Abstract
Significance: Ascorbate, this multifaceted small molecular weight carbohydrate derivative, plays important roles in a range of cellular processes in plant cells, from the regulation of cell cycle, through cell expansion and senescence. Beyond these physiological functions, ascorbate has a critical role in responses to abiotic stresses, such as high light, high salinity, or drought. The biosynthesis, recycling, and intracellular transport are important elements of the balancing of ascorbate level to the always-changing conditions and demands. Recent Advances: A bidirectional tight relationship was described between ascorbate biosynthesis and the mitochondrial electron transfer chain (mETC), since L-galactono-1,4-lactone dehydrogenase (GLDH), the enzyme catalyzing the ultimate step of ascorbate biosynthesis, uses oxidized cytochrome c as the only electron acceptor and has a role in the assembly of Complex I. A similar bidirectional relationship was revealed between the photosynthetic apparatus and ascorbate biosynthesis since the electron flux through the photosynthetic ETC affects the biosynthesis of ascorbate and the level of ascorbate could affect photosynthesis. Critical Issues: The details of this regulatory network of photosynthetic electron transfer, respiratory electron transfer, and ascorbate biosynthesis are still not clear, as are the potential regulatory role and the regulation of intracellular ascorbate transport and fluxes. Future Directions: The elucidation of the role of ascorbate as an important element of the network of photosynthetic, respiratory ETC and tricarboxylic acid cycle will contribute to understanding plant cell responses to different stress conditions. Antioxid. Redox Signal. 19, 1036–1044.
Introduction
Ascorbate. The molecule, that raises new issues and supplies new findings continuously. Our knowledge about its conventional but crucial role in antioxidant defense, as well as in stress protection has deepened (14, 47). Furthermore, the cellular and physiological role of the protagonist of this review has notably broadened in the last two decades. This repertoire includes functions in nearly every aspect of plant biology from mitosis and cell expansion (48), through programmed cell death (15), as well as protection against environmental stresses, including ozone, UV radiation (14), high temperatures (26), and high light intensity (35) to growth (40), hormone responses, flowering, senescence (5), and defense against pathogens (39).
Probably the paramount advances were the discovery of the link between the mitochondrial electron transport chain and ascorbate biosynthesis (7, 34, 41, 43), the regulatory role of photosynthetic electron transfer to ascorbate biosynthesis and accumulation (8, 55) and the possible role for ascorbate in coordinating the rates of respiration, the tricarboxylic acid (TCA) cycle and photosynthesis (38, 52, 16, 49).
Ascorbate. The molecule, a (plant) biologist cannot lose interest in.
Plant Mitochondrion: Ascorbate Biosynthesis
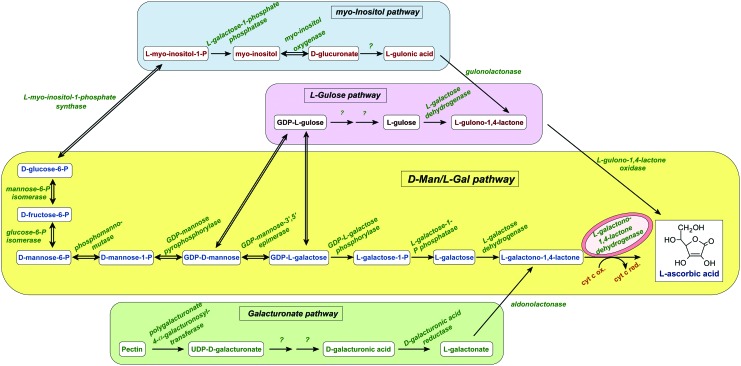
The first complete pathway of ascorbate biosynthesis in plant cells, the so called D-Man/L-Gal or Smirnoff-Wheeler pathway, was only unraveled in 1998 (53) (Fig. 1). In the mean time, all the genes and their products, involved in the pathway in Arabidopsis thaliana have been fully characterized (30). Shortly after the description of the Smirnoff-Wheeler pathway, the existence of alternative ascorbate biosynthetic pathways became apparent with the cloning and characterization of other ascorbate biosynthetic enzymes. It was shown that GDP-mannose-3′,5′-epimerase catalyzes at least two distinct epimerization reactions and releases GDP-L-gulose (GDP-L-Gul) in addition to the well known, Smirnoff-Wheeler pathway intermediate, GDP-L-galactose (GDP-L-Gal) (54). GDP-L-Gul can be channeled directly into the proposed L-Gulose branch of the biosynthetic pathway, in which the last step is the oxidation of L-gulono-1,4-lactone to ascorbate (54) (Fig. 1). The possible contribution of myo-inositol as a precursor of ascorbate biosynthesis via the formation of UDP-glucuronate by myo-inositol oxidase was also suggested in Arabidopsis (31). This route—similar to the L-Gulose branch one—provides L-gulono-1,4-lactone (Fig. 1). Finally, evidence was provided that D-galacturonic acid, a principal component of cell wall pectins, is produced by D-galacturonate reductase and contributes to the ascorbate pool in strawberry fruit (1). The latter route—similar to the classic D-Man/L-Gal pathway—provides L-galactono-1,4-lactone to the biosynthetic pathway (Fig. 1). Mutants in the D-Man/L-Gal pathway, for example, vtc-1 (an Arabidopsis mutant with defective GDP-mannose pyrophosphorylase activity) and vtc-2 (an Arabidopsis mutant with defective GDP-L-Gal phosphorylase activity), demonstrated that ascorbate derived from alternative pathways, could not compensate for the low ascorbate levels (13, 17).
FIG. 1.
Biosynthetic pathways to L-ascorbate in higher plants. The major ascorbate biosynthetic pathway is the D-Man/L-Gal or Smirnoff-Wheeler pathway (yellow). The minor L-gulose pathway is branched at GDP-Man 3′,5′-epimerase (purple). The last step of this alternative route is the oxidation of L-gulono-1,4-lactone to ascorbate. The myo-Inositol branch (blue); also provides L-gulono-1,4-lactone for the biosynthesis of ascorbate via the formation of UDP-glucuronate by myo-Inositol oxidase. The D-galacturonic acid route (green), uses D-galacturonic acid, a principal component of cell wall and similar to the classical D-Man/L-Gal pathway provides galactono-1,4-lactone via the formation of L-galactonate by D-galacturonate reductase. Question marks indicate reactions where the gene and the specific enzyme have not been identified yet. The conversion of L-galactono-1,4-lactone to ascorbate by GLDH coupled to mitochondrial ETC is shown in red (mitochondria-like shape) circle. ETC, electron transfer chain; GLDH, L-galactono-1,4-lactone dehydrogenase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Both the D-Man/L-Gal and the Galacturonate routes converge to L-galactono-1,4-lactone. The oxidation of L-galactono-1,4-lactone to ascorbate was attributed to mitochondrial L-galactono-1,4-lactone dehydrogenase (GLDH) already more than 50 years ago (32). However, the intimate link between the mitochondrial electron transfer chain (mETC) and the activity of this enzyme became clear only more recently (7). Subfractionation of mitochondria demonstrated that GLDH is largely associated with the inner-membrane fraction (7). Also, the latency of the enzyme is similar to that of cytochrome c oxidase (CCO), suggesting that the GLDH localization is similar to CCO. GLDH delivers electrons to oxidized cytochrome c (7, 28). The absolute requirement of the enzyme for oxidized cytochrome was proved by the stimulatory effect of antimycin A, an inhibitor of cytochrome c reductase (7) and by the observation of enhanced GLDH activity in the Arabidopsis ppr40-1 mutant, which has a block of electron flow at complex III (60). Therefore, L-galactono-1,4-lactone can be defined as a respiratory substrate in plants (7, 34) (Fig. 2).
