Abstract
Significance
Neutrophils are one of the most abundant cells of the immune system and they are extremely active during the repair of cutaneous wounds. In general, the antimicrobial activity of neutrophils is effective and allows these cells to carry out their primary function of preventing wounds from becoming infected.
Recent Advances
It is now known that in addition to sterilizing the wound, the weapons used by neutrophils to kill potential pathogens can also cause significant tissue damage to the host. This additional damage can lead to delayed healing and excessive scar formation.
Critical Issues
Much of the host damage caused by neutrophils results from the activity of proteases secreted by these cells. The clinical significance of this problem is highlighted by numerous studies showing that high levels of neutrophil-derived proteases are associated with chronic, non-healing wounds.
Future Directions
Studies are currently being performed to evaluate new ways of counteracting protease activity in chronic wounds. Additional studies will have to be carried out to determine whether neutralizing neutrophil proteases can improve the healing of chronic wounds without sacrificing the ability of neutrophils to eliminate pathogens and risking infection.

Traci A. Wilgus, PhD
Scope and Significance
Efficient wound repair requires the coordinated effort of many different cell types. A healing wound typically goes through phases of inflammation, proliferation, and remodeling/scar formation. The first of these phases, inflammation, is an important part of the wound-healing response. Inflammatory cells, such as neutrophils (or polymorphonuclear cells) are one of the first inflammatory cells to be recruited to the site of a wound. Their primary function is to prevent infection by attacking any microbes attempting to invade the body through the open skin wound. Neutrophils produce a collection of chemical weapons used to combat microbes that includes antimicrobial peptides, reactive oxygen species, and proteases. Unfortunately, there is often collateral tissue damage associated with the release of these protective mediators. In all likelihood, this is the reason that inflammation, a relatively early event in the repair process, can have long-lasting effects that influence not only the speed of repair, but also the quality of the healed wound (e.g., the amount of scar tissue produced). This article will provide an overview of neutrophils in wound healing, emphasizing the effects of neutrophil-derived proteases on wound repair.
Translational Relevance
As is the case with many types of inflammatory cells, there are advantages and disadvantages to eliciting a strong neutrophil response after injury. While the benefit of having a strong neutrophil response early after injury to fight off potential pathogens and reduce the chance of infection is appreciated, neutrophils can have harmful effects as well. Unfortunately, the activity of many enzymes and chemicals used by neutrophils to kill microbes is not specific to pathogens, so they can damage host tissues when released extracellularly. The multiple proteases released by neutrophils can be particularly problematic for healing wounds when extracellular levels are abnormally high. More work needs to be done to find ways of optimizing the antimicrobial effects of neutrophils while limiting the amount of tissue damage these cells cause to host tissues.
Clinical Relevance
In a normally healing wound, a robust neutrophil response is limited to the acute wound setting. However, active neutrophils are observed for an extended period of time in chronic wounds. It has been suggested that excessive protease production by activated neutrophils can cleave growth factors and growth factor receptors causing inactivation. These proteases can also degrade the extracellular matrix to a point where it becomes an ineffective substrate for cellular migration and enlarges the area in need of repair. Ultimately, this additional tissue destruction causes persistent inflammation and more tissue damage, which then prevents the wound from progressing through the proper stages of repair. Indeed, high levels of neutrophil-derived proteases, such as serine proteases and matrix metalloproteinases (MMPs), have been reported in chronic, nonhealing wounds and active research is being conducted to assess the potential clinical value of inhibiting protease activity in chronic wounds.
Discussion of Findings and Relevant Literature
Neutrophils and wound healing
Neutrophils are part of the innate immune system, and these cells carry out a variety of functions during the normal wound repair process (Fig. 1). Neutrophils are not frequently observed in normal skin, but they are recruited in high numbers after tissue injury (Fig. 2). They are the first circulating inflammatory cell to move to the site of the wound, which is consistent with their primary role in defending against infection. Neutrophils produce antimicrobial substances and proteases that help kill and degrade potential pathogens, but their ability to generate multiple cytokines and growth factors allows them to do more than simply minimize the risk of infection. In fact, studies have demonstrated that a variety of genes known to be important for wound healing are transcriptionally upregulated as neutrophils leave the bloodstream and reach the site of injury. For example, the expression of genes encoding proteins that recruit and activate more neutrophils and other inflammatory cells, promote angiogenesis, and stimulate keratinocyte and fibroblast proliferation is elevated in neutrophils that have been recruited to wounds compared to circulating neutrophils.1 While neutrophils are an integral part of the inflammatory response and can secrete signals which amplify inflammation at the early stages of healing, recent evidence suggests that these cells also act as a signal to shut down the inflammatory phase of healing.2 In a normally healing wound, neutrophils undergo apoptosis after performing their function at the wound site. Apoptotic neutrophils are eventually engulfed by macrophages, and the uptake of apoptotic cells by macrophages provides a strong signal for the resolution of inflammation.3,4 Presumably, this allows the wound to continue through the subsequent phases of healing. Therefore, continued recruitment of active neutrophils or a build up of apoptotic neutrophils due to dysregulated neutrophil apoptosis or reduced clearance by macrophages could then prolong inflammation and contribute to the development of chronic wounds.5,6 Thus, in addition to using their phagocytic capabilities to clear the wound of potential pathogens, neutrophils also help regulate inflammation and produce mediators that activate other cells important for the repair process.2
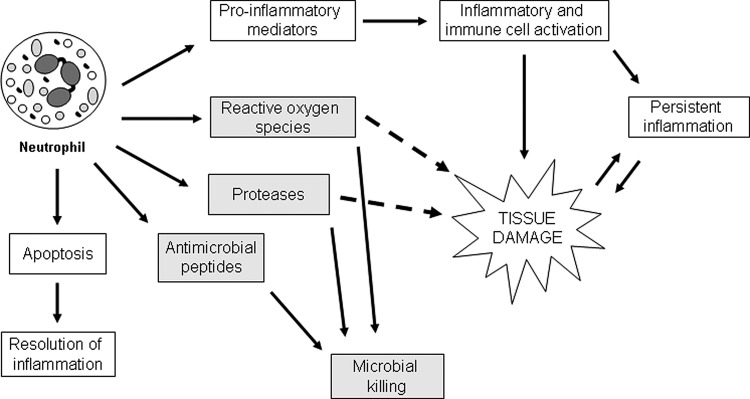
Figure 1.
