Abstract
Although Clostridium difficile (C. difficile) is the leading cause of infectious diarrhoea in hospitalized patients, the economic burden of this major nosocomial pathogen for hospitals, third-party payers and society remains unclear. We developed an economic computer simulation model to determine the costs attributable to healthcare-acquired C. difficile infection (CDI) from the hospital, third-party payer and societal perspectives. Sensitivity analyses explored the effects of varying the cost of hospitalization, C. difficile-attributable length of stay, and the probability of initial and secondary recurrences. The median cost of a case ranged from $9179 to $11 456 from the hospital perspective, $8932 to $11 679 from the third-party payor perspective, and $13 310 to $16 464 from the societal perspective. Most of the costs incurred were accrued during a patient’s primary CDI episode. Hospitals with an incidence of 4.1 CDI cases per 100 000 discharges would incur costs ≥$3.2 million (hospital perspective); an incidence of 10.5 would lead to costs ≥$30.6 million. Our model suggests that the annual US economic burden of CDI would be ≥$496 million (hospital perspective), ≥$547 million (third-party payer perspective) and ≥$796 million (societal perspective). Our results show that C. difficile infection is indeed costly, not only to third-party payers and the hospital, but to society as well. These results are consistent with current literature citing C. difficile as a costly disease.
Keywords: Burden, Clostridium difficile, cost, economics, stochastic model
Introduction
Although Clostridium difficile (C. difficile) is a major nosocomial pathogen and leading cause of infectious diarrhoea in hospitalized patients [1–3], its economic burden has not been fully characterized. Several retrospective studies and one prospective study have examined the healthcare costs of C. difficile [1, 4–10]. However, these studies explored specific cases or types of patients (e.g. IBS patients, surgical patients, intensive care unit (ICU) patients, or adults in tertiary care hospitals) and therefore may not be generalizable to other hospitals and circumstances. Moreover, their methodologies and included costs varied, as cost evaluation was not a priority for most. Separate systematic reviews by Dubberke et al. and Ghantoji et al. cite the limitations of these studies and call for a more accurate and comprehensive report of the true economic burden of C. difficile infection (CDI) [1, 6].
Better understanding of the economic burden of C. difficile can assist various decision makers. Hospital administrators, infection control practitioners and policy makers could use this information to determine how much to invest in C. difficile prevention and control measures. This information can help policy makers and third-party payers to make insurance coverage and reimbursement decisions. These reimbursement decisions may change as Centers for Medicare and Medicaid Services rules evolve. Manufacturers and drug companies can use such information to develop and price C. difficile tests and treatments. We developed an economic computational simulation model to determine the cost of healthcare-acquired CDI (for the duration of hospitalization) from the perspective of various decision makers and determine the annual United States (US) burden. Sensitivity analyses varied the key parameters of attributable length-of-stay (LOS), probability of first and second recurrences, and hospitalization cost.
Methods
Using TreeAgo Pro 2009 (Williamstown, MA, USA) we constructed a stochastic computer simulation model to determine the additional costs associated with healthcare-acquired CDI from the hospital, third-party payer and societal perspectives. We also determined the annual burden of hospital-acquired C. difficile in US hospitals and across the country. Fig. 1 shows the basic model structure. Patients were the typical age of CDI patients (≥65 years old) [2, 11]. An infected patient could have either mild or severe CDI. All patients had a probability of having recurrent CDI over the course of a year. Patients with severe CDI could progress to fulminant C. difficile, which required hospitalization in the ICU. Those with fulminant CDI could undergo surgery (i.e. total colectomy) or not undergo surgery, each accompanied with its own probability of death. This first CDI occurrence was experienced by all patients in the model. Recurrences could be either mild or severe, resulting in rehospitalization. Up to two CDI recurrences could occur in 1 year for a total of three possible episodes per patient, resulting in a progressively smaller fraction of patients that experienced any recurrence.
FIG. 1.