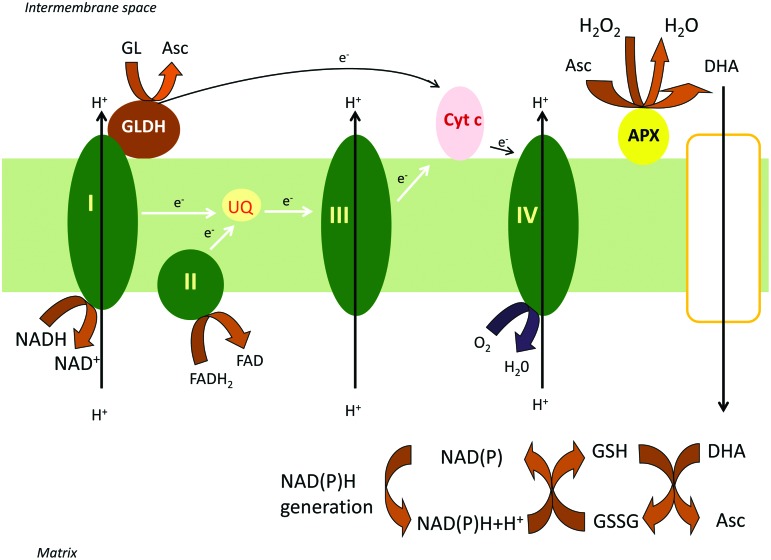
FIG. 2.
Ascorbate generation and regeneration in plant mitochondria. GLDH, embedded in the respiratory ETC, delivers electrons to oxidized cytochrome c by oxidizing GL to ascorbate. The enzyme shows absolute requirement for oxidized cytochrome c; hence, L-galactono-1,4-lactone can be defined as an alternative respiratory substrate in plants. DHA is generated in the IMS by the action of APX. DHA can reach the matrix by the aid of a GLUT-like transporter, where a glutathione-dependent ascorbate regenerating system (Foyer-Halliwell-Asada cycle) operates to reduce it to ascorbate. The supply of reductants is ensured in the form of NADH and NADPH to fuel these reactions from the TCA cycle. APX, ascorbate peroxidase; DHA, dehydroascorbate; IMS, intermembrane space; TCA, tricarboxylic acid. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Although three membrane spanning regions were predicted previously (7), no transmembrane regions were found in the mature AtGLDH in a recent study (28). A tight link between complex I and GLDH was predicted by the fact, that rotenone, a specific inhibitor of electron flow through complex I, inhibited ascorbate biosynthesis (34). Indeed, GLDH was present in the minor higher-mobility form (850 kDa) of complex I (22, 41). The 58 kDa GLDH protein did not attach to the 1000 kDa holo complex, but only to a slightly smaller version of complex I (34, 41). Interestingly, complex I and its associated NADH dehydrogenase activity were lacking in leaf and root mitochondria of gldh mutant Arabidopsis, although the other respiratory complexes were unaffected. This suggests that GLDH might represent an assembly factor in complex I biogenesis (41). Consistent with the reduction of cytochrome c, GLDH is believed to be located in the intermembrane space (IMS), associated with the membrane arm of complex I (25). A recent study described two new low abundance GLDH containing protein complexes with molecular mass of 470 and 420 kDa in Arabidopsis mitochondria (43). These complexes represent parts of the membrane arm of complex I and assembly intermediates. The authors suggest that GLDH binds to the 420 and 470 kDa complex I assembly intermediates, which at a later stage form the 850 kDa intermediate. Formation of the 1000 kDa holo complex I is preceded by detachment of GLDH (43).
The dual role of GLDH in ascorbate biosynthesis and in the assembly of complex I is indisputable; however, the connection between these two processes is still a mystery. GLDH belongs to the vanillyl-alcohol oxidase flavoprotein family, including L-gulono-lactone oxidase. The latter enzyme is responsible for ascorbate synthesis in several animal groups, generating H2O2 as a by-product. However, in contrast to related aldonolactone oxidoreductases, GLDH contains an alanine (Ala-113), instead of the conserved glycine or proline, at an essential position near the isoalloxazine flavin-ring of the cofactor. This substitution possibly prevents molecular oxygen from reaching the reduced flavin, avoiding the generation of superoxide or H2O2 (27, 28). GLDH contains noncovalently bound FAD, as it also lacks the conserved histidine involved in covalent FAD binding (28). The dehydrogenase activity of the enzyme—instead of a possible oxidase activity—may contribute to the reduction of the H2O2 load of mitochondria and avoid the possible inactivation of GLDH by the oxidation of its redox sensitive thiol (Cys-340) (29).
In addition to L-galactono-1,4-lactone, recombinant Arabidopsis GLDH, also oxidizes the isomer, L-gulono-1,4-lactone, at significant rates, although with a much higher Km value (0.17 mM vs. 13.1 mM) (28). Hence, it is possible that GLDH also plays a role in the oxidation of L-gulono-1,4-lactone, in an alternative, minor, ascorbate synthesizing pathway (Fig. 1). [Although L-gulono-lactone oxidase activities have also been described in plants (33).]
Tobacco plants overexpressing GLDH do not have elevated ascorbate content (23), and silencing of GLDH in tomato did not reduce ascorbate levels or its redox status, despite decreased mRNA, protein levels, and enzyme activity (2). These observations strongly suggest that the mitochondrial GLDH-catalyzed step is not rate limiting in ascorbate biosynthesis. [The GDP-L-Gal phosphorylase (encoded by vtc2 and vtc5) catalyzed conversion of GDP-L-Gal to L-galactose-1-P (Fig. 1) is supposed to be the rate-limiting step of ascorbate synthesis (17, 47).] The metabolic changes that were observed in GLDH-silenced plants (2) are probably explained by decreased functioning of the mitochondrial holo complex I in the absence of GLDH (41).
Plant Mitochondrion: Ascorbate Regeneration
The role of ascorbate recycling (i.e., reduction of its oxidized forms) in the maintenance of a sufficient level of ascorbate is generally accepted.