Overview of neutrophil activities during cutaneous repair. Circulating neutrophils are recruited to the wound site quickly after injury, where they carry out a variety of functions. They produce an array of proinflammatory mediators that recruit and activate other inflammatory cells, enhancing inflammation. In normal wounds, recruited neutrophils will eventually undergo apoptosis and be engulfed by macrophages, initiating a resolution program that terminates the inflammatory response. However, in non-healing wounds, inflammatory cells often continue to be recruited and activated, leading to persistent inflammation. Activated neutrophils help prevent wound infection by generating reactive oxygen species and producing proteases and antimicrobial peptides. These substances kill and degrade potentially pathogenic microbes, but reactive oxygen species and proteases, in particular, can cause tissue damage when released extracellularly. Excessive proteases can be especially harmful, causing unwanted degradation of the extracellular matrix and additional tissue damage. This can lead to a harmful cycle, whereby the damage caused by neutrophil-derived proteases causes even more inflammation. This leads to more tissue damage, eventually stalling the wound-healing process and preventing complete closure of the wound.
Figure 2.
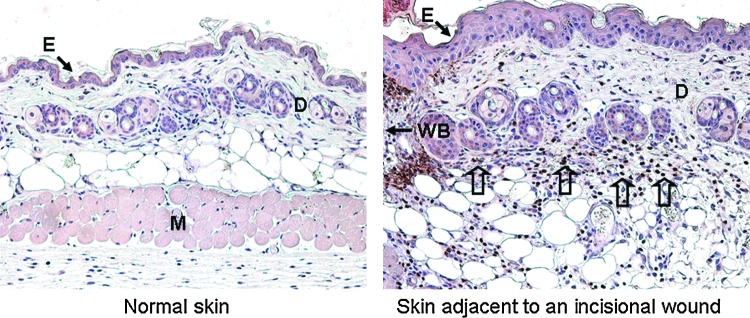
Neutrophil recruitment in the tissue adjacent to an acute murine skin wound. Ly-6G immunostaining is commonly used to detect tissue neutrophils (brown staining). Neutrophils are not frequently observed in normal skin of 6-week-old FVB mice (left). However, damage to the skin sets in motion a series of events causing neutrophils to travel from the bloodstream to the site of injury. This is a rapid process, and a large number of neutrophils can be seen at the wound margin, or the tissue immediately adjacent to the wound bed (WB), after injury (right). The image shows skin adjacent to a full-thickness incisional FVB mouse wound at 48 h post-injury. Open arrows are used to indicate areas heavily populated with neutrophils (brown cells). E, epidermis; D, dermis; M, muscle; WB, wound bed. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Traditionally, neutrophils have been considered important for efficient wound repair. Clinical observations support this idea, as neutropenic individuals or patients with diseases characterized by defects in neutrophil trafficking or function (deficiencies in adhesion or neutrophil granules, impaired respiratory burst activity, etc.)7 are at a higher risk for developing wound infections and often have difficulty healing wounds.2 However, the value of having a large number of activated neutrophils in a healing wound has been heavily debated in recent years. Studies in animal models have suggested that neutrophils are dispensable for cutaneous wound repair8 and that in wounds with a low risk of infection, neutrophils may actually impede re-epithelialization or increase scar tissue production.9,10 It should be noted that the percentage of circulating neutrophils in the blood differs between humans and animals like mice, which are commonly used to study wound healing.11 In theory, these species-specific alterations could mean there are differences with regard to the importance of neutrophils in the overall healing response between humans and animals. However, the prominence of neutrophils in chronic, nonhealing human wounds is in line with animal studies linking abundant neutrophils to delayed healing and suggests that an overactive or prolonged neutrophil response can be detrimental to wound healing.12,13 This has led to the idea that neutrophils play dual roles in wound healing. On one hand, neutrophils can kill invading microorganisms and stimulate other immune cells to effectively eliminate threats of infection. On the other hand, because the activity of toxic antimicrobial substances and proteases is not specific to pathogens, neutrophil-derived mediators can cause even more damage to host tissues and potentially delay the repair process.
Neutrophil granules: a rich source of proteases
To kill and degrade potential pathogens, neutrophils are armed with a variety of antimicrobial substances and proteases. The majority of these molecules are stored in cytoplasmic granules that can either be released into phagolysosomes after a microbe has been engulfed through phagocytosis or released into the extracellular space.2 Storage of these mediators in granules ensures that there are plenty of these proteins available and ready to be used if there is a threat of infection. Additionally, because many of these substances are nonspecific and can be toxic to the host as well as microorganisms, containment within granules also helps prevent these substances from damaging the neutrophil and host tissues. However, if neutrophils are highly stimulated or continually activated, granule contents can be released from the cell, risking unwanted damage to the extracellular matrix and surrounding host cells. The proteins contained within intracellular granules are produced at distinct stages of neutrophil development, leading to the generation of unique granule types enriched with different proteins. As a neutrophil matures in the bone marrow, it begins as an undifferentiated myeloblast with very few granules and gains a greater number of granules as it moves through the differentiation process in the bloodstream, moving from an immature promyelocyte to a segmented neutrophil (Fig. 3). In addition to the granules containing different subsets of proteins (Table 1), each granule type also differs in how quickly their contents can be released.
