Abstract
Significance: Antioxidant enzymes are thought to provide critical protection to cells against reactive oxygen species (ROS). However, many organisms can fully compensate for the loss of such enzymatic defenses by accumulating metabolites and Mn2+, which can form catalytic Mn-antioxidants. Accumulated metabolites can direct reactivity of Mn2+ with superoxide and specifically shield proteins from oxidative damage. Recent Advances: There is mounting evidence that Mn-Pi (orthophosphate) complexes act as potent scavengers of superoxide in all three branches of life. Moreover, it is evident that Mn2+ in complexes with carbonates, peptides, nucleosides, and organic acids can also form catalytic Mn-antioxidants, pointing to diverse metabolic routes to oxidative stress resistance. Critical Issues: What conditions favor utility of Mn-metabolites versus enzymatic means for removing ROS? Mn2+-metabolite defenses are critical for preserving the activity of repair enzymes in Deinococcus radiodurans exposed to intense radiation stress, and in Lactobacillus plantarum, which lacks antioxidant enzymes. In other microorganisms, Mn-antioxidants can serve as an auxiliary protection when enzymatic antioxidants are insufficient or fail. These findings of a critical role of Mn-antioxidants in the survival of prokaryotes under oxidative stress parallel the trends developing for the simple eukaryote Saccharomyces cerevisiae. Future Directions: Phosphates, peptides and organic acids are just a snapshot of the types of anionic metabolites that promote such reactivity of Mn2+. Their probable roles in pathogen defense against the host immune response and in ROS-mediated signaling pathways are also areas that are worthy of serious investigation. Moreover, it is clear that these protective chemical processes can be harnessed for practical purposes. Antioxid. Redox Signal. 19, 933–944.
Introduction
The evolution of manganese-accumulating photosynthetic cyanobacteria 2.8 billion years ago was accompanied by the whole-scale oxygenation of the Earth's atmosphere and oceans, and with that followed a magnificent explosion in the diversity and complexity of life (43). However, there were severe consequences of oxygenation. The ocean's bacteria became starved of soluble ferrous atoms (Fe2+), which reacted with dioxygen (O2) and precipitated as iron oxides (rust), and the adventitious (or sometimes intentional) production of reactive oxygen species (ROS) mandated that cells develop powerful mechanisms to neutralize ROS and repair oxidative cellular damage (28, 37). A variety of powerful enzymes evolved to remove ROS, including superoxide (O2•−) and hydrogen peroxide (H2O2), although no enzymes are kinetically capable of controlling the most reactive species of all, the hydroxyl radical (HO•).
The role of protein-based enzymes in neutralizing or eliminating ROS is well-documented. Diverse enzyme classes can handle peroxides, including catalases, glutathione peroxidases, and thiol peroxidases (peroxiredoxins) (58). However, the enzymatic means for removing O2•− appear limited. The single major enzyme type that reacts with O2•− is the superoxide dismutase (SOD), which oxidizes and reduces O2•− in one catalytic cycle, generating H2O2 and O2 (Fig. 1A). Superoxide poses a greater threat to cellular targets than does H2O2 or O2, because (i) the anion O2•− cannot easily cross membranes and can become trapped in cells (37, 57); and (ii) since O2•− is negatively charged, it specifically targets and can destroy active sites of many enzymes that bind positively charged Fe2+ atoms (Fig. 1) (37). In contrast, H2O2 is neutral, not nearly as reactive as O2•−, and does not build up in cells, because it is membrane permeable. SOD enzymes are found throughout nature in most prokaryotes, and all eukaryotes and their organelles. They include the manganese- and iron-containing SOD family, the copper/zinc-containing SODs, and the rare nickel-containing SOD enzymes (1). The ubiquitous nature of SODs helps meet the demands of scavenging O2•−, which is generated as a continuous by-product of aerobic metabolism. However, O2•− is generated spontaneously at high levels in cells that are exposed to ionizing (X-rays and γ-rays) and non-ionizing (UV) forms of radiation (72), and is also intentionally produced during the oxidative burst of the host immune response and during cell-signaling events in animal cells (78). Thus, the demand on cells and organisms to manage this radical can be great and, in some cases, may exceed the capacities of SOD enzymes. To this end, certain cells appear to have evolved reinforcement ROS-scavenging systems for handling O2•− and related ROS that involve non-protein complexes of manganese.
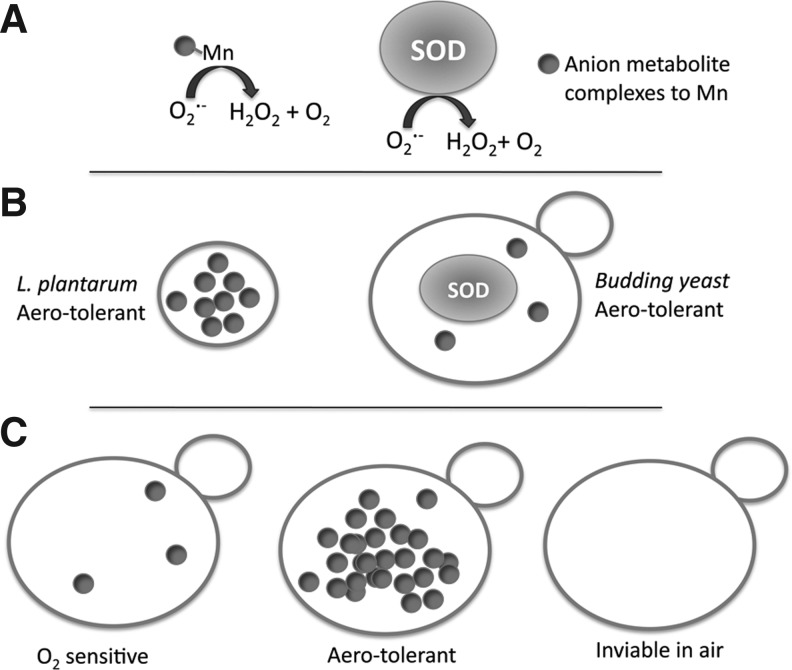
FIG. 1.
Aero tolerance provided by SOD enzymes and/or Mn-antioxidants that remove O2•−. (A) As is the case with SOD enzymes, certain manganese complexes to small metabolites (small ball attached to Mn) have the capacity to remove O2•−. Such metabolites include phosphate, carbonate, and organic acids (see text). (B) Lactobaccilus plantarum does not express SOD enzymes but is aero tolerant due to high accumulation of Mn antioxidants (small balls). Other organisms such as the Baker's yeast Saccharomyces cerevisiae largely remove O2•− by SOD enzymes, but can also use Mn-antioxidants as a secondary defense against O2•−. (C) In the absence of SOD enzymes, susceptibility to atmospheric oxygen is directly proportional to the level of Mn antioxidants that accumulate. O2•−, superoxide; SOD, superoxide dismutase.
There has been mounting evidence over the last 30 years for the widespread use of small metabolite complexes as antioxidant defense (21, 22). These include the accumulation of non-proteinaceous complexes of manganese (Mn) that remove O2•− (so-called Mn-antioxidants) and divalent manganese (Mn2+) complexes of small metabolites such as nucleosides and peptides which shield macromolecules from ROS (23). Manganous ions serve as the ideal metal for this purpose, because unlike ferrous and cuprous ions that can generate the highly toxic HO•, manganese is by comparison a poor Fenton reagent—Mn2+ does not react with H2O2. Manganese provides a beneficial antioxidant activity without the pro-oxidant side effects of other redox active metals (20, 21). The non-enzymatic, Mn-dependent artillery for combating oxidative stress has not only been best studied in microbes and appears particularly prevalent in aerobic radiation- and desiccation-resistant extremophiles (21, 22), but may also be relevant in multicellular organisms and important for pathogen defense. For the purposes of this review, we shall refer to these reactive manganese metabolites collectively as “Mn-antioxidants.”
Mn-Antioxidants Versus SOD Enzymes in Bacterial Aero Tolerance
The first studies implicating Mn2+ in the removal of O2•− were in the 1950s. Chloroplasts were shown to contain plentiful Mn, both free and bound, and the oxidation of Mn2+ by illuminated chloroplasts was found to be due to O2•− (45). In the late 1960s to mid 1970s, families of metal-containing SOD enzymes were turning up in all facets of life, many of which were discovered by Professor Irwin Fridovich at Duke University (40, 59, 65, 76, 79). Following these seminal discoveries, Fridovich along with this postdoc Fred Archibald asked the question: Is it possible to thrive in atmospheric oxygen without a SOD enzyme?
Three organisms at that time, namely Bacillus coagulans, Neisseria gonorrhoeae, and Lactobacillus plantarum, were thought to be devoid of SOD activity. Through biochemical studies, and later confirmed with the advent of genome sequencing, B. coagulans and N. gonorrhoeae were, in fact, found to encode SOD enzymes, leaving only L. plantarum devoid of SODs. Puzzled by the high oxygen tolerance of these organisms lacking detectable SODs, Archibald closely inspected the growth medium that had been designed back in the 1940s for lactate-fermenting bacteria. The medium was found to contain high levels of manganese that were essential for L. plantarum growth. In a careful series of studies that followed, Archibald and Fridovich found that L. plantarum accumulated millimolar quantities of intracellular manganese compared with the micromolar levels which were typical of most other organisms, and that this high manganese was essential for aerobic growth (6). A manganese-dependent O2•− scavenging activity was discovered in the lysates of L. plantarum that was dialyzable, heat and protease resistant, and sensitive to ethylenediaminetetraacetic acid (EDTA, a chelator of divalent metals) (5–7). Hence, the aero tolerance of L. plantarum was provided by non-SOD complexes of manganese that can remove O2•− (Fig. 1B).
