to lead independent and fulfilling lives, whereas for others the impact can be severe, interfering significantly with quality of life [Farley et al., 2009]. While the global burden of autism is currently unknown, in the United States and in the UK, the annual societal cost of the condition exceeds several billions [Ganz, 2007; Knapp, Romeo, & Beecham, 2007].
Increased recognition, understanding, and awareness of autism in the last few decades have been, in part, driven by the significant growth in research evidence. While many aspects of autism remain poorly understood, major advances have been made in terms of highlighting the genetic [Abrahams & Geschwind, 2008], biological [Belmonte et al., 2004], environmental [Currenti, 2009], and developmental [Elsabbagh & Johnson, 2010] origins of the condition. Large-scale and well controlled cohort studies (e.g., http://www.earlistudy.org) following-up pregnant mothers are likely to clarify the effects of some pre- and perinatal risk factors implicated in autism. Significant strides have also contributed towards developing and validating screening and diagnostic instruments, helping to reduce heterogeneity in clinical characterization in research studies. While some of these diagnostic tools remain highly resource intensive, they are increasingly used in clinical settings, as they provide rich and systematic information to inform service provisions where those are available. However, even in high-income countries, provisions for screening, diagnosis, and intervention are highly variable and many cases absent in community settings.
Such advances in autism research have contributed towards bridging the gap between evidence and practice in some countries, but there is little systematic information available with regards to the impact of the condition on most of the world's population. Frequently regarded as essential for advancing basic research and strategic for informing policy and developing services, epidemiological studies have emerged as a clear priority within several global initiatives. The charity Autism Speaks in partnership with the US Center for Disease Control (CDC) launched the International Autism Epidemiology Network (http://www.autismepidemiology.net), bringing together researchers worldwide and focusing specifically on service improvements in developing countries. According to the network, prevalence studies for PDD are ongoing in Australia, Mexico, Finland, Portugal, Iceland, India, Vietnam, Taiwan, South Africa, and Uganda. Focusing on a broader context than autism, the Movement for Global Mental Health has identified a clear treatment gap, particularly pronounced in low- and middle-income countries [http://www.globalmentalhealth.org; Patel et al., 2008]. Epidemiological data on the burden of mental and neurological disorders and systematic mapping of relevant services in low- and middle-income countries encouraged World Health Organization (WHO) to launch the mental health Gap Action Programme (mhGAP) [WHO, 2008]. Moreover, the Global Alliance for Chronic Disease, which groups several agencies including Australia's National Health Medical Research Council, the Canadian Institutes of Health Research, the Chinese Academy of Medical Science, the UK's Medical Research Council, and the United States' National Institutes of Health, announced a program to identify the world's “Grand Challenges in Global Mental Health” (http://www.grandchallenges.org). The reasons for why epidemiological surveys are viewed as a priority extend beyond the need for objective and robust estimates of prevalence. These provide additional valuable benefits as they often result in systematic information regarding existing services and may help in assessing the needs and priorities for each community. In the long run, the availability of comparable estimates from different geographic regions may also enable testing challenging hypotheses regarding the etiology of PDD.
Systematic Review Methodology
The primary aim of this report is to provide an up-to-date and systematic review of the methodological features and substantive results of epidemiological surveys of the prevalence of pervasive developmental disorders (PDDs), including autistic disorder (AD) worldwide. This is to our knowledge the first attempt to review available evidence from different world regions, including low- and middle-income countries. While the scope of the current review does not provide extensive coverage of the situation in all countries, every effort was made to represent objectively available evidence from regions where research capacity is limited.
Search Strategies
We adopted a systematic review methodology in the identification of epidemiological reports included in the current article. First, as a preliminary step, key findings from previous reviews of epidemiological surveys were incorporated into the current report [Fombonne, 2003a, 2005; Fombonne et al., 2011; Sun & Allison, 2010; Williams, Higgins, & Brayne, 2006]. Second, several authors or group of authors undertook a comprehensive review of available evidence from regions that were underrepresented in previous reviews. Different countries were grouped into subregions according to the WHO classification. The main search strategy was to perform extensive region and/or country-specific search of Medline publications. When the authors were familiar with additional local or regional search engines, these were also used. Additional search sources included IndMed search engine, indmed.nic.in national medical database, VIP.cn Chinese search engine, and http://arabpsynet.com regional engine. Search terms included Autis*, Asperger, Autistic Disorder and Child Development and Disorders, Pervasive (mesh term), or their other-language equivalent. In regions where it was difficult to identify epidemiological reports, authors also consulted other researchers and practitioners who supplied references or information. Using these strategies authors identified and reviewed over 600 studies typically not included in previous reviews (133 from South and Central America, and the Caribbean, 319 from South East Asia and the Western Pacific, and 130 from the Eastern Mediterranean, 54 from Africa). Languages of the identified articles included Arabic, Chinese, Dutch, English, French, Portuguese, and Spanish.
Inclusion and Exclusion Criteria of Prevalence Estimate
From the identified publications, the following criteria were used for selecting epidemiological surveys included in Tables I–III:
Table I.
Europe
| Region | Category | Year | Authors | Country | Area | Population | Age | Number affected | Diagnostic criteria | % with average IQ | Gender ratio | Prevalence/ 10 000 | 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Europe | AD | 1966 | Lotter | UK | Middlesex | 78 000 | 8–10 | 32 | Rating scale | 15.6 | 2.6 (23/9) | 4.1 | 2.7; 5.5 |
| AD | 1972 | Brask | Denmark | Aarhus County | 46 500 | 2–14 | 20 | Clinical | — | 1.4 (12/7) | 4.3 | 2.4; 6.2 | |
| AD | 1976 | Wing et al. | UK | Camberwell | 25 000 | 5–14 | 17a | 24-item rating scale of Lotter | 30 | 16 (16/1) | 4.8b | 2.1; 7.5 | |
| AD | 1983 | Bohman et al. | Sweden | County of Västerbotten | 69 000 | 0–20 | 39 | Rutter criteria | 20.5 | 1.6 (24/15) | 5.6 | 3.9; 7.4 | |
| AD | 1984 | McCarthy et al. | Ireland | East | 65 000 | 8–10 | 28 | Kanner | — | 1.33 (16/12) | 4.3 | 2.7; 5.9 | |
| AD | 1986 | Steinhausen et al. | Germany | West Berlin | 279 616 | 0–14 | 52 | Rutter | 55.8 | 2.25 (36/16) | 1.9 | 1.4; 2.4 | |
| AD | 1989 | Cialdella and Mamelle | France | Rhône | 135 180 | 3–9 | 61 | DSM-III like | — | 2.3 | 4.5 | 3.4; 5.6 | |
| AD | 1991 | Gillberg et al. | Sweden | South-West Gothenburg, Bohuslän | 78 106 | 4–13 | 74 | DSM-III-R | 18 | 2.7 (54/20) | 9.5 | 7.3; 11.6 | |
| AD | 1992 | Fombonne and du Mazaubrun | France | 4 regions | 274 816 | 9 & 13 | 154 | Clinical-ICD-10 like | 13.3 | 2.1 (105/49) | 4.9 | 4.1; 5.7 | |
| AD | 1997 | Fombonne et al. | France | 3 regions | 325 347 | 8–16 | 174 | Clinical ICD-10-like | 12.1 | 1.81 (112/62) | 5.35 | 4.6; 6.1 | |
| AD | 1997 | Webb et al. | UK | South Glamorgan, Wales | 73 301 | 3–15 | 53 | DSM-III-R | — | 6.57 (46/7) | 7.2 | 5.3; 9.3 | |
| AD | 1997 | Arvidsson et al. | Sweden | Mölnlycke | 1 941 | 3–6 | 9 | ICD-10 | 22.2 | 3.5 (7/2) | 46.4 | 16.1; 76.6 | |
| AD | 1998 | Sponheim & Skjeldal | Norway | Akershus County | 65 688 | 3–14 | 34 | ICD-10 | 47.1c | 2.09 (23/11) | 5.2 | 3.4; 6.9 | |
| AD | 1999 | Taylor et al. | UK | North Thames | 490 000 | 0–16 | 427 | ICD-10 | — | — | 8.7 | 7.9; 9.5 | |
| AD | 1999 | Kadesjö et al. | Sweden | Karlstad | 826 | 6.7–7.7 | 6 | DSM-III-R/ICD-10 Gillberg criteria for AS | 50.0 | 5.0 (5/1) | 72.6 | 14.7; 130.6 | |
| AD | 2000 | Baird et al. | UK | South-East Thames | 16 235 | 7 | 50 | ICD-10 | 60 | 15.7 (47/3) | 30.8 | 22.9; 40.6 | |
| AD | 2000 | Powell et al. | UK | West Midlands | 25 377 | 1–5 | 62 | Clinical/ICD10/DSM-IV | — | — | 7.8 | 5.8; 10.5 | |
| AD | 2000 | Kielinen et al. | Finland | North (Oulu et Lapland) | 27 572 | 5–7 | 57 | DSM-IV | 49.8d | 4.12 (156/50) | 20.7 | 15.3; 26.0 | |
| AD | 2001 | Davidovitch et al. | Israel | Haifa | 26 160 | 7–11 | 26 | DSM-III-R/DSM-IV | — | 4.2 (21/5) | 10.0 | 6.6; 14.4 | |
| AD | 2007 | Oliveira et al. | Portugal | Mainland and Azores | 67 795 | 6–9 | 115 | DSM-IV | 17 | 2.9 | 16.7 | 14.0; 20.0 | |
| AD | 2001 | Fombonne et al. | UK | England and Wales | 10 438 | 5–15 | 27 | DSM-IV/ICD-10 | 55.5 | 8.0 (24/3) | 26.1 | 16.2; 36.0 | |
| AD | 2001 | Magnússon and Saemundsen | Iceland | Whole Island | 43 153 | 5–14 | 57 | Mostly ICD-10 | 15.8 | 4.2 (46/11) | 13.2 | 9.8; 16.6 | |
| AD | 2001 | Chakrabarti and Fombonne | UK | Stafford | 15 500 | 2.5–6.5 | 26 | ICD10/DSM-IV | 29.2 | 3.3 (20/6) | 16.8 | 10.3; 23.2 | |
| AD | 2002 | Madsen et al. | Denmark | National register | 63 859 | 8 | 46 | ICD-10 | — | — | 7.2 | 5.0–10.0 | |
| AD | 2004 | Tebruegge et al. | UK | Kent | 2 536 | 8–9 | 6 | ICD-10 | — | (6/0) | 23.7 | 9.6; 49.1 | |
| AD | 2005 | Chakrabarti & Fombonne | UK | Stafford | 10 903 | 4–7 | 24 | ICD-10/DSM-IV | 33.3 | 3.8 (19/5) | 22.0 | 14.4; 32.2 | |
| AD | 2006 | Gillberg et al. | Sweden | Göteborg | 32 568 | 7–12 | 115 | Gillberg's criteria | — | 3.6 (90/25) | 35.3 | 29.2; 42.2 | |
| AD | 2006 | Baird et al. | UK | London (South Thames) | 56 946 | 9–10 | 81 | ICD-10 | 47 | 8.3 (≍ 72/9) | 38.9 | 29.9; 47.8 | |
| AD | 2007 | Ellefsen et al. | Denmark | Faroe Islands | 7 689 | 8–17 | 12 | ICD-10 Gillberg criteria for AS | — | 3.0 (9/3) | 16.0 | 7.0; 25.0 | |
| AD | 2007 | Latif and Williams | UK | Wales | 39 220 | 0–17 | 50 | Kanner | — | — | 12.7 | 9.0; 17.0 | |
| AD | 2008 | Williams et al. | UK | South West (Avon) | 14 062 | 11 | 30 | ICD-10 | 86.7 | 5.0 (25/5) | 21.6 | 13.9; 29.3 | |
| PDD | 2000 | Baird et al. | UK | London (South Thames) | 16 235 | 7 | 94 | ICD-10 | 60 | 15.7 (83/11) | 57.9 | 46.8; 70.9 | |
| PDD | 2001 | Chakrabarti and Fombonne | UK | Stafford | 15 500 | 4–7 | 96 | ICD-10 | 74.2 | 3.8 (77/20) | 61.9 | 50.2; 75.6 | |
| PDD | 2002 | Madsen et al. | Denmark | National register | — | 8 | 738 | ICD-10 | — | — | 30.0 | — | |
| PDD | 2002 | Scott et al. | UK | Cambridge | 33 598 | 5–11 | 196 | ICD-10 | — | 4.0 (—) | 58.3 * | 50; 67* | |
| PDD | 2004 | Tebruegge et al. | UK | Kent | 2 536 | 8–9 | 21 | ICD-10 | — | 6.0 (18/3) | 82.8 | 51.3; 126.3 | |
| PDD | 2005 | Chakrabarti and Fombonne | UK | Stafford | 10 903 | 4–6 | 64 | ICD-10 | 70.2 | 6.1 (55/9) | 58.7 | 45.2; 74.9 | |
| PDD | 2006 | Baird et al. | UK | South Thames | 56 946 | 9–10 | 158 | ICD-10 | 45 | 3.3 (121/37) | 116.1 | 90.4; 141.8 | |
| PDD | 2006 | Harrison et al. | UK | Scotland | 134 661 | 0–15 | 443e | ICD-10, DSMIV | — | 7.0 (369/53) | 44.2 | 39.5; 48.9 | |
| PDD | 2006 | Gillberg et al. | Sweden | Göteborg | 32 568 | 712 | 262 | DSM-IV | — | 3.6 (205/57) | 80.4 | 71.3; 90.3 | |
| PDD | 2007 | Latif and Williams | UK | South Wales | 39 220 | 0–17 | 240 | ICD-10, DSM-IV, Kanner's & Gillberg's criteria | — | 6.8 — | 61.2 | 54; 69* | |
| PDD | 2007 | Ellefsen et al. | Denmark | Faroe Islands | 7 689 | 8–17 | 41 | DSM-IV, Gillberg's criteria | 68.3 | 5.8 (35/6) | 53.3 | 36; 70 | |
| PDD | 2008 | Williams et al. | UK | Avon | 14 062 | 11 | 86 | ICD-10 | 85.3 | 6.8 (75/11) | 61.9 | 48.8; 74.9 | |
| PDD | 2009 | Baron—Cohen et al. | UK | Cambridge | 8 824 | 5–9 | 83 | ICD-10 | — | — | 94f | 75; 116 | |
| PDD | 2011 | Brugha et al. | UK | National | 7 333 | >16 | 72 | ADOS-4 | 100% | 3.8 — | 98 | 30; 165 |
Calculated by the author.