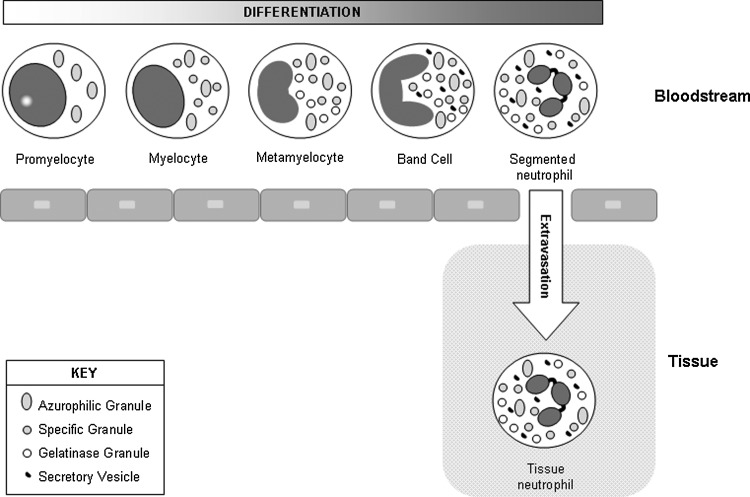
Figure 3.
Neutrophil maturation and granule formation. Mature neutrophils have a variety of granule types that contain distinct sets of mediators involved in neutrophil function. Neutrophil precursors originate in the bone marrow and undergo differentiation in the blood. The formation of different granule types coincides with specific stages of neutrophil development. Azurophilic granules begin to form at the promyelocyte stage, specific granule formation spans the myelocyte and metamyelocyte stages, and gelatinase granules are produced in metamyelocytes and band cells. Secretory vesicles form last through endocytosis. Because gene expression patterns change during the course of neutrophil development, each granule type contains a distinct set of proteins. During an inflammatory response, mature neutrophils leave the bloodstream by undergoing extravasation. After traversing the endothelial cell lining of blood vessels, neutrophils enter the tissue and migrate to the site of inflammation or injury.
Table 1.
Localization and function of prominent neutrophil proteases and antimicrobial proteins
| Granule Type | Sequence of Release | Protein | Function |
|---|---|---|---|
| Azurophilic granule (primary granule) | Fourth | α1-antitrypsin | Protease inhibitor |
| Azurocidin | Antimicrobial | ||
| Bactericidal/permeability-increasing protein | Antimicrobial | ||
| Cathepsin G | Antimicrobial; protease | ||
| Defensins | Antimicrobial | ||
| Elastase | Antimicrobial; protease | ||
| Lysozyme | Antimicrobial | ||
| Myeloperoxidase | Antimicrobial | ||
| Protease-3 | Antimicrobial; protease | ||
| Specific granule (secondary granule) | Third | hCAP-18/LL-37 (cathelicidin) | Antimicrobial |
| Lactoferrin | Antimicrobial | ||
| Lysozyme | Antimicrobial | ||
| MMP-8 (collagenase) | Protease | ||
| Secretory leukocyte protease inhibitor | Antimicrobial; protease inhibitor | ||
| Gelatinase granule (tertiary granule) | Second | Lysozyme | Antimicrobial |
| MMP-2 (gelatinase) | Protease | ||
| MMP-8 (collagenase) | Protease | ||
| MMP-9 (gelatinase) | Protease | ||
| MMP-25 (leukolysin) | Protease | ||
| Secretory vesicles | First | MMP-25 (leukolysin) | Protease |
hCAP, human cationic antimicrobial protein; MMP, matrix metalloproteinase.
Azurophilic granules (or primary granules) are the earliest granules to form and begin to appear at the promyelocyte stage.14 Azurophilic granules contain the highest concentrations of antimicrobial substances and are the only peroxidase-positive neutrophil granules. High levels of myeloperoxidase, an enzyme used to generate toxic reactive oxygen species, are present. In addition, azurocidin, bacterial permeability-increasing protein, lysozyme, defensins, and several serine proteases (cathepsin G, elastase, and protease 3), are found in these granules. Although azurophilic granules are the first to be synthesized, they are the last to undergo exocytosis in response to activating stimuli. For this reason, they are believed to be particularly important for intracellular killing of engulfed microbes, by fusing with and emptying their contents into the phagolysosome.14
Specific granules (or secondary granules) are the next to form during the myelocyte and metamyelocyte stages.14 They contain antimicrobial molecules such as the human cationic antimicrobial protein (hCAP-18; a cathelicidin family member) and lactoferrin. MMP-8 or collagenase-2, is also found in specific granules. Gelatinase granules (or tertiary granules), on the other hand, are produced during the metamyelocyte and band cell stages of neutrophil development. Like specific granules, gelatinase granules also contain some MMP-8, but they contain especially high levels of MMPs with the gelatinase activity, including MMP-2, MMP-9, and MMP-25. MMP-25 or leukolysin, is a relatively recently described membrane-bound enzyme found specifically in granulocytes.15 It has activity against collagen IV, fibronectin, and other extracellular matrix proteins.
Secretory vesicles are sometimes classified as a fourth type of neutrophil granule. Secretory vesicles are formed in segmented neutrophils by endocytosis after the other granules are produced, and their contents can be released very quickly after stimulation.14 This compartment contains MMP-25 as well as many receptors (i.e., integrins, growth factors, and cytokine receptors) that are translocated to the surface of the neutrophil as the vesicles fuse with the plasma membrane to release their contents.16
Neutrophil-derived proteases
In addition to containing an array of antimicrobial molecules, neutrophil granules are rich in proteases (Table 1). The primary function of these proteases is to help kill and degrade microbes, but they can also be used by the neutrophil to degrade components of the basement membrane and extracellular matrix (Fig. 4). This can aid in the movement of neutrophils from the circulation, into the tissue, and to the site of injury.2,17 Clinically, these proteases are also important because they are responsible for the added damage to host tissues caused by neutrophils when released into the extracellular space at high concentrations. Two main classes of proteases released by neutrophils are involved in wound healing: serine proteases and MMPs (Fig. 5).
