Abstract
Significance: The imino acid proline is utilized by different organisms to offset cellular imbalances caused by environmental stress. The wide use in nature of proline as a stress adaptor molecule indicates that proline has a fundamental biological role in stress response. Understanding the mechanisms by which proline enhances abiotic/biotic stress response will facilitate agricultural crop research and improve human health. Recent Advances: It is now recognized that proline metabolism propels cellular signaling processes that promote cellular apoptosis or survival. Studies have shown that proline metabolism influences signaling pathways by increasing reactive oxygen species (ROS) formation in the mitochondria via the electron transport chain. Enhanced ROS production due to proline metabolism has been implicated in the hypersensitive response in plants, lifespan extension in worms, and apoptosis, tumor suppression, and cell survival in animals. Critical Issues: The ability of proline to influence disparate cellular outcomes may be governed by ROS levels generated in the mitochondria. Defining the threshold at which proline metabolic enzyme expression switches from inducing survival pathways to cellular apoptosis would provide molecular insights into cellular redox regulation by proline. Are ROS the only mediators of proline metabolic signaling or are other factors involved? Future Directions: New evidence suggests that proline biosynthesis enzymes interact with redox proteins such as thioredoxin. An important future pursuit will be to identify other interacting partners of proline metabolic enzymes to uncover novel regulatory and signaling networks of cellular stress response. Antioxid. Redox Signal. 19, 998–1011.
Introduction
Almost three decades ago, the proline metabolic pathway was proposed to have a regulatory function in oxidation–reduction homeostasis and cell survival (95). Now, numerous laboratories have shown that the imino acid proline impacts a wide range of cellular processes, including bioenergetics, differentiation, growth, lifespan, and apoptosis (30, 70, 74, 75, 84, 95, 97, 99, 153). It is well established that proline metabolism leads to increased mitochondrial reactive oxygen species (ROS) production via the electron transport chain (ETC) and that proline metabolism impacts cell survival and cell death in different species (14, 30, 84, 97). In plants, the protective effect of proline during stress is especially well documented (123). Here, we review proline metabolic stress adaption in plants and examine the potential mechanisms of proline stress protection in different organisms.
Proline Metabolic Enzymes
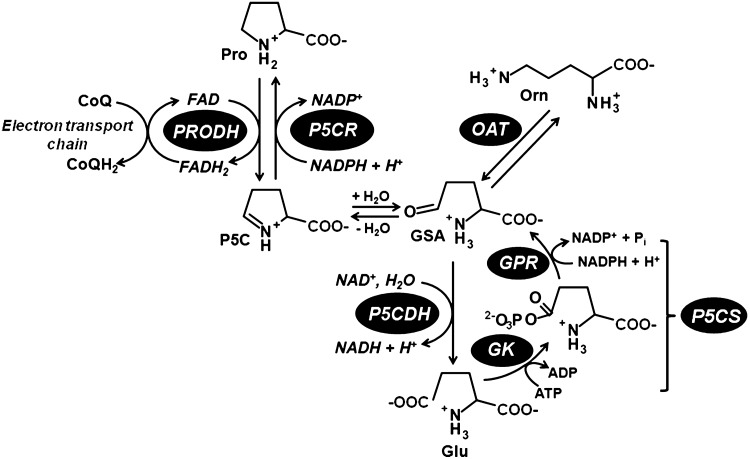
The reactions of the proline metabolic pathway are shown in Figure 1. Proline is synthesized from glutamate by the enzymes Δ1-pyrroline-5-carboxylate (P5C) synthetase (P5CS) and P5C reductase (P5CR). Alternatively, proline can be formed from ornithine, which is converted into P5C/GSA via ornithine-δ-aminotransferase (OAT) (2). The conversion of proline back to glutamate is catalyzed by proline dehydrogenase (PRODH) and P5C dehydrogenase (P5CDH). An overview of the proline metabolic enzymes is provided next.
FIG. 1.
Reactions of the proline metabolic pathway. Proline (Pro) is synthesized from glutamate (Glu) starting with the enzymes glutamate kinase (GK) and γ-glutamyl phosphate reductase (GPR), which in plants and animals are fused together in the bifunctional enzyme P5C synthetase (P5CS). The intermediate, γ-glutamate-semialdehyde (GSA), spontaneously cyclizes to Δ1-pyrroline-5-carboxylate (P5C), which is then reduced to proline by P5C reductase (P5CR). Alternatively, GSA/P5C can be generated from ornithine and ornithine-δ-aminotransferase (OAT). Proline is oxidized back to glutamate by proline dehydrogenase (PRODH) and P5C dehydrogenase (P5CDH) in the mitochondrion. PRODH couples proline oxidation to the reduction of ubiquinone (CoQ) in the electron transport chain (ETC). In Gram-negative bacteria, PRODH and P5CDH domains are fused together in the PutA protein.
P5C synthetase
P5CS catalyzes the NADPH-dependent reduction of glutamate to γ-glutamate-semialdehyde (GSA), which then spontaneously cyclizes to P5C (49, 110). The full-length cDNA encoding P5CS in multicellular eukaryotes was first cloned and characterized in plants (49). P5CS is a bifunctional adenosine triphosphate (ATP) and an NAD(P)H-dependent enzyme in higher eukaryotes that displays glutamate kinase (GK) and γ-glutamyl phosphate reductase (GPR) activities (49, 110). In primitive organisms such as bacteria and yeast, GK and GPR are monofunctional enzymes (6, 94). The structure of bifunctional P5CS has not been reported, but individual structures of GK and GPR are available from bacteria (79, 89). Important residues for glutamate binding in the GK domain are conserved among GK of different species (93, 94). Proline biosynthesis is feedback inhibited by proline binding to the GK domain and interfering with the glutamate binding site (93).
Recently, Engelhard et al. identified human P5CS as a potential target of mitochondrial thioredoxin 2 using an in situ kinetic trapping assay (32). This interesting finding indicates that P5CS is in the mitochondrial matrix and subject to redox regulation.
P5C reductase
The product of the P5CS reaction, P5C, is reduced to proline by P5CR using NAD(P)H as an electron donor (2). P5CR is conserved among bacteria, plants, insects, and vertebrates (82, 103). X-ray crystal structures of P5CR from different organisms, including humans, have been solved showing a conserved N-terminal Rossmann fold for NADPH binding (6, 82). In plants, P5CR is not only located in the cytosol but has also been shown to be expressed in chloroplasts (123, 131). There are three isoforms of P5CR in humans: PYCR1, PYCR2, and PYCRL. PYCR1 and PYCR2 are localized in mitochondria (24, 103), whereas PYCRL is cytosolic (24). P5CR is not only critical for synthesizing proline, but also has a critical role in cycling proline and P5C between cellular compartments and in maintaining proper NADP+/NAPDH levels in the cytosol to drive the pentose phosphate pathway (84, 95).
Ornithine-δ-aminotransferase
OAT catalyzes the interconversion of ornithine and GSA with the flux direction determined by nutritional needs such as in neonate, where the overall flux from proline to arginine is favored (98, 142). In yeast, OAT is cytoslic (55); whereas in plants and humans, OAT is localized in the mitochondria (65, 116, 123).
Recently, Jortzik et al. reported that OAT of the malaria parasite Plasmodium falciparum (PfOAT) interacts with thioredoxin via Cys154 and Cys163, which are highly conserved residues in Plasmodium OAT but absent in other organisms (56). PfOAT also interacts with other cellular redox proteins such as glutaredoxin and plasmoredoxin and is reversibly regulated by S-glutathionylation (56, 60). It is of interest to note that no homolog of P5CS or GK and GPR can be found in the genome of Plasmodium, indicating that proline synthesis in Plasmodium may only be through the degradation of ornithine (56). These data suggest that proline biosynthesis in Plasmodium is redox regulated via OAT, and they reveal new molecular linkages between redox homeostasis and proline metabolism.
Proline dehydrogenase/P5C dehydrogenase
PRODH and P5CDH are well conserved in eukarya and bacteria with PRODHs sharing a catalytic core domain of a distorted (αβ)8 barrel (117, 126). It should be noted that PRODH enzymes from Archaea have a structural fold that is distinct from eukarya/bacteria PRODHs (59, 126). In eukaryotes, PRODH and P5CDH are localized in the mitochondrial matrix with PRODH associated with the inner membrane of the mitochondria. In Gram-positive bacteria, PRODH binds peripherally to the cytoplasmic membrane, whereas P5CDH is cytosolic (126, 137). Interestingly, in Gram-negative bacteria, PRODH and P5CDH are combined into a single protein known as proline utilization A (PutA) with the PRODH domain linked N-terminal to the P5CDH domain (73, 83, 126). In some Gram-negative bacteria such as Escherichia coli, PutA also has an N-terminal DNA-binding domain (ribbon-helix-helix motif), enabling PutA to function as a transcriptional repressor and a proline metabolic enzyme (72, 155).
PRODH contains a noncovalently bound flavin adenine dinucleotide (FAD) and is responsible for catalyzing the first step of L-proline oxidation (6). The reaction catalyzed by PRODH results in the transfer of two electrons from proline to the flavin cofactor to generate P5C and reduced flavin. Next, PRODH transfers two electrons from reduced flavin to an electron acceptor such as ubiquinone in the inner membrane of the mitochondria (or cytoplasmic membrane in prokaryotes) (86, 135). After P5C spontaneously converts into GSA, GSA is oxidized to L-glutamate by P5C dehydrogenase (P5CDH) using nicotinamide adenine dinucleotide as an electron receptor (6, 121). Glutamate generated by proline oxidation enters the tricarboxylic acid cycle after enzymatic conversion to α-ketogluturate. The oxidation of one molecule of L-proline can yield approximately 30 ATP equivalents, thus providing important energy for the cell, particularly under nutrient deplete conditions (44, 96, 98).
Proline Metabolic Adaptation in Plants During Stress
Proline accumulation is a common phenomenon observed in response to environmental stress in bacteria, protozoa, algae, plants, and marine invertebrates (21, 81, 123, 131). In plants, intracellular proline levels have been found to increase by >100-fold during stress (42, 131). Proline accumulation in plants occurs during exposure to various stresses, including salt (150), drought (9, 19), UV radiation (108), heavy metal ions (18), pathogens (33), and oxidative stress (146). Proline accumulation and stress tolerance have been studied in plants by exogenously and endogenously manipulating proline levels (45). Under stress conditions (e.g., drought, cold shock, and biotic challenges), proline accumulation in plants involves reciprocal regulation of P5CS and PRODH (44, 45, 101, 131). In tobacco, overexpression of P5CS results in higher levels of proline, enhanced osmotolerance, root biomass, and flower development (45, 46). Here, we provide a summary of proline metabolic gene expression changes in plants in response to stress. For a more detailed description of metabolic changes in plants, readers are encouraged to see the excellent review by Szabados and Savouré (123).
Proline biosynthesis
Glutamate appears to be the main precursor in stress-induced proline accumulation in plants, as the ornithine pathway mainly facilitates nitrogen recycling from arginine to glutamate (41, 123). The rate-limiting enzyme for proline synthesis is P5CS, the increased expression of which correlates with proline accumulation in Arabidopsis (110). Changes in P5CR (P5CR; At5g14800) expression levels seem to associate less with proline accumulation, which is consistent with P5CS catalyzing the rate-limiting step of the pathway. However, there are a few reports that P5CR transcripts levels are moderately enhanced in the root of soybean and pea, and in the leaves of Arabidopsis in response to osmotic stress (26, 132, 138). In addition, Cecchini et al. recently showed that P5CR was up-regulated by the hypersensitive response (HR) in Arabidopsis after infection with an avirulent strain of Pseudomonas syringae (13). Thus, P5CR may have an important role in stress response that is not yet fully realized.