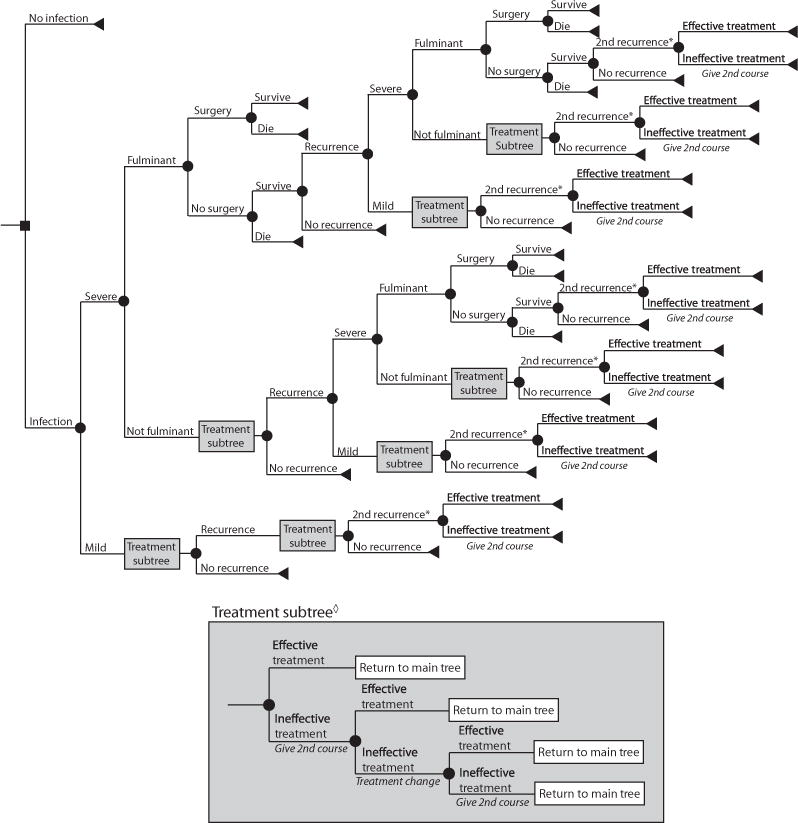
General decision model tree structure. *Give Tapered Vanomycin. ◊Use treatment strategy that was effective for prior episode. Treatment change: from metronidazole to vancomycin; no treatment change for vancomycin.
Treatment options differed by disease severity, prior treatments and number of recurrences. Patients with mild CDI received metronidazole (500 mg, three times a day for 10–14 days [2, 3]) as a first-line antibiotic treatment and vancomycin if two courses of metronidazole failed. Patients with severe disease received vancomycin (125 mg, four times as day for 10–14 days [2, 3]) as a first-line antibiotic treatment and received another course if the first failed. Patients with fulminant infection received vancomycin prior to surgery. In the event of a recurrence, the first-line treatment was the same antibiotic that worked for the initial episode as resistance to both drugs remains fairly low [3, 12]. A tapered course of vancomycin (four times a day for 14 days, two times a day for 7 days, once a day for 7 days, once every 2 days for 8 days, once every 3 days for 15 days [2, 3]) was used to treat patients with ≥2 recurrences. Additionally, patients who were candidates for surgery (i.e. fulminant CDI patients) had an abdominal computerized tomography CT scan performed to determine if surgery should occur; those proceeding to surgery required an additional peripheral intravenous line insertion and received metronizadole intravenously (500 mg, every 8 h for 10–14 days [2, 3]) in addition to oral vancomycin.
To determine the cost of a healthcare-acquired CDI case, each simulation sent 1000 adults (≥65 years old) through the model 1000 times for 1 000 000 total unique cost outcomes.
The three different perspectives accounted for different combinations of costs.
Hospital perspective: determined the opportunity cost of lost hospital bed days from an extended hospital LOS caused by CDI adapting a method described by Graves (extended LOS occupies beds that could have been used by other patients, costing hospitals potential revenue) [13]. This lost revenue for each case was determined by the cost of a bed-day for each CDI-attributable additional LOS day for each episode.
Third-party payer perspective: included only the direct costs of illness, such as the cost of hospitalization, diagnosis, treatment and surgery.
Societal perspective: included both direct (those included in the third-party payer perspective) and indirect costs. Indirect costs included productivity losses for the duration of illness (i.e. time missed from work for those working and/or caregivers and lost contributions to society for those retired) and lifelong productivity losses (i.e. lost wages from death for a patient 65 years old). Productivity losses due to illness were derived from wages lost for the LOS for each episode (not beyond) and those due to mortality were calculated from the annual wages lost for the years of life lost outlined by the Human Mortality Database [14], discounted 3% per year. We did not include any costs incurred after hospital discharge.
Data inputs
Table 1 lists the model inputs with their corresponding values, distribution types and sources. All costs were converted to 2010 $US using a 3% annual discount rate [15].
TABLE 1.