Chloroplasts are the major source of reactive oxygen species (ROS) in plant cells and ascorbate plays multiple roles in their elimination (4, 20, 35, 36, 47). It is therefore perhaps not surprising that enzymes for the reduction of oxidized forms of ascorbate, that is, monodehydroascorbate (MDHA) and dehydroascorbate (DHA), using GSH and NADPH (from photosystem I [PSI]) were first described in chloroplasts (i.e., the ascorbate-glutathione or Foyer-Halliwell-Asada cycle) (4, 20). Although the mitochondrial ROS production is considerably less than the ROS production in illuminated chloroplasts or in peroxisomes, in the dark, or in nongreen tissues, mitochondria are major sources of ROS (47).
After scattered reports on presence in mitochondria of the individual elements of the ascorbate-glutathione cycle (3, 18), all four enzymes, ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), and glutathione reductase (GR), were described in mitochondria and peroxisomes (24). The mitochondrial location for these proteins was confirmed by green fluorescent protein fusions (12). Furthermore, a dual import assay showed targeting of APX, MDHAR, and GR gene products to mitochondria and chloroplasts, while a putative DHAR was only imported into mitochondria (12). The subcellular localization and the topology of the enzymes were also determined by preparing submitochondrial fractions and performing enzyme latency measurements. APX and MDHAR activities were associated mainly with the inner membrane fraction. The low latency suggested a predominantly IMS side localization for APX, while the high latency of MDHAR suggested a predominantly matrix side localization (Fig. 2.) (24). GR and DHAR activities were prominent in the matrix, but could also be found in other submitochondrial fractions, suggesting they are partitioned between compartments and can be partially membrane associated (12). The predominant matrix location of MDHAR, DHAR, and GR is presumably related to the supply of reductants NADH and NADPH to fuel these reactions (from the TCA cycle). In this way the MDHA and DHA generated by the action of APX in plant mitochondria must pass through the inner mitochondrial membrane to the matrix for reduction back to ascorbate.
Hence the DHA transporter—located in the inner mitochondrial membrane—is an important member of the ascorbate regeneration machinery (45). Interestingly, uptake of glucose by tobacco mitochondria was also observed in the same study. The strong inhibition of DHA and glucose uptake by the known GLUT inhibitor genistein and the inhibitory effect of glucose on DHA transport suggested that the two molecules could be ligands for the same transporter. The uptake of both compounds is independent of mitochondrial respiration. Since the uptake of the reduced form, ascorbate—in contrast to the DHA transport—showed a rather low affinity it cannot be excluded that only DHA is taken up by these organelles. In that case, the intramitochondrial appearance of ascorbate may be merely due to the uptake and concomitant reduction of the oxidized form (45). Although components of the ascorbate-glutathione cycle have been demonstrated in mitochondria, GSH is certainly not the only possible electron donor for DHA reduction in these organelles. Results obtained from animal mitochondria indicate a role for the mETC in the reduction of DHA (46). Indeed, ascorbate generation (upon DHA addition) could be stimulated by the complex II substrate succinate. The involvement of complex II in DHA reduction was further supported by the inhibitory effect of the succinate dehydrogenase competitive inhibitor, malonate. Glucose, a competitive inhibitor of mitochondrial DHA uptake, interrupted the regeneration cycle and decreased ascorbate production in the absence and in the presence of succinate. At the same time, the role of complex I and III could be ruled out, since neither the substrates nor the inhibitors of these complexes had any effect on ascorbate generation (46).
In addition to succinate, potassium cyanide (KCN) showed a strong positive effect on ascorbate accumulation upon DHA addition, presumably due to the decreased ascorbate oxidation. The strong inhibitory effect of KCN on ascorbate oxidation could be verified by electron paramagnetic resonance measurements (46). The assumption was strengthened in Arabidopsis ppr40-1 mutant. Impaired electron transport through Complex III in the mitochondria isolated from ppr40-1 mutant is accompanied by enhanced CCO activity and ascorbate consumption (59). CCO activity and ascorbate consumption were increased in same manner. Furthermore ascorbate consumption could be inhibited also in this case by KCN treatment (59). These observations support that ascorbate can act as an alternative electron donor in vivo to sustain oxidative respiration in states with impaired electron flow through complex III (Fig. 3).
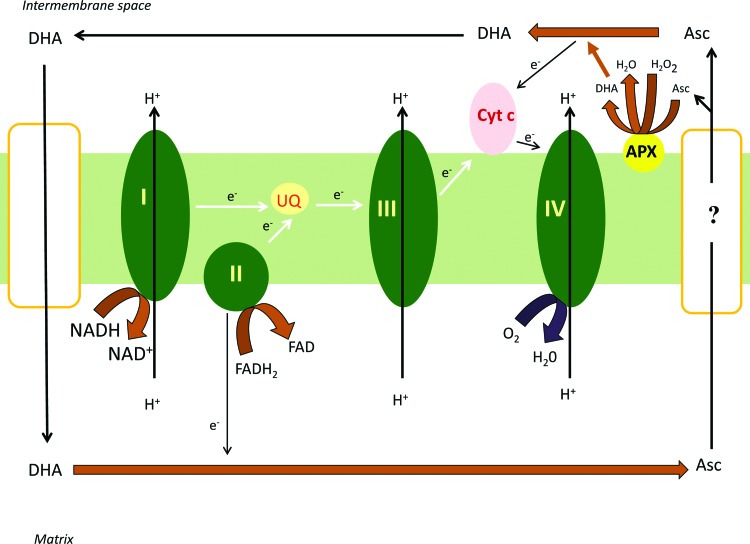
FIG. 3.
The complex II coupled reduction of DHA–a possible bypass of complex III by function of the Asc/DHA redox couple. DHA formed in the IMS, due to the activity of APX and cytochrome c oxidase, crosses the inner membrane with the mediation of a GLUT-like transporter. DHA is reduced to ascorbate in the matrix at complex II with exposure to the inner surface of the inner mitochondrial membrane (or by the ascorbate-glutathione cycle, see figure). A portion of ascorbate regenerated in the matrix leave the mitochondria. The efflux of ascorbate has not been characterized yet at molecular level. This electron route represented by the DHA/ascorbate redox couple may also gives survival value in case of complex III damage by taking up electrons at complex II (by the reduction of DHA to ascorbate) and providing electrons through a bypass way to complex IV (by the oxidation of ascorbate to DHA). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Based on these observations, the following model can be proposed, DHA, formed in the IMS due to the activity of APX, crosses the inner membrane through a glucose transporter. DHA is subsequently reduced to ascorbate in the mitochondrial matrix via the ascorbate-glutathione cycle or at complex II. A fraction of ascorbate can leave the mitochondrial matrix by a presently unidentified mechanism. Ascorbate in the IMS can be oxidized by supplying electrons to complex IV (Fig. 3). The less well characterized step of this hypothesis is the efflux of ascorbate from the matrix. This process is potentially rate limiting, since the ascorbate produced in the mitochondria appeared in the incubation medium with a significant delay compared to the matrix (Szarka A, unpublished). This electron route represented by the DHA/ascorbate redox couple may also provide an alterntive route in case of complex III damage by taking up electrons at complex II (by the reduction of DHA to ascorbate) and providing electrons through a bypass to complex IV (by the oxidation of ascorbate to DHA).
In conclusion, the plant mETC, and mitochondria, play an important role not only in the synthesis of ascorbate but also in its regeneration.