What was the nature of this new SOD-like complex of manganese? Archibald and Fridovich found that numerous phosphate and carboxylate complexes to manganese had the capacity to react with O2•−, with phosphate and lactate complexes being most efficient (8). With the high levels of lactate that accumulate in fermenting L. plantarum, lactate complexes to manganese seemed likely candidates for the Mn-antioxidant in this organism (5–8). Mn2+ can act as a stoichiometric scavenger of O2•− in pyrophosphate-buffered media but as a catalytic scavenger of O2•− in orthophosphate-buffered media. In summary, the hexaquo Mn2+ ion was found to be poorly reactive with O2•− on its own, but when coordinated to various anion metabolites, Mn2+ acted as a SOD mimic (8, 11, 12).
The role of Mn-antioxidants in stress resistance is not unique to L. plantarum. Independent studies which preceded those of Fridovich and Archibald showed that Mn2+ accumulation in bacteria correlated to UV resistance. In 1976, Deinococcus radiodurans, an extremely radiation- and desiccation-resistant bacterium, was shown to accumulate millimolar concentrations of Mn2+(49). Since then, a critical role for the accumulation of Mn2+ has been demonstrated in D. radiodurans in a mechanism toward surviving extreme doses of radiation and other oxidative stress conditions that is dependent on neither SOD nor catalase enzymes (details ahead) (21, 25). Remarkably, antioxidant enzymes in Mn-accumulating aerobic bacteria and archaea are dispensable, but not in prokaryotes, which lack Mn transport systems (22, 68). Evidently, the accumulation of intracellular Mn2+ complexes in D. radiodurans can provide levels of protection from ROS that equal or exceed the ROS-scavenging capacities of antioxidant enzymes (21, 25, 46, 72).
While the accumulation of Mn2+ complexes can fully compensate for the loss of antioxidant enzymes in some organisms, Mn-antioxidants also appear to act synergistically with SOD enzymes. It has been proposed that when Mn is abundant, surplus intracellular Mn2+ can form antioxidant complexes, whereas cells rely more heavily on the high efficiency of SODs when Mn is limited (1, 23). In N. gonorrhoeae, which accumulates Mn2+, Mn antioxidants are pivotal for oxidative stress protection in this organism that also expresses a single Fe SOD enzyme (71). In other SOD-expressing organisms, the efficacy of Mn antioxidants is best seen under conditions when the SODs have been deleted. For example, in SOD mutant strains of Escherichia coli and Bacillus, oxidative damage can be suppressed when cells accumulate high manganese from the growth medium (2, 38). Conversely, when Bacillus cells are starved for manganese, mutants lacking SOD cannot thrive in air (38). In diverse species, there appears to be widespread use of Mn antioxidants as a supplement or as a backup for SOD enzymes. It is noteworthy that these manganese-metabolite complexes are not as catalytically efficient as SODs; however, when abundantly concentrated and widely distributed in cells, they have the capacity to substitute functionally for SOD enzymes (12).
The Mn Antioxidant in the Eukaryotic Model Organism Saccharomyces cerevisiae
In most eukaryotes, the major SOD enzyme is the largely cytosolic Cu/Zn SOD1. In the case of Baker's yeast Saccharomyces cerevisiae, loss of this SOD1 leads to a number of aerobic defects that are associated with O2•− toxicity, including increases in mutation frequency, disruptions in amino acid biosynthetic pathways, and sensitivities to redox-cycling compounds and high oxygen. All these defects can be corrected by boosting intracellular levels of manganese, as illustrated in Figure 1C (14, 47, 48, 54, 70). As with prokaryotes, eukaryotic micro-organisms show a dependence on Mn antioxidants as an important backup or reinforcement to SOD enzymes. The Mn antioxidant appears conserved in all three branches of life.
Chang and Kosman were the first to note in the late 1980s that all the oxidative stress phenotypes associated with lack of Cu/Zn SOD1 in yeast could be suppressed by growing cells in high manganese salts. Yeast cells that accumulate high manganese exhibit a SOD-independent but an Mn-dependent O2•− scavenging activity in cell lysates which is heat resistant and EDTA sensitive (14). Using a series of genetic suppressor screens, the Culotta lab attempted in the 1990s to identify genes in S. cerevisiae that had the capacity to substitute for Cu/Zn SOD and in virtually every case, the genes identified were found to control uptake and accumulation of manganese and, hence, activity of Mn-antioxidants. These genes included Pmr1p—the Golgi transporting pump for manganese and calcium; Bsd2p and Atx2p—the negative regulators of the yeast Nramp transporters for manganese uptake; and Ccc1p—vacuolar transporter for manganese (47, 50, 52–54). In essence, the search for new anti-oxidant genes led instead to a discovery of pathways for manganese trafficking and, moreover, emphasized the essential role of Mn-antioxidants as a backup for Cu/Zn SOD1. When intracellular manganese levels are high (mM, as in the case of L. plantarum), the Mn-antioxidants can reverse all markers of oxidative damage in yeast cells lacking Cu/Zn SOD1 (14, 47, 50, 52–54, 70). It is important to note that the level of manganese required to fully compensate for the loss of Cu/Zn SOD1 is below the toxic threshold for the metal. Yeast cells can benefit from the Mn antioxidants without the side reactions of manganese toxicity. Even physiological levels of Mn antioxidants that accumulate with normal (μM) intracellular manganese are important. When manganese antioxidant levels drop too low, sod1Δ mutants are not viable in air (66, 67) (Fig. 1C).
Is the formation of Mn antioxidants simply a passive process of manganese binding to available metabolites, or does the cell control the assembly of Mn antioxidants with protein-based antioxidants? At least in the Baker's yeast S. cerevisiae, the latter seems to be true. The Culotta lab observed that Mn-antioxidant activity falls under the control of two major nutrient and stress-signaling pathways, namely the Pho80/Pho85p CDK/cyclin pathway and the pathway involving the Sch9p kinase (homologue to human Akt/S6K). When either of these pathways in yeast is disrupted by gene mutations, Mn-antioxidant activity is greatly reduced to virtually non-detectable levels (60, 66, 69). This loss in the Mn antioxidant is due to neither inhibition of manganese uptake nor accumulation; if anything, cells disrupted in the Pho80/Pho85p pathway hyperaccumulate manganese, yet this manganese cannot suppress oxidative damage and cells lacking Cu/Zn SOD1 are not viable in air (66, 69).
The parallel pathways involving Pho80/Pho85p and the Sch9p kinase converge at the level of the Rim15p stress response kinase, and it was shown that hyperactivation of Rim15p in pho80 or sch9 mutants abolishes the Mn antioxidant (66). Through a series of genetic studies, the transcription factor Gis1p was identified as the target of Rim15p that was responsible for controlling the Mn antioxidant (66) (Fig. 2). Gis1p is known to regulate a large battery of stress response genes for adaptation to changes in environmental conditions. The specific Gis1p target that controls the Mn antioxidant is currently unknown, but is the subject of ongoing investigations. In any case, these studies stress the physiological importance of the Mn antioxidant. These complexes are not just the product of passive interactions between the metals and metabolites; rather, the formation of Mn antioxidants is carefully regulated in yeast cells. The same nutrient and stress-signaling pathways that control formation of protein-based antioxidants such as SOD enzymes and catalases also control production of the Mn antioxidant.
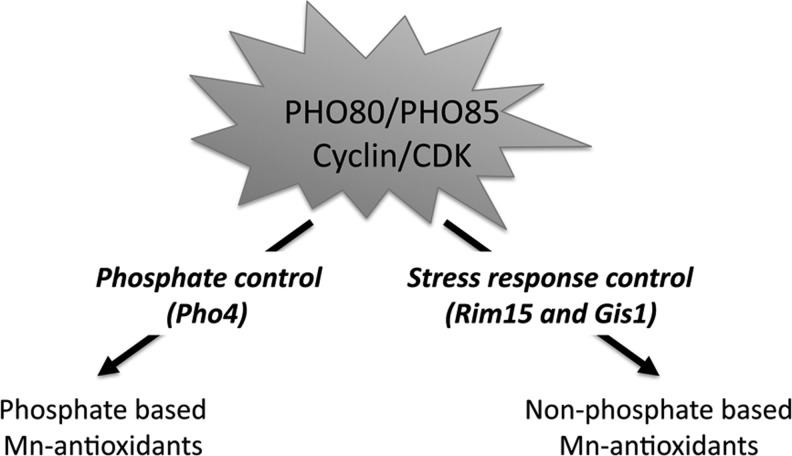
FIG. 2.
Effects of the Pho80p/Pho85p signaling pathways on the Mn antioxidant in yeast. Loss of the Pho80p cyclin or its CDK partner Pho85p causes a dramatic loss in Mn antioxidants by two methods. First, by disrupting Pho80p control of phosphate (via the Pho4p transcription factor), the levels of Mn-Pi antioxidants drop (60). Second, by disrupting the Pho80p stress response involving the Rim15p kinase and Gis1p transcription factor, non-phosphate-based antioxidants are also lost (66). The nature of these non-phosphate Mn-antioxidants is still unknown.
The Chemical Makeup of Mn Antioxidants that Remove O2•−
Strong lines of evidence from different laboratories have now converged on the conclusion that the accumulation of Mn2+ with orthophosphate (Pi) along with various organic metabolites represents a widespread strategy for combating oxidative stress. The nature of the manganese complexes, however, remains unknown, principally because it has not been possible to determine Mn speciation in a solution in which small Mn2+ complexes exchange their ligands rapidly, and standard analytical procedures that disrupt cells likely alter speciation (23). Until recently, this limitation has stymied in vivo investigations into cellular manganese. However, reconstituted prokaryotic complexes of Mn2+ have been characterized in vitro for their reactivity with ROS (23), and new spectroscopy techniques (electron nuclear double resonance [ENDOR]) (60) now hold the prospect of revealing their in vivo structures and roles.