Model input parameters
| Description (units) | Model perspective | Distribution type | Mean | SD or range | Source |
|---|---|---|---|---|---|
| Costs ($US) | |||||
| Median hourly wage | S | 16.43 | 25 | ||
| Mean annual wage | S | 44 764 | 25 | ||
| Hospitalization | T, S | Gamma | 7767.70 | 322.19 | 11 |
| Bed day | H | Gamma | 1220.30 | 53.70 | 11 |
| ICU bed day | H | Triangular | 4398 | 1000–8000 | 26 |
| Peripheral intravenous line insertion | T, S | 96.98 | 27 | ||
| CT scan | T, S | Gamma | 276.10 | 29.61 | 27 |
| Antibiotics (full course) | |||||
| Metronidazole (IV) | T, S | Gamma | 112.97 | 10.56 | 28 |
| Metronidazole (oral) | T, S | Gamma | 55.74 | 37.49 | 28 |
| Vancomycin (oral) | T, S | Gamma | 1308.15 | 75.26 | 28 |
| Vancomycin (tapered) | T, S | Gamma | 2008.94 | 115.57 | 28 |
| Colectomy | T, S | Gamma | 35 161.46 | 3905.92 | 11 |
| All-cause mortality | T, S | Triangular | 6921 | 5191–9025 | 29 |
| Probabilities (%) | |||||
| Given C. difficile infection | |||||
| Severe disease | All | Beta | 17.8 | 4.06 | 30–33 |
| Fulminant disease (ICU) | All | Uniform | 3.0–8.0 | 34,35 | |
| Fulminant mortality | All | 80.5 | 36,37 | ||
| Recurrence | All | Beta | 18.9 | 6.77 | 33,38,39 |
| Colectomy | All | Beta | 40.92 | 24.43 | 37,40 |
| Mortality from colectomy | All | Beta | 41.60 | 7.85 | 34,37,40–42 |
| C. difficile treatment efficacies | |||||
| Metronidazole | All | Beta | 87.7 | 5.22 | 3,43,44 |
| Vancomycin | All | Beta | 97.1 | 0.13 | 3,44 |
| Vancomycin (tapered) | All | Triangular | 68.97 | 45.52–92.41 | 45 |
H, hospital perspective; T, third-party payer perspective; S, societal perspective.
All, denotes parameters used from the hospital, third-party payer and societal perspectives.
Sensitivity analyses
Sensitivity analyses ranged key variables to determine their effects on CDI cost. We varied hospitalization cost from baseline (mean, $7 767.70 [11]) to baseline ±$1000 (SD times three). The probability of secondary recurrences has been debated in the literature and was varied from 20% (same as primary recurrences) to a distribution ranging from 33 to 45% (likeliest, 40%) [2, 16]. An additional set of sensitivity analyses varied the first recurrence probability (10–30%) and secondary recurrence probability (30–50%). CDI-attributable additional LOS for severe and fulminant CDI episodes was ranged from 6 to 14 days ([11, 17] and expert opinion); all mild episodes had a CDI-attributable LOS of 6 days. Additionally, probabilistic sensitivity analyses simultaneously varied all parameters throughout their ranges in Table 1.
Results
Table 2 shows the median cost and 95% confidence interval (CI) for all perspectives and scenarios explored. The cost of a healthcare-acquired CDI case ranged from $8932 to $16 464. Fig. 2 shows the impact that varying the model parameters over the ranges explored through our sensitivity analysis has on CDI’s burden.
TABLE 2.
Incremental cost of Clostridium difficile infection from each perspective for baseline and an increased secondary recurrence rate
| Baseline secondary recurrence rateb
|
Increased secondary recurrence ratec
|
|||
|---|---|---|---|---|
| Scenario | Median | 95% CI | Median | 95% CI |
| Hospital perspective | ||||
| 6-day LOS | 9179 | 7833–11 047 | 9401 | 8063–11 136 |
| 10-day LOS | 10 187 | 8684–12 249 | 10 424 | 8873–12 466 |
| 14-day LOS | 11 175 | 9398–13 651 | 11 456 | 9511–13 709 |
| Third-party payer perspective | ||||
| Baseline–$1000 | 8932 | 7542–10 865 | 9188 | 7838–11 056 |
| Baseline costa | 10 123 | 8711–12 369 | 10 499 | 8983–12 722 |
| Baseline + $1000 | 11 386 | 9837–13 820 | 11 679 | 10 107–13 983 |
| Societal perspective | ||||
| Baseline−$1000, 6-day LOS | 13 310 | 9431–19 840 | 13 740 | 9730–19 508 |
| Baseline−$1000, 10-day LOS | 13 589 | 9843–19 116 | 13 783 | 9967–20 204 |
| Baseline−$1000, 14-day LOS | 13 511 | 9832–19 740 | 13 851 | 9857–20 573 |
| Baseline, 6-day LOS | 14 726 | 10 491–20 724 | 14 926 | 10 853–21 500 |
| Baseline, 10-day LOS | 14 732 | 10 578–20 849 | 15 213 | 11 067–21 743 |
| Baseline, 14-day LOS | 14 783 | 10 662–21 065 | 15 257 | 11 334–21 878 |
| Baseline + $1000, 6-day LOS | 15 810 | 11 790–21 967 | 16 271 | 11 997–23 065 |
| Baseline + $1000, 10-day LOS | 15 851 | 11 752–22 541 | 16 355 | 12 082–23 245 |
| Baseline + $1000, 14-day LOS | 16 078 | 11 862–22 110 | 16 464 | 12 139–23 019 |
Baseline cost = mean, $7767.70 (SD, $322.19).