This number corresponds to the sample described in Wing and Gould [1979].
This rate corresponds to the first published paper on this survey and is based on 12 participants amongst children aged 5 to 14 years.
In this study, mild mental retardation was combined with average IQ, whereas moderate and severe mental retardation were grouped together.
These figures apply to the whole study sample of 206 participants with an autism spectrum disorder (ASD).
Estimated using a capture-recapture analysis, the number of cases used to calculate prevalence was estimated to be 596.
Rate based on Special Education Needs register. A figure of 99/10 000 is provided from a parental and diagnostic survey. Other estimates in this study vary from 47 to 165/10 000 deriving from various assumptions made by the authors.
AD, autistic disorder; AS, Asperger syndrome; DSM, Diagnostic and Statistical Manual of Mental Disorders; ICD, International Classification of Diseases. PDD, developmental disorder.
Table III.
America
| Region | Category | Year | Authors | Country | Area | Population | Age | Number affected | Diagnostic criteria | % with average IQ | Gender ratio | Prevalence/ 10 000 | 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| America | AD | 1970 | Treffert | USA | Wisconsin | 899 750 | 3–12 | 69 | Kanner | — | 3.06 (52/17) | 0.7 | 0.6; 0.9 |
| AD | 1987 | Burd et al. | USA | North Dakota | 180 986 | 2–18 | 59 | DSM-III | — | 2.7 (43/16) | 3.26 | 2.4; 4.1 | |
| AD | 1988 | Bryson et al. | Canada | Part of Nova-Scotia | 20 800 | 6–14 | 21 | New RDC | 23.8 | 2.5 (15/6) | 10.1 | 5.8; 14.4 | |
| AD | 1989 | Ritvo et al. | USA | Utah | 769 620 | 3–27 | 241 | DSM-III | 34 | 3.73 (190/51) | 2.47 | 2.1; 2.8 | |
| AD | 2001 | Bertrand et al. | USA | Brick Township, New Jersey | 8 896 | 3–10 | 36 | DSM-IV | 36.7 | 2.2 (25/11) | 40.5 | 28.0; 56.0 | |
| AD | 2002 | Croen et al. | USA | California DDS | 4 950 333 | 5–12 | 5 038 | CDER « Full syndrome » | 62.8a | 4.47 (4116 /921) | 11.0 | 10.7; 11.3 | |
| AD | 2005 | Barbaresi et al | USA | Minnesota (Olmstead County) | 37 726 | 0–21 | 112 | DSM-IV | — | — | 29.7 | 24.0; 36.0 | |
| AD | 2006 | Fombonne et al. | Canada | Montreal island | 27 749 | 5–17 | 60 | DSM-IV | — | 5.7 (51/9) | 21.6 | 16.5; 27.8 | |
| AD | 2008 | Montiel-Nava et al. | Venez-uela | Maracaibo | 254 905 | 3–9 | 430 | DSM-IV-TR | — | 3:1 | 17 | 15; 19* | |
| AD | 2009 | van Balkom et al. | Aruba | National | 13 109 | 0–13 | 25 | DSM-IV | 36.0 | 7.3 (22/3) | 19.1 | 12.3; 28.1 | |
| AD | 2010 | Lazoff et al. | Canada | Montreal | 23 635 | 5–17 | 68 | DSM-IV | — | 5.8 (58/10) | 25.4 | 19.0; 31.8 | |
| PDD | 2001 | Bertrand et al. | USA | New Jersey | 8 896 | 3–10 | 60 | DSM-IV | 51 | 2.7 (44/16) | 67.4 | 51.5; 86.7* | |
| PDD | 2003 | Yeargin-Allsopp et al. | USA | Atlanta | 289 456 | 3–10 | 987 | DSM-IV | 31.8 | 4.0 (787/197) | 34.0 | 32; 36 | |
| PDD | 2003 | Gurney et al. | USA | Minnesota | — | 8–10 | — | — | — | — | 52.0 - 66.0b | — — | |
| PDD | 2006 | Fombonne et al. | Canada | Montreal | 27 749 | 5–17 | 180 | DSM-IV | — | 4.8 (149/31) | 64.9 | 55.8; 75.0 | |
| PDD | 2007a | CDC | USA | 6 states | 187 761 | 8 | 1 252 | DSM-IV-TR | 38 to 60c | 2.8 to 5.5 | 67.0 | —d | |
| PDD | 2007b | CDC | USA | 14 states | 407 578 | 8 | 2 685 | DSM-IV-TR | 55.4e | 3.4 to 6.5 | 66.0 | 63; 68 | |
| PDD | 2008 | Nicholas et al. | USA | South Carolinaf | 47 726 | 8 | 295 | DSM-IV-TR | 39.6 | 3.1 (224/71) | 62.0 | 56; 70 | |
| PDD | 2009 | Kogan et al. | USA | Nationwide | 77 911 | 3–17 | 913 | — | — | 4.5 (746/167) | 110 | 94; 128 | |
| PDD | 2009 | CDC | USA | 11 states | 3307 790 | 8 | 2 757 | DSM-IV | 59 | 4.5 (—) | 89.6 | 86; 93 | |
| PDD | 2010 | Lazoff et al. | Canada | Montreal | 23 635 | 5–17 | 187 | DSM-IV | — | 5.4 (158/29) | 79.1 | 67.8; 90.4 | |
| South America & Caribbean | PDD | 2008 | Lejarraga et al. | Argentina | San Isidro | 839 | 0–5 | 11 | DSM-IV | — | — | 13.1 | 7.3; 23.6* |
| PDD | 2009 | Van Balkom et al. | Aruba | National | 13 109 | 0–13 | 69 | DSM-IV | 58.8 | 6.7 (60/9) | 52.6 | 41.0; 66.6 | |
| PDD | 2010 | Paula et al. | Brazil | Atibaia | 1 470 | 7–12 | 4 | DSM-IV | — | (4/0) | 27.2 | 17.6; 36.8 |
calculated by the author.
This proportion is likely to be overestimated and to reflect an underreporting of mental retardation in the CDER evaluations.
These are the highest prevalence estimated reported in this study of time trends. The prevalence in 10-year olds is for the 1991 birth cohort, and that for 8-year olds for the 1993 birth cohort. Both prevalences were calculated in the 2001–2002 school year.
Specific values for % with average IQ are available for each state prevalence.
Confidence intervals are available for each state prevalence.
Average across seven states.
Children aged 8, born either in 2000 and 2002, and included in the two CDC multisite reports.
AD, autistic disorder; CDER, California Department of Disabilities; DSM, Diagnostic and Statistical Manual of Mental Disorders; PDD, developmental disorder; RDC, Research Diagnostic Criteria.
A full article (published or otherwise) was available in a language that could be read by one of the authors.
The primary aim of the survey was to estimate prevalence of PDD and/or narrower AD. Studies exclusively focusing on other categories of PDD, including childhood disintegrative disorder and Asperger disorder (see details in the following) were excluded from the current review as these were very rare and have been reviewed elsewhere [Fombonne et al., 2011]. Other articles, including a wealth of case reports, surveys of more general intellectual or mental disabilities, and surveys estimating PDD prevalence among comorbid conditions, were also excluded from the current report.
The survey included a step of diagnostic confirmation regardless of the specific approach taken to verify caseness (see following discussion for further details). Those studies providing prevalence estimates without diagnostic confirmation, particularly from regions where the evidence base is overall limited, were reviewed in the following main text but are not presented in the tables.
The following information categories were included or could be ascertained based on information from the survey: the country and area where the survey was conducted, the size of the population based on which the prevalence estimate was ascertained, the age range of the participants, the number affected by AD or PDD, the diagnostic criteria used in case ascertainment, gender ratio within the affected sample, prevalence estimate (number per 10 000), and 95% confidence intervals (CI) for the estimate.
Tables I–III summarize the results of epidemiological surveys identified in different world regions. Studies estimating either AD or PDD as a spectrum of disorders from each region were grouped and ordered by publication year. Characteristics of samples surveyed in these studies are briefly summarized. A secondary aim of this report was to review evidence for the possible impact of geographic, economic, social, or cultural/ethnic factors on the clinical presentation and the prevalence of PDD. To that end, selected studies relevant for clinical presentation, particularly from underrepresented regions were reviewed. This included information on gender differences in the rates of PDD, cognitive characteristics of the identified cases, medical history, or socioeconomic and ethnic characteristics.