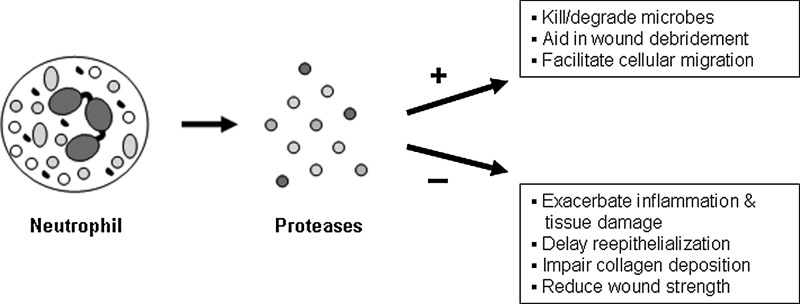
Figure 4.
Beneficial and detrimental effects of neutrophil-derived proteases on wound healing. Activated neutrophils produce a number of proteases that can have positive (+) or negative (−) effects on the cutaneous repair process. Neutrophil-derived proteases help kill and degrade microbes and break down components of the extracellular matrix, which can debride the wound and facilitate cellular migration when present at appropriate levels. Conversely, excessive levels of proteases in the tissue resulting from overly active neutrophils or prolonged neutrophil recruitment can be detrimental by causing additional tissue damage and further inflammation. Consequently, defective collagen deposition, reduced wound strength, and delayed re-epithelialization can occur.
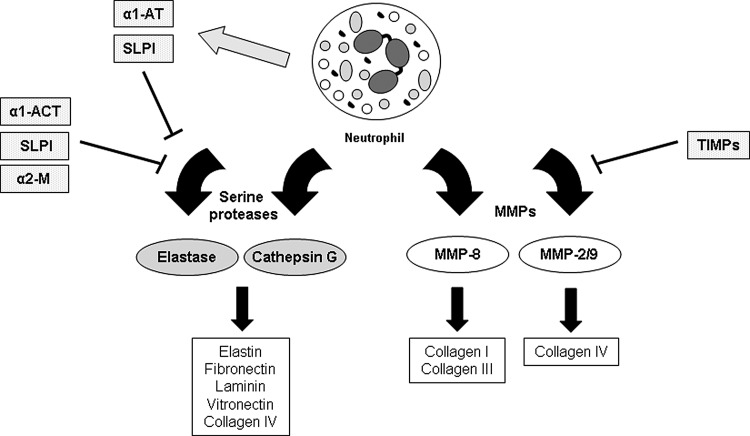
Figure 5.
Neutrophil proteases involved in wound healing and their inhibitors. Neutrophils produce two main classes of proteases relevant to tissue repair. Serine proteases like elastase and cathepsin G target a variety of extracellular matrix proteins, including elastin, fibronectin, laminin, vitronectin, and collagen IV. The action of serine proteases is balanced by several protease inhibitors produced by neutrophils (α1-AT, SLPI) and surrounding skin cells (α1-ACT, SLPI, α2-M). Neutrophils also produce several types of MMPs. MMP-8, which cleaves fibrillar collagen, and MMP-2/MMP-9, which cleave collagen IV (among other substrates), are involved in wound repair. The activity of MMPs is inhibited by a class of molecules called TIMPs produced by a variety of cells in the skin. If the activity of proteases and their inhibitors is not tightly regulated, the protease activity can become extreme and impair the healing process.
Serine proteases
Neutrophil-derived serine proteases include cathepsin G, elastase, and protease 3. All of these proteases contain a conserved histidine-aspartic acid-serine sequence in the active site, and all are stored in azurophilic granules.18 The majority of studies on neutrophil serine proteases have focused on elastase and cathepsin G. They have similar proteolytic activities and can cleave a variety of extracellular matrix proteins, including elastin, fibronectin, laminin, vitronectin, and collagen IV.18 Both elastase and cathepsin G are secreted at high levels by activated neutrophils early during an infection; as a result, a recent study has suggested that the activity of these proteases could be used as an early biomarker to detect wound infection.19 Since both of these proteases are capable of degrading basement membrane proteins, it has been suggested that they could potentially interfere with re-epithelialization and the formation of a new basement membrane in the dermo–epidermal junction by keratinocytes.20 This idea is supported by a study showing that the elastase inhibitor gramerin accelerates re-epithelialization rates.21 In this study, inflammation was also reduced in gramerin-treated wounds, suggesting a role for elastase in the regulation of wound inflammation.21 Similarly, high elastase levels have been observed in chronic wounds.22,23 Interestingly, elastase can also activate other classes of proteases (i.e., MMPs)24–26 and inactivate protease inhibitors,27 which could increase the total protease activity in a wound and further exacerbate host tissue damage. Elastase can also cleave and inactivate growth factors, such as hepatocyte growth factor, which becomes ineffective at stimulating keratinocyte migration after cleavage.28 This reduction in growth factor signaling efficiency could be another reason delayed healing is observed in the presence of high elastase levels. Elastase has also been shown to be important for the antimicrobial response of neutrophils. In a porcine model, Cole et al. demonstrated that inhibition of neutrophil elastase reduced the effectiveness of bacterial clearance from wounds.29 This was likely due to reduced activation of antimicrobial peptides when elastase activity was blocked.29 While several studies have suggested that high levels of elastase may be damaging and reduce the efficiency of wound repair, the studies by Cole et al. demonstrate that elastase may also help prevent wound infection.
Several studies have also examined the role of cathepsin G in wound repair. In one study, incisional wounds were shown to heal with reduced wound breaking strength in cathepsin G knockout mice.30 Wounds lacking cathepsin G also had elevated levels of myeloperoxidase and higher neutrophil numbers, suggesting that reduced degradation of neutrophil chemoattractants (tumor necrosis factor, interleukin-8, etc.) in cathepsin G knockout mice may have caused more neutrophils to populate the wound. Cathepsin G is also known to have antimicrobial effects independently of its protease activity,31 which could be important for microbial clearance. Overall, the studies on elastase and cathepsin G suggest that high levels of neutrophil-derived serine proteases can interfere with healing, but insufficient levels could leave a wound more vulnerable to infection.