Ascorbate Metabolism and the Chloroplast: Ascorbate as a Connection Between Mitochondrial Respiration and Photosynthesis
Ascorbate levels are dramatically increased in response to light (58), which is undoubtedly related to its multiple roles in photoprotection. On one hand, ascorbate has an important role in nonphotochemical quenching, since violaxanthin de-epoxidase uses ascorbate as its reductant in vitro and the availability of ascorbate could also limit the activity of the enzyme in vivo in vtc-2 mutants (35). On the other hand, ascorbate is also a key component of the water-water cycle in chloroplast (4) (Fig. 4).
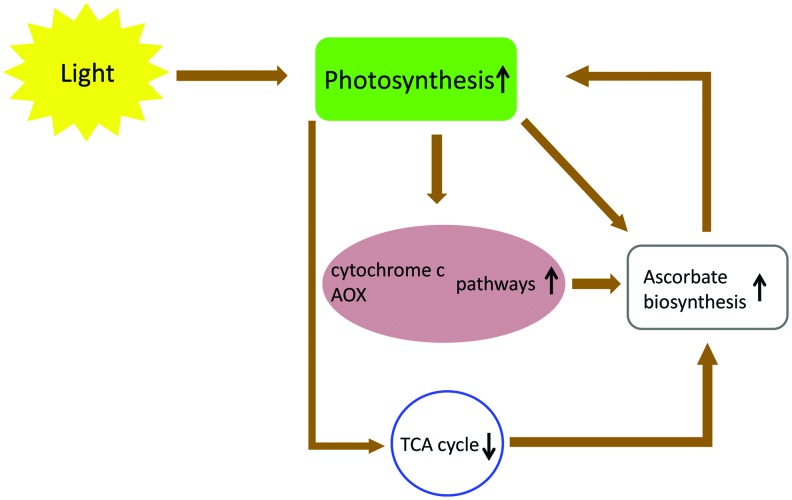
FIG. 4.
The photosynthesis - respiration - TCA-cycle - ascorbate network. The electron flux through the photosynthetic ETC enhances the mitochondrial cytochrome c, AOX pathways, and the biosynthesis of ascorbate. Furthermore, the activity of AOX itself enhances the biosynthetic rate of ascorbate. Since ascorbate is synthesized tightly coupled to the mitochondrial ETC, the oxidation of L-galactono-1,4-lactone to ascorbate provides electrons to complex IV in the case of the inhibition of TCA cycle. The reductant overload of the respiration can be reduced by light inhibition of TCA cycle and TCA cycle-linked respiration during light conditions. The inhibition of TCA cycle (by genetic approaches) accompanies by the dramatic increase in ascorbate levels. Ascorbate can elevate the intensity of photosynthesis by its multiple roles in photoprotection. Any changes in either of these elements of this network influence the others. AOX, alternative oxidase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
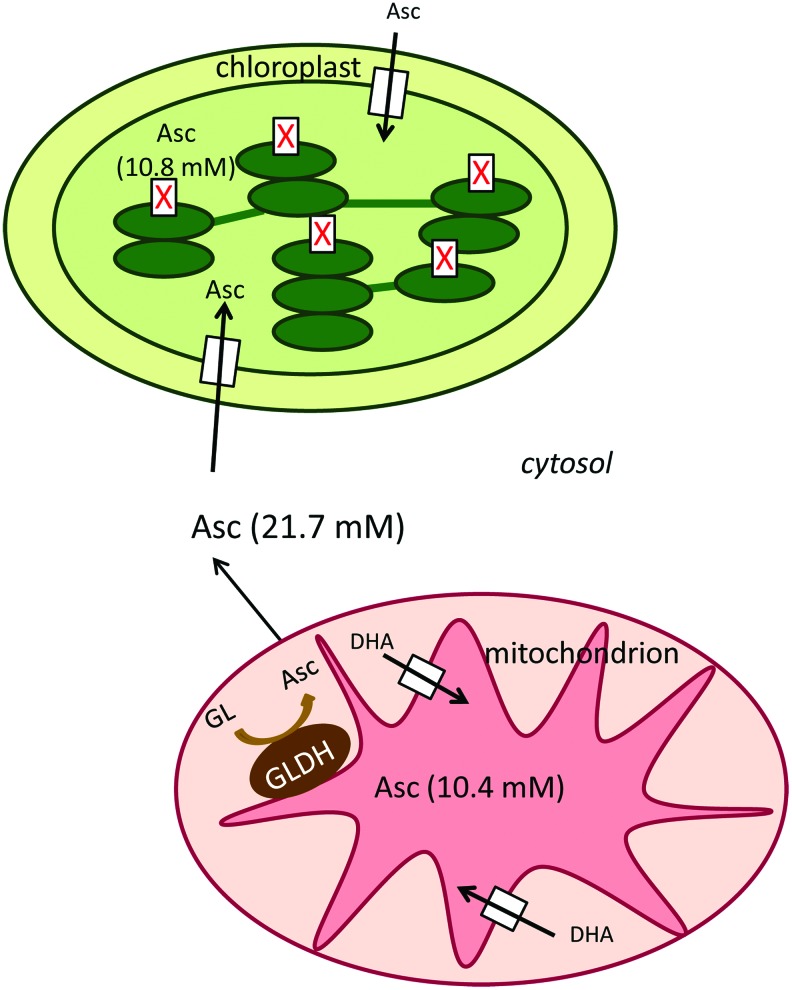
Since the ultimate step of ascorbate biosynthesis occurs in the mitochondria ascorbate must be transported into the chloroplasts. The chloroplast envelope contains an ascorbate translocator (9) (Fig. 5). In intact spinach chloroplasts ascorbate transport occurs through facilitated diffusion and has a relatively low affinity (Km∼20 mM) (9). In contrast, thylakoid membranes appear to have no transport system for ascorbate and uptake may result from diffusion alone (21). Recently, the subcellular distribution of ascorbate was determined by ascorbate-specific immunogold labeling (58). In both Arabidopsis and Nicotiana, highest levels of ascorbate were found in the cytosol (21.7 mM), whereas mitochondria (10.4 mM) and chloroplasts (10.8 mM) contained intermediate levels (The concentrations refer to Arabidopsis.) (58). Subcellular concentrations strengthened the idea that ascorbate uptake occurs by facilitated diffusion through the chloroplast envelope. Consistent with the transport experiments (21), ascorbate was only detected in the chloroplast stroma and mitochondrial matrix, but not within the lumen of thylakoids or cristae in nonstressed plants, ascorbate-specific labeling could be found in the lumen of thylakoids only during high light conditions (58) (Fig. 5).
FIG. 5.