Combining and Appraising Results
Each group of authors focusing on specific WHO regions entered their findings into the same template that included the following: (a) general context and background for the region covered; (b) key findings on prevalence estimates entered into the same format presented in Tables I–III; (c) key findings clinical characterization summarized qualitatively; and (d) commentary on opportunities and barriers in conducting research in the region covered. Studies included were appraised by each group of authors who decided on inclusion and exclusion based on the criteria outlined earlier. M.E. reviewed all entries and combined the results, and together with E.F. performed overall appraisal of the data included. All authors subsequently verified the information in the final article. The diversity in the methodological approaches precluded the possibility of any further analysis of these estimates included meta-analysis but average figures and basic analyses were included in later sections.
Methodological Issues and Interpretation of Prevalence Estimates
Before summarizing the key findings related to prevalence and clinical characterization, we will discuss some methodological issue that need to be accounted for when interpreting epidemiological data. These include characterization of PDD, changes in concepts over time, and case identification and evaluation.
Characterization of PDD
Over time, the definitions of autism have changed as illustrated by the numerous diagnostic criteria that were used in both epidemiological and clinical settings. Starting with the narrowly defined Kanner's autism, definitions progressively broadened in the criteria proposed by Rutter [Rutter, 1970], and then International Classification of Diseases (ICD)-9 [WHO, 1975], Diagnostic and Statistical Manual of Mental Disorders (DSM)-III [American Psychiatric Association, 1980] and DSM-III-R [American Psychiatric Association, 1987], and more recently in the two major nosographies used worldwide: ICD-10 [WHO, 1992] and DSM-IV [American Psychiatric Association, 1994]/DSM-IV-TR [American Psychiatric Association, 2000]. The first diagnostic criteria reflected the more qualitatively severe forms of the phenotype of autism, usually associated with severe delays in language and cognitive skills. It is only in the 1980s that less severe forms of autism were recognized, either as a qualifier for autism occurring without intellectual disability (so-called “high-functioning” autism), or as separate diagnostic categories (PDDs not otherwise specified [PDDNOS]) within a broader class of autism spectrum disorders (ASD) denominated “PDDs” (an equivalent to ASD) in current nosographies. Whilst it had been described in the literature as early as 1944, one type of PDD, Asperger disorder, appeared in official nosographies only in the 1990s and, then, with unclear validity, especially with respect to its differentiation from “high-functioning” autism. Subtypes of PDD that existed in DSM-III subsequently disappeared (i.e., autism—residual state). While there is generally high inter-rater reliability on the diagnosis of PDDs and commonality of concepts across experts, some differences persist between nomenclatures about the terminology and precise operationalized criteria of PDDs. For example, DSM-IV [American Psychiatric Association, 1994] has a broad category of PDDNOS, sometimes referred to loosely as “atypical autism,” whereas ICD-10 [WHO, 1992] has several corresponding diagnoses for clinical presentations that fall short of AD, which include atypical autism (F84.1, a diagnostic category that existed already in ICD-9), Other PDD (F84.8), and PDD, unspecified (F84.9).
In the recent years, the research definitions of syndromes falling on the autism spectrum have been expanded further with an increasing reliance on a dimensionalization of the autism phenotype. As no impairment or diagnostic criteria are available for these milder forms, the resulting boundaries with the spectrum of PDDs are left uncertain. Whether or not this plays a role in more recent epidemiological studies is difficult to know, but the possibility should be considered in assessing results for the new generation of surveys. While the vast majority of studies have relied on these common nosographies, culturally specific instruments and systems have been used. For example, the Chinese Classification of Mental Disorders (CCMD) has been used in some of the studies appearing in Chinese journals and often combined with other classification systems.
Case Identification
When an area or population has been identified for a survey, different strategies have been used to find participants matching the case definition retained for the study. Some studies have solely relied on existing service providers databases [Croen, Grether, Hoogstrate, & Selvin, 2002]; on special educational databases [Fombonne, Zakarian, Bennett, Meng, & McLean-Heywood, 2006; Gurney et al., 2003; Lazoff, L., Piperni, & Fombonne, 2010]; or on national registers [Madsen et al., 2002] for case identification. These studies have the common limitation of relying on a population group who happened to access the service provider or agencies rather than sampling from the population at large. As a result, participants with the disorder who are not in contact with these services are yet unidentified and not included as cases, leading to underestimation of prevalence proportion. This is a particularly significant issue when estimating prevalence using such methods in communities with recognized limitations in service provisions.
Other investigations have relied on a multistage approach to identify cases in underlying populations [e.g., Baird et al. 2006; Kim et al., ]. The aim of the first screening stage of these studies is to cast a wide net in order to identify participants possibly affected with a PDD, with the final diagnostic status being determined at a subsequent phase. The process used by researchers often consisted of sending letters or brief screening scales requesting school and health professionals and/or other data sources to identify possible cases of autism. Few investigations have relied on systematic sampling techniques that would ensure a near complete coverage of the target population. Moreover, each investigation differed in several key aspects of this screening stage. First, the thoroughness of the coverage of all relevant data sources varied enormously from one study to another. In addition, the surveyed areas were not comparable in terms of service development, reflecting the specific educational or health care systems of each country and of the period of investigation. Second, the type of information sent out to professionals invited to identify children varied from simple letters, including a few clinical descriptors of autism-related symptoms or diagnostic checklists rephrased in nontechnical terms, to more systematic screening strategy based on questionnaires or rating scales of known reliability and validity. Third, variable participation rates in the first screening stages provide another source of variation in the screening efficiency of surveys, although refusal rates tended, on average, to be very low.
Few studies provided an estimate of the reliability of the screening procedure. The sensitivity of the screening methodology is also difficult to gauge in autism surveys, and the proportion of children truly affected with the disorder but not identified in the screening stage (the “false negatives”) remains generally unmeasured. The usual approach, which consists of sampling at random screened negative participants in order to estimate the proportion of false negatives, has not been used in these surveys for the obvious reason that, because of the relatively low frequency of the disorder, it would be both imprecise and very costly to undertake such estimations. This approach may gradually change in view of recent prevalence studies suggesting that autism can no longer be regarded as a rare condition. In any case, the magnitude of this of under- or over-estimation of prevalence studies relative to “true” prevalence is currently unknown.
Case Evaluation
When the screening phase is completed, participants identified as positive screens go through the next step involving a more in-depth diagnostic evaluation to confirm their case status. Similar considerations about the methodological variability across studies apply to these more intensive assessment phases. Participation rates in second stage assessments within the studies reviewed were generally high. The source of information used to determine caseness usually involved a combination of data coming from different informants and data sources (medical records, educational sources, parents, teachers, health professionals, etc.), with a direct assessment of the person with autism being offered in some but not all studies. Obviously, surveys of very large populations as those conducted in the United States by the CDC [CDC, 2007a, 2007b, 2009] or in national registers [Madsen et al., 2002] did not include a direct diagnostic assessment of all participants by the research team. However, these investigators could generally confirm the accuracy of their final caseness determination by undertaking, on a randomly selected subsample, a more complete diagnostic work-up. The CDC surveys have established a methodology for surveys of large populations that relies on screening of population using multiple data sources, a systematic review and scoring system for the data gathered in the screening phase combined with, in the less obvious cases, input from experienced clinicians. This methodology is adequate for large samples, and is likely to be used in the future for surveillance efforts. When participants are directly examined, the assessments were conducted with various diagnostic instruments, ranging from an unstructured examination by a clinical expert to the use of batteries of standardized measures by trained research staff. The Autism Diagnostic Interview [ADI; Le Couteur et al., 1989] and/or the Autism Diagnostic Observational Schedule [ADOS; Lord et al., 2000] have been increasingly used in the most recent surveys in North America and some European countries. Against this background and in view of these diverse methodological features in the studies we report, we now turn to the available evidence from epidemiological surveys and studies on clinical characterization.
Europe
Older Prevalence Estimates
Prior to the modern conceptualization of PDD, epidemiological surveys presented in Table I conducted as early as the 1960s in Europe, mainly in Northern countries, provided useful information on rates of syndromes similar to autism but not meeting strict diagnostic criteria for AD then in use [Fombonne, 2003b, 2005]. At the time, different labels were used by authors to characterize these clinical pictures such as the triad of impairments involving deficits in reciprocal social interaction, communication, and imagination [Wing & Gould, 1979], or “autistic-like” syndromes [Burd, Fisher, & Kerbeshan, 1987]. These syndromes would be falling within our currently defined autistic spectrum, probably with diagnostic labels such as atypical autism and/or PDD-NOS. In surveys providing separate estimates of the prevalence of these developmental disorders, higher rates for the atypical forms were found compared with those for more narrowly defined AD [see Fombonne, 2003a, p.172]. This group received little attention in previous epidemiological studies and these participants were not counted in the numerators of prevalence calculations, thereby underestimating systematically the prevalence of what would be defined today as the spectrum of ADs. For example, in the surveys conducted by Lotter [Lotter, 1966] and Wing et al. [Wing, Yeates, Brierly, & Gould, 1976], prevalence figures would rise substantially if atypical forms had been included in the case definition. For purpose of historical comparisons, it is important to be attentive to this earlier figure, bearing in mind that the study was conducted in the early 1970s for the field work and that autism occurring in participants with an IQ within the normal range was not yet being investigated. Progressive recognition of the importance and relevance to autism of these less typical clinical presentations has led to changes in the design of more recent epidemiological surveys (see following discussion) that are now using case definitions that incorporate upfront these milder phenotypes.
Recent Prevalence Estimates
Available studies from Northern European countries (UK, Iceland, Denmark, Sweden) provide estimates for combined PDD as well as AD. Much less data are available from other European countries, namely from France, Germany, Portugal, and Israel. Sample sizes of multiple surveys estimating AD varied from 826 to 490 000 participants, with an age range of birth to adulthood. Prevalence rates varied from 1.9/10 000 to 72.6/10 000 with a median value of 10.0/10 000. In studies published since 1999, the median rate was 18.75/10 000. Studies providing estimate for combined PDD ranged in sample size from 2536 to 134 661 participants. All these surveys were published since 2000, and the majority since 2006. The diagnostic criteria reflect reliance on modern diagnostic schemes. There was high variation in prevalence proportions that ranged from a low 30.0/10 000 to a high of 116.1/10 000, with a median rate of 61.9/10 000.
The wide range of prevalence estimates reported in these studies may be attributed, at least in part, to methodological issues outlined earlier. Furthermore, such estimates should always be regarded in the context of the imperfect sensitivity of case ascertainment that results in downward biases in prevalence proportions. For example, in the Danish investigation [Madsen et al., 2002], case finding depended upon notification to a National Registry, a method that is usually associated with lower sensitivity for case finding. By contrast, case-finding techniques used in other surveys relied on multiple and repeated screening phases, involving both different informants at each phase and surveying the same cohorts at different ages, which certainly maximized the sensitivity of case identification [Baird et al., 2006; Chakrabarti & Fombonne, 2005].
Clinical Presentation
Clinical characterization in Europe has increasingly relied on standardized measures, serving to reduce heterogeneity in clinical judgment. Assessments in several of the studies summarized earlier were often performed with these diagnostic measures that match the more dimensional approach for case definition. Extensive discussion of clinical presentation has been previously presented based on these studies [Fombonne, 2003a, 2005]. To summarize, males consistently outnumbered females in the vast majority of studies, with a ratio ranging from 1.33 to 16.0 for AD and 3.3 to 15.7 for PDD. In studies where IQ scores were available, the proportion of participants with normal IQ was 15.6–86.7% for AD and 45–85.3% for PDD. A few studies provided information on the social class of the families of autistic children. Of these, two older studies [Brask, 1972; Lotter, 1966] suggested an association between autism and social class or parental education. Studies conducted thereafter provided no evidence for the association. Thus, the epidemiological results suggest that the earlier findings were probably due to artifacts in the availability of services and in the case-finding methods, as already shown in other samples [Schopler, Andrews, & Strupp, 2012; Wing, 1980].