There are two isoforms of P5CS in plants; in Arabidopsis, isoform 1 (P5CS1; At2g39800) is localized in the chloroplasts and is required for stress-induced proline accumulation (80, 124). Isoform 2 (P5CS2; At3g55610) is localized mainly in the cytosol and is essential for embryo and seedling development (80, 124). Disruption of P5CS1 by T-DNA insertion in Arabidopsis leads to significantly lower proline accumulation in plants during stress, resulting in hypersensitivity to salt stress and high levels of ROS (124). Disruption of P5CS2 does not significantly impact proline accumulation but impairs development of seedlings and fertile plants (124). Consistent with an important role in proline accumulation, P5CS1 expression is up-regulated in response to drought and salt stress (1, 122, 150).
Recently, Verslues et al. identified a splice variant of P5CS1 in Arabidopsis that resulted in a nonfunctional transcript (62). The alternatively spliced transcript led to reduced P5CS1 protein levels and proline accumulation (62). In a comprehensive study of how proline content and the abundance of the nonfunctional splice variant varied with climate, it was found that the nonfunctional P5CS1 transcript correlated better with climate variability than with proline content (62). These interesting findings suggest that proline accumulation may not be the sole factor for adaptation to environmental stress, but rather the proline biosynthesis pathway may have an important role in climate adaptation that is not yet fully realized (62).
The signaling mechanisms by which environmental stress induces proline biosynthesis in plants includes several molecules such as abscisic acid (ABA) (109, 122), calcium, and phospholipase C (91, 109, 148). Recently, Sharma et al. reported that proline metabolism is required for ABA-mediated growth protection in plants under water deficit (112). Evidence for ROS-mediated regulation of proline biosynthesis has also been found (33, 134, 146). Fabro et al. reported that in Arabidopsis, HR triggered by incompatible plant pathogen interaction results in proline accumulation via up-regulation of P5CS2 but not P5CS1 in a salicylic acid and an ROS-dependent manner (33). Later, Verslues et al. also reported that hydrogen peroxide (H2O2) may cause proline accumulation or promote ABA-induced proline accumulation (134). Recently, Yang et al. suggested that H2O2 may induce proline accumulation by up-regulation of P5CS and down-regulation of PRODH activity in coleoptiles and radicles of maize seedlings (146).
In addition to transcriptional regulation, plant P5CS is feedback inhibited by proline (154). The feedback inhibition of P5CS is similar to that of bacterial GKs and involves competitive inhibition by proline with regard to glutamate (93, 94). Structural analysis and site-directed mutagenesis of bacterial GKs suggest that proline partially occupies the glutamate binding site when bound to GK, thus interfering with glutamate binding and inhibiting GK activity (93). Incorporating a GK variant that is insensitive to proline inhibition has been used to overproduce proline in bacteria (23, 119) and yeast (125). In plants, expression of a P5CS variant lacking proline inhibition increased proline levels by two fold (46). In a recent review, Pérez-Arellano et al. (94) noted that under stress conditions, some bacteria and plants accumulate proline at a concentration (>100 mM) that is well above that which is needed to inhibit GK activity with KI values ranging from ∼0.2 to 1 mM proline for bacterial GK and plant P5CS enzymes, respectively (10, 27, 61, 93, 94). It has been proposed that under stress conditions, proline inhibition of GK activity is attenuated by the high levels of other solutes such as glutamate (61, 93, 94).
Proline degradation
During stress response, it is generally anticipated that along with up-regulation of proline biosynthesis, a corresponding decrease in the proline degradation pathway occurs that maximizes proline accumulation. Similar to proline biosynthesis, the first step in the pathway of proline degradation (i.e., PRODH) is rate determining. Arabidopsis has two functional PRODH isoforms, both of which are localized to the mitochondria: PRODH1 (PRODH1; At3g30775), also known as ERD5 gene (Early Responsive to Dehydration) (40, 64) and PRODH2 (PRODH2; At5g38710) (40, 123). PRODH1 is widely expressed in plants and is considered the predominant isoform (40). Expression of PRODH2 is significantly lower than PRODH1 with PRODH2 expressed mainly in the vasculature (40). PRODH1 and PRODH2 are up-regulated by exogenous proline but surprisingly, they respond differently to drought and salt stress (131, 136). It is well documented that PRODH1 expression decreases in response to cold, drought, and salt stress (57, 64). Drought-tolerant wheat (Tritium aestivum) cultivars were found to contain significantly lower PRODH activity than drought-sensitive plants (147). Moreover, when seedlings were exposed to lead (Pb(NO3)2), an elevation of PRODH activity was found in drought-sensitive wheat cultivars (Ningchun) but not in drought-tolerant ones (Xihan), which is consistent with down-regulation of proline degradation and providing a benefit to plants during stress (147).
In Arabidopsis, salt stress has been shown to induce PRODH2 expression, while PRODH1 expression is significantly down-regulated (40). Differential regulation of the two PRODH isoforms has also been reported in tobacco (104). Thus, it appears that PRODH1 and PRODH2 have distinct physiological roles, which will require further investigation to fully understand the benefits of proline in stress tolerance. Funck et al. suggest that proline degradation in the vasculature may provide important energy for the plant during stress exposure (40). Indeed, some tissues in plants have been found to maintain proline oxidation under stress. At low water potential (drought), PRODH1 expression was found to remain high in root apex and shoot meristem in Arabidopsis, whereas PRODH1 expression was reduced in the bulk of shoot tissue (112). A PRODH1 mutant in Arabidopsis exhibited significantly lower oxygen consumption in the root apex, indicating that proline catabolism is a important pathway for driving oxidative phosphorylation (112). Furthermore, the apical region of barley roots under NaCl stress had less free proline accumulation even though L-proline transporter and P5CS activities were increased (128). Thus, proline transport and utilization may vary in different tissues depending on the energy demands of different regions of the plant.
The second enzyme of the proline catabolic pathway in Arabidopsis, P5CDH (P5CDH; At5g62530), is up-regulated by exogenous proline, although the induction of P5CDH is much slower than that of PRODH (29). For the most part, P5CDH expression remains constant during stress. Interest in P5CDH has been in whether P5CDH expression levels attenuate the toxicity of proline, which is observed in plants at high proline levels (29). The adverse effect of exogenous proline has been postulated to be caused by the build-up of P5C/GSA due to low P5CDH activity (123, 131). P5C/GSA has been reported to increase intracellular ROS (88) and to react with other metabolites (35). Knockout of P5CDH in Arabidopsis generates mutant plants that are hypersensitive to exogenous proline, whereas P5CDH-overexpressing plants are more tolerant to exogenous proline treatment (28). However, Arabidopsis with limited PRODH activity still exhibits sensitivity to exogenous proline, suggesting that other mechanisms contribute to proline toxicity such as inhibition of endogenous proline synthesis (13, 44, 78, 123).
During the recovery phase after stress, proline is considered as serving as an important energy source (44, 123). Proline oxidative metabolism in the mitochondria helps drive oxidative phosphorylation and ATP synthesis in recovering tissues (44). Accordingly, PRODH and P5CDH expression are increased during rehydration (64). Accumulated proline has been shown to be rapidly degraded during stress recovery in cultured tomato cells (Lycopersicon esculentum cv VFNT-Cherry) (43).
Mechanisms of Proline Stress Protection
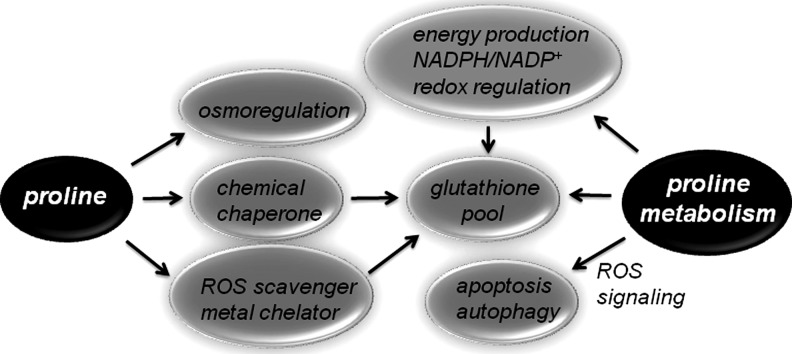
The molecular mechanisms of how proline protects cells during stress are not fully understood but appear to involve its chemical properties and effects on redox systems such as the glutathione (GSH) pool (Fig. 2). The function of proline in stress adaptation is often explained by its property as an osmolyte and its ability to balance water stress (27). However, adverse environmental conditions often perturb intracellular redox homeostasis, necessitating mechanisms that also work to balance oxidative stress. Thus, proline protective mechanisms have also been proposed to involve the stabilization of proteins and antioxidant enzymes, direct scavenging of ROS, balance of intracellular redox homeostasis (e.g., ratio of NADP+/NADPH and GSH/GSSG), and cellular signaling promoted by proline metabolism. The potential mechanisms by which proline provides stress protection are discussed next.
FIG. 2.
Potential functions of proline and proline metabolism in stress protection.
Osmolyte function
Proline is one of the several small molecules classified as an osmolyte or an osmoprotectant (22). Other biologically important osmolytes are glycerol, trehalose, sorbitol, sucrose, taurine, sarcosine, glycine betaine, and trimethylamine N-oxide (145). These osmolytes are accumulated in response to conditions of drought, salt, and temperature extremes. Osmolytes help mitigate water stress and balance turgor pressure during stress (22). Osmolytes are also excellent cryoprotectants (92). For example, proline has been shown to increase the freeze tolerance of yeast (85, 125) and plants (45, 123, 151), and to be a useful cryoprotector of protein crystals (92), fly larvae (67, 68), plant cells (140), and human stem cells (39). Thus, as an osmolyte, proline is an important molecule that is employed by various organisms to combat stress.
Chemical chaperone
Proline has been shown to act as a chemical protein chaperone and to prevent protein aggregation (71, 107). A thermosensitive dnaK-mutant strain of E. coli was rescued by increased intracellular proline levels using a GK variant that is insensitive to proline inhibition (15). The higher proline levels reduced protein aggregation and thermodenaturation. In an in vitro experiment, proline (1 M) protected nitrate reductase under osmotic, metal, and H2O2 stress (111). Ignatova et al. reported that proline can prevent the aggregation of P39A cellular retinoic acid-binding protein (an aggregation prone protein) under salt stress (51). Proline also diminished the aggregation of a cellular retinoic acid-binding protein that is fused to a pathogenic polyglutamine repeat of the human huntingtin protein (51).
Due to its chaperone properties, proline protection against oxidative stress has been proposed to involve enhancement and stabilization of redox enzymes. Exogenous application of proline to cell cultures has been found to increase the activity of different antioxidant enzymes under salt (47), cadmium (53, 54, 144), and oxidative stress (17), resulting in increased stress tolerance. These enzymes include superoxide dismutase (53, 144), catalase (17, 48, 53, 144), and GSH related or ascorbate (ASC)-GSH cycle-related enzymes (47, 54). All these are important antioxidant enzymes (52, 87) and in plants, the ASC-GSH cycle is especially critical for mitigating ROS (87). In the P5CS1 knockout of Arabidopsis, significantly lower activities of ASC-GSH cycle-related enzymes, including ascorbate peroxidase, GSH peroxidase, and GSH-S-transferase, were observed under NaCl stress conditions (124). Chen et al. reported that the addition of proline in the growth medium quenched ROS as efficiently as other ROS scavengers, such as N-acetyl cysteine in the fungal pathogen Colletotrichum trifolii (17). The decrease in ROS was shown to be due to an increase in catalase activity by proline treatment (17). Altogether, different groups have reported that increased proline levels enhance antioxidant enzyme activity.