The ascorbate transporters and ascorbate fluxes of mitochondria and chloroplast. The ultimate step of ascorbate biosynthesis is localized in the mitochondria. Hence, ascorbate must be transported into the other compartments of the plant cell. The chloroplast envelope contains a low affinity (Km∼20 mM) ascorbate translocator, which mediates the facilitated diffusion of ascorbate from the cytosol to the stroma. The thylakoid membranes appear to have no transport system for ascorbate. This system is supported by the recently determined intracellular distribution of ascorbate. In the investigated plants, highest levels of ascorbate specific labeling were found in the cytosol (21.7 mM), whereas mitochondria (10.4 mM) and chloroplasts (10.8 mM) contained intermediate levels. In concordance with the transport experiments within chloroplasts and mitochondria, ascorbate was only detected in the stroma and matrix, respectively, but not within the lumen of thylakoids or cristae (58). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The tight interaction of photosynthetic and respiratory ETCs is a key feature of plant cells (see for reviews Refs. 36, 37, 56). During high light conditions, NADP-malate dehydrogenase, the key enzyme of the malate valve, converts oxaloacetate to malate using NADPH, facilitating the regeneration of the NADP+ pool in the chloroplast. Malate then can be exported to the cytosol via the malate/oxaloacetate shuttle (51). The exported malate is oxidized by NAD-malate dehydrogenases (MDH) of the cytosol, peroxisomes, and mitochondria generating NADH. Finally, NADH may be consumed by mitochondrial respiration (42). During photorespiration a large amount of NADH is also produced by the glycine decarboxylase complex (GDC) in the mitochondria. Although a part of the generated NADH is transported to the peroxisomes through the malate/oxaloacetate shuttle of mitochondria, the rest of the photorespiratory NADH is oxidized by the respiratory chain (36). The induction of the leaf alternative oxidase (AOX) and internal-type II NAD(P)H dehydrogenase (NDin) genes by light (19, 57) presume that mitochondrial respiration and especially the nonphosphorylating pathways can be important elements of the dissipating system of reductants in the illuminated leaf (Fig. 4). The suppressed rate of photosynthesis in leaves of uncoupling protein-1 (UCP1)-deficient Arabidopsis suggests that UCP also allows for more efficient NADH consumption and can be a part of this dissipation system (44).
Increased capacities of the cytochrome c and AOX pathways, in Arabidopsis leaves grown under high light, were accompanied by an increase of the capacity for ascorbate synthesis and GLDH activities (8) (Fig. 4). However, high light had no detectable effects on GLDH transcript levels or on day/night variations in protein abundance (8). Similarly, leaves of AOX1a overexpressing lines also had higher rates of ascorbate production and accumulation, without affecting GLDH activity (Fig. 4). Elevated ascorbate production in these lines may be enhanced by an increased availability of oxidized cytochrome c, which is the electron acceptor in the last step of ascorbate synthesis (see above). On the other hand, an increased rate of the cytochrome pathway and an increased amount of cytochrome c, in high light, may also contribute to higher CCO activities and enhanced ascorbate synthesis (8) (Figs. 2, 4, and 5).
In contrast to the results of Bartoli et al. (8), the transcript level of GLDH (and GDP-mannose pyrophosphorylase, L-galactose 1-P phosphatase, and VTC2) changed in parallel with changes in the leaf ascorbate pool during dark and light periods in the study of Yabuta et al. (55). Overall, the correlation between the GLDH expression and activity, and foliar ascorbate concentrations seems to be quite loose. No significant changes were observed in the transcript levels of GLDH in tobacco leaves during the day-night cycles, whereas foliar ascorbate increased nearly fourfold in the light (40), and no correlation was found in the abundance and activity of GLDH and foliar ascorbate levels in a range of plant species (6). At the same time, an approximately two-fold increase of the GLDH transcript level was observed in young Arabidopsis rosette leaves during the daytime (50).
Since AOX is induced by light, and an AOX overexpression line showed elevated ascorbate production, the effects of photosynthesis inhibitors on ascorbate levels in leaves, was investigated. DCMU (3-(3,4-dichlorophenyl)-1,1-dimethylurea) and atrazine, which block electron flow to plastoquinones in PSII, mimic the effect of darkness by causing the downregulation of GLDH, and prevented the increase in leaf ascorbate (55) (Fig. 4). Unfortunately, in these experiments the transcription level and the activity of AOX were not determined. The importance of photosynthetic electron flow and of the plastoquinone redox status was further strengthened by the transfer of plants to a sucrose containing medium, which resulted in lower photosynthetic activity and leaf ascorbate levels, even under exposure to light (55). Together, these results underline the role of the redox status of the plastoquinone pool and photosynthesis itself in the control of ascorbate level in illuminated leaves.
For continuous scavenging of H2O2 generated in chloroplasts, ascorbate needs to be regenerated. With an estimated maximum production rate of H2O2 of 450 μM.s−1, and 30 mM ascorbate in chloroplasts (without regeneration), it was calculated that ascorbate would be consumed within 60 s (4). Hence, it is not surprising that the existence of an ascorbate regenerating system in chloroplasts was demonstrated earlier than its complete biosynthetic pathway (4, 12, 20). It was also shown that the capacity to regenerate ascorbate from its oxidized forms is enhanced in the high light-grown leaves, suggesting that amelioration of the recycling systems backs up the enhanced capacity for synthesis to ensure that the cellular ascorbate pool remains in the reduced state (8).
Plants show light inhibition of the TCA cycle and TCA cycle-linked respiration (52) (Fig. 4). The induction of GDC in mitochondria of illuminated leaves (during photorespiration) results in a more reduced redox status and increased adenylate phosphorylation level in the mitochondrial matrix (10). These changes cause restrictions at the pyruvate dehydrogenase complex and at other reactions within the TCA cycle. This way the reductant overload of the mETC can be reduced during light conditions. Hence, the elevated rates of photosynthesis, over a range of light intensities, could be reached by the generation of mitochondrial MDH antisense (38) or aconitase mutant (Aco1) (11) tomato plants. A dramatic increase in ascorbate could be observed in both transgenic lines (38). These observations further strengthened the link between the photosynthetic electron flow and ascorbate biosynthesis, accumulation (Fig. 4). However, a recent report of Tomaz et al. (52) reduces the clarity of this picture somewhat. These authors found higher mitochondrial respiratory rates in the double mutant mitochondrial MDH Arabidopsis line (52). Intriguingly, the amount of GLDH was lower, but the ascorbate content was doubled in the mitochondrial MDH deficient plant compared to the wild type (52).
The photoprotective role of ascorbate can be shown more pronounced if the electron sink of mitochondria is plugged in, thus the elimination of reductants is diminished. Both Antimycin A (inhibitor of the cytochrome c pathway) and salicylhydroxamic acid (SHAM) (inhibitor of the AOX pathway) caused marked reduction in photosynthetic carbon assimilation with concomitant rise in total cellular ROS. Furthermore, the restriction of mitochondrial electron transport decreased the ratio of the reduced form of both ascorbate and GSH (16). Accordingly, the application of these respiratory inhibitors in vtc-1 mutant Arabidopsis caused an extreme level of photoinhibition (83%) (49). However, the photoinhibition—even in the presence of antimycin A or SHAM—could be decreased significantly by the treatment with L-galactono-1,4-lactone. As was expected, the protective effect of L-galactono-1,4-lactone was maximal in vtc-1 mutants in the presence of SHAM (49).