Using yeast as a model system, the possible role of Mn-Pi as the key in vivo antioxidant was examined using a combination of ENDOR spectroscopy by Brian Hoffman's laboratory and genetics in the Culotta and Valentine laboratories. At the time, yeast cells were known to accumulate μM levels of manganese and mM levels of phosphate, yet it was not known whether these entities actually interacted in cells to form stable complexes. To address this, Hoffman's laboratory developed a method for monitoring manganese speciation in intact yeast cells through ENDOR spectroscopy. The studies showed that in wild-type yeast cells, approximately three quarters of cellular manganese is actually bound to phosphates, with the bulk represented as Mn-Pi (60). This level of intracellular Mn-Pi could be modulated by gene mutations that either increase (pmr1Δ null mutations) or decrease (smf2Δ null mutations) cellular manganese, and, most importantly, resistance to oxidative stress closely correlated with levels of Mn-Pi (Fig. 3) (60). These studies placed Mn-Pi as a lead candidate for the Mn antioxidant. However, not all findings were consistent with this notion. In particular, the aforementioned pho80 (or pho85) mutants exhibited a dramatic loss in oxidative stress resistance and the most severe loss of the Mn-antioxidant (Fig. 3). However, Mn-Pi levels in this strain were only decreased to about one-third that of wild-type cells, which could not by itself explain the complete loss in Mn-antioxidant activity (60). While Mn-Pi is one type of Mn-antioxidant in yeast cells, it is not the only one. In pho80 (or pho85) mutants, a non Mn-Pi complex is lost, which renders cells completely vulnerable to oxidative stress. Hence, this new Mn-antioxidant falls under control of the Pho80/Pho85 stress response pathway (Fig. 2).
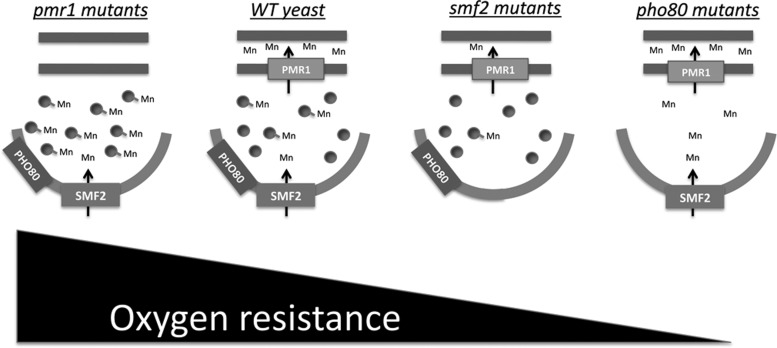
FIG. 3.
Yeast mutants that alter ability to form Mn antioxidants and their effect on oxygen tolerance. Shown are cartoons of four yeast strains, including WT yeast and the corresponding pmr1, smf2, and pho80 mutants that affect the Mn antioxidant. The small balls attached to Mn represent the collection of all the different species of Mn antioxidants, including Mn-Pi as well as other unknown complexes to Mn. PMR1 encodes the Golgi ATPase pump for Mn and in pmr1 mutants, Mn accumulates to very high levels (47). By electron nuclear double resonance spectroscopy, Mn-Pi is 10-fold higher in pmr1 mutants, and these cells are most resistant to oxidative stress (60). SMF2 encodes the Nramp metal transporter for Mn in yeast; smf2 mutants accumulate low Mn and as a result, Mn-Pi levels are ten-fold lower than WT cells (60). The smf2 mutants are more sensitive to oxygen than the WT strains (60). PHO80 encodes the cyclin involved in signaling phosphate control and key stress responses. Loss of pho80 (or pho85) only causes a 2–3-fold reduction in Mn-Pi levels (60), but, nevertheless, Mn can no longer function as an antioxidant in these cells and mutants of pho80 (or pho85) are highly oxygen sensitive. Without SOD enzymes, pho80 (or pho85) mutants cannot survive in air (60, 67). WT, wild-type.
What are possibilities for the non-phosphate Mn-antioxidant? Recent studies by Valentine and coworkers have demonstrated the Mn-carbonate has strong catalytic activity with O2•−, with rates that are even higher than Mn-Pi (12). Carbonate can be of very high levels in mammalian cells (12) and in yeast cells, carbonate production is particularly important for meiosis and other cellular processes (26). In addition, various manganese-based porphyrin complexes show reactivity with O2•−, and some can substitute for Cu/Zn SOD1 in yeast (61); the assimilation of similar compounds in living cells may contribute to oxidative stress resistance. An ideal tool to uncover new Mn antioxidants in yeast is the S. cerevisiae pho80/pho85 mutant, which is virtually devoid of Mn-antioxidant activity (66, 67). As mentioned earlier, the loss of Mn-antioxidant activity in this case results from activation of the Gis1p transcription factor (Fig. 2), and identification of the key Gis1p-targets will provide valuable information in this regard.
Cellular Targets of Mn Protection
Molecular insights into how ROS cause mutagenesis, disease, aging, and myriad biological processes ending in cell death were built on studies that revolve around the toxic effects of radiation (22). As a result, early models of cellular oxidative stress were based on the concept that ROS indiscriminately damage cellular macromolecules. This, however, is not the case. While HO• cause indiscriminate damage to all cellular macromolecules, O2•− and H2O2 do not react directly with DNA (22, 37). The most consequential damage by O2•− and H2O2 in cells is to proteins that contain exposed iron-sulfur or haem groups, to proteins which contain cysteine residues, and to proteins containing cation-binding sites where an iron-catalyzed site-specific oxidation occurs (37, 73). Since individual proteins in a cell typically exist at much higher levels than their corresponding genes, death in cells under oxidative stress was originally attributed mainly to DNA damage (22). However, extreme resistance to oxidative insult among bacteria and archaea that accumulate Mn consistently coincide with a greatly diminished susceptibility to protein oxidation, but with similar DNA lesion yields as in other organisms (22). Although those studies did not exclude the possibility that Mn2+ also prevents lipid peroxidation in cell membranes, the lowest regional Mn concentrations in Mn-accumulating bacteria are associated with the cell envelope (24), indicating that Mn2+ predominantly protects the cytosol. Moreover, earlier reports strongly support the idea that lipid peroxidation can be dissociated from lethal damage in mammalian and bacterial cells under oxidative stress [(24) and citations therein].
So far, all the most oxidative stress-resistant aerobic prokaryotes reported have been shown to accumulate Mn along with low-molecular-weight metabolites (22). It has been demonstrated that Mn2+ complexes accumulated in cells specifically protect enzymes and the functions they catalyze (23, 24). The argument that damaged proteins may be responsible for the lethal action of ROS was first developed by Walter M. Dale in the early 1940s (17–19). This view of oxidative stress was based on findings that enzymes in aqueous solution could be inactivated by small doses of X-rays. The possibility that resistance to ROS could be increased was supported by his studies which showed that the radiosensitivity of an enzyme is not a fixed entity but a variable, where oxidative inactivation could be prevented by the addition of nucleotides, sugars, amino acids, and a variety of other organic compounds. Dale's idea that damaged proteins might be most responsible for toxicity in cells under oxidative stress has endured. Indeed, for most oxidative stress conditions, DNA is no longer considered the principal target of ROS that accounts for their toxicity. Instead, cellular proteins are principal targets that mediate the lethal effects of ROS (22).
Two definitive insights into the reparability of damaged genomes in cells under oxidative stress were gained recently by comparisons of DNA and protein damage in irradiated bacteria. First, the yields of DNA damage per dose among naturally sensitive and extremely resistant bacteria, and for other cell types with very different antioxidant statuses, were relatively constant—this supports the fact that DNA damage in cells under oxidative stress is caused mainly by “non-scavengable indirect effects” (22). Second, the yields of protein oxidation in sensitive and resistant prokaryotes exposed to oxidative stress were highly variable and quantitatively related to survival (22)—this supports the fact that variations in oxidative sensitivity and efficiency of DNA repair in wild-type cells are caused mainly by protein oxidation, which is governed by the antioxidant status of a cell (24).
ROS scavenging has historically been assigned mainly to antioxidant enzymes, but D. radiodurans cells rely mostly on non-enzymatic antioxidants based on small metabolite complexes of Mn2+ (23–25). In prokaryotes, the action of Mn2+ in protecting cytosolic proteins from ROS appears to occur at two levels: (i) by replacing Fe2+ and other divalent cations (e.g., Mg2+ and Cu2+) with Mn2+ as mononuclear cofactors in enzymes, active sites are protected from oxidative damage (4); and (ii) surplus Mn2+ (i.e., the portion of a cell's Mn2+ budget that is not bound to proteins) forms ROS-scavenging complexes with various metabolites (23), which provide global protein protection and preserve the quaternary structures of irradiated enzymes. It is important to note, based on in vitro enzyme studies, that high (not extreme) levels of oxidative stress resistance are predicted to occur in cells which accumulate secondary metabolites even without significantly elevated Mn2+; Mn2+ accumulation is not a singular determinant of oxidative stress resistance. Rather, Mn2+ appears to boost protein protection in cells by interacting synergistically with the pool of small-molecule metabolites built up in cells (23).
Mn Antioxidants in Multicellular Organisms and in Pathogenesis
Thus far, the bulk of what is known about O2•− reactive Mn-antioxidants has stemmed from work in microbes. However, it is also likely that these complexes function in multicellular species. For example, in the nematode Caenorhabditis elegans, elevated manganese accumulation can enhance oxidative stress resistance and extend life span (15, 51). High levels of manganese can also protect against the oxidative damage during cryopreservation of the sperm (10). Moreover, in mitochondria and chloroplasts, where organellar Mn budgets appear to exceed the demands of their enzymes (34), Mn might also form antioxidant complexes with proton-donor ligands such as carboxylic acids generated by the tricarboxylic acid (TCA) cycle (8). Another setting in which Mn antioxidants may become critical is with microbial pathogenesis.
During infection, the host responds with an oxidative burst to kill pathogens. The O2•− producing NADPH oxidase becomes activated in macrophages and neutrophils, and concomitant rises in O2•− in the locale of invading microbes help block pathogen invasion (Fig. 4). In addition to this oxidative burst, a rising concept in host defense is “nutritional immunity,” a phrase recently coined by Eric Skaar to describe the molecular starvation of pathogens for the vital metal ions iron, zinc, and manganese (13, 42). In the case of manganese, the metal is depleted from the macrophage phagolysosomes via the Nramp1 divalent metal transporter, and is also removed at the site of an infected abscess through neutrophil production of calprotectin, a metal chelating molecule (13, 16, 31, 33, 41).