Studies comparing the ability of different biological osmolytes to stabilize proteins have provided insights into the chaperone properties of proline. Proline stabilizes protein structures by driving burial of the peptide backbone and protein folding (7, 130, 143). This is different than protein folding in the absence of osmolytes, which is driven by favorable burying of nonpolar side chains (7, 130, 143). Relative to other osmolytes, proline is categorized as a weak stabilizer of protein folding and ranks lower in ability to induce protein folding (7, 11). Thus, although proline helps stabilize proteins, besides facilitating protein folding, additional mechanisms likely contribute to the protective effect of proline during stress.
Metal chelator
Another mechanism by which proline protects cells against stress has been suggested to involve the chelation of metals. High proline content in metal-tolerant plants is not unusual (113). One of the major toxicities of heavy metals is perturbation of cellular redox balance by ROS production (114). A potent oxidizing agent of biological macromolecules in the cell is the hydroxyl radical (OH•), which is formed by the reduction of H2O2 by transition metal ions such as Cu+ and Fe2+ (114). The function of proline as a metal chelator was suggested by Sharma et al., who reported that proline can protect enzymes from zinc- and cadmium-induced inhibition by forming proline-metal complexes (115). A copper–proline complex was also reported in the copper-tolerant Armeria maritima (34).
ROS scavenger
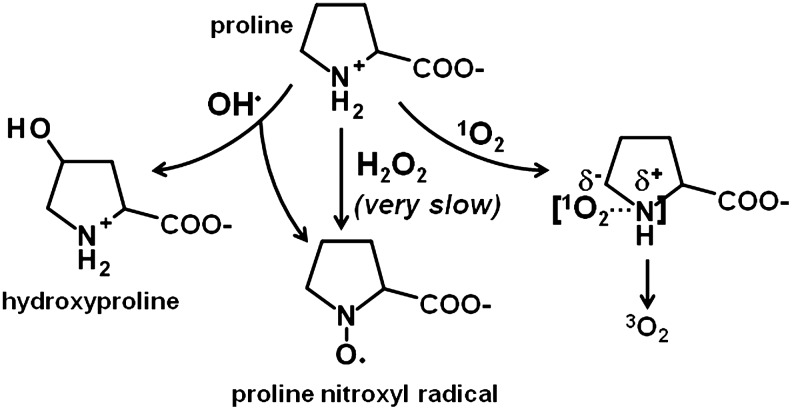
The ability of proline to directly react with ROS has been investigated by numerous laboratories (58, 114). Previous studies have shown that free and polypeptide-bound proline can react with H2O2 and OH• (pH 7–8) to form stable free radical adducts of proline and hydroxyproline derivatives as shown in Figure 3 (e.g., 4-hydroxyproline and 3-hydroxyproline) (38, 58, 102, 106, 127). Although Floyd and Nagy (38) observed that nitroxyl radicals accumulate during the incubation of proline with H2O2, the reaction is very slow relative to that of proline and OH• (5.4×108 M−1s−1) (3). Recently, the ability of proline to scavenge H2O2 was compared with pyruvate, a well-established scavenger of H2O2. At 30 min, H2O2 levels were diminished by >90% in cell medium supplemented with 1 mM pyruvate, whereas no significant decrease was observed with proline (5 mM) (70). This observation further indicates that a direct reaction between H2O2 and proline does not significantly contribute to the scavenging of cellular H2O2 (48). Proline has also been shown not to directly scavenge O2•− (58).
FIG. 3.
Potential reactive oxygen species (ROS) scavenging mechanisms of proline.
An ROS-scavenging mechanism that is important to proline stress protection is the facile reaction of proline with singlet oxygen (1O2). In cultured skin fibroblasts, exogenously added proline has been shown to diminish 1O2 levels (141). Proline has been shown to protect human skin cells from photo-induced apoptosis, suggesting that proline suppresses photo-oxidative stress and skin carcinogenesis (141). In plants, Alia et al. reported that during strong illumination, the production of 1O2 in the thylakoids from the cotyledons of Brassica juncea was dramatically suppressed by proline (5).
The five-membered ring of proline, pyrrolidine, has a low ionization potential that effectively quenches 1O2 most likely through a charge transfer mechanism in which molecular oxygen returns to the ground triplet state (3O2) (Fig. 3) (20, 81, 152). Alia and coworkers used irradiation of various photosensitizers to produce 1O2, which is detected by measuring the formation of 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) by EPR (4). TEMPO formation was completely inhibited by adding 20 mM proline to the reaction, indicating that proline directly scavenged or quenched 1O2 (4). Quenching of 1O2 is also documented for other secondary amine compounds as well, such as spermine (63). Due to its action as a 1O2 quencher, proline may help stabilize proteins, DNA, and membranes (81). Prolyl residues in proteins also provide protection against oxidative stress caused by 1O2. For example, the epithelial small proline-rich protein, which is a precursor of the cornified envelope of the epidermal skin, is strongly induced after exposure of the skin to UV radiation (37, 133). Figure 3 summarizes the reactivity of proline with different ROS.
Energy homeostasis and NADP+/NADPH
In addition to the chemical properties of proline discussed earlier, changes in proline metabolic flux can also impact stress tolerance. The effects of proline metabolism on the intracellular redox state have been well studied by Phang and coworkers, who first proposed that the proline-P5C cycle can help maintain proper NADP+/NADPH levels in the cytosol and drive the oxidative pentose phosphate pathway (95). The cycling of proline and P5C via PRODH and P5CR results in the transfer of reducing equivalents from the cytosol into the mitochondria (84, 95). Electrons are passed into the mitochondrial ETC directly from PRODH via ubiquinone, leading to increases in oxidative phosphorylation and mitochondrial ROS production (84, 95). The proline-P5C cycle is, thus, thought to help maintain a proper NADP+/NADPH ratio (84, 95). The proline-P5C cycle may especially be important when increased PRODH activity is not balanced with P5CDH activity (84, 149). Currently, it is not known how P5C shuttles in/out of the mitochondria. An excellent review on the proline-P5C cycle and the wide ranging effects of proline metabolism was recently provided by Phang (97).
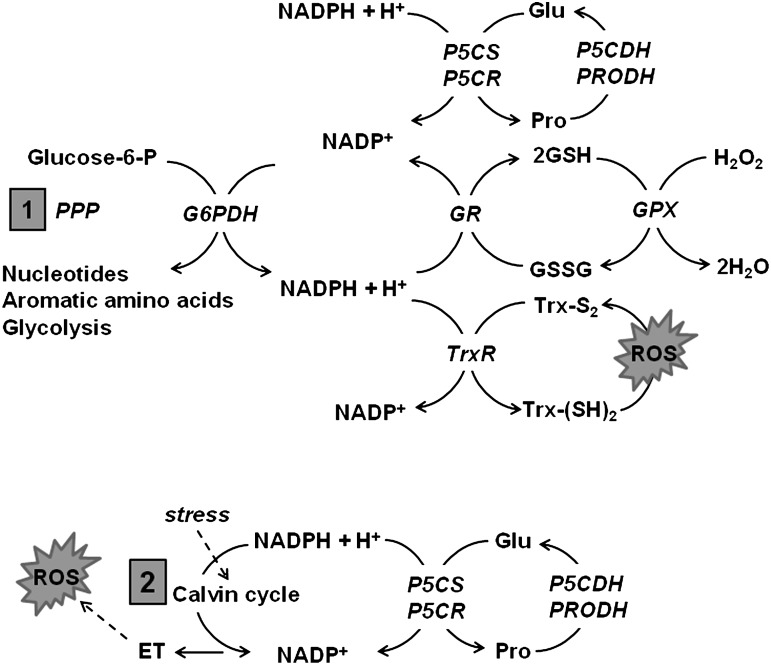
Evidence for proline metabolic flux influencing the NADP+/NADPH ratio in plants has been reported by several groups and summarized in Figure 4 (44, 66, 112). Proline metabolic cycling was found to increase oxidation of NADPH in the soybean nodule, thereby enhancing the oxidative pentose phosphate pathway (66). Increased flux through the oxidative pentose phosphate pathway would support purine nucleotide biosynthesis during stress recovery (Fig. 4) (44, 66). Up-regulation of proline synthesis has also been proposed to maintain the NADP+/NADPH ratio at normal levels during photoinduced stress (44, 123). Significant decreases in the NADP+/NADPH ratio has been reported under different stress conditions due to decreased Calvin cycle activity (44, 123). Without sufficient levels of NADP+ available for electron transfer, photosynthetic cells under stress conditions produce more 1O2 when exposed to high light (16, 123). Light exposure, however, promotes P5CS expression, leading to increased proline biosynthesis and NADP+ levels, which ultimately diminishes 1O2 production in the chloroplasts (Fig. 4) (110, 124). These observations suggest a link between enhanced proline synthesis and photoinduced oxidative stress. Hare et al. suggest that the redox modulation accompanying proline synthesis may be more important than proline accumulation (44).
FIG. 4.
Proposed mechanisms by which proline metabolism mediates redox homeostasis and energy production via NADP+/NADPH. (1) NADP+ produced by proline biosynthesis may stimulate the pentose phosphate pathway (PPP), thereby supporting energy production and the biosynthesis of key molecules such as nucleotides. NADPH is utilized for reductive biosynthesis pathways and is critical for glutathione (GSH) and thioredoxin (Trx) antioxidant systems. (2) In plant chloroplasts, NADP+ produced from proline biosynthesis may replenish depleted NADP+ pools caused by inhibition of the Calvin cycle during stress. Maintaining adequate levels of NADP+ for electrons transfer to the ETC would help minimize ROS generation during stress. Dashed line indicates inhibition. GR, glutathione reductase; GPx, glutathione peroxidase; GSSG, oxidized glutathione; GSH, reduced glutathione; TrxR, thioredoxin reductase; Trx-(SH)2, reduced thioredoxin; Trx-S2, oxidized thioredoxin; NADP+, nicotinamide adenine dinucleotide phosphate; NADPH, nicotinamide adenine dinucleotide phosphate reduced form; Glucose-6-P, glucose-6-phosphate; G6PDH, glucose-6-phosphate dehydrogenase.
Manipulation of proline metabolic enzyme expression has also provided evidence for proline metabolism influencing NADP+ levels in plants. A comparison of sense-orientated and antisense-orientated P5CR gene transgenic soybean plants showed that sense plants had higher NADP+ levels and higher stress tolerance relative to antisense plants (25). Antisense knockdown of P5CR resulted in lower NADP+ levels and higher sensitivity to stress (25). Recently, Sharma and coworkers reported that under low water stress, plants deficient in P5CS1 or PRODH1 exhibit a lower NADP+/NADPH ratio than wild-type plants (112). In addition, L-proline catabolism was suggested to be important for maintaining the NADP+/NADPH ratio, as the NADP+/NADPH ratio is significantly lower in the prodh mutant of Arabidopsis than wild-type Arabidopsis (112). Although PRODH1 activity apparently declines during stress, a low level of cycling between proline and P5C may be enough to support the maintenance of proper NADP+/NADPH (44).