Matrix metalloproteinases
In addition to serine proteases, neutrophils also store various MMPs in their granules and secretory vesicles. MMPs are a family of enzymes that contain conserved pro-domains and catalytic zinc-binding domains.17 These proteases are stored in neutrophil granules in their latent form and must be activated after they are released by the cell. Of the MMPs present in neutrophil granules, the functions of MMP-2, MMP-8, and MMP-9 have been studied in the context of wound repair.
Several studies have examined the role of MMP-8 in wound healing. MMP-8, also known as collagenase-2, cleaves fibrillar collagen and is expressed primarily by neutrophils. In normal acute wounds, mRNA expression levels of MMP-8 are low and MMP-8 protein is primarily present in its inactive form; however, increased MMP-8 expression and high levels of active MMP-8 are associated with chronic wounds.32–34 Two studies suggest that MMP-8 is functionally important for normal wound healing using mouse models. Gutierrez-Fernandez et al. examined wound healing in MMP-8 knockout mice.5 They showed a delay in wound closure in MMP-8 knockout mice and reduced neutrophil infiltration early in the repair process, suggesting that MMP-8 may aid in neutrophil trafficking. However, at later stages, they found persistent inflammation with lower levels of neutrophil apoptosis. Apoptotic neutrophils are an important signal for the resolution of inflammation, so a reduction in neutrophil apoptosis could lead to persistent inflammation. Another study used an adenoviral vector to drive MMP-8 expression in the skin, which led to impaired healing with reduced collagen deposition and breaking strength in incisional wounds.35 The authors also observed reduced neutrophil numbers in wounds with high levels of MMP-8, which was likely due to an increase in neutrophil apoptosis.
MMP-2 and MMP-9 are also stored in neutrophil granules, although they are not as closely tied to neutrophils as MMP-8 since they are also produced by other cell types. MMP-2 and MMP-9 are gelatinase enzymes that cleave collagen IV, a primary component of basement membranes. High gelatinase activity has been described in chronic wounds,13,36,37 which may be enhanced by low levels of active MMP inhibitors in these wounds.36 MMP-2 does not appear to play a major role in the normal wound-healing process, as MMP-2-deficient mice display similar rates of wound re-epithelialization, granulation tissue formation, and collagen content.38 However, high MMP-2 levels are found in chronic wounds.37 MMP-9, on the other hand, is expressed at the advancing front of the wound epithelium39 and findings from animal studies evaluating the importance of MMP-9 in wound healing have varied. In MMP-9 knockout mice, accelerated closure of both corneal and skin wounds was reported by Mohan et al.,39 while a delay in re-epithelialization and collagen organization was described by Kyriakides et al.40 In another study, Reiss et al. showed that treatment of wounds with active MMP-9 alters the structure of the collagen IV-containing basement membrane and delays re-epithelialization.41 Like MMP-2, abundant active MMP-9 is associated with chronic wounds.13,37,42 Taken together, the studies suggest that precisely balanced levels of MMP-9 may be needed for proper repair.
Counteracting protease activity: protease inhibitors
In addition to various proteases, several different protease inhibitors (Fig. 5 and Table 1) are stored in neutrophil granules. These inhibitors likely serve as a way to limit some of the host damage caused by neutrophil-derived proteases. Several of the protease inhibitors produced by neutrophils, such as α1-antitrypsin and secretory leukocyte protease inhibitor (SLPI), block the activity of serine proteases. Interestingly, α1-antitrypsin has been shown to be active in fluid collected from acute wounds, but is degraded in chronic wounds.22,43 The degradation and inactivity of α1-antitrypsin likely contributes to excessive serine protease activity in chronic wounds. SLPI is another protease inhibitor produced by neutrophils as well as epithelial cells. It counteracts the activity of elastase, but it also has anti-inflammatory and antimicrobial effects. Higher levels of elastase and inflammation have been described in wounds from SLPI-deficient mice, which correlated with delayed healing.44 In addition to neutrophils, epithelial cells are a rich source of protease inhibitors, including SLPI44 and another protease inhibitor, α1-antichymotrypsin (α1-ACT). α1-ACT inhibits serine proteases, primarily cathepsin G, and is upregulated in keratinocytes during the repair process.45 Treatment of diabetic wounds with α1-ACT in a mouse model was able to accelerate re-epithelialization and increase granulation tissue formation.45 Additionally, elastase from chronic wounds was found to cleave and inactivate α1-ACT.45 Aside from inhibiting serine protease activity, α1-ACT can also inhibit the activation of MMP-9. MMP-9 activation has been shown to be low in acute wounds due to high α1-ACT levels, whereas chronic wounds have degraded α1-ACT and high active MMP-9 levels.46,47
Take-Home Messages.
Neutrophils are an important part of the innate immune response in injured skin. They are among the first circulating immune cells recruited to a wound, where they defend against infection. Neutrophils produce cytokines, growth factors, and other soluble mediators that activate inflammatory cells, keratinocytes, endothelial cells, fibroblasts, and other cells present in the wound. Neutrophils also generate reactive oxygen species, antimicrobial peptides, and proteases to kill and degrade potential pathogens.
The activity of many molecules used by neutrophils to sterilize the wound is not specific to extrinsic organisms, so in addition to killing microbes, they can also target the host. Although a certain amount of protease activity is needed during wound repair, high levels of protease release by neutrophils can be harmful, causing degradation of the extracellular matrix and additional tissue damage beyond that associated with the initial injury.