Some investigators have mentioned the possibility that rates of autism might be higher among immigrants in Northern Europe [Gillberg, 1987; Gillberg, Schaumann, & Gillberg, 1995; Gillberg, Steffenburg, & Schaumann, 1991; Wing, 1980]. A meta analysis including five studies found weak (nonsignificant) association between autism and mother's birthplace [in the country where the study was conducted vs. abroad; Gardener, Spiegelman, & Buka, 2009]. Serious methodological caveats related to these comparisons have been extensively discussed, including small samples, variation in rate of immigration in the areas samples, and the lack of a reasoned biologically plausible hypothesis linking immigration to autism [Fombonne, 2005].
Complex sociobiological [Gillberg et al., 1995; Gillberg et al., 1991] and environmental routes [Dealberto, #b1001] have been suggested to explain the differences reported across a range of studies but none have been formally tested. In trying to ascertain factors that might explain differences in rates among immigrants and nonimmigrants, a recent study in the Netherlands [Begeer, Bouk, Boussaid, Terwogt, & Koot, 2009] showed that ethnic minorities were underrepresented among cases referred to autism institutions. Yet, the study also found that bias in clinical judgment among pediatricians may lead to under-referral. Moreover, the same bias was no longer apparent when pediatricians were asked to provide explicit structured ratings of the probability of autism. Taken together, in view of methodological limitations related to low numbers of identified cases, appropriateness of the comparison data that were used and large heterogeneity in the definition of immigrant status these findings cannot support a difference in PDD rates among immigrants and nonimmigrants.
Western Pacific, South East Asia, and the Eastern Mediterranean
Prevalence Estimates
The oldest and most comprehensive prevalence surveys of AD in the Western Pacific Region come from Japan and China. While studies from Japan have tended to be published in English and have been reported in previous reviews, Chinese search engines provided a substantial body of studies published in local journals. Studies in this region had population size ranging from 3606 to 609 848. Since 2000, prevalence rates varied from 2.8/10 000 to 94/10 000 with a median value of 11.6/10 000. The median is somewhat similar (13/10 000) when older studies are included. The median value does not differ when older studies are excluded. This rate of AD is lower than current estimates from a comparable number of studies in Northern Europe but resembles estimates from older studies in that region. Only one estimate of AD was available from South East Asia. The study conducted in Indonesia estimated the rate of AD to be 11.7/10 000 [Wignyosumarto, Mukhlas, & Shirataki, 1992]. No estimates of AD were found in the Eastern Mediterranean.
In relation to combined PDD, three were available from the Western Pacific Region. One study in Australia estimated prevalence of PDD to be 39.2/10000 [Icasiano, Hewson, Machet, Cooper, & Marshall, 2004]. The highest rate of PDD internationally comes from a recent Korean study estimating the prevalence to be 189/10 000 [Kim et al., ] followed by a Japanese study estimating the prevalence to be 181.1/10 000 [Kawamura, Takahashi, & Ishii, 2008]. In fact, the former study [Kim et al., ] also reports a high probability estimate of 264/10 000 in addition to the general population estimate. By contrast, a study from China [Wong & Hui, 2008] reported a much lower estimate of 16.1/10 000. The Chinese study also reported that ASD incidence based on registry data in Hong Kong has been rather stable during 20 years. Estimated rates of 5-year incidence increased slightly from 5.19 (CI: 4.43–5.94) to 7.86 (CI: 6.68–9.05) per 10 000 over the study period.
Four estimate of PDD was available from South East Asia and the Eastern Mediterranean. These were 100/10 000 in Sri Lanka [Perera, Wijewardena, & Aluthwelage, 2011], 29/10 000 in the United Arab Emirates [Eapen, Mabrouk, Zoubeidi, & Yunis, 2007], 1.4/10 000 in Oman [Al-Farsi et al., 2010], and 6.3/10 000 in Iran [Samadi, Mahmoodizadeh, & McConkey, ]. Another estimate (not included in Table II) from Iran [1.9%, Ghanizadeh, 2008] was available but the study did not confirm diagnosis in the identified group. In addition to these estimates, ASD incidence based on referral statistics in Bangkok were reported to have increased from 1.43 per 10 000 in 1998 to 6.94 per 10 000 in 2002 [Plubrukarn, Piyasil, Moungnoi, Tanprasert, & Chutchawalitsakul, 2005]. Factors related to broadening of the diagnostic concepts and increasing service seeking discussed earlier are likely to account for the variability in estimates as well as the observed increase. In this study, most of incidence changes stemmed from the increase of the AD cases, not from an increase of PDD-NOS. Additionally, incidence in children older than five did not change (1.52 to 1.66) during the same period [Plubrukarn et al. 2005]. These findings suggest that younger and more severe cases of PDD rather than older and milder cases of children over five years account for the increase. By contrast, the relatively low prevalence in the Omani and Iranian studies was attributed to prevalence caused by underdiagnosis and underreporting of cases resulting from limited access to service centers [Al-Farsi et al., 2010].
Table II.
Western Pacific, South East Asia, and the Eastern Mediterranean
| Region | Category | Year | Authors | Country | Area | Population | Age | Number affected | Diagnostic criteria | % with average IQ | Gender ratio | Prevalence/ 10 000 | 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Western Pacific | AD | 1982 | Hoshino et al. | Japan | Fukushima-Ken | 609 848 | 0–18 | 142 | Kanner's criteria | — | 9.9 (129/13) | 2.33 | 1.9; 2.7 |
| AD | 1987 | Matsuishi et al. | Japan | Kurume City | 32 834 | 4–12 | 51 | DSM-III | — | 4.7 (42/9) | 15.5 | 11.3; 19.8 | |
| AD | 1988 | Tanoue et al. | Japan | Southern Ibaraki | 95 394 | 7 | 132 | DSM-III | — | 4.07 (106/26) | 13.8 | 11.5; 16.2 | |
| AD | 1989 | Sugiyama et Abe | Japan | Nagoya | 12 263 | 3 | 16 | DSM-III | — | — | 13.0 | 6.7; 19.4 | |
| AD | 1996 | Honda et al. | Japan | Yokohama | 8 537 | 5 | 18 | ICD-10 | 50.0 | 2.6 (13.5) | 21.08 | 11.4; 30.8 | |
| AD | 2000 | Luo | China | Fujian | 10 802 | 0–14 | 3 | ABC, CCMD-2-R & DSM-III-R | — | 2:1 | 2.8 | 0.6; 8.1* | |
| AD | 2002 | Wang et al. | China | JiangSu | 3 912 | 2–6 | 7 | CABS, CARS,CCMD2-R | — | 2.5:1 | 17.9 | 7.2; 36.8* | |
| AD | 2003 | Wang et al. | China | JiangSu | 7 344 | 2–6 | 9 | CABS, CARS, CCMD2-R, | — | 2:1 | 12.3 | 5.6; 23.3* | |
| AD | 2004 | Guo et al. | China | Tianjin | 3 606 | 2–6 | 5 | CARS,CCMD-2-R,CABS | — | All boys | 13.9 | 4.5; 32.3* | |
| AD | 2004 | Zhang et al. | China | Tianjin | 7 316 | 2–6 | 8 | CARS, DSM–IV | 0 | (7:1) | 10.9 | 4.7; 21.5* | |
| AD | 2005 | Zhang & Ji | China | Tianjin | 7 345 | 2–6 | 8 | DSM-IV, CARS | — | 7 | 10.9 | 4.7; 21.4* | |
| AD | 2005 | Honda et al.a | Japan | Yokohama | 32 791 | 5 | 123 | ICD-10 | 25.3 | 2.5 (70/27) | 37.5 | 31.0; 45.0 | |
| AD | 2007 | Yang et al. | China | Zunyi city | 10 412 | 3–12 | 6 | DSM-IV, ABC | — | (5/1) | 5.8 | 2.1; 12.5* | |
| AD | 2007 | Chen et al. | China | Taiwan | 329 539 | 3–8 | 1115 | ICD-10 | — | 4.13 | 34* | 32; 36* | |
| AD | 2008 | Zhang et al. | China | Wuxi city | 25 521 | 1–6 | 25 | CARS/DSM-IV | — | 3.2 | 9.8 | 6.3; 14.5* | |
| AD | 2009 | Zhang et al. | China | GuiZhou | 5 000 | 1–6 | 5 | CCMD-2-R, CARS, CABS | — | 4:1 | 10.0 | 3.2; 23.3* | |
| AD | 2011 | Kim et al. | South Korea | Goyang | 55 266 | 7–12 | 27 | DSM-IV | 55.6 | 4.4 | 94 | 56; 134 | |
| PDD | 2004 | Icasiano et al. | Australia | Barwon | 45 153 * | 2–17 | 177 | DSM-IV | 53.4 | 8.3 (158/19) | 39.2 | 34; 45* | |
| PDD | 2008 | Wong and Hui | China | Hong Kong | 4 247 206 | 0–14 | 682 | DSM-IV | 30 | 6.6 (592/90) | 16.1 | 14.9; 17.3* | |
| PDD | 2008 | Kawamura et al. | Japan | Toyota | 12 589 | 5–8 | 228 | DSM-IV | 66.4 | 2.8 (168/60) | 181.1 | 158.5; 205.9* | |
| PDD | 2011 | Kim et al. | South Korea | Goyang | 55 266 | 7–12 | 104 | DSM-IV | 72 | 2.4 | 189 | 143; 236 | |
| South East Asia | AD | 1992 | Wignyo-sumarto et al. | Indo-nesia | Yogyakarita | 5 120 | 4–7 | 6 | CARS | 0 | 2.0 (4/2) | 11.7 | 2.3; 21.1 |
| PDD | 2009 | Perera et al. | Sri Lanka | Semi urban area | 374 | 1.5–2 | 4 | DSM-IV, Parent report | — | All boys | 107 | 2.9.2; 271.6* | |
| Eastern Medi- terranean | PDD | 2007 | Eapen et al. | UAE | Three regions | 694 | 3.5 | 2 | DSM-IV | — | 5.14 (—) | 29 | 3.5; 103.7* |
| PDD | 2010 | Al-Farsi et al. | Oman | Country-wide | 798 913 | 0–14 | 113 | DSM-IV | — | 2.9 (84/29) | 1.4 | 1.2; 1.7 | |
| PDD | 2012 | Samadi et al. | Iran | Country-wide | 1 320 334 | 5 | 826 | ADI-R | — | 4.3 | 6.26 | 5.84; 6.70 |
Calculated by the author
This figure was calculated by the author and refers to prevalence data (not cumulative incidence) presented in the paper (the M/F ratio is based on a subsample).
AD, autistic disorder; ADI-R, Autism Diagnostic Interview-Revised; CARS, Childhood Autism Rating Scale; CABS, Clancy Autism Behavior Scale; CCMD, Chinese Classification of Mental Disorders; DSM, Diagnostic and Statistical Manual of Mental Disorders; ICD, International Classification of Diseases. PDD, developmental disorder.