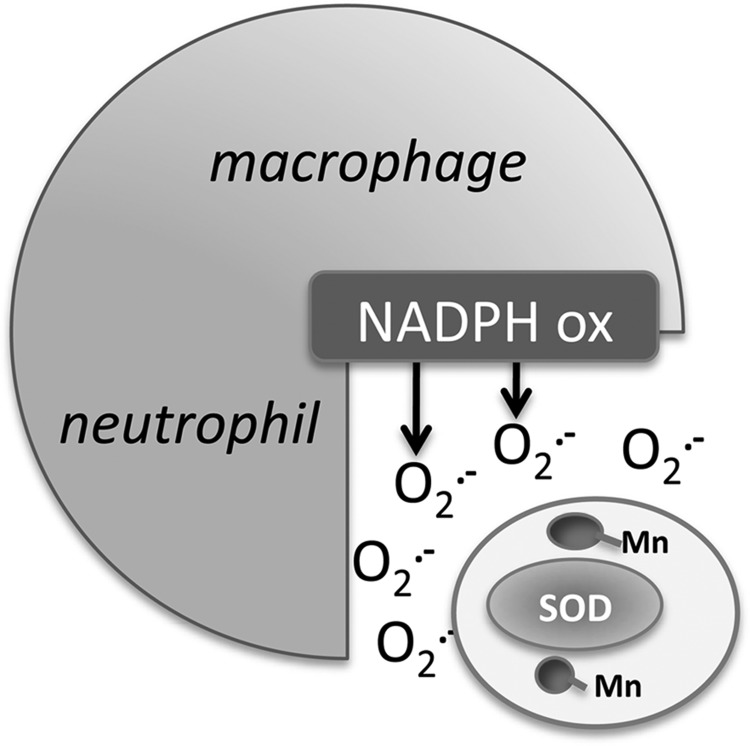
FIG. 4.
The role of Mn antioxidants and SOD enzymes in microbial pathogenesis. During infection, the host defense response includes the oxidative burst by macrophages and neutrophils involving the NADPH oxidase production of O2•−. To evade the host response, microbes (white circle) employ SOD enzymes as well as Mn antioxidants (see main text). Since O2•− is not membrane permeable, extracellular/periplasmic microbial antioxidant defenses may be most critical.
The efficacy of manganese starvation in microbial killing depends on the degree to which the metal is required for pathogen survival. Indeed, a number of microbes have shown a dependence on manganese for virulence. Manganese uptake is required for virulence of Staphylococcus aureus (16, 35, 71), and N. gonorrhoeae expresses a ATP-binding cassette manganese uptake system that is necessary for protection against ROS (74). Moreover, cell surface manganese uptake through various manganese transporter types is required for virulence of Brucella abortus, Salmonella enterica, Enterococcus faecalis, S. pneumoniae, and Yersinia pestis [reviewed in ref. (13)]. The Lyme disease pathogen Borrelia burgdorferi exhibits a particularly strong requirement for manganese, as this “iron-free” pathogen has replaced iron in many cellular reactions with manganese (63).
In pathogens, manganese can promote oxidative stress resistance in two ways: either through activation of manganese-containing SOD molecules, as has been shown in S. aureus (41) and B. burgdorferi (27), or through formation of Mn antioxidants. For example, the manganese-dependent O2•− resistance of S. aureus is not totally dependent on Mn-SOD, strongly implicating an Mn antioxidant (35, 36). Accordingly, the calprotectin produced by the host to starve S. aureaus of manganese is believed to target both Mn-SOD and a SOD-independent antioxidant activity (i.e., Mn-antioxidant) (41). The resistance of N. gonorrhoeae to O2•− killing may be entirely due to Mn-antioxidants, because this organism does not express an Mn-SOD (71, 74). Even in the related N. meningitides that does express Mn-SOD, a SOD-independent O2•− scavenging activity can be detected, presumably representing the Mn antioxidant (71) (Fig. 4).
These aforementioned examples of non-SOD Mn antioxidants in pathogenic bacteria may just be the tip of the iceberg. The exploitation of small manganese-metabolite complexes for handling O2•− may be widespread throughout the microbial world, particularly in pathogens where the O2•− burden from the host defense becomes challenging. It will be intriguing to determine the makeup of the various manganese complexes that have evolved in these distinct organisms. Is Mn-phosphate a widely used antioxidant, or does the specialized metabolism of diverse microbes mandate new metabolite complexes to manganese that promote reactivity with O2•−? Investigations of this type can lead to new insights into microbial pathogenesis and the development of anti-microbial strategies that target manganese or its partner metabolites.
Mn2+ Antioxidants and Radiation Protection: D. radiodurans
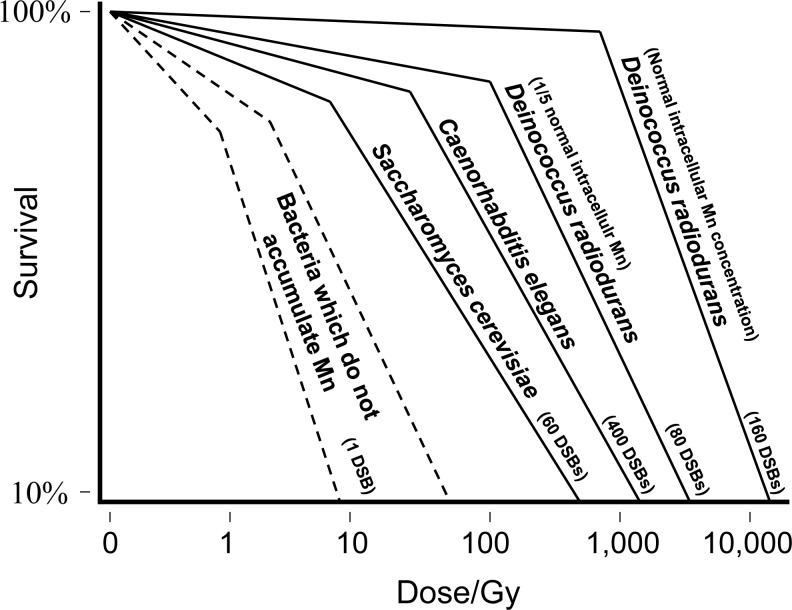
The Mn antioxidant has been well studied in the extremely radiation- and desiccation-resistant bacterium D. radiodurans (25). D. radiodurans was first reported in 1956, capable of surviving huge doses of X-rays or γ-rays (12,000 Gy) (Fig. 5), 100 times greater than most bacteria grown under comparable conditions, and 3000 times greater than most human cells in liquid culture (21). The survival curves for D. radiodurans display very large shoulders, but the mutation frequency of the cells does not increase significantly with dose. Reasoning that DNA in D. radiodurans might be protected, early studies compared the amount of DNA damage in D. radiodurans and E. coli exposed to ionizing radiation or UV light, and later with other organisms (22, 72). For a given dose of X-rays or γ-rays, or UVC (254 nm) radiation, the relatively small differences in DNA breaks and DNA base damages between the bacteria were not nearly sufficient to explain the great differences in their resistance. Since then, the remarkable survival of irradiated D. radiodurans cells has been rationalized under the hypothesis of enhanced DNA repair. Recent progress clearly implicates intracellular Mn-complexes of D. radiodurans as responsible for preserving the high efficiency of its DNA repair proteins (21, 72). In contrast, most other organisms are killed by relatively low exposures to oxidative stress, which causes many enzyme systems to malfunction, including those responsible for metabolism and DNA repair/replication.
FIG. 5.
Survival curves of Mn-accumulating organisms exposed to γ-radiation. The numbers in parentheses indicate the approximate number of survivable DSBs inflicted per haploid genome at the dose that kills 90% of the organisms (22). Note that the ability of Mn-accumulating organisms to mend DSBs after exposure to γ-rays is much greater than in most bacteria which do not accumulate significant Mn (25). The high efficiency of repair enzymes in Mn-accumulating organisms under oxidative stress is attributed to the formation of Mn antioxidants that scavenge ROS and prevent the inactivation of enzymes. Mn-accumulating organisms: Bacterium—Deinococcus radiodurans (ATCC BAA-816) grown under Mn-depleted or Mn-replete conditions (25); Diploid yeast—S. cerevisiae (JW1777) (9); Nematode worm—Caenorhabditis elegans (39). Many explanations for the cause of the long shoulders of survival curves of Mn-accumulating organisms have been proposed. The most favored hypotheses begin with the premise that the yield of DSBs is linear with dose (0.004 DSB/Gy/Mbp) and that the non-linearity of the survival curves is caused by dose-dependent changes in the efficiency/accuracy of enzymatic DNA repair, which in many cell types appears to be dependent on the accumulation of Mn antioxidants (22). Note that the x-axis is a logarithmic scale. Radiation source: 60Co γ-radiation. DSBs, DNA double-strand breaks; ROS, reactive oxygen species.
A few researchers can say that they are working on a whole-genome sequence acquired during the last century. Thanks to J. Craig Venter, then at The Institute for Genomic Research (Rockville, MD), and the U. S. Department of Energy, those of us now working on D. radiodurans can continue to do so (77). D. radiodurans was one of the first completed genomes to be published, revealing one of the great paradoxes in biology. There it was, for all to see and ponder, the genome of the most radiation-resistant organism yet discovered, D. radiodurans, encodes just about the same number and types of DNA repair proteins as most other organisms. Further, D. radiodurans encodes a standard set of antioxidant enzymes (30, 56). It was clear that the extreme radiation-, desiccation-, and oxidative stress-resistance phenotypes of Deinococcus bacteria would need to be explained in a new way, which meant revisiting some of its peculiar phenotypes. Certainly, Mn accumulation and the myriad of metabolic defects in D. radiodurans all pointed to a role of Mn antioxidants in its phenomenal resistance to oxidative stress (30). If Mn antioxidants in D. radiodurans protected and preserved the activity of conventional repair enzymes, then novel and highly ROS-resistant repair enzymes would not need to be evolved (25).