GSH pool
Different studies have shown that proline addition to the cell medium and up-regulation of endogenous proline biosynthesis leads to increased total GSH and protection of intracellular reduced GSH (47, 118, 144). Guarding reduced GSH is especially important in heavy metal stress, as heavy metal ion toxicity is often associated with depletion of GSH (114). The ability of proline to protect GSH during metal ion stress was tested in Chlamydomonas reinhardtii in which transgenic algae expressing the mothbean P5CS gene had 80% higher intracellular proline levels relative to wild-type algae (118). After exposing cells to 50 μM Cd2+, the GSH:0.5GSSG ratio was four-fold higher in transgenic algae relative to wild-type cells, indicating that proline prevents GSH depletion during heavy metal stress (118). The higher GSH levels in the P5CS transgenic algae were suggested to increase phytochelatin synthesis and the formation of Cd-thiolate complexes in the vacuole, thereby protecting against heavy metal stress (118). The manner in which proline protects the GSH pool is not clear, but it has been proposed that proline directly scavenges OH• and 1O2 generated by heavy metal stress and helps stabilize ROS detoxifying enzymes (47, 118, 144).
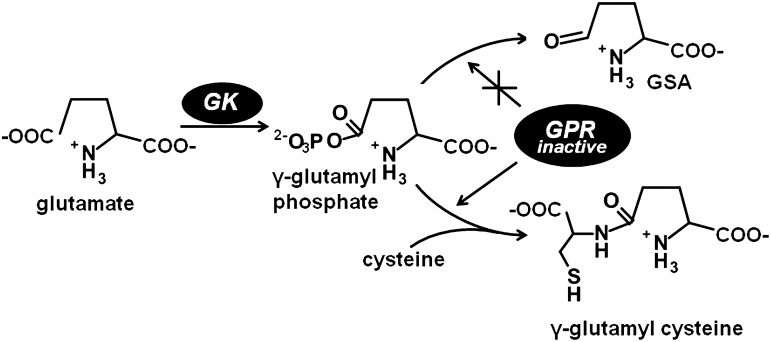
The proline and GSH synthesis pathways share the intermediate, γ-glutamyl phosphate, suggesting possible crosstalk between these pathways. Evidence of proline biosynthesis contributing to GSH synthesis has been recently shown using a GSH-deficient mutant strain of E. coli that lacks GshA (glutamate-cysteine ligase), the enzyme which links glutamate and cysteine to form γ-glutamylcysteine and is, thus, auxotrophic for GSH (129). Using a random mutagenesis screen for mutants that rescue GSH auxotrophy, Veeravalli et al. discovered that a mutant having both proB (GK) and proA (GPR) mutations may repress the GSH auxotrophy of gshA mutation (129). The mutation in proB resulted in a GK mutant that lacks proline feedback inhibition, and the mutation in proA generated a GPR mutant which lacks NADPH dehydrogenase activity. The E. coli strain with both proA and proB mutations rescued the GSH auxotropy of the gshA mutant by providing an alternative route for generating γ-glutamylcysteine as shown in Figure 5. The mechanism is thought to involve L-cysteine reacting with γ-glutamyl phosphate bound to GPR via an S-to-N acyl shift reaction (129).
FIG. 5.
Formation of γ-glutamylcysteine from the proline biosynthesis pathway. Lack of NADPH dehydrogenase activity in GPR allows cysteine to react with γ-glutamyl phosphate and to generate γ-glutamylcysteine.
Intriguingly, bioinformatic analysis of several bacterial genomes showed that in prokaryotes which synthesize GSH, some lack GshA. This suggests that in certain organisms, proline biosynthesis is partly diverted toward GSH production via γ-glutamyl phosphate (129). In yeast, it was found that a specific mutation in PRO2 (GPR) is the only suppressor of the GSH auxotrophy of the gsh1 (homolog of gshA in bacteria) null mutant (120). The rescue of a GSH auxotroph by a pro2 mutant was due to a trace amount of GSH synthesis by wild-type PRO1 and the PRO2 mutant enzyme (120). Whether proline biosynthesis significantly contributes to GSH pools during stress is not yet known, but these studies demonstrate that γ-glutamyl phosphate derived from the proline biosynthetic pathway is a sufficient precursor for GSH synthesis.
ROS signaling
Overproduction of ROS is well known to cause intracellular damage of biological molecules and to contribute to pathological mechanisms. ROS (e.g., H2O2), however, is also an important physiological signaling molecule that triggers adaptive and survival responses by regulating cell death, proliferation, and apoptosis (36, 139). Various studies have shown evidence that proline metabolism leads to increased endogenous ROS (75, 84, 97, 123, 153). Thus, the wide array of effects reported for proline on cellular processes may be due, in part, to the numerous signaling roles of ROS as illustrated in Figure 6.
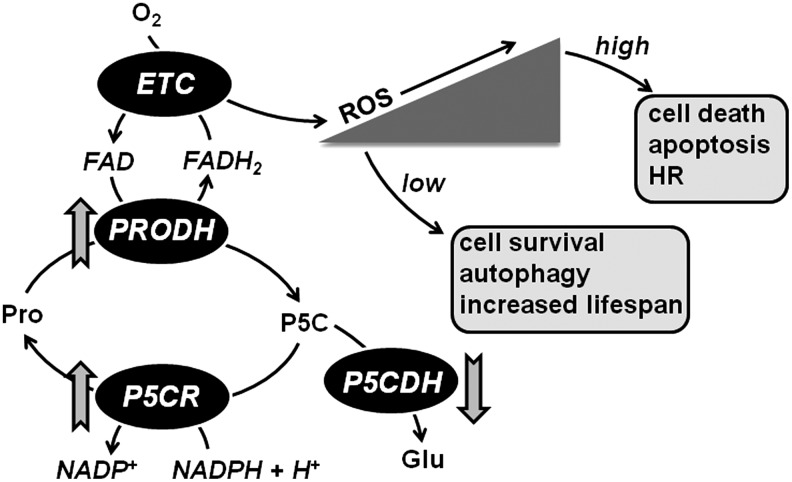
FIG. 6.
Proline metabolism and ROS formation. PRODH activity leads to ROS formation in mitochondria by coupling proline oxidation to reduction of the ETC. Increases in PRODH and P5CR activities along with down-regulation of P5CDH are predicted to increase proline-P5C cycling and ROS levels. ROS levels flucuate according to changes in proline metabolism and activate diverse signaling pathways, thereby enabling proline to influence different cellular processes.
The first evidence for proline metabolic signaling via ROS was provided by Phang's group (30, 97). They have shown that in mammalian cells, PRODH activity increases mitochondrial ROS production, leading to induction of intrinsic and extrinsic apoptotic cell death pathways (50, 76). The PRODH1 gene, which encodes PRODH, is a p53-inducible gene (PIG6) (100). Up-regulation of PRODH by p53 increases mitochondrial superoxide (O2•−) production most likely through complex III, leading to cytochrome c release and caspase 9 activation (50, 75). Increased ROS levels due to PRODH have also been implicated in activating apoptotic pathways through the Ca2+/calcineurin-NFAT cascade (76). Phang and colleagues have also shown that PRODH is activated by peroxisome proliferator-activated receptor γ (PPARγ) with PRODH-dependent ROS being an important mediator of apoptosis in cancer cells treated with PPARγ ligands (31, 90, 96, 156). Furthermore, tumor growth is significantly inhibited by overexpression of PRODH in mice (77). Recently, Phang et al., showed that the oncogenic transcription factor c-MYC down-regulates PRODH expression through miR-23b* and increases the expression of proline biosynthesis enzymes (75). Altogether, the studies by Phang's group have implicated PRODH as an important tumor suppressor protein (97).
ROS signaling stimulated by PRODH has also been implicated in cell proliferation, survival, and autophagy (96–98, 153). Recently, proline and PRODH were found to extend lifespan in Caenorhabditis elegans (153). In a C. elegans daf-2 mutant with impaired insulin and IGF1 signaling, knockdown of PRODH significantly decreased lifespan (153). Complementary to the effect of PRODH knockdown on lifespan, proline treatment extended the lifespan of wild-type worms expressing PRODH. The mechanism by which PRODH increased lifespan was shown to involve transient ROS signals generated by PRODH via the mitochondrial ETC (153). Increased mitochondrial ROS production by proline metabolism has been proposed to activate the worm homologues of p38 MAP kinase and Nrf2, leading to increased expression of antioxidant enzymes and lifespan (153). In tumor cells grown under hypoxic conditions, PRODH and proline metabolism generate a protective effect that involves ROS and autophagic signaling (74). Exogenous addition of proline has also been shown to protect mammalian cells against oxidative stress (69). PRODH was recently shown to be essential for proline protection against oxidative stress with the mechanism of protection involving activation of the Akt survival pathway (70). Whether ROS mediates the effects of PRODH on Akt is not yet known.
Increased endogenous ROS formation due to proline metabolism has an important cell signaling role in plants as well (123). In contrast to abiotic stress response described earlier, PRODH1 expression has been observed to increase in Arabidopsis on infection by a nonvirulant strain of P. syringae (13). The increase in PRODH1 levels is dependent on salicylic acid and is considered a part of the initial HR in infected tissues (13). Interestingly, increased PRODH activity correlated with the oxidative burst of the HR (13). Plants in which PRODH expression was silenced exhibited increased susceptibility to infection relative to wild-type plants. These results suggest that PRODH may participate in the HR by helping to induce cell death and to prevent pathogen growth in plants (13).
Exogenous proline application has also been shown to lead to increased mitochondrial ROS in Arabidopsis, especially in p5cdh mutant plants (84). The higher levels of ROS in p5cdh plants were suggested to be due to increased proline-P5C cycling, resulting in more flux through the mitochondrial ETC (84). The increased ROS production resulting from exogenous proline addition is proposed to contribute to proline toxicity that is often observed with plants treated with high levels of proline (123). P5CDH was proposed to be an important regulator of ROS production in plants by controlling flow through the proline-P5C cycle to avoid overproduction of mitochondrial ROS. Consistent with this, the expression of P5CDH is down-regulated by 24-nt SRO5-P5CDH natural silencing RNAs during salt treatment, leading to an increase in ROS production (12, 131). Furthermore, it was found in flax (Linum usitatissimum) that reduced expression of the flax homologue of Arabidopsis P5CDH, FIS1, resulted in increased sensitivity to exogenous proline and higher levels of H2O2 (8, 105).
Conclusion
The ability of proline to protect different organisms during stress involves a plethora of molecular mechanisms, each of which contribute differently according to the physiological and metabolic contexts. Deciphering the mechanisms of how proline influences stress response and redox homeostasis is also complicated by the fact that proline is a proteinogenic amino acid. Thus, the effects of proline metabolism during stress need to be carefully distinguished from potential impacts on protein synthesis that may perturb normal cellular processes and cell survival.
The mechanisms by which proline abates stress can be divided into two general strategies. One strategy is for the organism to accumulate proline via up-regulation of proline biosynthesis with proline serving as an osmolyte, a chemical chaperone, and a direct scavenger of OH• or 1O2. A second strategy depends on active proline metabolic flux and linkages to other metabolic pathways. Proline metabolic flux leads to cell protection by helping maintain cellular energy and NADP+/NADPH balance, activating signaling pathways that promote cell survival, and contributing to other pathways such as the tricarboxylic acid cycle and GSH biosynthesis.