Clinical Presentation—Western Pacific
While detailed medical conditions and ASD phenotype data were not available, several studies reported similarities in the PDD phenotypes within this region. Excluding older studies from Japan, AD and PDD were more prevalent in boys than girls in the epidemiological studies in Table II. In Japan, 66.4% of participants in the study with the highest prevalence estimate had IQ scores within the normal range. By contrast, all cases with AD identified in the population-based prevalence study in China had intellectual disabilities. Zhang and Ji, [2005] speculated that autistic children with average cognitive function or mild intellectual disabilities might be neglected during the screening and case-ascertainment process. The Tanjin study [Zhang & Ji, 2005] reported that the AD prevalence was higher among urban children than children in suburban area (1.37% vs. 0.80%, respectively). Authors also noted that parents in urban areas had higher education and higher family income, leading to better accessibility for clinical care and rehabilitation services in these families.
Clinical Presentation—South East Asia
In addition to the two prevalence studies in Table II, a few studies characterized relatively small samples of children in tertiary care settings in India among a larger literature on intellectual disabilities from the region. The studies had a high but variable male-to-female ratios [Juneja, Mukherjee, & Sharma, 2004; Kalra, Seth, & Sapra, 2005; Malhi & Singhi, 2005; Singhi & Malhi, 2001]. Across studies, the concern that most commonly led to referral from medical professionals was language delay or regression in language skills, followed by social difficulties and hyperactivity. Most children received the diagnosis of ASD between 3 and 6 years [Juneja et al., 2004; Kalra et al., 2005]. The time between recognition of symptoms by caregivers and diagnosis averaged about 2 years. Three Indian studies noted that the majority of families were from middle-class backgrounds [Daley, 2004; Juneja et al., 2004; Singhi & Malhi, 2001] and postulated that the higher socioeconomic families do not attend state-run facilities while the lower socioeconomic groups may not access care unless the child is acutely ill. Regression of skills was found in 25% of the children in one study [Singhi & Malhi, 2001]. Seizures were associated with ASD in 6.8–31% of children [Daley, 2004; Juneja et al., 2004; Kalra et al., 2005]. Intellectual disability was the most common co-morbid condition ranging from 24 to 95% of children. Perinatal events were examined in three studies [Juneja et al., 2004; Kalra et al., 2005; Malhi & Singhi, 2005]; two reported such events in up to 25% of the children whereas the third found no significant perinatal events.
Clinical Presentation—Eastern Mediterranean
Similar to other regions, a number of studies report a high but variable male-to-female ratios [Al-Farsi et al., 2010; Al-Salehi, Al-Hifthy, & Ghaziuddin, 2009; Eapen et al., 2007; Seif Eldin et al., 2008]. The gender ratio in the Iranian study was more equally distributed between males and females [Ghanizadeh, 2008], but the rate reported in this study most likely reflects that of PDD symptoms among school-aged children rather than estimates of the disorder because a stage of diagnostic confirmation was not conducted [Ghanizadeh, 2008]. One study from Saudi Arabia reported that girls were older than boys in a tertiary referral center [Al-Salehi et al., 2009].
Parallel findings were observed across a number of studies from neighboring Arabic-speaking countries [Al-Salehi et al., 2009; Eapen et al., 2007], including one study that combined groups of children from nine countries [Seif Eldin et al., 2008]. These studies suggest high rates of behavioral, cognitive, and/or medical problems among children with PDD. One study highlighted that communication difficulties were a main reason for referral and reported a regressive profile in 10% of cases [Al-Farsi et al., 2010; Al-Salehi et al., 2009]. Two studies examining family characteristics of groups of children with PDD reported that the rate of consanguinity did not differ from a control group [Seif Eldin et al., 2008] or from population estimates [Al-Salehi et al., 2009]. Yet, the affected groups typically had higher parental age, incidence of pregnancy and labor complications [Seif Eldin et al., 2008], and incidence of familial mental health problems [Eapen et al., 2007; Seif Eldin et al., 2008]. In the Omani study, most cases identified were in the capital Muscat and the rates were variable across the other regions examined [Al-Farsi et al., 2010].
America
Prevalence Estimates
The vast majority of data from this region (Table III) comes from the United States and Canada from as early as the 1970s. The sample sizes of studies estimating AD were fairly large ranging from 8896 to 4 950 333 individuals. Estimates of AD varied from 0.7–40.5/10 000, with a median of 11/10 000. Studies done since 2000, however, yield estimates ranging from 11 to 50.5/10 000, with a median of 21.6/10 000. Studies estimating combined PDD, all of which were conducted since 2000 ranged from 8896 to 407 578 individuals in the target population. PDD estimates varied from 34 to 90/10 000, with a median of 65.5/10 000. Despite huge variation in ranges, both AD and PDD median estimates correspond closely to those derived from Northern Europe. Similar to the latter studies, a likely underestimation of the true population rates holds in this region as well. For example, the Atlanta survey by the CDC [Yeargin-Allsopp et al., 2003] was based on a very large population and included younger age groups than subsequent CDC surveys, and age specific rates were in fact in the 40–45/10 000 range in some birth cohorts [Fombonne, 2003b].
Less data were available from other countries in America. Two published studies from Argentina [Lejarraga et al., 2008] and Venezuela [Montiel-Nava & Peña, 2008] along with unpublished reports from Brazil [Paula, Ribeiro, Fombonne, & Mercadante, ] and Mexico [Marcín, personal communication] provided prevalence data (Table III). There was also one study from the Caribbean (Aruba) [van Balkom et al., 2009]. All of the studies had relatively small sample sizes. The two studies from Venezuela and Aruba provided similar AD estimates of 13 and 19/10 000. With the exception of one study conducted in 1982, the remaining estimates of PDD ranged from 27 to 39.2/10 000. Administrative prevalence was available from Mexico through a registry of minors with disabilities reporting the number of children identified with autism, combined with Mexican census figures. Based on these figures the rate for childhood autism in Mexico was estimated to be 14.3 per 10 000 [Marcín, personal communication]. The estimates in those studies are smaller than those reviewed earlier but it is difficult to compare the findings given the limited data and methodological differences among studies.
Clinical Presentation
Similar to Northern Europe, clinical characterization of PDD in the United States and Canada has increasingly relied on standardized measures and have been previously reviewed [Fombonne, 2003a, 2005]. Across all studies reported in Table III, the male-to-female ratio ranged from 2.2 to 5.8 for AD and from 2.7 to 6.7 for PDD. In studies reporting IQ scores, 23.8–62.5% of participants with AD and 31.8–60.0% of participants with PDD were reported to have normal IQ, with the higher estimate corresponding to the United States (South Carolina) and Aruba.
The largest studies internationally to utilize comparable methodology for examining variation of prevalence among neighboring geographical regions were conducted by the CDC in the United States. Findings from geographic regions falling within 10 American states found a threefold variation of rate by state, where Alabama had the lowest rate (3.3/1000) whereas New Jersey had the highest (10.6/1000) [CDC, 2007b]. Subsequent CDC surveys found a similar variation of rates but the lowest was in Florida (4.2/1000) and highest in Missouri and Arizona (12.1/1000) [CDC, 2009]. The CDC surveys found that prevalence estimates were lower in sites that relied solely on health sources to identify cases compared with sites that also had access to education records, and hence a less rich database for those areas where ASD indicators could be found. As surveillance efforts continue, it is likely that awareness and services will develop in states that were lagging behind, resulting in a predictable increase in the average rate for the United States as time elapses.
CDC surveys also monitor variation in prevalence estimates among different ethnic communities. In the last survey [CDC, 2009] prevalence among white children was greater than that among black and Hispanic children. Similar to other world regions, service availability may account for the observed differences. In support of this, prevalence has increased over time across all sex, racial/ethnic, and cognitive functioning subgroups, making it unlikely that one group is differentially affected. Variability of estimates as a function of income level has also been noted. As an example, a study conducted in Texas showed that the top decile of income had a 6 times greater administrative prevalence than the bottom decile [Palmer, Blanchard, Jean, & Mandell, 2005].
Africa
Prevalence Estimates
As early as 1970s, a report describing autism among children with mental handicaps who were known to authorities in six central and south African countries reported a preliminary observation that the number of cases of autism maybe smaller than those observed in the UK [Lotter, 1978]. Findings from this preliminary report, which was not designed as an epidemiological survey, were never corroborated. At the time, however, this served as crucial evidence that autism can be identified across varied geographic region. Similar to the conclusion of a recent review on autism in Africa [Bakare & Munir, 2011], we could not identify published data on population-based estimates of prevalence of PDD from African region.
Clinical Presentation
A few studies [reviewed in Ametepee & Chitiyo, 2009] including Lotter's [Lotter, 1978], documented unambiguous recognition of autism among African children, albeit raising some questions about the relative frequency of some of the manifestations [Lotter, 1978; Mankoski et al., 2006]. A higher male-to-female ratio was consistent [Khan & Hombarume, 1996; Lotter, 1978; Mankoski et al., 2006]. Similar to observations from other regions, those studies suggested an over representation of higher socioeconomic background and higher frequency of cases with intellectual difficulties, i.e., the children identified were mainly nonverbal. Yet, none of the studies used appropriate tools for the evaluation of cognitive abilities and similar arguments related to service accessibility apply here as well. In one study, 3/14 cases described were described as having developed autism after the second year subsequent to a severe malaria infection [Mankoski et al., 2006]. The findings, however, were never replicated with appropriate sample sizes or with a prospective rather than retrospective methodology to account for the possibility of biased recall. In contrast to the dearth of evidence, there has been an abundance of claims regarding the prevalence and etiology of the autism in Africa [reviewed in Ametepee & Chitiyo, 2009; Bakare & Munir, 2011]. The clinical presentation of the condition in this region remains elusive.
PDD in the Global Context: Prevalence, Determinants, and Burden
Global Variation in Prevalence Estimates
Epidemiological surveys of autism and AD and PDD (summarized inFigs 1 and 2) have now been conducted in several countries. Methodological differences in case definition and case-finding procedures make between survey comparisons difficult to perform. However, most studies conducted since the year 2000 in different geographical regions and by different teams converge to estimates to a median of 17/10 000 for AD and 62/10 000 for all PDDs combined (Table IV). This is currently the best estimate for the prevalence of PDDs available. The estimate represents an average figure and there is substantial variability across studies.
Figure 1.
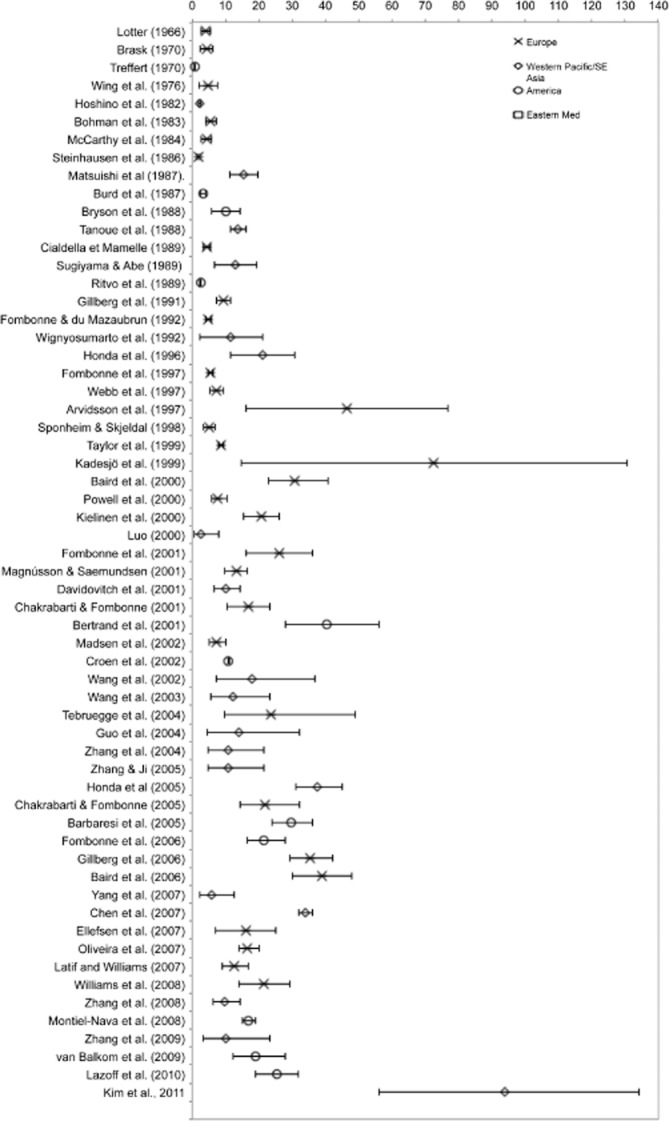
Prevalence of autistic disorder from Tables I and II (rate/10 000 and 95% confidence interval).