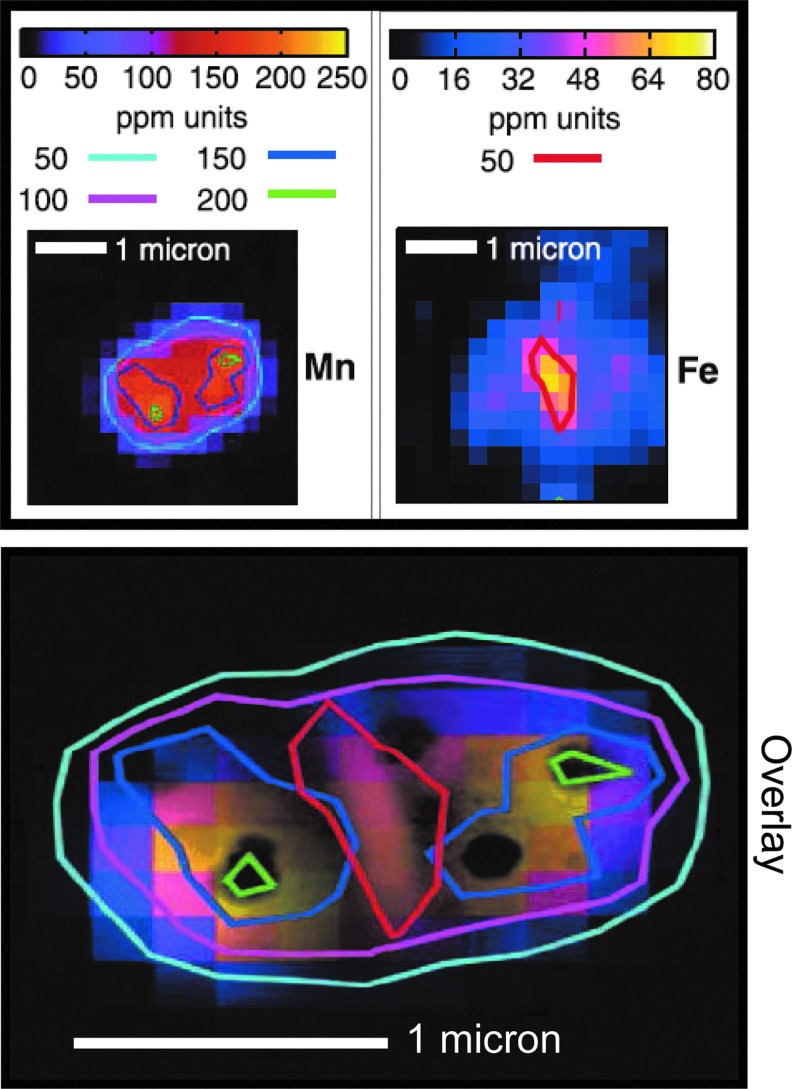
Defined minimal media compositions for D. radiodurans list Mn as an essential ingredient. In 1976, Bruce and colleagues first reported a large depot of Mn in D. radiodurans, which contained ∼100 times more Mn than the radiation-sensitive E. coli, and that Mn-depletion decreased the UV resistance of D. radiodurans (49). Using neutron activation analysis (NAA), they showed that D. radiodurans normally accumulated about 5 mM Mn. In 2004, the NAA results were corroborated using inductively coupled plasma mass spectrometry (25); and other studies showed that when D. radiodurans was incubated in defined minimal medium containing the radioisotope 54Mn, the cells accumulated about 2 mM Mn (25). More recently, X-ray fluorescence microspectroscopy revealed that Mn is distributed throughout D. radiodurans cells, but with regional intracellular Mn concentrations ranging from ∼0.5 to 2 mM (Fig. 6) (24). In contrast, most of the Fe in D. radiodurans was sequestered outside of the cytosol, in the septa of dividing cells (Fig. 6). Based on electron paramagnetic resonance spectroscopy and X-ray-absorption near-edge structure analyses, the dominant form of manganese in D. radiodurans cells is Mn2+, with no significant levels detected of Mn3+ or Mn4+, which are strong oxidants (24, 25). When D. radiodurans cells were grown under conditions that limited Mn accumulation, their cellular Mn concentration decreased markedly along with their resistance to γ-rays (Fig. 5). However, in vitro studies showed that Mn2+ alone did not significantly protect DNA from ROS, and only moderate levels of protection of proteins were observed compared with the level of protein protection in D. radiodurans cells. Something was missing in the in vitro Mn2+ studies (24).
FIG. 6.
XRF maps of the qualitative distribution and concentration gradients of manganese and iron in D. radiodurans. Transparent image overlay assembled from XRF microspectroscopy analyses (top) and transmission electron microscopy (not shown) of a single desiccated D. radiodurans diplococcus (22). Depth-average abundance of Mn (green, 200 ppm: 2 mM; dark blue, 150 ppm: 1.5 mM; mauve, 100 ppm: 1 mM; light blue, 50 ppm: 0.5 mM) and Fe (red, 50 ppm: 0.5 mM) are shown [data from ref. (24)]. XRF, X-ray fluorescence.
Over the last 50 years, members of the family Deinococcaceae have been isolated worldwide, from very diverse nutrient-poor environments (21). It was, therefore, surprising to find that extremely oxidative stress-resistant deinococcal isolates display numerous metabolic defects (22, 30, 75). D. radiodurans cells are not able to grow and are killed in radioactive nutritionally restricted environments in which luxuriant growth occurs in the absence of irradiation (75). Under nutrient-poor conditions, DNA repair in irradiated D. radiodurans is limited by its metabolic capabilities and not by any nutritionally induced defect in genetic repair (30). The question followed—could radiation resistance develop through the loss of metabolic functions? A striking informatic and experimentally verified feature of wild-type D. radiodurans is its inability to utilize inorganic sulfate (CysJ is missing), which results in its dependence on an exogenous source of sulfur-containing amino acids for growth (30). D. radiodurans growth and resistance also is dependent on methionine and nicotinamide adenine dinucleotide (oxidized form). Moreover, the cells contain highly expanded gene families encoding phosphatases, proteases, and peptide transporters (30), and they display a remarkable shift in the regulation of metabolic flux through the TCA cycle after irradiation (55). Collectively, the metabolic configuration of D. radiodurans is predicted to promote the accumulation of small molecules, and this raised the possibility that a major route to extreme resistance in cells which express Mn2+ uptake systems is via metabolite regulation (30). Numerous organisms that accumulate “compatible solutes” fit this model, including representative archaea, cyanobacteria, lichens, black yeast and fungi, and tardigrades (22), which are well known for their extreme radiation and desiccation resistance.
Intermediary organic metabolites are ordinarily present at extremely low intracellular concentrations. However, mutations that block biosynthetic reactions present themselves as routes to oxidative stress resistance by promoting the constitutive accumulation of precursors which precede the defective reactions. This idea links not only naturally resistant prokaryotes, but also highly resistant mutants evolved from sensitive bacteria in the laboratory. For bacteria, directed evolution of extremely radiation-resistant mutants, for example, has been achieved by the successive passage of cells through fractionated sublethal doses of γ-rays. Remarkably, the most resistant mutants consistently displayed a variety of growth defects (64) that resemble auxotrophic nutritional modes manifested in many Deinococcus bacteria (75). The idea that oxidative stress resistance may develop through the loss of metabolic functions was reinforced by a whole-genome sequence analysis of D. radiodurans, where gene loss squarely accounted for its unusual growth defects (see above) (56, 75). It followed that selective oxidative inactivation of biosynthetic enzymes, such as those involved in amino acid production in normal cells, could contribute to oxidative stress resistance by promoting the accumulation of precursors. As ROS levels in cells increase, the most sensitive enzymes would be the first to fail, and predicted to cause metabolite accumulation. One example of a class of metabolic enzymes that is highly sensitive to ROS are dehydratases (37).
In 2010, Daly's groups showed that protein-free cell extracts of D. radiodurans are highly enriched in peptides, free amino acids, nucleosides, and their analogues containing two carbonyl oxygen groups (C=O) separated by one NH3 group—a configuration which forms stable Mn2+ complexes when deprotonated (23). Moreover, D. radiodurans activated proteases when under oxidative stress, which led to further accumulation of peptides. In contrast, metabolite profiling of oxidative stress-sensitive bacteria did not display small-molecule accumulation or evidence of protease induction (23). Based on D. radiodurans protein-free extracts, potent antioxidant complexes consisting of Mn2+, Pi, peptides, and nucleosides were reconstituted in the laboratory and shown to specifically protect proteins from oxidation (23). This reinforced the hypothesis that a major route to oxidative stress resistance in cells which express Mn transport systems is via metabolite regulation, which also appears to be the case in the model yeast S. cerevisiae (66) and model bacterium E. coli (3). Environmental isolates of ROS-resistant organisms that amass Mn and compatible solutes include spores of Bacillus species, which accumulate Mn2+ and dipicolinic acid (32); cyanobacteria accumulate Mn2+ and the non-reducing disaccharide trehalose (43, 44); and similarly for other Mn-accumulating prokaryotes which accumulate small organic molecules such as mycosporin-like amino acids (62) or mannosyl glycerate (68).