Neutrophil-derived proteases are clinically significant, and have been repeatedly shown to be elevated in chronic, non-healing wounds. It is believed that a prolonged neutrophil presence and protease release causes additional tissue damage, which augments inflammation, which leads to even more protease production and further tissue damage. This creates a vicious cycle that eventually prevents the wound from healing appropriately.
It is likely that the correct balance of multiple proteases and protease inhibitors is needed to effectively clear microbes from the wound and facilitate cell migration without causing more damage to the tissue and delayed healing. Currently, it is still unclear whether measuring wound protease activity could be used clinically for early detection of wound infection, to predict healing rates, or to guide decisions for therapy. Additionally, more work will be needed to determine whether products designed to neutralize protease activity can be used to accelerate healing in chronic wounds.
Conclusions
While neutrophils play a key role in preventing infection, excessive neutrophil activity or the persistence of neutrophils at the wound site can contribute to the development of chronic wounds. Although some level of neutrophil-derived serine proteases and MMPs are needed for efficient microbial clearance and wound repair, data from multiple basic science and clinical studies suggest that excessive neutrophil activity can increase the amount of damage at the site of injury, leading to prolonged inflammation, further tissue damage, and ultimately, a wound that does not heal properly. The overabundance of neutrophils in chronic wounds suggests that reducing the number or limiting the activity of neutrophils may be beneficial for the treatment of recalcitrant wounds. Possible strategies for dampening the neutrophil response include neutrophil depletion or the induction of neutrophil apoptosis to reduce the overall number of wound neutrophils; however, the critical role of neutrophils in fighting infection and the difficulty of inducing apoptosis specifically in only one cell type limit the potential feasibility of these broad approaches. Because there are several proteases associated with chronic wounds for which neutrophils appear to be the primary source, neutralization of neutrophil-derived proteases may be a more useful approach to limit the harmful effects of neutrophils. Compounds that neutralize extracellular proteases, while leaving the activity of proteases contained within the neutrophil undisturbed would be ideal, sparing the ability of the neutrophil to kill microbes after they have undergone phagocytosis. Overall, the studies examining neutrophil-derived proteases and their inhibitors suggest that tight regulation of protease activity is needed for optimal healing.
Abbreviations and Acronyms
- α1-ACT
α1-antichymotrypsin
- α1-AT
α1-antitrypsin
- α2-M
α2-macroglobulin
- hCAP-18
human cationic antimicrobial protein
- MMP
matrix metalloproteinase
- SLPI
secretory leukocyte protease inhibitor
- TIMPs
tissue inhibitor of metalloproteinases
Acknowledgments and Funding Sources
The authors are supported, in part, by funding from the following sources: National Institutes of Health grants CA127109 and ES020462 (T.A.W.), DK076566 (S.R.), and NIH Clinical and Translational Science Award to The Ohio State University, Award Number UL1RR025755 (J.C.M.).
Author Disclosure and Ghostwriting
The authors declare that they have no competing financial interests to disclose. The content of this article was expressly written by the authors listed and no ghostwriters were used.
About the Authors
Traci Wilgus, PhD, is currently an Assistant Professor in the Department of Pathology at The Ohio State University. Her research efforts focus on understanding the role of inflammation and angiogenesis in wound healing and skin carcinogenesis, and she also uses a fetal wound-healing model to study scarless repair. Sashwati Roy, PhD, is an Associate Professor of Surgery at The Ohio State University, Columbus, Ohio. She received her PhD in 1994 in Physiology and Environmental Sciences. She completed her postdoctoral training from the University of California, Berkeley. Dr. Roy has over 150 peer review publications. Her research interests include wound inflammation and macrophages, mechanisms of resolution of diabetic wound inflammation, and the role of miRNA in tissue repair processes. She is an expert in the significance of inflammation in chronic wounds. Jodi McDaniel, PhD, is an Assistant Professor in the College of Nursing at The Ohio State University. Dr. McDaniel is involved in clinical research, primarily examining the effects of n-6 and n-3 polyunsaturated fatty acids on the neutrophil activity, inflammation, and healing of chronic venous leg ulcers.
References
- 1.Theilgaard-Monch K. Knudsen S. Follin P. Borregaard N. The transcriptional activation program of human neutrophils in skin lesions supports their important role in wound healing. J Immunol. 2004;172:7684. doi: 10.4049/jimmunol.172.12.7684. [DOI] [PubMed] [Google Scholar]
- 2.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 3.Serhan CN. Chiang N. Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Widgerow AD. Cellular resolution of inflammation—catabasis. Wound Repair Regen. 2012;20:2. doi: 10.1111/j.1524-475X.2011.00754.x. [DOI] [PubMed] [Google Scholar]