Figure 2.
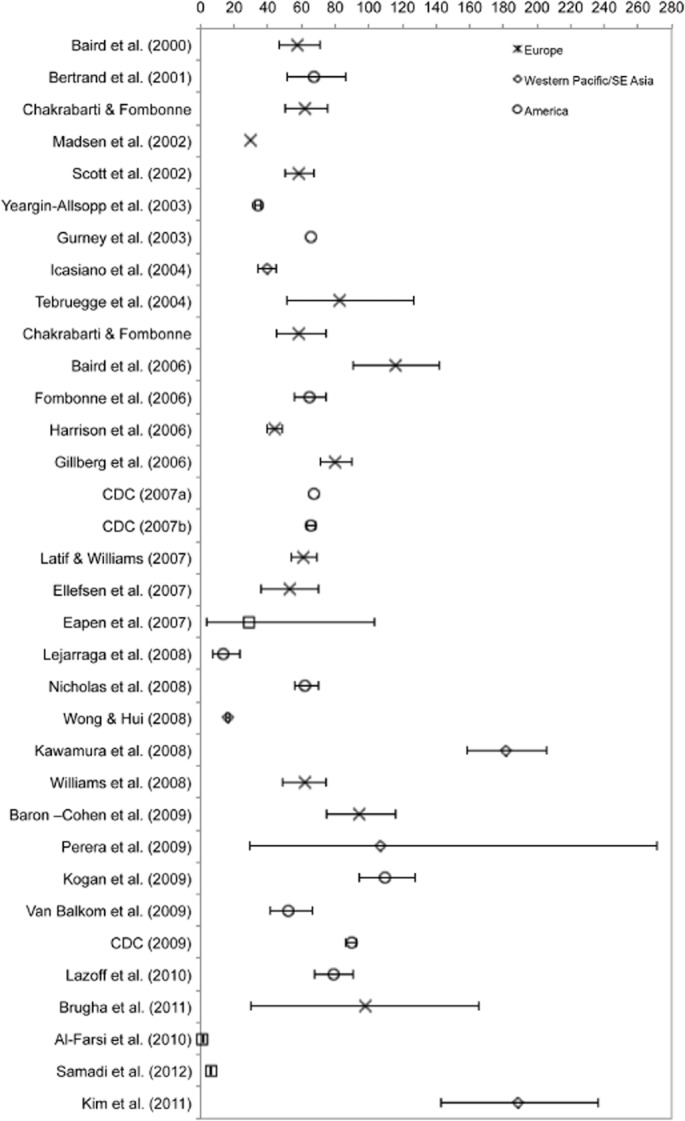
Prevalence of PDD from Tables I and II (rate/10 000 and 95% confidence interval).
Table IV.
Summary of Prevalence Estimates of AD and PDD Across World Regions Since the Year 2000. Medians are not Presented When there were Too Few Estimates Available Within a Given Region
| AD estimates | PDD estimates | |||||
|---|---|---|---|---|---|---|
| Region | Median | Range | Number of estimates | Median | Range | Number of estimates |
| Europe | 19 | 7–39 | 16 | 62 | 30–116 | 14 |
| America | 22 | 11–40 | 7 | 65 | 13–110 | 12 |
| Western Pacific | 12 | 2.8–94 | 12 | — | — | 3 |
| South East Asia | — | — | 1 | — | — | 1 |
| Eastern Mediterranean | — | — | 0 | — | — | 3 |
| Africa | — | — | 0 | — | — | 0 |
| All | 17 | 2.8–94 | 36 | 62 | 1–189 | 33 |
AD, autistic disorder; PDD, developmental disorder.
Notwithstanding considerable variability across and within geographical regions, estimates of AD collected since the year 2000 in America, Western Pacific, and Europe do not differ statistically (P = 0.3). While considerably less studies have been conducted on PDD, those estimates do not differ between America and Europe. The global mean prevalence of 62/10 000 translates into one child out of 160 with a PDD. Some well-controlled studies have, however, reported rates that are two to three times higher [Baird et al., 2006; Kawamura et al., 2008; Kim et al., ]. By contrast, in many regions of the world including Africa, prevalence estimates are either unavailable or preliminary. Moreover, within each of the regions covered, the majority of studies were conducted in circumscribed regions, namely, in northern Europe, Japan, China, and the United States. With the exception of China, countries where a relatively large evidence base exists are high-income countries. A few studies have been conducted in middle-income countries and no prevalence estimates were available from any low-income country.
Our minimal criteria for inclusion of prevalence estimates described in the previous section were motivated by the need for a systematic and comprehensive account of available estimates. However, given the significant variability in resulting prevalence estimates, and the variability in methodological features outlined earlier, the estimates should be considered with caution. Specifically, these should not be considered as reflecting “world-wide” estimates, but rather, a general reflection of the current state of evidence from various regions. As we note in the final section, our findings offer critical public health messages regarding the need for scaling of services as well as research in most of the world's regions.
Phenotypic presentation of PDD
As early as the 1970s, the phenotypic characteristics of autism have been observed and described across a wide range of geographical, economic, social and cultural settings. Based on available evidence world-wide, the higher ratio in males relative to females is consistently observed. It is also clear that PDD is associated with a degree of intellectual impairment among a significant portion of those affected. Yet, the range of variation in gender ratio and intellectual functioning is vast. By contrast, very few studies examined the impact geographical, economic, social and cultural factors directly and, on the whole, the findings indicate little impact. Differences in such factors reported retrospectively in large-scale surveys such as those conducted by the CDC [CDC, 2007a, 2007b] may be accounted for entirely by differences in service provisions, richness of registered data, and community awareness. Moreover, there is a striking absence of controlled studies examining the potential impact of such factors on the understanding, experience, expression, and help-seeking for autism, masking the true global burden of the condition. Some positive indicators suggest improved identification of autism in low-resource countries, as inferred from the increase in referrals and identified cases [e.g., Plubrukarn et al., 2005]. Yet, it has also been suggested that regional variation in parental education may still determine their help seeking for autism [Seif Eldin et al., 2008] and that it is predominantly severe cases that are currently being identified in poor communities [Daley, 2004; Juneja et al., 2004; Singhi & Malhi, 2001]. Such possibilities require confirmation in future large-scale controlled studies.
The data we reviewed is also relevant for the ongoing debate regarding time trends in autism. Considering all studies conducted since the 1960s, prevalence data suggest that there is a correlation between AD estimates and year of publication (r = 0.4, P < 0.01, R2 = 18.5%). While this simple test is limited by the considerable variation, it does confirm that when data is considered from several world regions there is indeed a rise in prevalence over time, confirming previous conclusions. Such time trends have given rise to the hypothesis that there is a secular increase in rates of autism. However in view of a number of conceptual and methodological reasons, no inference related to trends in the incidence of PDDs can be derived from a simple comparison of prevalence rates over time. In particular, it is crucial to differentiate prevalence (the proportion of individuals in a population who suffer from a defined disorder at any one point in time) from incidence (the number of new cases occurring in a population over a period of time). Prevalence is useful to estimate needs and plan services; only incidence rates can be used for causal research. Both prevalence and incidence estimates will increase when case definition is broadened and case ascertainment is improved. Time trends in rates can therefore only be gauged in investigations that hold these parameters under strict control over time. Several factors accounting for this rise have been proposed and discussed extensively in previous studies. These factors include: (a) the broadening of diagnostic criteria; (b) the increased efficiency over time in case identification methods used in surveys as well as changes in diagnostic practices; and (c) the diagnostic substitution (or switching) when some diagnostic categories become increasingly familiar to health professionals and/or when access to better services is insured by using a new diagnostic category.
The Way Forward
Prospects and Challenges in Global Epidemiological Research
Because significant costs are associated with prevalence studies, some have questioned whether country-specific estimates should be a priority, especially where resources are limited and priorities include preventable life-threatening conditions. Such epidemiological studies are useful, however, for assessing needs and priorities within each community. Epidemiological studies often provide systematic information regarding the availability, quality, and accessibility of existing services. These studies also require development of valid tools for systematic clinical characterization, including screening and/or diagnostic instruments that can be useful for immediate improvements in training, services, and awareness, as well as facilitating future research. As such, epidemiological research may be viewed as a systematic framework for unveiling local burden and informing policy and research.
The power of epidemiological research extends well beyond the communities in which it is conducted, particularly in view of mounting speculation regarding time trends and the potential impact of geographical and socioeconomic factors on the prevalence and possibly on the incidence of autism. While research within a global context is likely to help address such perplexing puzzles, currently, available evidence is extremely limited. There is also a clear need for better theoretical and experimental modelling to test the possible mechanisms through which various factors may have an impact on incidence, clinical presentation, or both. Studies using comparable methodology to estimate prevalence across different geographical regions and to monitor changes to it over time provide a powerful approach. Such findings may address whether certain factors, environmental or otherwise, operating in specific regions, have a disproportionate impact on prevalence over time as well as ascertain the true global burden of the condition. Moreover, epidemiological studies can be potentially translated into public health practices through developing or validating screening and diagnostic methods and though strengthening capacity for services.
Rapid developments in the public policy context for autism in various regions have given rise to promising opportunities for furthering research in this area. A number of parent and professional led groups as well as governmental agencies have been established around the world, who are actively working on creating awareness, services, and advocacy for people with PDD. While the critical need for population-based studies of the epidemiology of PDD may be evident, it is essential to also consider the likely reasons for the current lack of such data.
Funding for research in some countries is disproportionate to the burden of disease they face (referred to as the 10/90 gap, that is, only 10% of global spending on health research is directed towards the problems that primarily affect the poorest 90% of the world's population). There is limited local capacity to conduct research in these settings, let alone research in field of childhood autism. Despite this situation being common to several low- and middle-income countries, our review highlighted that regional or country-specific economic and development capacity only one factor contributing to the scarcity of data. More general contextual difficulties may be encountered in conducting epidemiological surveys worldwide. First, the epidemiological research concept of PDD is relatively new, thus little cumulative information on epidemiology of PDD are available. Second, service availability/accessibility in many regions remains low, which creates problems for case finding. Third, awareness of PDD among the public as well as clinical experts remains limited. Such contextual factors may help explain why epidemiological data is limited in some countries within Western Europe, where psychoanalytic frameworks for management of PDD may still be operative, as they were in the 1960s and 1970s in other regions of Europe and North America (and remain operative is some parts of Europe). Hence, there is a need for continued educational, training, and awareness programs with specific targets tailored to the needs and resources available for each community.