Deinococcus Mn-Peptide Complexes
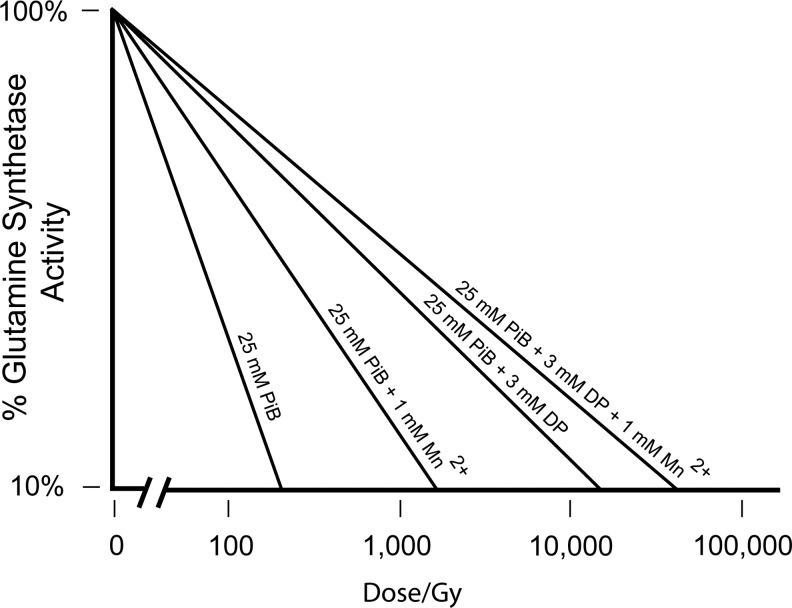
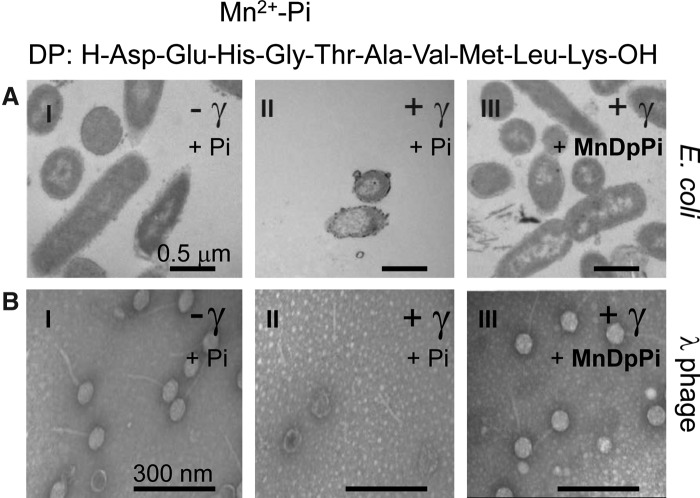
The most striking feature of the metabolite profile comparison between D. radiodurans and radiation-sensitive bacteria was the high concentration of peptides (2–22 amino acids in length) in D. radiodurans (23). The D. radiodurans peptide pool was derived mainly from its own proteins. Other peptides identified in D. radiodurans were yeast derived, presumably from the Yeast Extract growth medium. Based on sequence analyses of peptides derived from D. radiodurans, a consensus decapeptide (H-Asp-Glu-His-Gly-Thr-Ala-Val-Met-Leu-Lys-OH) was designed and tested for its ability to form ROS-scavenging Mn-complexes in Pi buffer (23). When reconstituted in vitro, these constituents interacted synergistically in preventing the inactivation of enzymes during supralethal irradiation. For example, at 50,000 Gy, the Mn2+-decapeptide-Pi complex preserved 50% in vitro activity of the dodecameric enzyme glutamine synthetase, which is normally inactivated by just 50 Gy. When the decapeptide was applied at physiologically relevant concentrations (3 mM Mn2+, 25 mM Pi, 3 mM decapeptide), the quaternary structures of proteins and their functions were preserved in aqueous solution at doses of γ-radiation that obliterated similarly treated DNA (23) (Fig. 7). Radioprotection assays were then extended to whole cells and viruses (29). The Mn-peptide complexes did not prevent genome damage, rendering the preparations sterile, but the bacteria and viruses exposed to massive doses of γ-rays remained morphologically intact down to the scale of individual surface proteins (Fig. 8); this approach has now been harnessed in preparing protective vaccines (29). In contrast, bacteria and viruses exposed to supralethal doses in the absence of the Mn-decapeptide complex were extremely damaged (Fig. 8). Among the Mn-complexes tested, Mn-peptide complexes were the most protective (23). Collectively, these findings validated the idea that Mn-peptide-based protein protection in D. radiodurans is key to its extreme oxidative stress resistance, and that its expanded family of proteases and peptide transports likely provide the core ingredients of the Mn-complexes (23, 30). Since then, it has been shown that the composition of the decapetide is critical to ROS scavenging, but the sequence is not (R.L. Levine et al., unpublished). For example, the presence of His in the decapeptide is very important to its ability to scavenge ROS in the presence of Mn2+, but the position of His in the decapeptide is not.
FIG. 7.
In vitro radioprotection of the dodecameric metabolic enzyme glutamine synthetase by Mn-antioxidants. Glutamine synthetase (622 kDa) was irradiated in the presence of Mn2+and/or the decapeptide (DP: H-Asp-Glu-His-Gly-Thr-Ala-Val-Met-Leu-Lys-OH) in potassium phosphate buffer (PiB), pH 7.4. Note that the x-axis is a logarithmic scale. Radiation source: 60Co γ-radiation (23).
FIG. 8.
Structural integrity of irradiated Escherichia coli cells and bacteriophage lambda viruses assessed by transmission electron microscopy after exposure to γ-radiation in the presence or absence of a reconstituted Deinococcus Mn2+-decapeptide complex (MnDpPi). (A) E. coli. (I), E. coli in PiB (non-irradiated control); (II) E. coli irradiated in PiB to 25 kGy; (III) E. coli irradiated in MnDpPi to 25 kGy. (B) Lambda phage. (I) λ phage in PiB (non-irradiated control); (II), λ phage irradiated in PiB to 40 kGy; (III) λ phage irradiated in MnDpPi to 40 kGy. PiB, 25 mM phosphate buffer pH 7.4; MnDpPi, 3 mM MnCl2+3 mM Dp:H-Asp-Glu-His-Gly-Thr-Ala-Val-Met-Leu-Lys-OH+25 mM PiB (29). Radiation source: 60Co γ-radiation (23).
Conclusion
The list of non-enzymatic methods for protection against oxidative stress is on the rise. Metabolites previously thought to function only in catabolic or anabolic pathways can now find a place in the cells battery for antioxidant defense. By complexing with cellular divalent manganese ions, specific anionic metabolites can enhance and focus the reactivity of the metal toward O2•−, and perhaps H2O2 as well (11, 12, 21, 22, 60). These metal-metabolite complexes are powerful shields for proteins against the ionizing effects of radiation and other origins of oxidative damage. Mn antioxidants are best known for their role in promoting survival of extremophiles such as the radiation-resistant Deinococcus and of organisms that lack SOD enzymes, such as L. plantarum, which is also radiation resistant (25), and now also in baker's yeast and perhaps in multi-cellular organisms as well (10, 51). These metal-metabolite complexes are becoming increasingly evident in all branches of life and, in particular, may play an important role in microbial pathogenesis. Thus far, only a handful of metabolites have been identified that function as partners for Mn2+ in antioxidant defense, including phosphate, carbonate, organic acids, free amino acids, and peptides. In the future, the list is likely to expand and become as diverse as the organisms that use these molecules for promoting survival in the aerobic atmosphere.
Abbreviations Used
- DSB
DNA double strand break repair
- EDTA
ethylenediaminetetraacetic acid
- ENDOR
electron nuclear double resonance
- H2O2
hydrogen peroxide
- NAA
neutron activation analysis
- O2•−
superoxide
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TCA
tricarboxylic acid
- WT
wild-type
- XRF
X-ray fluorescence
Acknowledgments
The authors are indebted to Dr. Fred Archibald for sharing his personal accounts on the discovery of the Mn antioxidant in L. plantarum. The work of M.J.D. is supported by the Air Force Office of Scientific Research (AFOSR), the Defense Advanced Research Projects Agency (DARPA), and the Defense Threat Reduction Agency (DTRA). V.C.C. is supported by NIH grants ES 08996 and R37 GM 50016.