Figure 6 shows that the ability of proline metabolism to influence various signaling pathways resulting in either cell survival or cell death may be mediated by ROS. Proline metabolism feeds electrons directly to the ETC via PRODH, which leads to superoxide anion formation and H2O2. The amount of ROS generated depends on the availability of proline and the level of PRODH activity in the mitochondria. Low ROS generation (i.e., constitutive PRODH expression) would be predicted to lead to protective effects such as activation of Nrf2 and lifespan extension as found in C. elegans. High ROS generation due to increased PRODH expression would lead to apoptosis and cell death and contribute to physiological processes such as the HR in plants. Mitochondrial ROS production linked to proline would depend not only on PRODH but also on the activities of P5CR and P5CDH. An increased ratio of PRODH/P5CDH activity, for example, would be predicted to increase proline metabolic cycling with P5C being converted back to proline via P5CR and NADPH. Thus, proline metabolic flux determined by the activities of PRODH, P5CR, and P5CDH will have a profound impact on ROS-mediated signaling and ultimately, cell fate. In the future, it will be important to understand not only the regulation of proline metabolism, but also how the activities of these key enzymes are correlated with cell survival and cell death. Potentially, this cycle could be exploited to further improve plant stress behavior and as Phang has already suggested, as a novel target of cancer therapy (97).
Abbreviations Used
- ABA
abscisic acid
- ASC
ascorbate
- ATP
adenosine triphosphate
- ETC
electron transport chain
- FAD
flavin adenine dinucleotide
- GK
glutamate kinase
- GPR
γ-glutamyl phosphate reductase
- GPx
glutathione peroxidase;
- GSA
γ-glutamate semialdehyde
- GSH
glutathione
- GSSG
oxidized glutathione
- H2O2
hydrogen peroxide
- HR
hypersensitive response
- NADP+
nicotinamide adenine dinucleotide phosphate
- OAT
ornithine-δ-aminotransferase
- P5C
Δ1-pyrroline-5-carboxylate
- P5CDH
P5C dehydrogenase
- P5CR and PYCR
P5C reductase
- P5CS
P5C synthetase
- PPARγ
peroxisome proliferator-activated receptor γ
- PRODH
proline dehydrogenase
- put
proline utilization
- PutA
proline utilization A
- ROS
reactive oxygen species
- TEMPO
2,2,6,6-tetramethylpiperidine-1-oxyl
- Trx
thioredoxin
- TrxR
thioredoxin reductase
- Trx-S2
oxidized thioredoxin
- Trx-(SH)2
reduced thioredoxin
Acknowledgments
The work was supported in part by grants GM079393, P20 RR-017675, and P30GM103335 from National Institutes of Health.
References
- 1.Abraham E. Rigo G. Szekely G. Nagy R. Koncz C. Szabados L. Light-dependent induction of proline biosynthesis by abscisic acid and salt stress is inhibited by brassinosteroid in Arabidopsis. Plant Mol Biol. 2003;51:363–372. doi: 10.1023/a:1022043000516. [DOI] [PubMed] [Google Scholar]
- 2.Adams E. Metabolism of proline and of hydroxyproline. Int Rev Connect Tissue Res. 1970;5:1–91. doi: 10.1016/b978-0-12-363705-5.50007-5. [DOI] [PubMed] [Google Scholar]
- 3.Akashi K. Miyake C. Yokota A. Citrulline, a novel compatible solute in drought-tolerant wild watermelon leaves, is an efficient hydroxyl radical scavenger. FEBS Lett. 2001;508:438–442. doi: 10.1016/s0014-5793(01)03123-4. [DOI] [PubMed] [Google Scholar]
- 4.Alia Mohanty P. Matysik J. Effect of proline on the production of singlet oxygen. Amino Acids. 2001;21:195–200. doi: 10.1007/s007260170026. [DOI] [PubMed] [Google Scholar]
- 5.Alia Saradhi PP. Mohanty P. Involvement of proline in protecting thylakoid membranes against free radical-induced photodamage. J Photochem and Photobiol B-Biol. 1997;38:253–257. [Google Scholar]
- 6.Arentson BW. Sanyal N. Becker DF. Substrate channeling in proline metabolism. Front Biosci. 2012;17:375–388. doi: 10.2741/3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auton M. Bolen DW. Predicting the energetics of osmolyte-induced protein folding/unfolding. Proc Natl Acad Sci U S A. 2005;102:15065–15068. doi: 10.1073/pnas.0507053102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayliffe MA. Roberts JK. Mitchell HJ. Zhang R. Lawrence GJ. Ellis JG. Pryor TJ. A plant gene up-regulated at rust infection sites. Plant Physiol. 2002;129:169–180. doi: 10.1104/pp.010940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnett NM. Naylor AW. Amino Acid and protein metabolism in bermuda grass during water stress. Plant Physiol. 1966;41:1222–1230. doi: 10.1104/pp.41.7.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binzel ML. Hasegawa PM. Rhodes D. Handa S. Handa AK. Bressan RA. Solute accumulation in tobacco cells adapted to NaCl. Plant Physiol. 1987;84:1408–1415. doi: 10.1104/pp.84.4.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolen DW. Baskakov IV. The osmophobic effect: natural selection of a thermodynamic force in protein folding. J Mol Biol. 2001;310:955–963. doi: 10.1006/jmbi.2001.4819. [DOI] [PubMed] [Google Scholar]
- 12.Borsani O. Zhu J. Verslues PE. Sunkar R. Zhu JK. Endogenous siRNAs derived from a pair of natural cis-antisense transcripts regulate salt tolerance in Arabidopsis. Cell. 2005;123:1279–1291. doi: 10.1016/j.cell.2005.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cecchini NM. Monteoliva MI. Alvarez ME. Proline dehydrogenase contributes to pathogen defense in Arabidopsis. Plant Physiol. 2011;155:1947–1959. doi: 10.1104/pp.110.167163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cecchini NM. Monteoliva MI. Alvarez ME. Proline dehydrogenase is a positive regulator of cell death in different kingdoms. Plant Signal Behav. 2011;6:1195–1197. doi: 10.4161/psb.6.8.15791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chattopadhyay MK. Kern R. Mistou MY. Dandekar AM. Uratsu SL. Richarme G. The chemical chaperone proline relieves the thermosensitivity of a dnaK deletion mutant at 42 degrees C. J Bacteriol. 2004;186:8149–8152. doi: 10.1128/JB.186.23.8149-8152.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaves MM. Flexas J. Pinheiro C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot. 2009;103:551–560. doi: 10.1093/aob/mcn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen C. Dickman MB. Proline suppresses apoptosis in the fungal pathogen Colletotrichum trifolii. Proc Natl Acad Sci U S A. 2005;102:3459–3464. doi: 10.1073/pnas.0407960102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen CT. Chen L. Lin CC. Kao CH. Regulation of proline accumulation in detached rice leaves exposed to excess copper. Plant Sci. 2001;160:283–290. doi: 10.1016/s0168-9452(00)00393-9. [DOI] [PubMed] [Google Scholar]
- 19.Choudhary NL. Sairam RK. Tyagi A. Expression of delta1-pyrroline-5-carboxylate synthetase gene during drought in rice (Oryza sativa L.) Indian J Biochem Biophys. 2005;42:366–370. [PubMed] [Google Scholar]
- 20.Clennan EL. Noe LJ. Wen T. Szneler E. Solvent effects on the ability of amines to physically quench singlet oxygen as determined by time-resolved infrared emission studies. J Org Chem. 1989;54:3581–3584. [Google Scholar]
- 21.Csonka LN. Proline over-production results in enhanced osmotolerance in Salmonella typhimurium. Mol Gen Genet. 1981;182:82–86. doi: 10.1007/BF00422771. [DOI] [PubMed] [Google Scholar]
- 22.Csonka LN. Physiological and genetic responses of bacteria to osmotic-stress. Microbiol Rev. 1989;53:121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Csonka LN. Gelvin SB. Goodner BW. Orser CS. Siemieniak D. Slightom JL. Nucleotide-sequence of a mutation in the proB gene of Escherichia-coli that confers proline overproduction and enhanced tolerance to osmotic-stress. Gene. 1988;64:199–205. doi: 10.1016/0378-1119(88)90335-6. [DOI] [PubMed] [Google Scholar]
- 24.De Ingeniis J. Ratnikov B. Richardson AD. Scott DA. Aza-Blanc P. De SK. Kazanov M. Pellecchia M. Ronai Z. Osterman AL. Smith JW. Functional specialization in proline biosynthesis of melanoma. PloS ONE. 2012;9:e45190. doi: 10.1371/journal.pone.0045190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Ronde JA. Cress WA. Kruger GHJ. Strasser RJ. Van Staden J. Photosynthetic response of transgenic soybean plants, containing an Arabidopsis P5CR gene, during heat and drought stress. J Plant Physiol. 2004;161:1211–1224. doi: 10.1016/j.jplph.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 26.Delauney AJ. Verma DPS. A soybean gene encoding delta-1-pyrroline-5-carboxylate reductase was isolated by functional complementation in Escherichia-coli and is found to be osmoregulated. Mol Gen Genet. 1990;221:299–305. doi: 10.1007/BF00259392. [DOI] [PubMed] [Google Scholar]
- 27.Delauney AJ. Verma DPS. Proline biosynthesis and osmoregulation in plants. Plant J. 1993;4:215–223. [Google Scholar]
- 28.Deuschle K. Funck D. Forlani G. Stransky H. Biehl A. Leister D. van der Graaff E. Kunze R. Frommer WB. The role of [Delta]1-pyrroline-5-carboxylate dehydrogenase in proline degradation. Plant Cell. 2004;16:3413–3425. doi: 10.1105/tpc.104.023622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deuschle K. Funck D. Hellmann H. Daschner K. Binder S. Frommer WB. A nuclear gene encoding mitochondrial Delta-pyrroline-5-carboxylate dehydrogenase and its potential role in protection from proline toxicity. Plant J. 2001;27:345–356. doi: 10.1046/j.1365-313x.2001.01101.x. [DOI] [PubMed] [Google Scholar]
- 30.Donald SP. Sun XY. Hu CA. Yu J. Mei JM. Valle D. Phang JM. Proline oxidase, encoded by p53-induced gene-6, catalyzes the generation of proline-dependent reactive oxygen species. Cancer Res. 2001;61:1810–1815. [PubMed] [Google Scholar]
- 31.Elrod HA. Sun SY. PPARgamma and apoptosis in cancer. PPAR Res. 2008;2008:704165. doi: 10.1155/2008/704165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engelhard J. Christian BE. Weingarten L. Kuntz G. Spremulli LL. Dick TP. In situ kinetic trapping reveals a fingerprint of reversible protein thiol oxidation in the mitochondrial matrix. Free Radic Biol Med. 2011;50:1234–1241. doi: 10.1016/j.freeradbiomed.2011.01.035. [DOI] [PubMed] [Google Scholar]
- 33.Fabro G. Kovacs I. Pavet V. Szabados L. Alvarez ME. Proline accumulation and AtP5CS2 gene activation are induced by plant-pathogen incompatible interactions in Arabidopsis. Mol Plant Microbe Interact. 2004;17:343–350. doi: 10.1094/MPMI.2004.17.4.343. [DOI] [PubMed] [Google Scholar]
- 34.Farago ME. Mullen WA. Plants which accumulate metals. Part IV. A possible copper–proline complex from the roots of Armeria maritima. Inorg Chim Acta. 1979;32:L93–L94. [Google Scholar]