Concluding Remarks
Ascorbate was initially considered to be a simple antioxidant or cofactor for hydroxylation reactions. However, this view has been radically changed by observations of the last two decades.
The enzyme, catalyzing the ultimate step of ascorbate biosynthesis, is deeply embedded into the mETC (Fig. 2). This tight relationship seems to be bidirectional, since GLDH has a role in the assembly of Complex I (41, 43). It is still an enigma whether the ascorbate generating function of the GLDH protein is a requirement for its assembly function. Despite this question, it is more than interesting that the electron flow through complex I has a regulatory role on the ascorbate biosynthesis capacity (34) and the enzyme responsible for ascorbate biosynthesis has a role in the assembly of complex I holo complex (43). It may have a role in balancing the electron flows under different (environmental) circumstances.
It became clear that the regeneration of ascorbate is as important in the maintenance of its level (redox status) as its biosynthesis. Furthermore, the intensity of the regeneration (beside the biosynthesis) is also influenced by the changing (redox) environment both in the mitochondria (60) and in the chloroplast (8) or rather its regulation seemed to be coupled in these two compartments (12). However, the contribution of mitochondrial recycling of ascorbate to the whole cell ascorbate pool is still a matter of future research.
Ascorbate can be an important element of the photosynthetic ETC, mETC, and TCA cycle regulatory network since (i) the level of ascorbate can influence the intensity of photosynthesis (35, 38, 49); (ii) the electron flux through the photosynthetic ETC influences the biosynthesis of ascorbate (55); (iii) ascorbate is synthesized tightly coupled to the mETC by providing electrons to complex IV (7) in case of the inhibition of TCA cycle (38) and (iv) the activity of AOX also influences the biosynthetic rate of ascorbate (8). Any changes in either of these elements of this network influence the others (Fig. 4). However, the details of this transmitter function of ascorbate between the plastidic and mitochondrial functions so far remains a mystery and needs further investigations.
Abbreviations Used
- AOX
alternative oxidase
- APX
ascorbate peroxidase
- CCO
cytochrome c oxidase
- DHA
dehydroascorbate
- DHAR
dehydroascorbate reductase
- GDC
glycine decarboxylase complex
- GDP-L-Gal
GDP-L-galactose
- GDP-L-Gul
GDP-L-gulose
- GLDH
L-galactono-1,4-lactone dehydrogenase
- GR
glutathione reductase
- IMS
intermembrane space
- KCN
potassium cyanide
- MDH
malate dehydrogenase
- MDHA
monodehydroascorbate
- MDHAR
monodehydroascorbate reductase
- mETC
mitochondrial electron transfer chain
- NDin
internal type II NAD(P)H dehydrogenases
- PSI
photosystem I
- ROS
reactive oxygen species
- SHAM
salicylhydroxamic acid
- TCA
tricarboxylic acid
- UCP
uncoupling protein
Acknowledgments
This work was supported by the Hungarian Scientific Research Fund (77826). This work is connected to the scientific program of the “Development of quality-oriented and harmonized R+D+I strategy and functional model at BME” project. The project was supported by the New Széchenyi Plan (Project ID: TÁMOP-4.2.1/B-09/1/KMR-2010-0002 and TÁMOP-4.2.1./B-09/1/KMR-2010-0001). Thanks are due to the János Bolyai Research Scholarship of the Hungarian Academy of Sciences for supporting A. Sz. This work was also supported by the Flemish Fund for Scientific Research, FWO (grant G.0118.07N to H.A.).
References
- 1.Agius F. González-Lamothe R. Caballero JL. Muñoz-Blanco J. Botella MA. Valpuesta V. Engineering increased vitamin C levels in plants by overexpression of a D-galacturonic acid reductase. Nat Biotechnol. 2003;21:177–181. doi: 10.1038/nbt777. [DOI] [PubMed] [Google Scholar]
- 2.Alhagdow M. Mounet F. Gilbert L. Nunes-Nesi A. Garcia V. Just D. Petit J. Beauvoit B. Fernie AR. Rothan C. Baldet P. Silencing of the mitochondrial ascorbate synthesizing enzyme L-galactono-1,4-lactone dehydrogenase affects plant and fruit development in tomato. Plant Physiol. 2007;145:1408–1422. doi: 10.1104/pp.107.106500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arrigoni O. Dipierro S. Borraccino G. Ascorbate free radical reductase: a key enzyme of the ascorbic acid system. FEBS Lett. 1981;125:242–244. [Google Scholar]
- 4.Asada K. The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:601–639. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- 5.Barth C. De Tullio M. Conklin PL. The role of ascorbic acid in the control of flowering time and the onset of senescence. J Exp Bot. 2006;57:1657–1665. doi: 10.1093/jxb/erj198. [DOI] [PubMed] [Google Scholar]
- 6.Bartoli CG. Guiamet JJ. Kiddle G. Pastori G. Di Cagno R. Theodoulou FL. Foyer CH. The relationship between L-galactono-1,4-lactone dehydrogenase (GalLDH) and ascorbate content in leaves under optimal and stress conditions. Plant Cell Environ. 2005;28:1073–1081. [Google Scholar]
- 7.Bartoli CG. Pastori GM. Foyer CH. Ascorbate biosynthesis in mitochondria is linked to the electron transport chain between complexes III and IV. Plant Physiol. 2000;123:335–344. doi: 10.1104/pp.123.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartoli CG. Yu J. Gómez F. Fernández L. McIntosh L. Foyer CH. Inter-relationships between light and respiration in the control of ascorbic acid synthesis and accumulation in Arabidopsis thaliana leaves. J Exp Bot. 2006;57:1621–1631. doi: 10.1093/jxb/erl005. [DOI] [PubMed] [Google Scholar]
- 9.Beck E. Burkert A. Hofmann M. Uptake of l-ascorbate by intact spinach chloroplasts. Plant Physiol. 1983;73:41–45. doi: 10.1104/pp.73.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bykova NV. Keerberg O. Pärnik T. Bauwe H. Gardeström P. Interaction between photorespiration and respiration in transgenic potato plants with antisense reduction in glycine decarboxylase. Planta. 2005;222:130–140. doi: 10.1007/s00425-005-1505-9. [DOI] [PubMed] [Google Scholar]
- 11.Carrari F. Nunes-Nesi A. Gibon Y. Lytovchenko A. Loureiro ME. Fernie AR. Reduced expression of aconitase results in an enhanced rate of photosynthesis and marked shifts in carbon partitioning in illuminated leaves of wild species tomato. Plant Physiol. 2003;133:1322–1335. doi: 10.1104/pp.103.026716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chew O. Whelan J. Millar AH. Molecular definition of the ascorbate-glutathione cycle in Arabidopsis mitochondria reveals dual targeting of antioxidant defenses in plants. J Biol Chem. 2003;278:46869–46877. doi: 10.1074/jbc.M307525200. [DOI] [PubMed] [Google Scholar]