References
- 1.Aguirre JD. Culotta VC. Battles with iron: manganese in oxidative stress protection. J Biol Chem. 2012;287:13541–13548. doi: 10.1074/jbc.R111.312181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Maghrebi M. Fridovich I. Benov L. Manganese supplementation relieves the phenotypic deficits seen in superoxide-dismutase-null Escherichia coli. Arch Biochem Biophys. 2002;402:104–109. doi: 10.1016/S0003-9861(02)00065-6. [DOI] [PubMed] [Google Scholar]
- 3.Anjem A. Imlay JA. Mononuclear iron enzymes are primary targets of hydrogen peroxide stress. J Biol Chem. 2012;287:15544–15456. doi: 10.1074/jbc.M111.330365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anjem A. Varghese S. Imlay JA. Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Mol Microbiol. 2009;72:844–858. doi: 10.1111/j.1365-2958.2009.06699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Archibald FS. Fridovich I. Manganese, superoxide dismutase and oxygen tolerance in some lactic acid bacteria. J Bacteriol. 1981;146:928–936. doi: 10.1128/jb.146.3.928-936.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Archibald FS. Fridovich I. Manganese and defenses against oxygen toxicity in Lactobacillus plantarum. J Bacteriol. 1981;145:422–451. doi: 10.1128/jb.145.1.442-451.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Archibald FS. Fridovich I. Investigations on the state of the manganese in Lactobacillus plantarum. Arch Biochim Biophys. 1982;215:589–596. doi: 10.1016/0003-9861(82)90120-5. [DOI] [PubMed] [Google Scholar]
- 8.Archibald FS. Fridovich I. The scavenging of superoxide radical by manganous complexes in vitro. Arch Biochem Biophys. 1982;214:452–463. doi: 10.1016/0003-9861(82)90049-2. [DOI] [PubMed] [Google Scholar]
- 9.Argueso JL. Westmoreland J. Mieczkowski PA. Gawel M. Petes TD. Resnick MA. Double-strand breaks associated with repetitive DNA can reshape the genome. Proc Natl Acad Sci U S A. 2008;105:11845–11850. doi: 10.1073/pnas.0804529105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bansal AK. Kaur AR. Cooperative functions of manganese and thiol redox system against oxidative stress in human spermatozoa. J Hum Reprod Sci. 2009;2:76–80. doi: 10.4103/0974-1208.57227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnese K. Gralla EB. Cabelli DE. Valentine JS. Manganous phosphate acts as a superoxide dismutase. J Am Chem Soc. 2008;130:4604–4606. doi: 10.1021/ja710162n. [DOI] [PubMed] [Google Scholar]
- 12.Barnese K. Gralla EB. Valentine JS. Cabelli DE. Biologically relevant mechanism for catalytic superoxide removal by simple manganese compounds. Proc Natl Acad Sci U S A. 2012;109:6892–6897. doi: 10.1073/pnas.1203051109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cassat JE. Skaar EP. Metal ion acquisition in Staphylococcus aureus: overcoming nutritional immunity. Semin Immunopathol. 2012;34:215–235. doi: 10.1007/s00281-011-0294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang EC. Kosman DJ. Intracellular Mn(II)-associated superoxide scavenging activity protects Cu,Zn superoxide dismutase-deficient Saccharomyces cerevisiae against dioxygen stress. J Biol Chem. 1989;264:12172–12178. [PubMed] [Google Scholar]
- 15.Cho JH. Ko KM. Singaravelu G. Ahnn J. Caenorhabditis elegans PMR1, a P-type calcium ATPase, is important for calcium/manganese homeostasis and oxidative stress response. FEBS Lett. 2005;579:778–782. doi: 10.1016/j.febslet.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 16.Corbin BD. Seeley EH. Raab A. Feldmann J. Miller MR. Torres VJ. Anderson KL. Dattilo BM. Dunman PM. Gerads R. Caprioli RM. Nacken W. Chazin WJ. Skaar EP. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science. 2008;319:962–965. doi: 10.1126/science.1152449. [DOI] [PubMed] [Google Scholar]
- 17.Dale WM. The effect of X-rays on enzymes. Biochem J. 1940;34:1367–1373. doi: 10.1042/bj0341367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dale WM. The effect of X-rays on the conjugated protein d-amino-acid oxidase. Biochem J. 1942;36:80–85. doi: 10.1042/bj0360080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dale WM. Effects of X-rays on acetylcholine solutions showing the dilution and protection phenomena, found for enzymes. J Physiol. 1943;102:50–54. doi: 10.1113/jphysiol.1943.sp004013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daly MJ. Modulating radiation resistance: insights based on defenses against reactive oxygen species in the radioresistant bacterium Deinococcus radiodurans. Clin Lab Med. 2006;26:491–504. doi: 10.1016/j.cll.2006.03.009. x. [DOI] [PubMed] [Google Scholar]
- 21.Daly MJ. A new perspective on radiation resistance based on Deinococcus radiodurans. Nat Rev Microbiol. 2009;7:237–245. doi: 10.1038/nrmicro2073. [DOI] [PubMed] [Google Scholar]
- 22.Daly MJ. Death by protein damage in irradiated cells. DNA Repair (Amst) 2012;11:12–21. doi: 10.1016/j.dnarep.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 23.Daly MJ. Gaidamakova EK. Matrosova VY. Kiang JG. Fukumoto R. Lee DY. Wehr NB. Viteri GA. Berlett BS. Levine RL. Small-molecule antioxidant proteome-shields in Deinococcus radiodurans. PLoS One. 2010;5:e12570. doi: 10.1371/journal.pone.0012570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daly MJ. Gaidamakova EK. Matrosova VY. Vasilenko A. Zhai M. Leapman RD. Lai B. Ravel B. Li SM. Kemner KM. Fredrickson JK. Protein oxidation implicated as the primary determinant of bacterial radioresistance. PLoS Biol. 2007;5:e92. doi: 10.1371/journal.pbio.0050092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daly MJ. Gaidamakova EK. Matrosova VY. Vasilenko A. Zhai M. Venkateswaran A. Hess M. Omelchenko MV. Kostandarithes HM. Makarova KS. Wackett LP. Fredrickson JK. Ghosal D. Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance. Science. 2004;306:1025–1028. doi: 10.1126/science.1103185. [DOI] [PubMed] [Google Scholar]
- 26.Dickinson JR. Dawes IW. Boyd AS. Baxter RL. 13C NMR studies of acetate metabolism during sporulation of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1983;80:5847–5851. doi: 10.1073/pnas.80.19.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esteve-Gassent MD. Elliott NL. Seshu J. sodA is essential for virulence of Borrelia burgdorferi in the murine model of Lyme disease. Mol Microbiol. 2009;71:594–612. doi: 10.1111/j.1365-2958.2008.06549.x. [DOI] [PubMed] [Google Scholar]
- 28.Fridovich I. Oxygen toxicity: a radical explanation. J Exp Biol. 1998;201:1203–1209. doi: 10.1242/jeb.201.8.1203. [DOI] [PubMed] [Google Scholar]
- 29.Gaidamakova EK. Myles IA. McDaniel DP. Fowler CJ. Valdez PA. Naik S. Gayen M. Gupta P. Sharma A. Glass PJ. Maheshwari RK. Datta SK. Daly MJ. Preserving immunogenicity of lethally irradiated viral and bacterial vaccine epitopes using a radio- protective mn(2+)-Peptide complex from Deinococcus. Cell Host Microbe. 2012;12:117–124. doi: 10.1016/j.chom.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghosal D. Omelchenko MV. Gaidamakova EK. Matrosova VY. Vasilenko A. Venkateswaran A. Zhai M. Kostandarithes HM. Brim H. Makarova KS. Wackett LP. Fredrickson JK. Daly MJ. How radiation kills cells: survival of Deinococcus radiodurans and Shewanella oneidensis under oxidative stress. FEMS Microbiol Rev. 2005;29:361–375. doi: 10.1016/j.femsre.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Govoni G. Gros P. Microphage NRAMP1 and its role in resistance to microbial infections. Inflamm Res. 1998;47:277–284. doi: 10.1007/s000110050330. [DOI] [PubMed] [Google Scholar]
- 32.Granger AC. Gaidamakova EK. Matrosova VY. Daly MJ. Setlow P. Effects of Mn and Fe levels on Bacillus subtilis spore resistance and effects of Mn2+, other divalent cations, orthophosphate, and dipicolinic acid on protein resistance to ionizing radiation. Appl Environ Microbiol. 2011;77:32–40. doi: 10.1128/AEM.01965-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gruenheid S. Pinner E. Desjardins M. Gros P. Natural resistance to infection with intracellular pathogens: the Nramp1 protein is recruited to the membrane of the phagosome. J Exp Med. 1997;185:717–730. doi: 10.1084/jem.185.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gunter TE. Gavin CE. Aschner M. Gunter KK. Speciation of manganese in cells and mitochondria: a search for the proximal cause of manganese neurotoxicity. Neurotoxicology. 2006;27:765–776. doi: 10.1016/j.neuro.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Horsburgh MJ. Wharton SJ. Cox AG. Ingham E. Peacock S. Foster SJ. MntR modulates expression of the PerR regulon and superoxide resistance in Staphylococcus aureus through control of manganese uptake. Mol Microbiol. 2002;44:1269–1286. doi: 10.1046/j.1365-2958.2002.02944.x. [DOI] [PubMed] [Google Scholar]
- 36.Horsburgh MJ. Wharton SJ. Karavolos M. Foster SJ. Manganese: elemental defence for a life with oxygen. Trends Microbiol. 2002;10:496–501. doi: 10.1016/s0966-842x(02)02462-9. [DOI] [PubMed] [Google Scholar]
- 37.Imlay JA. Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem. 2008;77:755–776. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inaoka T. Matsumura Y. Tsuchido T. SodA and manganese are essential for resistance to oxidative stress in growing and sporulating cells of Bacillus subtilis. J Bacteriol. 1999;181:1939–1943. doi: 10.1128/jb.181.6.1939-1943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson TE. Hartman PS. Radiation effects on life span in Caenorhabditis elegans. J Gerontol. 1988;43:B137–B141. doi: 10.1093/geronj/43.5.b137. [DOI] [PubMed] [Google Scholar]
- 40.Keele BB. McCord JM. Fridovich I. Superoxide dismutase from Escherichia coli B: a new manganese containing enzyme. J Biol Chem. 1970;245:6176–6181. [PubMed] [Google Scholar]