Baseline secondary recurrence rate = mean, 18.9% (SD, 6.77%).
Increased secondary recurrence rate = range, 33–45% (likeliest, 40%).
FIG. 2.
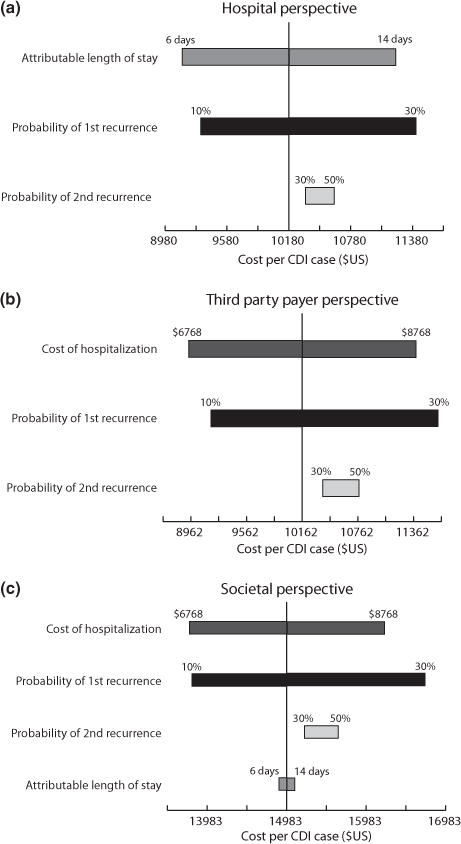
Tornado diagrams showing the effect of each variable on the cost per CDI case from (a) the hospital perspective, (b) the third-party payer perspective, and (c) the societal perspective.
Hospital perspective
Assuming all CDI cases had a 6-day CDI-attributable additional LOS, one CDI case cost a median $9197 (CI, $7833–$11 047). When the severe and fulminant CDI cases experienced a 10-day CDI-attributable additional LOS, the cost was $10 187 (Table 2). Increasing the probability of secondary recurrences (likeliest, 40%) increased costs, but did not have a large effect (range, $9401–$11 456). Compared with the baseline recurrence rate (18.9%), varying the first (10–30%) and second (30–50%) recurrence probabilities did not substantially affect CDI costs (range, $8438–$10 795 for 6-day LOS; data not shown) as relatively few patients experienced a recurrence. Fig. 2(a) shows which variables had the largest effect on CDI cost.
Most of the burden was attributed to a patient’s first CDI occurrence, with median cost $7511 (CI, $6868–$8210), $8485 (CI, $7562–$9427) and $9539 (CI, $8342–$10 994) for a 6, 10 and 14-day CDI-attributable additional LOS, respectively (data not shown).
Third-party payer perspective
As Table 2 shows, one CDI case from the third-party payer perspective cost a median $10 123 (CI, $8711–$12 369) at baseline. Increasing the probability of secondary recurrences (likeliest, 40%) had a marginal effect on cost (median, $10 499; CI, $8983–$12 722). Varying hospitalization cost had an effect on CDI cost. Decreasing hospitalization cost by $1000 resulted in median costs of $8932 and increasing by $1000 resulted in a cost of $11 386 (Table 2). Changing first and second recurrence probabilities resulted in costs ranging from $9392 (first recurrence probability 10%, probability second recurrence 30%) to $12 257 (30% first recurrence rate, 50% second recurrence rate) at the baseline hospitalization cost (data not shown). Even though these costs were similar to those at the baseline recurrence rate (18.9%), changing the first recurrence probability did have a substantial effect on cost. Fig. 2(b) shows the major cost drivers.
A major portion of these costs occurred during a patient’s first episode. The primary episode cost $8237 (CI, $7563–$9014) at baseline hospitalization cost, $7262 with a decreased hospitalization cost and $9271 with an increased hospitalization cost (data not shown).