- 13.Conklin PL. Norris SR. Wheeler GL. Williams EH. Smirnoff N. Last RL. Genetic evidence for the role of GDP-mannose in plant ascorbic acid (vitamin C) biosynthesis. Proc Natl Acad Sci U S A. 1999;96:4198–4203. doi: 10.1073/pnas.96.7.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conklin PL. Williams EH. Last RL. Environmental stress sensitivity of an ascorbic acid-deficient Arabidopsis mutant. Proc Natl Acad Sci U S A. 1996;93:9970–9974. doi: 10.1073/pnas.93.18.9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Pinto MC. Paradiso A. Leonetti P. De Gara L. Hydrogen peroxide, nitric oxide and cytosolic ascorbate peroxidase at the crossroad between defence and cell death. Plant J. 2006;48:784–795. doi: 10.1111/j.1365-313X.2006.02919.x. [DOI] [PubMed] [Google Scholar]
- 16.Dinakar C. Abhaypratap V. Yearla SR. Raghavendra AS. Padmasree K. Importance of ROS and antioxidant system during the beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Planta. 2010;231:461–474. doi: 10.1007/s00425-009-1067-3. [DOI] [PubMed] [Google Scholar]
- 17.Dowdle J. Ishikawa T. Gatzek S. Rolinski S. Smirnoff N. Two genes in Arabidopsis thaliana encoding GDP-L-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. Plant J. 2007;52:673–689. doi: 10.1111/j.1365-313X.2007.03266.x. [DOI] [PubMed] [Google Scholar]
- 18.Edwards EA. Rawsthorne S. Mullineux PM. Subcellular distribution of multiple forms of glutathione reductase in leaves of pea (Pisum sativum L.) Planta. 1990;180:278–284. doi: 10.1007/BF00194008. [DOI] [PubMed] [Google Scholar]
- 19.Escobar MA. Franklin KA. Svensson AS. Salter MG. Whitelam G. Rasmusson AG. Light regulation of the Arabidopsis respiratory chain. Multiple discrete photoreceptor responses contribute to induction of type II NAD(P)H dehydrogenase genes. Plant Physiol. 2004;136:2710–2721. doi: 10.1104/pp.104.046698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foyer CH. Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 1976;133:21–25. doi: 10.1007/BF00386001. [DOI] [PubMed] [Google Scholar]
- 21.Foyer CH. Lelandais M. A Comparison of the relative rates of transport of ascorbate and glucose across the thylakoid, chloroplast and plasmalemma membranes of pea leaf mesophyll cells. J Plant Physiol. 1996;148:391–398. [Google Scholar]
- 22.Heazlewood JL. Howell KA. Millar AH. Mitochondrial complex I from Arabidopsis and rice: orthologs of mammalian and fungal components coupled with plant-specific subunits. Biochim Biophys Acta. 2003;1604:159–169. doi: 10.1016/s0005-2728(03)00045-8. [DOI] [PubMed] [Google Scholar]
- 23.Imai T. Niwa M. Ban Y. Hirai M. Oba K. Moriguchi T. Importance of the L-galactonolactone pool for enhancing the ascorbate content revealed by L-galactonolactone dehydrogenase-overexpressing tobacco plants. Plant Cell Tissue Organ Cult. 2009;96:105–112. [Google Scholar]
- 24.Jimenez A. Hernandez JA. Del Rio LA. Sevilla F. Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol. 1997;114:275–284. doi: 10.1104/pp.114.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klodmann J. Sunderhaus S. Nimtz M. Jänsch L. Braun HP. Internal architecture of mitochondrial complex I from Arabidopsis thaliana. Plant Cell. 2010;22:797–810. doi: 10.1105/tpc.109.073726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larkindale J. Hall JD. Knight MR. Vierling E. Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol. 2005;138:882–897. doi: 10.1104/pp.105.062257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leferink NG. Fraaije MW. Joosten HJ. Schaap PJ. Mattevi A. van Berkel WJ. Identification of a gatekeeper residue that prevents dehydrogenases from acting as oxidases. J Biol Chem. 2009;284:4392–4397. doi: 10.1074/jbc.M808202200. [DOI] [PubMed] [Google Scholar]
- 28.Leferink NG. van den Berg WA. van Berkel WJ. l-Galactono-gamma-lactone dehydrogenase from Arabidopsis thaliana, a flavoprotein involved in vitamin C biosynthesis. FEBS J. 2008;275:713–726. doi: 10.1111/j.1742-4658.2007.06233.x. [DOI] [PubMed] [Google Scholar]
- 29.Leferink NG. van Duijn E. Barendregt A. Heck AJ. van Berkel WJ. Galactonolactone dehydrogenase requires a redox-sensitive thiol for optimal production of vitamin C. Plant Physiol. 2009;150:596–605. doi: 10.1104/pp.109.136929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linster CL. Clarke SG. L-Ascorbate biosynthesis in higher plants: the role of VTC2. Trends Plant Sci. 2008;13:567–573. doi: 10.1016/j.tplants.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lorence A. Chevone BI. Mendes P. Nessler CL. myo-inositol oxygenase offers a possible entry point into plant ascorbate biosynthesis. Plant Physiol. 2004;134:1200–1205. doi: 10.1104/pp.103.033936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mapson LW. Breslow E. Biological synthesis of ascorbic acid: L-galactono-gamma-lactone dehydrogenase. Biochem J. 1958;68:395–406. doi: 10.1042/bj0680395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maruta T. Ichikawa Y. Mieda T. Takeda T. Tamoi M. Yabuta Y. Ishikawa T. Shigeoka S. The contribution of Arabidopsis homologs of L-gulono-1,4-lactone oxidase to the biosynthesis of ascorbic acid. Biosci Biotechnol Biochem. 2010;74:1494–1497. doi: 10.1271/bbb.100157. [DOI] [PubMed] [Google Scholar]