Further challenges face the research community in particular. Diagnosis of PDD still relies on behavioral observation, leaving room for wide variation in clinical judgment, and in cultural and social impact in the identification of biological conditions. While these challenges have been addressed in some countries with strong research capacity through the development and standardization of diagnostic tools, progress has been slow in using such instruments widely within those countries themselves, let alone, within a global context, where significant cultural and linguistic variation across and within communities needs to be accounted for. A number of instruments have been or are in the process of being translated and/or validated. Translation of instruments has also been identified as a priority within the International Epidemiology Network (http://www.autismepidemiology.net). Yet the biggest challenge to describing the true burden of PDD is the availability of low cost tools for epidemiological surveys, which would allow those to be conducted with adequate samples in different world regions.
Further questions are often raised as to whether research efforts need to focus on PDD in particular or incorporate a wider range of disabilities. For example, a group of scientists and practitioners, led by INCLEN-India (http://www.inclentrust.org), have been developing and validating a neurodevelopmental screening test for use by community health workers for the detection of 10 neurodevelopmental disabilities (including ASD). This work is leading to the emergence of a collaborative framework for research on neurodevelopmental disorders in the region to carry out multicenter epidemiological studies with a common protocol. The availability of these tools will provide a new opportunity for studies of the burden, determinants and needs of families affected by PDD in the region. Epidemiological studies themselves may also eventually provide revealing findings regarding the manifestation of PDD across cultures. In view of the increased awareness of autism worldwide and the interest from a wide range of stakeholders, research in this relatively narrow area may become a vehicle for broader improvements in evidence, and consequently in practice standards, in child mental health in general.
Public Health Implications
Based on already available knowledge indicating that PDDs are not limited to high-income countries; the need for services especially in low and middle-income countries is felt more than before. It is imperative to engage community resources and more peripheral extensions of health systems as well. The situation in low- and middle- income countries appears to be that child health programs focus mainly on child survival issues. Very little attention is paid to developmental disabilities at policy and implementation level and as a result budget allocations and human resource deployment is directed away from these programs. For example, some regions in Africa face a dual burden of communicable and noncommunicable conditions, including childhood disabilities [Murray, Lopez, Mathers, & Stein, 2001]. Dysfunctional health systems contribute further to lack of service delivery for children with developmental disabilities. Where they exist, access to these facilities is also hindered by lack of effective identification and referral programs. An even bigger barrier has been the lack of an evidence-based, affordable, package of care for people with PDD, which could be delivered if such facilities were available. Only recently WHO's mhGAP has included evidence-based and simple interventions for PPD and intellectual disability in a package that needs to be pilot tested and evaluated in low- and middle-income countries. This review indicates that increase in prevalence estimates over time most likely represent broadening of the diagnostic concepts, diagnostic switching from other developmental disabilities to PDD, service availability, and awareness of autistic spectrum disorders in both the lay and professional public. This has clear public health implications that services for different categories of developmental disorders should not be segregated prematurely and health systems need to consider overarching programmes to fill the gap for all developmental disorders.
Acknowledgments
WHO commissioned and supported this review. However, the views expressed in this article do not necessarily represent the decisions, policy, or views of WHO. We appreciate the Shirley Foundation, Autism Speaks, and Autistica for supporting this work. We would like to thank Eileen Hopkins from Autistica, Andy Shih, Michael Rosanoff, and Jessica Napolitano from Autism Speaks and all the professionals and researchers who provided a wealth of information included in the current review. We are also grateful to Rachel Wu for valuable assistance in preparing this article.
References
- Abrahams BS, Geschwind DH. Advances in ASD genetics: on the threshold of a new neurobiology. Nature Reviews Genetics. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Farsi Y, Al-Sharbati M, Al-Farsi O, Al- Shafaee M, Brooks D, Waly M. Brief report: prevalence of autistic spectrum disorders in the Sultanate of Oman. 2010. [DOI] [PubMed]
- Al-Salehi SM, Al-Hifthy EH, Ghaziuddin M. Autism in Saudi Arabia: presentation, clinical correlates and comorbidity. Transcultural Psychiatry. 2009;46:340–347. doi: 10.1177/1363461509105823. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed. Arlington, VA: American Psychiatric Association; 1980. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed. Arlington, VA: American Psychiatric Association; 1987. Revised. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Arlington, VA: American Psychiatric Association; 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Arlington, VA: American Psychiatric Association; 2000. [Google Scholar]
- Ametepee LK, Chitiyo M. What we know about autism in Africa: a brief research synthesis. The Journal of the International Association of Special Education. 2009;10:11–13. [Google Scholar]
- Arvidsson T, Danielsson B, Forsberg P, Gillberg C, Johansson M, Kjellgren G. Autism in 3–6 year-old in a suburb of Goteborg, Sweden. Autism. 1997;2:163–173. [Google Scholar]
- Baird G, Simonoff E, Pickles A, Chandler S, Loucas T, et al. Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: the special needs and autism project (SNAP) Lancet. 2006;368:210–215. doi: 10.1016/S0140-6736(06)69041-7. [DOI] [PubMed] [Google Scholar]
- Bakare MO, Munir KM. Autism spectrum disorders (ASD) in Africa: a perspective. African Journal of Psychiatry (Johannesbg) 2011;14:208–210. doi: 10.4314/ajpsy.v14i3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Balkom IDC, Bresnahan M, Vogtländer MF, van Hoeken D, Minderaa R, et al. Prevalence of treated autism spectrum disorders in Aruba. Journal of Neurodevelopmental Disorders. 2009;1:197–204. doi: 10.1007/s11689-009-9011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begeer S, Bouk SE, Boussaid W, Terwogt MM, Koot HM. Underdiagnosis and referral bias of autism in ethnic minorities. Journal of Autism and Developmental Disorders. 2009;39:142–148. doi: 10.1007/s10803-008-0611-5. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Cook EHJ, Anderson GM, Rubenstein JL, Greenough WT, et al. Autism as a disorder of neural information processing: directions for research and targets for therapy. Molecular Psychiatry. 2004;9:646–663. doi: 10.1038/sj.mp.4001499. [DOI] [PubMed] [Google Scholar]
- Brask B. 1972. A prevalence investigation of childhood psychoses. Paper presented at the Nordic Symposium on the Care of Psychotic Children.
- Burd L, Fisher W, Kerbeshan J. A prevalence study of pervasive developmental disorders in North Dakota. Journal of the American Academy of Child and Adolescent Psychiatry. 1987;26:700–703. doi: 10.1097/00004583-198709000-00014. [DOI] [PubMed] [Google Scholar]
- CDC. Center for Disease Control: Prevalence of autism spectrum disorder—autism and developmental disabilities monitoring network, six sites, United States (2000) MMWR Surveillance Summary. 2007a;56:1–11. [PubMed] [Google Scholar]
- Center for Disease Control. Prevalence of autism spectrum disorders—autism and developmental disabilities monitoring network, 14 sites, United States (2002) MMWR Surveillance Summary. 2007b;56:12–28. [PubMed] [Google Scholar]
- Center for Disease Control. Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring network, United States, 2006. Morbidity and Mortality Weekly Report Surveillance Summary. 2009;58:1–14. [PubMed] [Google Scholar]
- Chakrabarti S, Fombonne E. Pervasive developmental disorders in preschool children. Journal of the American Medical Association. 2001;285:3093–3099. doi: 10.1001/jama.285.24.3093. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Fombonne E. Pervasive developmental disorders in preschool children: confirmation of high prevalence. American Journal. 2005;162:1133–1141. doi: 10.1176/appi.ajp.162.6.1133. [DOI] [PubMed] [Google Scholar]
- Croen LA, Grether JK, Hoogstrate J, Selvin S. The changing prevalence of autism in California. Journal of Autism and Developmental Disorders. 2002;32:207–215. doi: 10.1023/a:1015453830880. [DOI] [PubMed] [Google Scholar]
- Currenti SA. Understanding and determining the etiology of autism. Cellular & Molecular Neurobiology. 2009;30:161–171. doi: 10.1007/s10571-009-9453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley TC. From symptom recognition to diagnosis: children with autism in urban India. Social Science and Medicine. 2004;58:1323–1335. doi: 10.1016/S0277-9536(03)00330-7. [DOI] [PubMed] [Google Scholar]
- Dealberto MJ. Prevalence of autism according to maternal immigrant status and ethnic origin. Acta Psychiatrica Scandinavica. 2011;123:339–348. doi: 10.1111/j.1600-0447.2010.01662.x. [DOI] [PubMed] [Google Scholar]
- Eapen V, Mabrouk AA, Zoubeidi T, Yunis F. Prevalence of pervasive developmental disorders in preschool children in the UAE. Journal of Tropical Pediatrics. 2007;53:202–205. doi: 10.1093/tropej/fml091. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Johnson MH. Getting answers from babies about autism. Trends in Cognitive Science. 2010;14:81–87. doi: 10.1016/j.tics.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Farley M, McMahon W, Fombonne E, Jenson WR, Miller J, et al. Twenty-year outcome for individuals with autism and average or near-average cognitive abilities. Autism Research. 2009;2:109–118. doi: 10.1002/aur.69. [DOI] [PubMed] [Google Scholar]
- Fombonne E. Epidemiological surveys of autism and other pervasive developmental disorders: an update. Journal of Autism and Developmental Disorders. 2003a;33:365–382. doi: 10.1023/a:1025054610557. [DOI] [PubMed] [Google Scholar]
- Fombonne E. The prevalence of autism. Journal of the American Medical Association. 2003b;289:1–3. [Google Scholar]
- Fombonne E. Epidemiological studies of pervasive developmental disorders. In: Volkmar FR, Paul R, Klin A, Cohen D, editors. Handbook of autism and pervasive developmental disorders. Hoboken, NJ: Wiley; 2005. pp. 42–69. [Google Scholar]
- Fombonne E, Quirke S, Hagen A. Epidemiology of pervasive developmental disorders. In: Amaral DG, Dawson G, Geschwind DH, editors. Autism spectrum disorders. Oxford: Oxford University Press; 2011. [Google Scholar]
- Fombonne E, Zakarian R, Bennett A, Meng L, McLean-Heywood D. Pervasive developmental disorders in Montreal, Quebec, Canada: prevalence and links with immunizations. Pediatrics. 2006;118:e139–e150. doi: 10.1542/peds.2005-2993. [DOI] [PubMed] [Google Scholar]