Societal perspective
One CDI case cost $14 726 (CI, $10 491–$20 724) with the baseline hospitalization cost and 6-day additional LOS for all CDI patients (Table 2). Across all recurrence rates, lower hospitalization cost decreased CDI cost (range, $13 740–$16 079), and higher hospitalization cost increased CDI cost (range, $15 810–$16 464). Unlike the hospital perspective, additional LOS did not have much effect (Fig. 2c). Median costs for a 6-day CDI-attributable additional LOS ranged from $13 310 (cost, baseline −$1000, baseline recurrence) to $16 271 (cost, baseline +$1000, increased recurrence). An increased recurrence rate (likeliest, 40%) yielded higher costs (median, $14 926; CI, $10 853–$21 500) for CDI cases at baseline cost and 6-day CDI-attributable additional LOS. Costs increased for all combinations of hospitalization cost and additional LOSs at a higher recurrence rate. Although ranging the first (10–30%) and second (30–50%) recurrence probabilities showed little difference in costs, the first recurrence probability was a cost driver (Fig. 2c).
Again, most costs were accrued during the primary CDI episode; at baseline hospitalization cost, costs varied from $12 339 (6-day LOS) to $12 607 (14-day LOS). The primary episode cost $11 375 (6-day LOS) to $11 573 (14-day LOS) at a lower hospitalization cost and $13 149 (all cases 6-day LOS) to $15 590 (severe and fulminant 14-day LOS) at a higher hospitalization cost (data not shown).
Hospital burden
A recent multicentre surveillance study determined the incidence of C. difficile in six hospitals across the US using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes [18]. Table 3 gives the incidence and number of cases for each of their reported hospitals and gives the calculated burden for each. Costs are for these six hospitals over the study period from each perspective. Costs from the hospital perspective ranged from $3.2 million (Hospital D) to $30.6 million (Hospital A) and are even more substantial with an increased additional LOS and secondary recurrence rate. From the third-party payer and societal perspectives, costs are even more substantial, with an increased hospitalization cost, CDI-attributable additional LOS, and secondary recurrence rate.
TABLE 3.
Hospital and annual US burden (in $millions) from each perspective
| Burden by perspective (in $ US millions)
|
|||||
|---|---|---|---|---|---|
| Population | Incidence (per 1000 inpatients) | Number of cases | Hospital | Third-party payer | Societal |
| Hospital burdena | |||||
| Hospital A | 10.5 | 3334 | 30.6 | 33.7 | 49 |
| Hospital B | 8.3 | 915 | 8.3 | 9.3 | 13.5 |
| Hospital C | 4.9 | 1253 | 11.5 | 12.7 | 18.5 |
| Hospital D | 4.1 | 345 | 3.2 | 3.5 | 5.1 |
| Hospital E | 8.6 | 1404 | 12.9 | 14.2 | 20.7 |
| Annual US burden | |||||
| US Hospital inpatientsb | 13.1 | 1443 | 13 | 15 | 21 |
| Diagnosis of CDI (Patients | – | 54 046 | 496 | 547 | 796 |
| aged 65–84 years)c | |||||
Hospital perspective: 6-day CDI-attributable LOS and baseline secondary recurrence rate. Third-party payer perspective: baseline hospitalization cost. Societal perspective: baseline hospitalization cost and 6-day CDI-attributable LOS. Incidence trends for CDI for each hospital can be found in Dubberke et al.
Source: Dubberke et al. [18].
Source: Jarvis et al. [19].
Source: HCUP facts and figures: statistics on hospital-based care in the United States, 2008 [http://www.hcup-us.ahrq.gov/reports.jsp, 11].
Annual US burden
Table 3 also reports annual CDI costs for the US. In 2008, the national point prevalence of C. difficile in US hospital inpatients was 1443 out of 110 550 inpatients [19], and would generate the costs seen in Table 3. In 2008, 54 046 patients between the ages of 65 and 84 were discharged with a diagnosis of C. difficile[11]. Costs would be ≥$496 million (Table 3).
Discussion
To our knowledge, this is the first study to develop an economic model to determine the cost of CDI from different perspectives and demonstrate how cost may vary with different parameters. As Dubberke et al. and Ghantoji et al. indicated, most studies that reported CDI costs did not focus on economic analyses and therefore did not include several costs, had cost reporting limitations, and had varying calculation methods and populations (and country of origin) [1, 6]. In addition, several of these studies were completed before the emergence of the hypervirulent BI/NAP1 C. difficile strain [1]. This strain has increased in frequency, severity and morbidity [2], all of which lead to potentially increased CDI costs for tests, treatment and procedures.
Our results do fall within the range of previously reported CDI costs, which range from $2000 to $72 000 per case [1, 6] and vary with coexisting conditions (range, $11 000–$24 000) [5, 7, 8]. Regardless of severity, there are significant associated costs, with most incurred during the initial CDI episode. Even with first recurrence rates as low as 10%, costs were still substantial from all perspectives. Along with the first recurrence probability, additional LOS (hospital perspective) and hospitalization costs (third-party payer and societal perspectives) appeared to be the main CDI cost drivers. Nationally, Kyne et al. estimated CDI to be a $1.1 billion annual cost to the US, where as O’Brien et al. estimated the annual cost to be approximately $3.2 billion [9, 10]. Our estimates are closer to that reported by Kyne et al.