- 34.Millar AH. Mittova V. Kiddle G. Heazlewood JL. Bartoli CG. Theodoulou FL. Foyer CH. Control of ascorbate synthesis by respiration and its implications for stress responses. Plant Physiol. 2003;133:443–447. doi: 10.1104/pp.103.028399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Müller-Moulé P. Conklin PL. Niyogi KK. Ascorbate deficiency can limit violaxanthin de-epoxidase activity in vivo. Plant Physiol. 2002;128:970–977. doi: 10.1104/pp.010924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noguchi K. Yoshida K. Interaction between photosynthesis and respiration in illuminated leaves. Mitochondrion. 2008;8:87–99. doi: 10.1016/j.mito.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Nunes-Nesi A. Araújo WL. Fernie AR. Targeting mitochondrial metabolism and machinery as a means to enhance photosynthesis. Plant Physiol. 2011;155:101–107. doi: 10.1104/pp.110.163816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nunes-Nesi A. Carrari F. Lytovchenko A. Smith AM. Loureiro ME. Ratcliffe RG. Sweetlove LJ. Fernie AR. Enhanced photosynthetic performance and growth as a consequence of decreasing mitochondrial malate dehydrogenase activity in transgenic tomato plants. Plant Physiol. 2005;137:611–622. doi: 10.1104/pp.104.055566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pavet V. Olmos E. Kiddle G. Mowla S. Kumar S. Antoniw J. Alvarez ME. Foyer CH. Ascorbic acid deficiency activates cell death and disease resistance responses in Arabidopsis. Plant Physiol. 2005;139:1291–1303. doi: 10.1104/pp.105.067686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pignocchi C. Fletcher JE. Barnes J. Foyer CH. The function of ascorbate oxidase (AO) in tobacco (Nicotiana tabacum L.) Plant Physiol. 2003;132:1631–1641. doi: 10.1104/pp.103.022798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pineau B. Layoune O. Danon A. De Paepe R. L-galactono-1,4-lactone dehydrogenase is required for the accumulation of plant respiratory complex I. J Biol Chem. 2008;283:32500–32505. doi: 10.1074/jbc.M805320200. [DOI] [PubMed] [Google Scholar]
- 42.Scheibe R. Backhausen JE. Emmerlich V. Holtgrefe S. Strategies to maintain redox homeostasis during photosynthesis under changing conditions. J Exp Bot. 2005;56:1481–1489. doi: 10.1093/jxb/eri181. [DOI] [PubMed] [Google Scholar]
- 43.Schertl P. Sunderhaus S. Klodmann J. Gergoff Grozeff GE. Bartoli CG. Braun HP. L-galactono-1,4-lactone dehydrogenase (GLDH) forms part of three subcomplexes of mitochondrial complex I in Arabidopsis thaliana. J Biol Chem. 2012;287:14412–14419. doi: 10.1074/jbc.M111.305144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sweetlove LJ. Lytovchenko A. Morgan M. Nunes-Nesi A. Taylor NL. Baxter CJ. Eickmeier I. Fernie AR. Mitochondrial uncoupling protein is required for efficient photosynthesis. Proc Natl Acad Sci U S A. 2006;103:19587–19592. doi: 10.1073/pnas.0607751103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szarka A. Horemans N. Bánhegyi G. Asard H. Facilitated glucose and dehydroascorbate transport in plant mitochondria. Arch Biochem Biophys. 2004;428:73–80. doi: 10.1016/j.abb.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 46.Szarka A. Horemans N. Kovács Z. Gróf P. Mayer M. Bánhegyi G. Dehydroascorbate reduction in plant mitochondria is coupled to the respiratory electron transfer chain. Physiol Plant. 2007;129:225–232. [Google Scholar]
- 47.Szarka A. Tomasskovics B. Bánhegyi G. The ascorbate-glutathione-α-tocopherol triad in abiotic stress response. Int J Mol Sci. 2012;13:4458–4483. doi: 10.3390/ijms13044458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tabata K. Oba K. Suzuki K. Esaka M. Generation and properties of ascorbic acid-deficient transgenic tobacco cells expressing antisense RNA for L-galactono-1,4-lactone dehydrogenase. Plant J. 2001;27:139–148. doi: 10.1046/j.1365-313x.2001.01074.x. [DOI] [PubMed] [Google Scholar]
- 49.Talla S. Riazunnisa K. Padmavathi L. Sunil B. Rajsheel P. Raghavendra AS. Ascorbic acid is a key participant during the interactions between chloroplasts and mitochondria to optimize photosynthesis and protect against photoinhibition. J Biosci. 2011;36:163–173. doi: 10.1007/s12038-011-9000-x. [DOI] [PubMed] [Google Scholar]
- 50.Tamaoki M. Mukai F. Asai N. Nakajima N. Kubo A. Aono M. Saji H. Light-controlled expression of a gene encoding L-galactono-γ-lactone dehydrogenase which affects ascorbate pool size in Arabidopsis thaliana. Plant Sci. 2003;164:1111–1117. [Google Scholar]
- 51.Taniguchi M. Taniguchi Y. Kawasaki M. Takeda S. Kato T. Sato S. Tabata S. Miyake H. Sugiyama T. Identifying and characterizing plastidic 2-oxoglutarate/malate and dicarboxylate transporters in Arabidopsis thaliana. Plant Cell Physiol. 2002;43:706–717. doi: 10.1093/pcp/pcf109. [DOI] [PubMed] [Google Scholar]
- 52.Tomaz T. Bagard M. Pracharoenwattana I. Lindén P. Lee CP. Carroll AJ. Ströher E. Smith SM. Gardeström P. Millar AH. Mitochondrial malate dehydrogenase lowers leaf respiration and alters photorespiration and plant growth in Arabidopsis. Plant Physiol. 2010;154:1143–1157. doi: 10.1104/pp.110.161612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wheeler GL. Jones MA. Smirnoff N. The biosynthetic pathway of vitamin C in higher plants. Nature. 1998;393:365–369. doi: 10.1038/30728. [DOI] [PubMed] [Google Scholar]
- 54.Wolucka BA. Van Montagu M. GDP-mannose 3',5'-epimerase forms GDP-L-gulose, a putative intermediate for the de novo biosynthesis of vitamin C in plants. J Biol Chem. 2003;278:47483–47490. doi: 10.1074/jbc.M309135200. [DOI] [PubMed] [Google Scholar]
- 55.Yabuta Y. Mieda T. Rapolu M. Nakamura A. Motoki T. Maruta T. Yoshimura K. Ishikawa T. Shigeoka S. Light regulation of ascorbate biosynthesis is dependent on the photosynthetic electron transport chain but independent of sugars in Arabidopsis. J Exp Bot. 2007;58:2661–2671. doi: 10.1093/jxb/erm124. [DOI] [PubMed] [Google Scholar]
- 56.Yoshida K. Terashima I. Noguchi K. How and why does mitochondrial respiratory chain respond to light? Plant Signal Behav. 2011;6:864–866. doi: 10.4161/psb.6.6.15224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoshida K. Watanabe CK. Hachiya T. Tholen D. Shibata M. Terashima I. Noguchi K. Distinct responses of the mitochondrial respiratory chain to long- and short-term high-light environments in Arabidopsis thaliana. Plant Cell Environ. 2011;34:618–628. doi: 10.1111/j.1365-3040.2010.02267.x. [DOI] [PubMed] [Google Scholar]
- 58.Zechmann B. Subcellular distribution of ascorbate in plants. Plant Signal Behav. 2011;6:360–363. doi: 10.4161/psb.6.3.14342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zsigmond L. Rigó G. Szarka A. Székely G. Otvös K. Darula Z. Medzihradszky KF. Koncz C. Koncz Z. Szabados L. Arabidopsis PPR40 connects abiotic stress responses to mitochondrial electron transport. Plant Physiol. 2008;146:1721–1737. doi: 10.1104/pp.107.111260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zsigmond L. Tomasskovics B. Deák V. Rigó G. Szabados L. Bánhegyi G. Szarka A. Enhanced activity of galactono-1,4-lactone dehydrogenase and ascorbate–glutathione cycle in mitochondria from Complex III deficient Arabidopsis. Plant Physiol Biochem. 2011;49:809–815. doi: 10.1016/j.plaphy.2011.04.013. [DOI] [PubMed] [Google Scholar]