- 35.Farrant RD. Walker V. Mills GA. Mellor JM. Langley GJ. Pyridoxal phosphate de-activation by pyrroline-5-carboxylic acid. Increased risk of vitamin B6 deficiency and seizures in hyperprolinemia type II. J Biol Chem. 2001;276:15107–15116. doi: 10.1074/jbc.M010860200. [DOI] [PubMed] [Google Scholar]
- 36.Finkel T. Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 37.Fischer DF. Backendorf C. Promoter analysis in the human SPRR gene family. Methods Mol Biol. 2005;289:303–314. doi: 10.1385/1-59259-830-7:303. [DOI] [PubMed] [Google Scholar]
- 38.Floyd RA. Zsnagy I. Formation of long-lived hydroxyl free-radical adducts of proline and hydroxyproline in a fenton reaction. Biochim Biophys Acta. 1984;790:94–97. doi: 10.1016/0167-4838(84)90337-6. [DOI] [PubMed] [Google Scholar]
- 39.Freimark D. Sehl C. Weber C. Hudel K. Czermak P. Hofmann N. Spindler R. Glasmacher B. Systematic parameter optimization of a Me(2)SO- and serum-free cryopreservation protocol for human mesenchymal stem cells. Cryobiology. 2011;63:67–75. doi: 10.1016/j.cryobiol.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Funck D. Eckard S. Muller G. Non-redundant functions of two proline dehydrogenase isoforms in Arabidopsis. BMC Plant Biol. 2010;10:70. doi: 10.1186/1471-2229-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Funck D. Stadelhofer B. Koch W. Ornithine-delta-aminotransferase is essential for arginine catabolism but not for proline biosynthesis. BMC Plant Biol. 2008;8:40. doi: 10.1186/1471-2229-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Handa S. Bressan RA. Handa AK. Carpita NC. Hasegawa PM. Solutes contributing to osmotic adjustment in cultured plant cells adapted to water stress. Plant Physiol. 1983;73:834–843. doi: 10.1104/pp.73.3.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Handa S. Handa AK. Hasegawa PM. Bressan RA. Proline accumulation and the adaptation of cultured plant cells to water stress. Plant Physiol. 1986;80:938–945. doi: 10.1104/pp.80.4.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hare PD. Cress WA. Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul. 1997;21:79–102. [Google Scholar]
- 45.Hare PD. Cress WA. Staden Jv. Proline synthesis and degradation: a model system for elucidating stress-related signal transduction. J Exp Bot. 1999;50:413–434. [Google Scholar]
- 46.Hong Z. Lakkineni K. Zhang Z. Verma DPS. Removal of feedback inhibition of pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol. 2000;122:1129–1136. doi: 10.1104/pp.122.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoque MA. Banu MN. Nakamura Y. Shimoishi Y. Murata Y. Proline and glycinebetaine enhance antioxidant defense and methylglyoxal detoxification systems and reduce NaCl-induced damage in cultured tobacco cells. J Plant Physiol. 2008;165:813–824. doi: 10.1016/j.jplph.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 48.Hoque MA. Okuma E. Banu MN. Nakamura Y. Shimoishi Y. Murata Y. Exogenous proline mitigates the detrimental effects of salt stress more than exogenous betaine by increasing antioxidant enzyme activities. J Plant Physiol. 2007;164:553–561. doi: 10.1016/j.jplph.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 49.Hu CA. Delauney AJ. Verma DP. A bifunctional enzyme (delta 1-pyrroline-5-carboxylate synthetase) catalyzes the first two steps in proline biosynthesis in plants. Proc Natl Acad Sci U S A. 1992;89:9354–9358. doi: 10.1073/pnas.89.19.9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu CA. Donald SP. Yu J. Lin WW. Liu Z. Steel G. Obie C. Valle D. Phang JM. Overexpression of proline oxidase induces proline-dependent and mitochondria-mediated apoptosis. Mol Cell Biochem. 2007;295:85–92. doi: 10.1007/s11010-006-9276-6. [DOI] [PubMed] [Google Scholar]
- 51.Ignatova Z. Gierasch LM. Inhibition of protein aggregation in vitro and in vivo by a natural osmoprotectant. Proc Natl Acad Sci U S A. 2006;103:13357–13361. doi: 10.1073/pnas.0603772103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Imlay JA. Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem. 2008;77:755–776. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Islam MM. Hoque MA. Okuma E. Banu MN. Shimoishi Y. Nakamura Y. Murata Y. Exogenous proline and glycinebetaine increase antioxidant enzyme activities and confer tolerance to cadmium stress in cultured tobacco cells. J Plant Physiol. 2009;166:1587–1597. doi: 10.1016/j.jplph.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 54.Islam MM. Hoque MA. Okuma E. Jannat R. Banu MN. Jahan MS. Nakamura Y. Murata Y. Proline and glycinebetaine confer cadmium tolerance on tobacco bright yellow-2 cells by increasing ascorbate-glutathione cycle enzyme activities. Biosci Biotechnol Biochem. 2009;73:2320–2323. doi: 10.1271/bbb.90305. [DOI] [PubMed] [Google Scholar]
- 55.Jauniaux JC. Urrestarazu LA. Wiame JM. Arginine metabolism in Saccharomyces cerevisiae: subcellular localization of the enzymes. J Bacteriol. 1978;133:1096–1107. doi: 10.1128/jb.133.3.1096-1107.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jortzik E. Fritz-Wolf K. Sturm N. Hipp M. Rahlfs S. Becker K. Redox regulation of Plasmodium falciparum ornithine delta-aminotransferase. J Mol Biol. 2010;402:445–459. doi: 10.1016/j.jmb.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 57.Kaplan F. Kopka J. Sung DY. Zhao W. Popp M. Porat R. Guy CL. Transcript and metabolite profiling during cold acclimation of Arabidopsis reveals an intricate relationship of cold-regulated gene expression with modifications in metabolite content. Plant J. 2007;50:967–981. doi: 10.1111/j.1365-313X.2007.03100.x. [DOI] [PubMed] [Google Scholar]
- 58.Kaul S. Sharma SS. Mehta IK. Free radical scavenging potential of L-proline: evidence from in vitro assays. Amino Acids. 2008;34:315–320. doi: 10.1007/s00726-006-0407-x. [DOI] [PubMed] [Google Scholar]
- 59.Kawakami R. Satomura T. Sakuraba H. Ohshima T. L-proline dehydrogenases in hyperthermophilic archaea: distribution, function, structure, and application. Appl Microbiol Biotechnol. 2012;93:83–93. doi: 10.1007/s00253-011-3682-8. [DOI] [PubMed] [Google Scholar]
- 60.Kehr S. Jortzik E. Delahunty C. Yates JR., 3rd Rahlfs S. Becker K. Protein S-glutathionylation in malaria parasites. Antioxid Redox Signal. 2011;15:2855–2865. doi: 10.1089/ars.2011.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kempf B. Bremer E. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch Microbiol. 1998;170:319–330. doi: 10.1007/s002030050649. [DOI] [PubMed] [Google Scholar]
- 62.Kesari R. Lasky JR. Villamor JG. Des Marais DL. Chen YJ. Liu TW. Lin W. Juenger TE. Verslues PE. Intron-mediated alternative splicing of Arabidopsis P5CS1 and its association with natural variation in proline and climate adaptation. Proc Natl Acad Sci U S A. 2012;109:9197–9202. doi: 10.1073/pnas.1203433109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khan AU. Mei YH. Wilson T. A proposed function for spermine and spermidine: protection of replicating DNA against damage by singlet oxygen. Proc Natl Acad Sci U S A. 1992;89:11426–11427. doi: 10.1073/pnas.89.23.11426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kiyosue T. Yoshiba Y. YamaguchiShinozaki K. Shinozaki K. A nuclear gene encoding mitochondrial proline dehydrogenase, an enzyme involved in proline metabolism, is upregulated by proline but downregulated by dehydration in Arabidopsis. Plant Cell. 1996;8:1323–1335. doi: 10.1105/tpc.8.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kobayashi T. Nishii M. Takagi Y. Titani K. Matsuzawa T. Molecular cloning and nucleotide sequence analysis of mRNA for human kidney ornithine aminotransferase. An examination of ornithine aminotransferase isozymes between liver and kidney. FEBS Lett. 1989;255:300–304. doi: 10.1016/0014-5793(89)81110-x. [DOI] [PubMed] [Google Scholar]
- 66.Kohl DH. Schubert KR. Carter MB. Hagedorn CH. Shearer G. Proline metabolism in N2-fixing root nodules: energy transfer and regulation of purine synthesis. Proc Natl Acad Sci U S A. 1988;85:2036–2040. doi: 10.1073/pnas.85.7.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kostal V. Simek P. Zahradnickova H. Cimlova J. Stetina T. Conversion of the chill susceptible fruit fly larva (Drosophila melanogaster) to a freeze tolerant organism. Proc Natl Acad Sci U S A. 2012;109:3270–3274. doi: 10.1073/pnas.1119986109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kostal V. Zahradnickova H. Simek P. Hyperprolinemic larvae of the drosophilid fly, Chymomyza costata, survive cryopreservation in liquid nitrogen. Proc Natl Acad Sci U S A. 2011;108:13041–13046. doi: 10.1073/pnas.1107060108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krishnan N. Dickman MB. Becker DF. Proline modulates the intracellular redox environment and protects mammalian cells against oxidative stress. Free Radic Biol Med. 2008;44:671–681. doi: 10.1016/j.freeradbiomed.2007.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kumar Natarajan S. Zhu W. Liang X. Zhang L. Demers AJ. Zimmerman MC. Simpson MA. Becker DF. Proline dehydrogenase is essential for proline protection against hydrogen peroxide-induced cell death. Free Radic Biol Med. 2012;53:1181–1191. doi: 10.1016/j.freeradbiomed.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kumar TK. Samuel D. Jayaraman G. Srimathi T. Yu C. The role of proline in the prevention of aggregation during protein folding in vitro. Biochem Mol Biol Int. 1998;46:509–517. doi: 10.1080/15216549800204032. [DOI] [PubMed] [Google Scholar]
- 72.Larson JD. Jenkins JL. Schuermann JP. Zhou Y. Becker DF. Tanner JJ. Crystal structures of the DNA-binding domain of Escherichia coli proline utilization A flavoprotein and analysis of the role of Lys9 in DNA recognition. Protein Sci. 2006;15:2630–2641. doi: 10.1110/ps.062425706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ling M. Allen SW. Wood JM. Sequence analysis identifies the proline dehydrogenase and delta 1-pyrroline-5-carboxylate dehydrogenase domains of the multifunctional Escherichia coli PutA protein. J Mol Biol. 1994;243:950–956. doi: 10.1006/jmbi.1994.1696. [DOI] [PubMed] [Google Scholar]
- 74.Liu W. Glunde K. Bhujwalla ZM. Raman V. Sharma A. Phang JM. Proline oxidase promotes tumor cell survival in hypoxic tumor microenvironments. Cancer Res. 2012;72:3677–3686. doi: 10.1158/0008-5472.CAN-12-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu W. Le A. Hancock C. Lane AN. Dang CV. Fan TW. Phang JM. Reprogramming of proline and glutamine metabolism contributes to the proliferative and metabolic responses regulated by oncogenic transcription factor c-MYC. Proc Natl Acad Sci U S A. 2012;109:8983–8988. doi: 10.1073/pnas.1203244109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu Y. Borchert GL. Surazynski A. Hu CA. Phang JM. Proline oxidase activates both intrinsic and extrinsic pathways for apoptosis: the role of ROS/superoxides, NFAT and MEK/ERK signaling. Oncogene. 2006;25:5640–5647. doi: 10.1038/sj.onc.1209564. [DOI] [PubMed] [Google Scholar]