Compared with other types of infection, C. difficile can be an expensive pathogen. Acinetobacter baumannii costs range from $5000 to ≥$44 000 per case [20], one norovirus case can cost hospitals $6237 [21], vancomycin-resistant enterococcal bloodstream infections cost ≥$4000 [22], and methicillin-resistant Staphylococcus aureus can potentially cost between $5000 and $40 000 [23].
Our model may be conservative about estimating CDI costs. We only evaluated costs for the hospitalization duration; costs to third-party payers and society would be higher if they were captured for a longer duration to include lost productivity and other treatments. It included only the more common and less expensive procedures needed for each condition and excluded relatively rare CDI complications and exacerbations of co-morbid conditions. Patients with certain co-morbidities such as IBS or immunosuppressed patients may have more costly CDI episodes and elderly patients may be discharged to a nursing home, presenting additional costs. We did not include costs for potential antimicrobial resistance to vancomycin and its impact on colonization treatment and spread of vancomycin-resistant enterococci. Also, hospitals may have additional costs if pay for performance arrangements are in place [24]. Finally, a CDI case may require enhanced surveillance and requires infection control measures (e.g. contact isolation precautions) that incur additional costs not accounted for in the model. Costs may change if C. difficile becomes more virulent, if resistance develops, and if it becomes more increasingly common in the community and younger persons.
Limitations
Models, by nature, are simplifications and cannot completely represent every possible event and outcome, nor the full spectrum of socio-demographic and clinical heterogeneity among patients. Data inputs for our model derived from different studies of varying quality and were based on published data only, thus inputs may change as new data emerge. We also did not consider alternate or new forms of therapy for CDI, which may vary from clinician to clinician.
Conclusions
Our study quantifies the substantial costs of hospital-acquired CDI from the hospital, third-party payer and societal perspectives. Most costs incurred were accrued during a patient’s primary episode of CDI, with costs as much as $12 607 (14-day LOS, baseline hospitalization cost from the societal perspective). Varying the secondary recurrence probability showed little affect on costs, as they occurred in relatively few patients. Costs may change if CDI becomes increasingly common in the community. Efforts to prevent CDI, through infection control, vaccine development or new therapies may have the potential to be cost-saving as they may reduce CDI’s incidence, duration, severity and transmission.
Acknowledgments
This study was supported by the National Institute General Medical Sciences Models of Infectious Disease Agent Study (MIDAS) (grant number 5U54GM088491-02) and the Pennsylvania Department of Health (DOH). The funders had no role in the design and conduct of the study, collection, management, analysis and interpretation of the data, and preparation, review or approval of the manuscript.
Footnotes
Transparency Declaration
The authors have no conflicts of interest to declare.
References
- 1.Dubberke ER, Wertheimer AI. Review of current literature on the economic burden of Clostridium difficile infection. Infect Control Hosp Epidemiol. 2009;30:57–66. doi: 10.1086/592981. [DOI] [PubMed] [Google Scholar]
- 2.Kelly CP, LaMont JT. Clostridium difficile – more difficult than ever. N Engl J Med. 2008;359:1932–1940. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 3.Leffler DA, LaMont JT. Treatment of Clostridium difficile-associated disease. Gastroenterology. 2009;136:1899–1912. doi: 10.1053/j.gastro.2008.12.070. [DOI] [PubMed] [Google Scholar]
- 4.Song X, Bartlett JG, Speck K, Naegeli A, Carroll KC, Perl TM. Rising economic impact of Clostridium difficile-associated diesease in adult hopsitalized patient population. Infect Control Hosp Epidemiol. 2008;29:823–828. doi: 10.1086/588756. [DOI] [PubMed] [Google Scholar]
- 5.Ananthakrishnan AN, McGinley EL, Binion DG. Excess hospitalisation burden with Clostridium difficile in patients with inflammatory bowel disease. Gut. 2008;57:205–210. doi: 10.1136/gut.2007.128231. [DOI] [PubMed] [Google Scholar]