- 5.Gutierrez-Fernandez A. Inada M. Balbin M. Fueyo A. Pitiot AS. Astudillo A. Hirose K. Hirata M. Shapiro SD. Noel A. Werb Z. Krane SM. Lopez-Otin C. Puente XS. Increased inflammation delays wound healing in mice deficient in collagenase-2 (MMP-8) FASEB J. 2007;21:2580. doi: 10.1096/fj.06-7860com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khanna S. Biswas S. Shang Y. Collard E. Azad A. Kauh C. Bhasker V. Gordillo GM. Sen CK. Roy S. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One. 2010;5:e9539. doi: 10.1371/journal.pone.0009539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lekstrom-Himes JA. Gallin JI. Immunodeficiency diseases caused by defects in phagocytes. N Engl J Med. 2000;343:1703. doi: 10.1056/NEJM200012073432307. [DOI] [PubMed] [Google Scholar]
- 8.Simpson DM. Ross R. The neutrophilic leukocyte in wound repair a study with antineutrophil serum. J Clin Invest. 1972;51:2009. doi: 10.1172/JCI107007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dovi JV. He LK. DiPietro LA. Accelerated wound closure in neutrophil-depleted mice. J Leukoc Biol. 2003;73:448. doi: 10.1189/jlb.0802406. [DOI] [PubMed] [Google Scholar]
- 10.Martin P. D'Souza D. Martin J. Grose R. Cooper L. Maki R. McKercher SR. Wound healing in the PU.1 null mouse—tissue repair is not dependent on inflammatory cells. Curr Biol. 2003;13:1122. doi: 10.1016/s0960-9822(03)00396-8. [DOI] [PubMed] [Google Scholar]
- 11.Mestas J. Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 12.Yager DR. Kulina RA. Gilman LA. Wound fluids: a window into the wound environment? Int J Low Extrem Wounds. 2007;6:262. doi: 10.1177/1534734607307035. [DOI] [PubMed] [Google Scholar]
- 13.Moor AN. Vachon DJ. Gould LJ. Proteolytic activity in wound fluids and tissues derived from chronic venous leg ulcers. Wound Repair Regen. 2009;17:832. doi: 10.1111/j.1524-475X.2009.00547.x. [DOI] [PubMed] [Google Scholar]
- 14.Faurschou M. Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003;5:1317. doi: 10.1016/j.micinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 15.English WR. Velasco G. Stracke JO. Knauper V. Murphy G. Catalytic activities of membrane-type 6 matrix metalloproteinase (MMP25) FEBS Lett. 2001;491:137. doi: 10.1016/s0014-5793(01)02150-0. [DOI] [PubMed] [Google Scholar]
- 16.Borregaard N. Sorensen OE. Theilgaard-Monch K. Neutrophil granules: a library of innate immunity proteins. Trends Immunol. 2007;28:340. doi: 10.1016/j.it.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Parks WC. Wilson CL. Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 18.Witko-Sarsat V. Rieu P. Descamps-Latscha B. Lesavre P. Halbwachs-Mecarelli L. Neutrophils: molecules, functions and pathophysiological aspects. Lab Invest. 2000;80:617. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]
- 19.Hasmann A. Gewessler U. Hulla E. Schneider KP. Binder B. Francesko A. Tzanov T. Schintler M. Van der Palen J. Guebitz GM. Wehrschuetz-Sigl E. Sensor materials for the detection of human neutrophil elastase and cathepsin G activity in wound fluid. Exp Dermatol. 2011;20:508. doi: 10.1111/j.1600-0625.2011.01256.x. [DOI] [PubMed] [Google Scholar]
- 20.Briggaman RA. Schechter NM. Fraki J. Lazarus GS. Degradation of the epidermal-dermal junction by proteolytic enzymes from human skin and human polymorphonuclear leukocytes. J Exp Med. 1984;160:1027. doi: 10.1084/jem.160.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SK. Lee SS. Song IS. Kim YS. Park YW. Joo JY. Um HS. Kim JW. Kim KY. Choi SJ. Jung KH. Chung SI. Paradoxical effects of elastase inhibitor guamerin on the tissue repair of two different wound models: sealed cutaneous and exposed tongue wounds. Exp Mol Med. 2004;36:259. doi: 10.1038/emm.2004.35. [DOI] [PubMed] [Google Scholar]
- 22.Grinnell F. Zhu M. Fibronectin degradation in chronic wounds depends on the relative levels of elastase, alpha1-proteinase inhibitor, and alpha2-macroglobulin. J Invest Dermatol. 1996;106:335. doi: 10.1111/1523-1747.ep12342990. [DOI] [PubMed] [Google Scholar]
- 23.Herrick S. Ashcroft G. Ireland G. Horan M. McCollum C. Ferguson M. Up-regulation of elastase in acute wounds of healthy aged humans and chronic venous leg ulcers are associated with matrix degradation. Lab Invest. 1997;77:281. [PubMed] [Google Scholar]
- 24.Ferry G. Lonchampt M. Pennel L. de Nanteuil G. Canet E. Tucker GC. Activation of MMP-9 by neutrophil elastase in an in vivo model of acute lung injury. FEBS Lett. 1997;402:111. doi: 10.1016/s0014-5793(96)01508-6. [DOI] [PubMed] [Google Scholar]
- 25.Okada Y. Nakanishi I. Activation of matrix metalloproteinase 3 (stromelysin) and matrix metalloproteinase 2 (‘gelatinase’) by human neutrophil elastase and cathepsin G. FEBS Lett. 1989;249:353. doi: 10.1016/0014-5793(89)80657-x. [DOI] [PubMed] [Google Scholar]
- 26.Shamamian P. Schwartz JD. Pocock BJ. Monea S. Whiting D. Marcus SG. Mignatti P. Activation of progelatinase A (MMP-2) by neutrophil elastase, cathepsin G, and proteinase-3: a role for inflammatory cells in tumor invasion and angiogenesis. J Cell Physiol. 2001;189:197. doi: 10.1002/jcp.10014. [DOI] [PubMed] [Google Scholar]
- 27.Itoh Y. Nagase H. Preferential inactivation of tissue inhibitor of metalloproteinases-1 that is bound to the precursor of matrix metalloproteinase 9 (progelatinase B) by human neutrophil elastase. J Biol Chem. 1995;270:16518. doi: 10.1074/jbc.270.28.16518. [DOI] [PubMed] [Google Scholar]