- 77.Liu YM. Borchert GL. Donald SP. Diwan BA. Anver M. Phang JM. Proline oxidase functions as a mitochondrial tumor suppressor in human cancers. Cancer Res. 2009;69:6414–6422. doi: 10.1158/0008-5472.CAN-09-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mani S. Van De Cotte B. Van Montagu M. Verbruggen N. Altered levels of proline dehydrogenase cause hypersensitivity to proline and its analogs in Arabidopsis. Plant Physiol. 2002;128:73–83. [PMC free article] [PubMed] [Google Scholar]
- 79.Marco-Marin C. Gil-Ortiz F. Perez-Arellano I. Cervera J. Fita I. Rubio V. A novel two-domain architecture within the amino acid kinase enzyme family revealed by the crystal structure of Escherichia coli glutamate 5-kinase. J Mol Biol. 2007;367:1431–1446. doi: 10.1016/j.jmb.2007.01.073. [DOI] [PubMed] [Google Scholar]
- 80.Mattioli R. Falasca G. Sabatini S. Altamura MM. Costantino P. Trovato M. The proline biosynthetic genes P5CS1 and P5CS2 play overlapping roles in Arabidopsis flower transition but not in embryo development. Physiol Plant. 2009;137:72–85. doi: 10.1111/j.1399-3054.2009.01261.x. [DOI] [PubMed] [Google Scholar]
- 81.Matysik J. Alia Bhalu B. Mohanty P. Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr Sci. 2002;82:525–532. [Google Scholar]
- 82.Meng Z. Lou Z. Liu Z. Li M. Zhao X. Bartlam M. Rao Z. Crystal structure of human pyrroline-5-carboxylate reductase. J Mol Biol. 2006;359:1364–1377. doi: 10.1016/j.jmb.2006.04.053. [DOI] [PubMed] [Google Scholar]
- 83.Menzel R. Roth J. Regulation of the genes for proline utilization in Salmonella typhimurium: autogenous repression by the putA gene product. J Mol Biol. 1981;148:21–44. doi: 10.1016/0022-2836(81)90233-3. [DOI] [PubMed] [Google Scholar]
- 84.Miller G. Honig A. Stein H. Suzuki N. Mittler R. Zilberstein A. Unraveling delta1-pyrroline-5-carboxylate-proline cycle in plants by uncoupled expression of proline oxidation enzymes. J Biol Chem. 2009;284:26482–26492. doi: 10.1074/jbc.M109.009340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morita Y. Nakamori S. Takagi H. Effect of proline and arginine metabolism on freezing stress of Saccharomyces cerevisiae. J Biosci Bioeng. 2002;94:390–394. doi: 10.1016/s1389-1723(02)80214-6. [DOI] [PubMed] [Google Scholar]
- 86.Moxley MA. Tanner JJ. Becker DF. Steady-state kinetic mechanism of the proline:ubiquinone oxidoreductase activity of proline utilization A (PutA) from Escherichia coli. Arch Biochem Biophys. 2011;516:113–120. doi: 10.1016/j.abb.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Noctor G. Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- 88.Nomura M. Takagi H. Role of the yeast acetyltransferase Mpr1 in oxidative stress: regulation of oxygen reactive species caused by a toxic proline catabolism intermediate. Proc Natl Acad Sci U S A. 2004;101:12616–12621. doi: 10.1073/pnas.0403349101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Page R. Nelson MS. von Delft F. Elsliger MA. Canaves JM. Brinen LS. Dai X. Deacon AM. Floyd R. Godzik A. Grittini C. Grzechnik SK. Jaroszewski L. Klock HE. Koesema E. Kovarik JS. Kreusch A. Kuhn P. Lesley SA. McMullan D. McPhillips TM. Miller MD. Morse A. Moy K. Ouyang J. Robb A. Rodrigues K. Schwarzenbacher R. Spraggon G. Stevens RC. van den Bedem H. Velasquez J. Vincent J. Wang X. West B. Wolf G. Hodgson KO. Wooley J. Wilson IA. Crystal structure of gamma-glutamyl phosphate reductase (TM0293) from Thermotoga maritima at 2.0 A resolution. Proteins. 2004;54:157–161. doi: 10.1002/prot.10562. [DOI] [PubMed] [Google Scholar]
- 90.Pandhare J. Cooper SK. Phang JM. Proline oxidase, a proapoptotic gene, is induced by troglitazone: evidence for both peroxisome proliferator-activated receptor gamma-dependent and -independent mechanisms. J Biol Chem. 2006;281:2044–2052. doi: 10.1074/jbc.M507867200. [DOI] [PubMed] [Google Scholar]
- 91.Parre E. Ghars MA. Leprince AS. Thiery L. Lefebvre D. Bordenave M. Richard L. Mazars C. Abdelly C. Savoure A. Calcium signaling via phospholipase C is essential for proline accumulation upon ionic but not nonionic hyperosmotic stresses in Arabidopsis. Plant Physiol. 2007;144:503–512. doi: 10.1104/pp.106.095281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pemberton TA. Still BR. Christensen EM. Singh H. Srivastava D. Tanner JJ. Proline: Mother nature's cryoprotectant applied to protein crystallography. Acta Crystallogr D Biol Crystallogr. 2012;68:1010–1018. doi: 10.1107/S0907444912019580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Perez-Arellano I. Carmona-Alvarez F. Gallego J. Cervera J. Molecular mechanisms modulating glutamate kinase activity. Identification of the proline feedback inhibitor binding site. J Mol Biol. 2010;404:890–901. doi: 10.1016/j.jmb.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 94.Perez-Arellano I. Carmona-Alvarez F. Martinez AI. Rodriguez-Diaz J. Cervera J. Pyrroline-5-carboxylate synthase and proline biosynthesis: from osmotolerance to rare metabolic disease. Protein Sci. 2010;19:372–382. doi: 10.1002/pro.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Phang JM. The regulatory functions of proline and pyrroline-5-carboxylic acid. Curr Top Cell Regul. 1985;25:91–132. doi: 10.1016/b978-0-12-152825-6.50008-4. [DOI] [PubMed] [Google Scholar]
- 96.Phang JM. Donald SP. Pandhare J. Liu Y. The metabolism of proline, a stress substrate, modulates carcinogenic pathways. Amino Acids. 2008;35:681–690. doi: 10.1007/s00726-008-0063-4. [DOI] [PubMed] [Google Scholar]
- 97.Phang JM. Liu W. Hancock C. Christian KJ. The proline regulatory axis and cancer. Front Oncol. 2012;2:60. doi: 10.3389/fonc.2012.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Phang JM. Liu W. Zabirnyk O. Proline metabolism and microenvironmental stress. Annu Rev Nutr. 2010;30:441–463. doi: 10.1146/annurev.nutr.012809.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pistollato F. Persano L. Rampazzo E. Basso G. L-Proline as a modulator of ectodermal differentiation in ES cells. Focus on L-Proline induces differentiation of ES cells: a novel role for an amino acid in the regulation of pluripotent cells in culture. Am J Physiol Cell Physiol. 2010;298:C979–C981. doi: 10.1152/ajpcell.00072.2010. [DOI] [PubMed] [Google Scholar]
- 100.Polyak K. Xia Y. Zweier JL. Kinzler KW. Vogelstein B. A model for p53-induced apoptosis. Nature. 1997;389:300–305. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- 101.Raymond MJ. Smirnoff N. Proline metabolism and transport in maize seedlings at low water potential. Annals Bot. 2002;89:813–823. doi: 10.1093/aob/mcf082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Requena JR. Chao CC. Levine RL. Stadtman ER. Glutamic and aminoadipic semialdehydes are the main carbonyl products of metal-catalyzed oxidation of proteins. Proc Natl Acad Sci U S A. 2001;98:69–74. doi: 10.1073/pnas.011526698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reversade B. Escande-Beillard N. Dimopoulou A. Fischer B. Chng SC. Li Y. Shboul M. Tham PY. Kayserili H. Al-Gazali L. Shahwan M. Brancati F. Lee H. O'Connor BD. Schmidt-von Kegler M. Merriman B. Nelson SF. Masri A. Alkazaleh F. Guerra D. Ferrari P. Nanda A. Rajab A. Markie D. Gray M. Nelson J. Grix A. Sommer A. Savarirayan R. Janecke AR. Steichen E. Sillence D. Hausser I. Budde B. Nurnberg G. Nurnberg P. Seemann P. Kunkel D. Zambruno G. Dallapiccola B. Schuelke M. Robertson S. Hamamy H. Wollnik B. Van Maldergem L. Mundlos S. Kornak U. Mutations in PYCR1 cause cutis laxa with progeroid features. Nat Genet. 2009;41:1016–1021. doi: 10.1038/ng.413. [DOI] [PubMed] [Google Scholar]
- 104.Ribarits A. Abdullaev A. Tashpulatov A. Richter A. Heberle-Bors E. Touraev A. Two tobacco proline dehydrogenases are differentially regulated and play a role in early plant development. Planta. 2007;225:1313–1324. doi: 10.1007/s00425-006-0429-3. [DOI] [PubMed] [Google Scholar]
- 105.Roberts JK. Pryor A. Isolation of a flax (Linum usitatissimum) gene induced during susceptible infection by flax rust (Melampsora lini) Plant J. 1995;8:1–8. doi: 10.1046/j.1365-313x.1995.08010001.x. [DOI] [PubMed] [Google Scholar]
- 106.Rustgi S. Joshi A. Moss H. Riesz P. E.s.r. of spin-trapped radicals in aqueous solutions of amino acids. Reactions of the hydroxyl radical. Int J Radiat Biol Relat Stud Phys Chem Med. 1977;31:415–440. doi: 10.1080/09553007714550521. [DOI] [PubMed] [Google Scholar]
- 107.Samuel D. Kumar TK. Ganesh G. Jayaraman G. Yang PW. Chang MM. Trivedi VD. Wang SL. Hwang KC. Chang DK. Yu C. Proline inhibits aggregation during protein refolding. Protein Sci. 2000;9:344–352. doi: 10.1110/ps.9.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Saradhi PP. Alia Arora AS. Prasad KVSK. Proline accumulates in plants exposed to UV radiation and protects them against UV induced peroxidation. Biochem Biophys Res Comm. 1995;209:1–5. doi: 10.1006/bbrc.1995.1461. [DOI] [PubMed] [Google Scholar]
- 109.Savoure A. Hua XJ. Bertauche N. Van Montagu M. Verbruggen N. Abscisic acid-independent and abscisic acid-dependent regulation of proline biosynthesis following cold and osmotic stresses in Arabidopsis thaliana. Mol Gen Genet. 1997;254:104–109. doi: 10.1007/s004380050397. [DOI] [PubMed] [Google Scholar]
- 110.Savoure A. Jaoua S. Hua XJ. Ardiles W. Van Montagu M. Verbruggen N. Isolation, characterization, and chromosomal location of a gene encoding the delta 1-pyrroline-5-carboxylate synthetase in Arabidopsis thaliana. FEBS Lett. 1995;372:13–9. doi: 10.1016/0014-5793(95)00935-3. [DOI] [PubMed] [Google Scholar]
- 111.Sharma P. Dubey RS. Modulation of nitrate reductase activity in rice seedlings under aluminium toxicity and water stress: role of osmolytes as enzyme protectant. J Plant Physiol. 2005;162:854–864. doi: 10.1016/j.jplph.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 112.Sharma S. Villamor JG. Verslues PE. Essential role of tissue-specific proline synthesis and catabolism in growth and redox balance at low water potential. Plant Physiol. 2011;157:292–304. doi: 10.1104/pp.111.183210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sharma SS. Dietz KJ. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J Exp Bot. 2006;57:711–726. doi: 10.1093/jxb/erj073. [DOI] [PubMed] [Google Scholar]