- 6.Ghantoji SS, Sail K, Lairson D, DuPont HL, Garey KW. Economic healthcare costs of Clostridium difficile infection: a systematic review. J Hosp Infect. 2010;74:309–318. doi: 10.1016/j.jhin.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence SJ, Puzniak LA, Shadel BN, Gillespie KN, Kollef MH, Mundy LM. Clostridium difficile in the intensive care unit: epidemiology, costs, and colonization pressure. Infect Control Hosp Epidemiol. 2007;28:123–130. doi: 10.1086/511793. [DOI] [PubMed] [Google Scholar]
- 8.Zerey M, Paton BL, Lincourt AE, Gersin KS, Kercher KW, Heniford BT. The burden of Clostridium difficile in surgical patients in the United States. Surg Infect. 2007;8:557–566. doi: 10.1089/sur.2006.062. [DOI] [PubMed] [Google Scholar]
- 9.Kyne L, Hamel MB, Polavaram R, Kelly CP. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis. 2002;34:346–353. doi: 10.1086/338260. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien JA, Lahue BJ, Caro JJ, Davidson DM. The emerging infectious challenge of Clostridium difficile-associated disease in Massachusetts hospitals: clinical and economic consequences. Infect Control Hosp Epidemiol. 2007;28:1219–1227. doi: 10.1086/522676. [DOI] [PubMed] [Google Scholar]
- 11.United States Department of Health & Human Services. HCUP facts and figures: statistics on hospital-based care in the United States. Rockville, MD: AHRQ: Agency for Healthcare Research and Quality; 2008. Available at: http://www.hcup-us.ahrq.gov/reports.jsp(last accessed 16 March 2011) [PubMed] [Google Scholar]
- 12.Huang H, Weintraub A, Fang H, Nord CE. Antimicrobial resistance in Clostridium difficile. Int J Antimicrob Agents. 2009;34:516–522. doi: 10.1016/j.ijantimicag.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Graves N. Economics and preventing hospital-acquired infection. Emerg Infect Dis. 2004;10:561–566. doi: 10.3201/eid1004.020754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Human mortality database [database on the Internet] University of California; Berkeley (USA): Max Planck Institue for Demographic Reseach; Germany: 2008. Available at: http://www.mortality.org(last accessed 7 January 2011) [Google Scholar]
- 15.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-effectiveness in health and medicine. New York: Oxford University Press, Inc.; 1996. [Google Scholar]
- 16.Johnson S. Recurrent Clostridium difficile infection: causality and therapeutic approaches. Int J Antimicrob Agents. 2009;33:S33–S36. doi: 10.1016/S0924-8579(09)70014-7. [DOI] [PubMed] [Google Scholar]
- 17.Pepin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. Can Med Assoc J. 2005;173:1037. doi: 10.1503/cmaj.050978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubberke ER, Butler AM, Yokoe DS, et al. Multicenter study of surveillance for hospital-onset Clostridium difficile infection by the use of ICD-9-CM diagnosis codes. Infect Control Hosp Epidemiol. 2010;31:262–268. doi: 10.1086/650447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarvis WR, Schlosser J, Jarvis AA, Chinn RY. National point prevalence of Clostridium difficile in US health care facility inpatients, 2008. Am J Infect Control. 2009;37:263–270. doi: 10.1016/j.ajic.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Lee BY, McGlone SM, Doi Y, Bailey RR, Harrison LH. Economic impact of Acinetobacter baumannii infection in the intensive care unit. Infect Control Hosp Epidemiol. 2010;31:1087–1089. doi: 10.1086/656378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee BY, Wettstein ZS, McGlone SM, et al. Economic value of norovirus outbreak control measures in healthcare settings. Clin Microbiol Infect. 2011;17:640–646. doi: 10.1111/j.1469-0691.2010.03345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butler AM, Olsen MA, Guth RM, Woeltje KF, Camins BC, Fraser VJ. Attributable costs of enterococcal bloodstream infections in a nonsurgical hospital cohort. Infect Control Hosp Epidemiol. 2010;31:28–35. doi: 10.1086/649020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lodise TP, McKinnon PS. Burden of methicillin-resistant Staphylococcus aureus: focus on clinic and economic outcomes. Pharmacotherapy. 2007;27:1001–1012. doi: 10.1592/phco.27.7.1001. [DOI] [PubMed] [Google Scholar]
- 24.Maynard A, Bloor K. Will financial incentives and penalties improve hospital care? BMJ. 2010;340:297–298. doi: 10.1136/bmj.c88. [DOI] [PubMed] [Google Scholar]
- 25.Bureau of Labor Statistics. Occupational employment statistics: May 2009 national occupational employment and wage estimates, United States. Washington, D.C.: U.S. Bureau of Labor Statistics Division of Occupational Employment Statistics; 2010. Available at: http://stat.bls.gov/oes/2008/may/oes_nat.htm#b00-0000(last accessed 5 January 2011) [Google Scholar]