- 41.Kehl-Fie TE. Chitayat S. Hood MI. Damo S. Restrepo N. Garcia C. Munro KA. Chazin WJ. Skaar EP. Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus. Cell Host Microbe. 2011;10:158–164. doi: 10.1016/j.chom.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kehl-Fie TE. Skaar EP. Nutritional immunity beyond iron: a role for manganese and zinc. Curr Opin Chem Biol. 2010;14:218–224. doi: 10.1016/j.cbpa.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keren N. Kidd MJ. Penner-Hahn JE. Pakrasi HB. A light-dependent mechanism for massive accumulation of manganese in the photosynthetic bacterium Synechocystis sp. PCC 6803. Biochemistry. 2002;41:15085–15092. doi: 10.1021/bi026892s. [DOI] [PubMed] [Google Scholar]
- 44.Klahn S. Hagemann M. Compatible solute biosynthesis in cyanobacteria. Environ Microbiol. 2011;13:551–562. doi: 10.1111/j.1462-2920.2010.02366.x. [DOI] [PubMed] [Google Scholar]
- 45.Kono Y. Takahashi MA. Asada K. Oxidation of manganous pyrophosphate by superoxide radicals and illuminated spinach chloroplasts. Arch Biochem Biophys. 1976;174:454–462. doi: 10.1016/0003-9861(76)90373-8. [DOI] [PubMed] [Google Scholar]
- 46.Krisko A. Radman M. Protein damage and death by radiation in Escherichia coli and Deinococcus radiodurans. Proc Natl Acad Sci U S A. 2010;107:14373–14377. doi: 10.1073/pnas.1009312107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lapinskas PJ. Cunningham KW. Liu XF. Fink GR. Culotta VC. Mutations in PMR1 suppress oxidative damage in yeast cells lacking superoxide dismutase. Mol Cell Biol. 1995;15:1382–1388. doi: 10.1128/mcb.15.3.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lapinskas PJ. Lin SJ. Culotta VC. The role of the Saccharomyces cerevisiae CCC1 gene in the homeostasis of manganese ions. Mol Microbiol. 1996;21:519–528. doi: 10.1111/j.1365-2958.1996.tb02561.x. [DOI] [PubMed] [Google Scholar]
- 49.Leibowitz PJ. Schwartzberg LS. Bruce AK. The in vivo association of manganese with the chromosome of Micrococcus radiodurans. Photochem Photobiol. 1976;23:45–50. doi: 10.1111/j.1751-1097.1976.tb06769.x. [DOI] [PubMed] [Google Scholar]
- 50.Lin SJ. Culotta VC. Suppression of oxidative damage by Saccharomyces cerevisiae ATX2, encoding a manganese trafficking protein that localizes to Golgi-like vesicles in yeast. Mol Cell Biol. 1996;16:6303–6312. doi: 10.1128/mcb.16.11.6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin YT. Hoang H. Hsieh SI. Rangel N. Foster AL. Sampayo JN. Lithgow GJ. Srinivasan C. Manganous ion supplementation accelerates wild type development, enhances stress resistance, and rescues the life span of a short-lived Caenorhabditis elegans mutant. Free Radic Biol Med. 2006;40:1185–1193. doi: 10.1016/j.freeradbiomed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 52.Liu XF. Culotta VC. The requirement for yeast superoxide dismutase is bypassed through mutations in BSD2, a novel metal homeostasis gene. Mol Cell Biol. 1994;14:7037–7045. doi: 10.1128/mcb.14.11.7037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu XF. Elashvili I. Gralla EB. Valentine JS. Lapinskas P. Culotta VC. Yeast lacking superoxide dismutase: isolation of genetic suppressors. J Biol Chem. 1992;267:18298–18302. [PubMed] [Google Scholar]
- 54.Liu XF. Supek F. Nelson N. Culotta VC. Negative control of heavy metal uptake by the Saccharomyces cerevisiae BSD2 gene. J Biol Chem. 1997;272:11763–11769. doi: 10.1074/jbc.272.18.11763. [DOI] [PubMed] [Google Scholar]
- 55.Liu Y. Zhou J. Omelchenko MV. Beliaev AS. Venkateswaran A. Stair J. Wu L. Thompson DK. Xu D. Rogozin IB. Gaidamakova EK. Zhai M. Makarova KS. Koonin EV. Daly MJ. Transcriptome dynamics of Deinococcus radiodurans recovering from ionizing radiation. Proc Natl Acad Sci U S A. 2003;100:4191–4196. doi: 10.1073/pnas.0630387100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Makarova KS. Aravind L. Wolf YI. Tatusov RL. Minton KW. Koonin EV. Daly MJ. Genome of the extremely radiation-resistant bacterium Deinococcus radiodurans viewed from the perspective of comparative genomics. Microbiol Mol Biol Rev. 2001;65:44–79. doi: 10.1128/MMBR.65.1.44-79.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mao GD. Poznansky MJ. Electron spin resonance study on the permeability of superoxide radicals in lipid bilayers and biological membranes. FEBS Lett. 1992;305:233–236. doi: 10.1016/0014-5793(92)80675-7. [DOI] [PubMed] [Google Scholar]
- 58.McCord JM. Oxygen-derived free radicals. New Horiz. 1993;1:70–76. [PubMed] [Google Scholar]
- 59.McCord JM. Fridovich I. Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 60.McNaughton RL. Reddi AR. Clement MH. Sharma A. Barnese K. Rosenfeld L. Gralla EB. Valentine JS. Culotta VC. Hoffman BM. Probing in vivo Mn2+ speciation and oxidative stress resistance in yeast cells with electron-nuclear double resonance spectroscopy. Proc Natl Acad Sci U S A. 2010;107:15335–15339. doi: 10.1073/pnas.1009648107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Munroe W. Kingsley C. Durazo A. Gralla EB. Imlay JA. Srinivasan C. Valentine JS. Only one of a wide assortment of manganese-containing SOD mimicking compounds rescues the slow aerobic growth phenotypes of both Escherichia coli and Saccharomyces cerevisiae strains lacking superoxide dismutase enzymes. J Inorg Biochem. 2007;101:1875–1882. doi: 10.1016/j.jinorgbio.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oren A. Gunde-Cimerman N. Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol Lett. 2007;269:1–10. doi: 10.1111/j.1574-6968.2007.00650.x. [DOI] [PubMed] [Google Scholar]
- 63.Ouyang Z. He M. Oman T. Yang XF. Norgard MV. A manganese transporter, BB0219 (BmtA), is required for virulence by the Lyme disease spirochete, Borrelia burgdorferi. Proc Natl Acad Sci U S A. 2009;106:3449–3454. doi: 10.1073/pnas.0812999106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parisi A. Antoine AD. Increased radiation resistance of vegetative Bacillus pumilus. Appl Microbiol. 1974;28:41–46. doi: 10.1128/am.28.1.41-46.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ravindranath SD. Fridovich I. Isolation and characterization of a manganese-containing superoxide dismutase from yeast. J Biol Chem. 1975;250:6107–6112. [PubMed] [Google Scholar]
- 66.Reddi AR. Culotta VC. Regulation of manganese antioxidants by nutrient sensing pathways in Saccharomyces cerevisiae. Genetics. 2011;189:1261–1270. doi: 10.1534/genetics.111.134007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reddi AR. Jensen LT. Naranuntarat A. Rosenfeld L. Leung E. Shah R. Culotta VC. The overlapping roles of manganese and Cu/Zn SOD in oxidative stress protection. Free Radic Biol Med. 2009;46:154–162. doi: 10.1016/j.freeradbiomed.2008.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robinson CK. Webb K. Kaur A. Jaruga P. Dizdaroglu M. Baliga NS. Place A. Diruggiero J. A major role for nonenzymatic antioxidant processes in the radioresistance of Halobacterium salinarum. J Bacteriol. 2011;193:1653–1662. doi: 10.1128/JB.01310-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rosenfeld L. Reddi AR. Leung E. Aranda K. Jensen LT. Culotta VC. The effect of phosphate accumulation on metal ion homeostasis in Saccharomyces cerevisiae. J Biol Inorg Chem. 2010;15:1051–1062. doi: 10.1007/s00775-010-0664-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sanchez RJ. Srinivasan C. Munroe WH. Wallace MA. Martins J. Kao TY. Le K. Gralla EB. Valentine JS. Exogenous manganous ion at millimolar levels rescues all known dioxygen-sensitive phenotypes of yeast lacking CuZnSOD. J Biol Inorg Chem. 2005;10:913–923. doi: 10.1007/s00775-005-0044-y. [DOI] [PubMed] [Google Scholar]
- 71.Seib KL. Tseng HJ. McEwan AG. Apicella MA. Jennings MP. Defenses against oxidative stress in Neisseria gonorrhoeae and Neisseria meningitidis: distinctive systems for different lifestyles. J Infect Dis. 2004;190:136–147. doi: 10.1086/421299. [DOI] [PubMed] [Google Scholar]
- 72.Slade D. Radman M. Oxidative stress resistance in Deinococcus radiodurans. Microbiol Mol Biol Rev. 2011;75:133–191. doi: 10.1128/MMBR.00015-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stadtman ER. Levine RL. Protein modifications to cellular dysfunction and diseases. In: Dalle-Donne I, editor; Scaloni A, editor; Butterfield DA, editor. Redox Proteomics. Hoboken, NJ: John Wiley & Sons, Inc.; 2006. pp. 123–168. [Google Scholar]
- 74.Tseng HJ. Srikhanta Y. McEwan AG. Jennings MP. Accumulation of manganese in Neisseria gonorrhoeae correlates with resistance to oxidative killing by superoxide anion and is independent of superoxide dismutase activity. Mol Microbiol. 2001;40:1175–1186. doi: 10.1046/j.1365-2958.2001.02460.x. [DOI] [PubMed] [Google Scholar]
- 75.Venkateswaran A. McFarlan SC. Ghosal D. Minton KW. Vasilenko A. Makarova K. Wackett LP. Daly MJ. Physiologic determinants of radiation resistance in Deinococcus radiodurans. Appl Environ Microbiol. 2000;66:2620–2626. doi: 10.1128/aem.66.6.2620-2626.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weisiger RA. Fridovich I. Mitochondrial superoxide dismutase. J Biol Chem. 1973;248:4793–4796. [PubMed] [Google Scholar]
- 77.White O. Eisen JA. Heidelberg JF. Hickey EK. Peterson JD. Dodson RJ. Haft DH. Gwinn ML. Nelson WC. Richardson DL. Moffat KS. Qin H. Jiang L. Pamphile W. Crosby M. Shen M. Vamathevan JJ. Lam P. McDonald L. Utterback T. Zalewski C. Makarova KS. Aravind L. Daly MJ. Minton KW. Fleischmann RD. Ketchum KA. Nelson KE. Salzberg S. Smith HO. Venter JC. Fraser CM. Genome sequence of the radioresistant bacterium Deinococcus radiodurans R1. Science. 1999;286:1571–1577. doi: 10.1126/science.286.5444.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Winterbourn CC. Kettle AJ. Redox reactions and microbial killing in the neutrophil phagosome. Antioxid Redox Signal. 2012;18:642–660. doi: 10.1089/ars.2012.4827. [DOI] [PubMed] [Google Scholar]
- 79.Yost FJ. Fridovich I. An iron-containing superoxide dismutase from Escherichia coli. J Biol Chem. 1973;248:4905–4908. [PubMed] [Google Scholar]