- 114.Sharma SS. Dietz KJ. The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci. 2009;14:43–50. doi: 10.1016/j.tplants.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 115.Sharma SS. Schat H. Vooijs R. In vitro alleviation of heavy metal-induced enzyme inhibition by proline. Phytochemistry. 1998;49:1531–1535. doi: 10.1016/s0031-9422(98)00282-9. [DOI] [PubMed] [Google Scholar]
- 116.Simmaco M. John RA. Barra D. Bossa F. The primary structure of ornithine aminotransferase. Identification of active-site sequence and site of post-translational proteolysis. FEBS Lett. 1986;199:39–42. doi: 10.1016/0014-5793(86)81219-4. [DOI] [PubMed] [Google Scholar]
- 117.Singh RK. Tanner JJ. Unique structural features and sequence motifs of proline utilization A (PutA) Front Biosci. 2012;17:556–568. doi: 10.2741/3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Siripornadulsil S. Traina S. Verma DP. Sayre RT. Molecular mechanisms of proline-mediated tolerance to toxic heavy metals in transgenic microalgae. Plant Cell. 2002;14:2837–2847. doi: 10.1105/tpc.004853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Smith LT. Characterization of a gamma-glutamyl kinase from Escherichia coli that confers proline overproduction and osmotic tolerance. J Bacteriol. 1985;164:1089–1093. doi: 10.1128/jb.164.3.1088-1093.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Spector D. Labarre J. Toledano MB. A genetic investigation of the essential role of glutathione: mutations in the proline biosynthesis pathway are the only suppressors of glutathione auxotrophy in yeast. J Biol Chem. 2001;276:7011–7016. doi: 10.1074/jbc.M009814200. [DOI] [PubMed] [Google Scholar]
- 121.Srivastava D. Singh RK. Moxley MA. Henzl MT. Becker DF. Tanner JJ. The three-dimensional structural basis of type II hyperprolinemia. J Mol Biol. 2012;420:176–189. doi: 10.1016/j.jmb.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Strizhov N. Abraham E. Okresz L. Blickling S. Zilberstein A. Schell J. Koncz C. Szabados L. Differential expression of two P5CS genes controlling proline accumulation during salt-stress requires ABA and is regulated by ABA1, ABI1 and AXR2 in Arabidopsis. Plant J. 1997;12:557–569. doi: 10.1046/j.1365-313x.1997.00557.x. [DOI] [PubMed] [Google Scholar]
- 123.Szabados L. Savoure A. Proline: a multifunctional amino acid. Trends Plant Sci. 2010;15:89–97. doi: 10.1016/j.tplants.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 124.Szekely G. Abraham E. Cseplo A. Rigo G. Zsigmond L. Csiszar J. Ayaydin F. Strizhov N. Jasik J. Schmelzer E. Koncz C. Szabados L. Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant J. 2008;53:11–28. doi: 10.1111/j.1365-313X.2007.03318.x. [DOI] [PubMed] [Google Scholar]
- 125.Takagi H. Proline as a stress protectant in yeast: physiological functions, metabolic regulations, and biotechnological applications. Appl Microbiol Biot. 2008;81:211–223. doi: 10.1007/s00253-008-1698-5. [DOI] [PubMed] [Google Scholar]
- 126.Tanner JJ. Structural biology of proline catabolism. Amino Acids. 2008;35:719–730. doi: 10.1007/s00726-008-0062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Trelstad RL. Lawley KR. Holmes LB. Nonenzymatic hydroxylations of proline and lysine by reduced oxygen derivatives. Nature. 1981;289:310–312. doi: 10.1038/289310a0. [DOI] [PubMed] [Google Scholar]
- 128.Ueda A. Yamamoto-Yamane Y. Takabe T. Salt stress enhances proline utilization in the apical region of barley roots. Biochem Biophys Res Commun. 2007;355:61–66. doi: 10.1016/j.bbrc.2007.01.098. [DOI] [PubMed] [Google Scholar]
- 129.Veeravalli K. Boyd D. Iverson BL. Beckwith J. Georgiou G. Laboratory evolution of glutathione biosynthesis reveals natural compensatory pathways. Nat Chem Biol. 2011;7:101–105. doi: 10.1038/nchembio.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Venkatesu P. Lee MJ. Lin HM. Thermodynamic characterization of the osmolyte effect on protein stability and the effect of GdnHCl on the protein denatured state. J Phys Chem B. 2007;111:9045–9056. doi: 10.1021/jp0701901. [DOI] [PubMed] [Google Scholar]
- 131.Verbruggen N. Hermans C. Proline accumulation in plants: a review. Amino Acids. 2008;35:753–759. doi: 10.1007/s00726-008-0061-6. [DOI] [PubMed] [Google Scholar]
- 132.Verbruggen N. Villarroel R. Van Montagu M. Osmoregulation of a pyrroline-5-carboxylate reductase gene in Arabidopsis thaliana. Plant Physiol. 1993;103:771–781. doi: 10.1104/pp.103.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vermeij WP. Florea BI. Isenia S. Alia A. Brouwer J. Backendorf C. Proteomic identification of in vivo interactors reveals novel function of skin cornification proteins. J Proteome Res. 2012 doi: 10.1021/pr300310b. [DOI] [PubMed] [Google Scholar]
- 134.Verslues PE. Kim YS. Zhu JK. Altered ABA, proline and hydrogen peroxide in an Arabidopsis glutamate: glyoxylate aminotransferase mutant. Plant Mol Biol. 2007;64:205–217. doi: 10.1007/s11103-007-9145-z. [DOI] [PubMed] [Google Scholar]
- 135.Wanduragala S. Sanyal N. Liang X. Becker DF. Purification and characterization of Put1p from Saccharomyces cerevisiae. Arch Biochem Biophys. 2010;498:136–142. doi: 10.1016/j.abb.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Weltmeier F. Ehlert A. Mayer CS. Dietrich K. Wang X. Schutze K. Alonso R. Harter K. Vicente-Carbajosa J. Droge-Laser W. Combinatorial control of Arabidopsis proline dehydrogenase transcription by specific heterodimerisation of bZIP transcription factors. EMBO J. 2006;25:3133–3143. doi: 10.1038/sj.emboj.7601206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.White TA. Krishnan N. Becker DF. Tanner JJ. Structure and kinetics of monofunctional proline dehydrogenase from Thermus thermophilus. J Biol Chem. 2007;282:14316–14327. doi: 10.1074/jbc.M700912200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Williamson CL. Slocum RD. Molecular cloning and evidence for osmoregulation of the delta 1-pyrroline-5-carboxylate reductase (proC) gene in pea (Pisum sativum L.) Plant Physiol. 1992;100:1464–1470. doi: 10.1104/pp.100.3.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol. 2008;4:278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 140.Withers LA. King PJ. Proline: A novel cryoprotectant for the freeze preservation of cultured cells of Zea mays L. Plant Physiol. 1979;64:675–678. doi: 10.1104/pp.64.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wondrak GT. Jacobson MK. Jacobson EL. Identification of quenchers of photoexcited states as novel agents for skin photoprotection. J Pharmacol Exp Ther. 2005;312:482–491. doi: 10.1124/jpet.104.075101. [DOI] [PubMed] [Google Scholar]
- 142.Wu G. Bazer FW. Datta S. Johnson GA. Li P. Satterfield MC. Spencer TE. Proline metabolism in the conceptus: implications for fetal growth and development. Amino Acids. 2008;35:691–702. doi: 10.1007/s00726-008-0052-7. [DOI] [PubMed] [Google Scholar]
- 143.Wu P. Bolen DW. Osmolyte-induced protein folding free energy changes. Proteins. 2006;63:290–296. doi: 10.1002/prot.20868. [DOI] [PubMed] [Google Scholar]
- 144.Xu J. Yin H. Li X. Protective effects of proline against cadmium toxicity in micropropagated hyperaccumulator, Solanum nigrum L. Plant Cell Rep. 2009;28:325–333. doi: 10.1007/s00299-008-0643-5. [DOI] [PubMed] [Google Scholar]
- 145.Yancey PH. Clark ME. Hand SC. Bowlus RD. Somero GN. Living with water stress: evolution of osmolyte systems. Science. 1982;217:1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- 146.Yang SL. Lan SS. Gong M. Hydrogen peroxide-induced proline and metabolic pathway of its accumulation in maize seedlings. J Plant Physiol. 2009;166:1694–1699. doi: 10.1016/j.jplph.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 147.Yang Y. Zhang Y. Wei X. You J. Wang W. Lu J. Shi R. Comparative antioxidative responses and proline metabolism in two wheat cultivars under short term lead stress. Ecotoxicol Environ Saf. 2011;74:733–740. doi: 10.1016/j.ecoenv.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 148.Yoo JH. Park CY. Kim JC. Heo WD. Cheong MS. Park HC. Kim MC. Moon BC. Choi MS. Kang YH. Lee JH. Kim HS. Lee SM. Yoon HW. Lim CO. Yun DJ. Lee SY. Chung WS. Cho MJ. Direct interaction of a divergent CaM isoform and the transcription factor, MYB2, enhances salt tolerance in Arabidopsis. J Biol Chem. 2005;280:3697–3706. doi: 10.1074/jbc.M408237200. [DOI] [PubMed] [Google Scholar]
- 149.Yoon KA. Nakamura Y. Arakawa H. Identification of ALDH4 as a p53-inducible gene and its protective role in cellular stresses. J Hum Genet. 2004;49:134–140. doi: 10.1007/s10038-003-0122-3. [DOI] [PubMed] [Google Scholar]
- 150.Yoshiba Y. Kiyosue T. Katagiri T. Ueda H. Mizoguchi T. Yamaguchishinozaki K. Wada K. Harada Y. Shinozaki K. Correlation between the induction of a gene for delta(1)-pyrroline-5-carboxylate synthetase and the accumulation of proline in Arabidopsis thaliana under osmotic-stress. Plant J. 1995;7:751–760. doi: 10.1046/j.1365-313x.1995.07050751.x. [DOI] [PubMed] [Google Scholar]
- 151.Yoshiba Y. Kiyosue T. Nakashima K. Yamaguchi-Shinozaki K. Shinozaki K. Regulation of levels of proline as an osmolyte in plants under water stress. Plant Cell Physiol. 1997;38:1095–1102. doi: 10.1093/oxfordjournals.pcp.a029093. [DOI] [PubMed] [Google Scholar]
- 152.Young RH. Martin RL. Feriozi D. Brewer D. Kayser R. On the mechanism of quenching of singlet oxygen by amines-III. Evidence for a charge-transfer-like complex. Photochem Photobiol. 1973;17:233–244. [Google Scholar]
- 153.Zarse K. Schmeisser S. Groth M. Priebe S. Beuster G. Kuhlow D. Guthke R. Platzer M. Kahn CR. Ristow M. Impaired insulin/IGF1 signaling extends life span by promoting mitochondrial L-proline catabolism to induce a transient ROS signal. Cell Metab. 2012;15:451–465. doi: 10.1016/j.cmet.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang CS. Lu Q. Verma DP. Removal of feedback inhibition of delta 1-pyrroline-5-carboxylate synthetase, a bifunctional enzyme catalyzing the first two steps of proline biosynthesis in plants. J Biol Chem. 1995;270:20491–20496. doi: 10.1074/jbc.270.35.20491. [DOI] [PubMed] [Google Scholar]
- 155.Zhang W. Zhou Y. Becker DF. Regulation of PutA-membrane associations by flavin adenine dinucleotide reduction. Biochemistry. 2004;43:13165–13174. doi: 10.1021/bi048596g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zou W. Liu X. Yue P. Khuri FR. Sun SY. PPARgamma ligands enhance TRAIL-induced apoptosis through DR5 upregulation and c-FLIP downregulation in human lung cancer cells. Cancer Biol Ther. 2007;6:99–106. doi: 10.4161/cbt.6.1.3555. [DOI] [PubMed] [Google Scholar]