- 26.Cooke CR, Kahn JM, Watkins TR, Hudson LD, Rubenfeld GD. Cost-effectiveness of implementing low-tidal volume ventilation in patients with acute lung injury. Chest. 2009;136:79–88. doi: 10.1378/chest.08-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Medical Association. CPT code/relative value search. 2010 Available at: https://catalog.ama-assn.org/Catalog/cpt/cpt_search.jsp?_requestid=1175050(last accessed 1 September 2010)
- 28.PDR. Red book pharmacy’s fundamental reference. Montvale, NJ: Thompson Reuters (Healthcare) Inc.; 2010. [Google Scholar]
- 29.Gould MK, Dembitzer AD, Sanders GD, Garber AM. Low-molecular-weight heparins compared with unfractionated heparin for treatment of acute deep venous thrombosis. A cost-effectiveness analysis. Ann Intern Med. 1999;130:789–799. doi: 10.7326/0003-4819-130-10-199905180-00002. [DOI] [PubMed] [Google Scholar]
- 30.Henrich TJ, Krakower D, Bitton A, Yokoe DS. Clinical risk factors for severe Clostridium difficile-associated disease. Emerg Infect Dis. 2009;15:415–422. doi: 10.3201/eid1503.080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McFarland LV, Mulligan ME, Kowk RYY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320:204–210. doi: 10.1056/NEJM198901263200402. [DOI] [PubMed] [Google Scholar]
- 32.Pepin J, Valiquette L, Alary M-E, et al. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. Can Med Assoc J. 2004;171:466–472. doi: 10.1503/cmaj.1041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barbut F, Gariazzo B, Bonne L, et al. Clinical features of Clostridium difficile-associated infections and molecular characterization of strains: results of a retrospective study, 2000–2004. Infect Control Hosp Epidemiol. 2007;28:131–139. doi: 10.1086/511794. [DOI] [PubMed] [Google Scholar]
- 34.Jaber MR, Olfsson S, Fung WL, Reeves ME. Clinical review of the management of fulminant Clostridium difficile infection. Am J Gastroenterol. 2008;103:3195–3203. doi: 10.1111/j.1572-0241.2008.02198.x. [DOI] [PubMed] [Google Scholar]
- 35.Olivias AD, Umanskiy K, Zuckerbraun B, Alverdy JC. Avoiding colectomy during surgical management of fulminant Clostridium difficile colitis. Surg Infect. 2010;11:299–305. doi: 10.1089/sur.2010.026. [DOI] [PubMed] [Google Scholar]
- 36.Morris JB, Zollinger RMJ, Stellato TA. Role of surgery in antibiotic-induced pseudommbranous enterocolitis. Am J Surg. 1990;160:535–539. doi: 10.1016/s0002-9610(05)81024-4. [DOI] [PubMed] [Google Scholar]
- 37.Lamontagne F, Labbe A-C, Haeck O, et al. Impact of emergency colectomy on survival of patients with fulminant Clostridium difficile colitis during an epidemic caused by a hypervirulent strain. Ann Surg. 2007;245:267–272. doi: 10.1097/01.sla.0000236628.79550.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barbut F, Richard A, Hamadi K, Chomette V, Burghoffer B, Petit JC. Epidemiology of recurrences or reinfections of Clostridium difficile-associated diarrhea. J Clin Microbiol. 2000;38:2386–2388. doi: 10.1093/gao/9781884446054.article.t031141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fekety R, McFarland LV, Surawicz CM, Greenberg RN, Elmer GW, Mulligan ME. Recurrent Clostridium difficile diarrhea: characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blinded trial. Clin Infect Dis. 1997;24:324–333. doi: 10.1093/clinids/24.3.324. [DOI] [PubMed] [Google Scholar]
- 40.Dallal RM, Harbrecht BG, Boujoukas AJ, et al. Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications. Ann Surg. 2002;235:363–372. doi: 10.1097/00000658-200203000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Byrn JC, Maun DC, Gingold DS, Baril DT, Ozao JJ, Divino CM. Predictors of mortality after colectomy for fulminant Clostridium difficile colitis. Arch Surg. 2008;143:150–154. doi: 10.1001/archsurg.2007.46. [DOI] [PubMed] [Google Scholar]
- 42.Longo WE, Mazuski JE, Virgo KS, Lee P, Bahadursingh AN, Johnson FE. Outcome after colectomy for Clostridium difficle colitis. Dis Colon Rectum. 2004;47:1620–1626. doi: 10.1007/s10350-004-0672-2. [DOI] [PubMed] [Google Scholar]
- 43.Belmares J, Gerding DN, Parada JP, Miskevics S, Weaver F, Johnson S. Outcome of metronidazole therapy for Clostridium difficile disease and correlation with a scoring system. J Infect. 2007;55:495–501. doi: 10.1016/j.jinf.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 44.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302–307. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- 45.McFarland LV, Elmer GW, Surawucz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97:1769–1775. doi: 10.1111/j.1572-0241.2002.05839.x. [DOI] [PubMed] [Google Scholar]