- 28.Buchstein N. Hoffmann D. Smola H. Lang S. Paulsson M. Niemann C. Krieg T. Eming SA. Alternative proteolytic processing of hepatocyte growth factor during wound repair. Am J Pathol. 2009;174:2116. doi: 10.2353/ajpath.2009.080597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cole AM. Shi J. Ceccarelli A. Kim YH. Park A. Ganz T. Inhibition of neutrophil elastase prevents cathelicidin activation and impairs clearance of bacteria from wounds. Blood. 2001;97:297. doi: 10.1182/blood.v97.1.297. [DOI] [PubMed] [Google Scholar]
- 30.Abbott RE. Corral CJ. MacIvor DM. Lin X. Ley TJ. Mustoe TA. Augmented inflammatory responses and altered wound healing in cathepsin G-deficient mice. Arch Surg. 1998;133:1002. doi: 10.1001/archsurg.133.9.1002. [DOI] [PubMed] [Google Scholar]
- 31.Bangalore N. Travis J. Onunka VC. Pohl J. Shafer WM. Identification of the primary antimicrobial domains in human neutrophil cathepsin G. J Biol Chem. 1990;265:13584. [PubMed] [Google Scholar]
- 32.Pirila E. Korpi JT. Korkiamaki T. Jahkola T. Gutierrez-Fernandez A. Lopez-Otin C. Saarialho-Kere U. Salo T. Sorsa T. Collagenase-2 (MMP-8) and matrilysin-2 (MMP-26) expression in human wounds of different etiologies. Wound Repair Regen. 2007;15:47. doi: 10.1111/j.1524-475X.2006.00184.x. [DOI] [PubMed] [Google Scholar]
- 33.Nwomeh BC. Liang HX. Diegelmann RF. Cohen IK. Yager DR. Dynamics of the matrix metalloproteinases MMP-1 and MMP-8 in acute open human dermal wounds. Wound Repair Regen. 1998;6:127. doi: 10.1046/j.1524-475x.1998.60206.x. [DOI] [PubMed] [Google Scholar]
- 34.Nwomeh BC. Liang HX. Cohen IK. Yager DR. MMP-8 is the predominant collagenase in healing wounds and nonhealing ulcers. J Surg Res. 1999;81:189. doi: 10.1006/jsre.1998.5495. [DOI] [PubMed] [Google Scholar]
- 35.Danielsen PL. Holst AV. Maltesen HR. Bassi MR. Holst PJ. Heinemeier KM. Olsen J. Danielsen CC. Poulsen SS. Jorgensen LN. Agren MS. Matrix metalloproteinase-8 overexpression prevents proper tissue repair. Surgery. 2011;150:897. doi: 10.1016/j.surg.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 36.Bullen EC. Longaker MT. Updike DL. Benton R. Ladin D. Hou Z. Howard EW. Tissue inhibitor of metalloproteinases-1 is decreased and activated gelatinases are increased in chronic wounds. J Invest Dermatol. 1995;104:236. doi: 10.1111/1523-1747.ep12612786. [DOI] [PubMed] [Google Scholar]
- 37.Wysocki AB. Staiano-Coico L. Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 and MMP-9. J Invest Dermatol. 1993;101:64. doi: 10.1111/1523-1747.ep12359590. [DOI] [PubMed] [Google Scholar]
- 38.Frossing S. Rono B. Hald A. Romer J. Lund LR. Skin wound healing in MMP2-deficient and MMP2/plasminogen double-deficient mice. Exp Dermatol. 2010;19:e234. doi: 10.1111/j.1600-0625.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- 39.Mohan R. Chintala SK. Jung JC. Villar WV. McCabe F. Russo LA. Lee Y. McCarthy BE. Wollenberg KR. Jester JV. Wang M. Welgus HG. Shipley JM. Senior RM. Fini ME. Matrix metalloproteinase gelatinase B (MMP-9) coordinates and effects epithelial regeneration. J Biol Chem. 2002;277:2065. doi: 10.1074/jbc.M107611200. [DOI] [PubMed] [Google Scholar]
- 40.Kyriakides TR. Wulsin D. Skokos EA. Fleckman P. Pirrone A. Shipley JM. Senior RM. Bornstein P. Mice that lack matrix metalloproteinase-9 display delayed wound healing associated with delayed reepithelization and disordered collagen fibrillogenesis. Matrix Biol. 2009;28:65. doi: 10.1016/j.matbio.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reiss MJ. Han YP. Garcia E. Goldberg M. Yu H. Garner WL. Matrix metalloproteinase-9 delays wound healing in a murine wound model. Surgery. 2010;147:295. doi: 10.1016/j.surg.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rayment EA. Upton Z. Shooter GK. Increased matrix metalloproteinase-9 (MMP-9) activity observed in chronic wound fluid is related to the clinical severity of the ulcer. Br J Dermatol. 2008;158:951. doi: 10.1111/j.1365-2133.2008.08462.x. [DOI] [PubMed] [Google Scholar]
- 43.Rao CN. Ladin DA. Liu YY. Chilukuri K. Hou ZZ. Woodley DT. Alpha 1-antitrypsin is degraded and non-functional in chronic wounds but intact and functional in acute wounds: the inhibitor protects fibronectin from degradation by chronic wound fluid enzymes. J Invest Dermatol. 1995;105:572. doi: 10.1111/1523-1747.ep12323503. [DOI] [PubMed] [Google Scholar]
- 44.Ashcroft GS. Lei K. Jin W. Longenecker G. Kulkarni AB. Greenwell-Wild T. Hale-Donze H. McGrady G. Song XY. Wahl SM. Secretory leukocyte protease inhibitor mediates non-redundant functions necessary for normal wound healing. Nat Med. 2000;6:1147. doi: 10.1038/80489. [DOI] [PubMed] [Google Scholar]
- 45.Hoffmann DC. Textoris C. Oehme F. Klaassen T. Goppelt A. Romer A. Fugmann B. Davidson JM. Werner S. Krieg T. Eming SA. Pivotal role for alpha1-antichymotrypsin in skin repair. J Biol Chem. 2011;286:28889. doi: 10.1074/jbc.M111.249979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han YP. Yan C. Garner WL. Proteolytic activation of matrix metalloproteinase-9 in skin wound healing is inhibited by alpha-1-antichymotrypsin. J Invest Dermatol. 2008;128:2334. doi: 10.1038/jid.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reiss MJ. Han YP. Garner WL. Alpha1-antichymotrypsin activity correlates with and may modulate matrix metalloproteinase-9 in human acute wounds. Wound Repair Regen. 2009;17:418. doi: 10.1111/j.1524-475X.2009.00476.x. [DOI] [PubMed] [Google Scholar]