- Ganz ML. The lifetime distribution of the incremental societal costs of autism. Archives of Pediatric and Adolescent Medicine. 2007;161:343–349. doi: 10.1001/archpedi.161.4.343. [DOI] [PubMed] [Google Scholar]
- Gardener H, Spiegelman D, Buka SL. Prenatal risk factors for autism: comprehensive meta-analysis. British Journal of Psychiatry. 2009;195:7–14. doi: 10.1192/bjp.bp.108.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanizadeh A. A preliminary study on screening prevalence of pervasive developmental disorder in school children in Iran. Journal of Autism and Developmental Disorders. 2008;38:759–763. doi: 10.1007/s10803-007-0445-6. [DOI] [PubMed] [Google Scholar]
- Gillberg C. Infantile autism in children of immigrant parents: a population-based study from Göteborg, Sweden. British Journal of Psychiatry. 1987;150:856–858. doi: 10.1192/bjp.150.6.856. [DOI] [PubMed] [Google Scholar]
- Gillberg C, Schaumann H, Gillberg IC. Autism in immigrants: children born in Sweden to mothers born in Uganda. Journal of Intellectual Disability Research. 1995;39:141–144. doi: 10.1111/j.1365-2788.1995.tb00482.x. [DOI] [PubMed] [Google Scholar]
- Gillberg C, Steffenburg S, Schaumann H. Is autism more common now than ten years ago? British Journal of Psychiatry. 1991;158:403–409. doi: 10.1192/bjp.158.3.403. [DOI] [PubMed] [Google Scholar]
- Gurney JG, Fritz MS, Ness KK, Sievers P, Newschaffer CJ, Shapiro EG. Analysis of prevalence trends of autism spectrum disorder in Minnesota [comment] Archives of Pediatrics and Adolescent Medicine. 2003;157:622–627. doi: 10.1001/archpedi.157.7.622. [DOI] [PubMed] [Google Scholar]
- Honda H, Shimizu Y, Misumi K, Niimi M, Ohashi Y. Cumulative incidence and prevalence of childhood autism in children in Japan. British Journal of Psychiatry. 1996;169:228–235. doi: 10.1192/bjp.169.2.228. [DOI] [PubMed] [Google Scholar]
- Honda H, Shimizu Y, Rutter M. No effect of MMR withdrawal on the incidence of autism: a total population study. Journal of Child Psychology and Psychiatry. 2005;46:572–579. doi: 10.1111/j.1469-7610.2005.01425.x. [DOI] [PubMed] [Google Scholar]
- Icasiano F, Hewson P, Machet P, Cooper C, Marshall A. Childhood autism spectrum disorder in the Barwon region: a community based study. Journal of Paediatric Child Health. 2004;40:696–701. doi: 10.1111/j.1440-1754.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- Juneja M, Mukherjee SB, Sharma S. A descriptive hospital based study of children with autism. Indian Pediatrics. 2004;42:453–458. [PubMed] [Google Scholar]
- Kalra V, Seth R, Sapra S. Autism—experiences in a tertiary care hospital. Indian Journal of Pediatrics. 2005;72:227–230. [PubMed] [Google Scholar]
- Kawamura Y, Takahashi O, Ishii T. Reevaluating the incidence of pervasive developmental disorders: impact of elevated rates of detection through implementation of an integrated system of screening in Toyota, Japan. Psychiatry and Clinical Neuroscience. 2008;62:152–159. doi: 10.1111/j.1440-1819.2008.01748.x. [DOI] [PubMed] [Google Scholar]
- Khan N, Hombarume J. Levels of autistic behavior among the mentally handicapped children in Zimbabwe. Central African Journal of Medicine. 1996;42:36–39. [PubMed] [Google Scholar]
- Kielinen M, Linna SL, Moilanen I. Autism in northern Finland. European Child and Adolescent Psychiatry. 2000;9:162–167. doi: 10.1007/s007870070039. [DOI] [PubMed] [Google Scholar]
- Kim YS, Leventhal BL, Koh YJ, Fombonne E, Laska E, et al. Prevalence of autism spectrum disorders in a total population sample. American Journal of Psychiatry. 2011;168:904–912. doi: 10.1176/appi.ajp.2011.10101532. [DOI] [PubMed] [Google Scholar]
- Knapp M, Romeo R, Beecham J. The economic consequences of autism in the UK. London: Foundation for People with Learning Disabilities; 2007. [Google Scholar]
- Lazoff TL, Piperni T, Fombonne E. Prevalence of pervasive developmental disorders among children at the English Montreal School Board. Canadian Journal of Psychiatry. 2010;55:715–720. doi: 10.1177/070674371005501105. [DOI] [PubMed] [Google Scholar]
- Le Couteur A, Rutter M, Lord C, Rios P, Robertson S, et al. Autism Diagnostic Interview: a standardized investigator-based instrument. Journal of Autism and Developmental Disorders. 1989;19:363–387. doi: 10.1007/BF02212936. [DOI] [PubMed] [Google Scholar]
- Lejarraga H, Menendez AM, Menzano E, Guerra L, Silvia Biancato S, et al. Screening for developmental problems at primary care level: a field programme in SanIsidro, Argentina. Pediatric and Perinatal Epidemiology. 2008;22:180–187. doi: 10.1111/j.1365-3016.2007.00897.x. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lotter V. Epidemiology of autistic conditions in young children: I. Prevalence. Social Psychiatry. 1966;1:124–137. [Google Scholar]
- Lotter V. Childhood autism in Africa. Journal of Child Psychology and Psychiatry. 1978;19:231–244. doi: 10.1111/j.1469-7610.1978.tb00466.x. [DOI] [PubMed] [Google Scholar]
- Madsen KM, Hviid A, Vestergaard M, Schendel D, Wohlfahrt J, et al. A population-based study of measles, mumps, and rubella vaccination and autism. New England Journal of Medicine. 2002;347:1477–1482. doi: 10.1056/NEJMoa021134. [DOI] [PubMed] [Google Scholar]
- Magnússon P, Saemundsen E. Prevalence of autism in Iceland. Journal of Autism and Developmental Disorders. 2001;31:153–163. doi: 10.1023/a:1010795014548. [DOI] [PubMed] [Google Scholar]
- Malhi P, Singhi S. Patterns of development in young children with autism. Indian Journal of Pediatrics. 2005;72:553–556. doi: 10.1007/BF02724176. [DOI] [PubMed] [Google Scholar]
- Mankoski RE, Collins M, Ndosi NK, Mgalla EH, Sarwatt VV, Folstein SE. Etiologies of autism in a case series from Tanzania. Journal of Autism Developmental Disorders. 2006;36:1039–1051. doi: 10.1007/s10803-006-0143-9. [DOI] [PubMed] [Google Scholar]
- Montiel-Nava C, Peña J. Epidemiological findings of pervasive developmental disorders in a Venezuelan Study. Autism. 2008;12:191–202. doi: 10.1177/1362361307086663. [DOI] [PubMed] [Google Scholar]
- Murray CJL, Lopez AD, Mathers CD, Stein C. The Global Burden of Disease 2000 project: aims, methods and data sources. Geneva: World Health Organization; 2001. [Google Scholar]
- Palmer F, Blanchard S, Jean CR, Mandell DS. School district resources and identification of children with autistic disorder. American Journal of Public Health. 2005;95:125–130. doi: 10.2105/AJPH.2003.023077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V, Garrison P, de Jesus Mari J, Minas H, Prince M, Saxena S. Advisory group of the Movement for Global Mental Health. The Lancet's series on global mental health: 1 year on. Lancet. 2008;372:1354–1357. doi: 10.1016/S0140-6736(08)61556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paula CS, Ribeiro S, Fombonne E, Mercadante MT. Brief report: Prevalence of pervasive developmental disorder in Brazil: a pilot study. Journal of Autism and Developmental Disorders. 2011;41:1738–1741. doi: 10.1007/s10803-011-1200-6. [DOI] [PubMed] [Google Scholar]
- Perera H, Wijewardena K, Aluthwelage R. Screening of 18-24-month-old children for autism in a semi-urban community in Sri Lanka. Journal of Tropical Pediatrics. 2009;55:402–405. doi: 10.1093/tropej/fmp031. [DOI] [PubMed] [Google Scholar]
- Plubrukarn R, Piyasil V, Moungnoi P, Tanprasert S, Chutchawalitsakul V. Trend study of autistic spectrum disorders at Queen Sirikit National Institute of Child Health. Journal of Medical Association of Thailand. 2005;88:891–897. [PubMed] [Google Scholar]
- Powell J, Edwards A, Edwards M, Pandit B, Sungum-Paliwal S, Whitehouse W. Changes in the incidence of childhood autism and other autistic spectrum disorders in preschool children from two areas of the West Midlands, UK. Developmental Medicine and Child Neurology. 2000;42:624–628. doi: 10.1017/s001216220000116x. [DOI] [PubMed] [Google Scholar]
- Rutter M. Autistic children: infancy to adulthood. Seminars in Psychiatry. 1970;2:435–450. [PubMed] [Google Scholar]
- Samadi SA, Mahmoodizadeh A, McConkey R. A national study of the prevalence of autism among five-year-old children in Iran. Autism. 2012;16:5–14. doi: 10.1177/1362361311407091. [DOI] [PubMed] [Google Scholar]
- Schopler E, Andrews CE, Strupp K. Do autistic children come from upper-middle-class parents. Journal of Autism and Developmental Disorders. 1979;9:139–151. doi: 10.1007/BF01531530. [DOI] [PubMed] [Google Scholar]
- Seif Eldin A, Habib D, Noufal A, Farrag S, Bazaid K, et al. Use of M-CHAT for a multinational screening of young children with autism in the Arab countries. International Review of Psychiatry. 2008;20:281–289. doi: 10.1080/09540260801990324. [DOI] [PubMed] [Google Scholar]
- Singhi P, Malhi P. Clinical and neurodevelopmental profile of young children with autism. Indian Pediatrics. 2001;38:384–390. [PubMed] [Google Scholar]
- Sun X, Allison C. A review of the prevalence of Autism Spectrum Disorder in Asia. Research in Autism Spectrum Disorders. 2010;4:156–167. [Google Scholar]
- Webb E, Lobo S, Hervas A, Scourfield J, Fraser W. The changing prevalence of autistic disorder in a Welsh health district. Developmental Medicine and Child Neurology. 1997;39:150–152. doi: 10.1111/j.1469-8749.1997.tb07402.x. [DOI] [PubMed] [Google Scholar]
- Wignyosumarto S, Mukhlas M, Shirataki S. Epidemiological and clinical study of autistic children in Yogyakarta, Indonesia. Kobe Journal of Medical Sciences. 1992;38:1–19. [PubMed] [Google Scholar]
- Williams JG, Higgins JP, Brayne CE. Systematic review of prevalence studies of autism spectrum disorders. Archives of Disease in Childhood. 2006;91:8–15. doi: 10.1136/adc.2004.062083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing L. Childhood autism and social class: a question of selection? British Journal of Psychiatry. 1980;137:410–417. doi: 10.1192/bjp.137.5.410. [DOI] [PubMed] [Google Scholar]
- Wing L, Gould J. Severe impairments of social interaction and associated abnormalities in children: epidemiology and classification. Journal of Autism and Developmental Disorders. 1979;9:11–29. doi: 10.1007/BF01531288. [DOI] [PubMed] [Google Scholar]
- Wing L, Yeates S, Brierly L, Gould J. The prevalence of early childhood autism: comparison of administrative and epidemiological studies. Psychological Medicine. 1976;6:89–100. doi: 10.1017/s0033291700007522. [DOI] [PubMed] [Google Scholar]
- Wong VC, Hui SL. Epidemiological study of autism spectrum disorder in China. Journal of Child Neurology. 2008;23:67–72. doi: 10.1177/0883073807308702. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) The ICD-9 Classification of Mental and Behavioural Disorders. 1975. Clinical descriptions and diagnostic guidelines. Geneva.
- World Health Organization (WHO) The ICD-10 Classification of Mental and Behavioural Disorders. 1992. Clinical descriptions and diagnostic guidelines. Geneva.
- World Health Organization (WHO) Geneva: 2008. mhGAP Mental Health Gap Action Programme Scaling up care for mental, neurological, and substance use disorders. [PubMed] [Google Scholar]
- Yeargin-Allsopp M, Rice C, Karapurkar T, Doernberg N, Boyle C, Murphy C. Prevalence of autism in a US metropolitan area [comment. Journal of the American Medical Association. 2003;289:49–55. doi: 10.1001/jama.289.1.49. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ji CY. Autism and mental retardation of young children in China. Biomedical and Environmental Sciences. 2005;18:334–340. [PubMed] [Google Scholar]


