Abstract
Nitrosative stress, where nitrosylation of tyrosine (Tyr) leading to 3-nitrotyrosine proteins or free 3-nitrotyrosine is the most prominent change, has been proposed as a pathogenic mechanism in Parkinson's disease (PD). Levels of 3-nitrotyrosine proteins in serum and cerebrospinal fluid (CSF) of patients with PD have not been studied. Nitrosative stress-induced protein changes in serum and CSF were analyzed in patients with PD (n=54) and controls (n=40). Herein, we demonstrate the presence of nitrosative stress in serum and CSF of patients with early PD leading to selective increase of 3-nitrotyrosine proteins other than nitroalbumin, without free 3-nitrotyrosine (Hoehn-Yahr stage 1, p<0.05; stage 2, p<0.01). Among 3-nitrotyrosine proteins, nitro-α-synuclein (N-αSyn) was detected in serum, not CSF, and the sites of Tyr nitrosylation were observed to be modified in patients with early PD. Thus, the intensity of nitrosylation of Tyr125/136 residues is enhanced (stage 1, p<0.05; stage 2, p<0.01), and that of the Tyr39 site is reduced (stage 1, p<0.05), and the ratio between both parameters (α-synuclein with nitrosylated tyrosines 125 and 136 [N-αSyn-Tyr125/136]:α-synuclein with nitrosylated tyrosine 39 [N-αSyn-Tyr39] ratio) is significantly higher in patients with early PD (p<0.01). These observations lead to the hypothesis that evaluating nitrosative stress through enhanced levels of 3-nitrotyrosine proteins in serum and CSF without changes in nitroalbumin, together with the profile of tyrosine nitrosylation of serum αSyn characterized by dominant nitrosylation of Tyr125/136, could serve for the diagnosis of sporadic PD. Antioxid. Redox Signal. 19, 912–918.
Introduction
Oxidative stress is considered as a pathogenic mechanism in Parkinson's disease (PD) (6). Nitrosylation is a type of oxidative stress where modifications of proteins and free amines are due to excess of nitric oxide. Nitrosylation of tyrosine (Tyr) leading to 3-nitrotyrosine proteins or free 3-nitrotyrosine is the most prominent change. Excess nitrosylation is recognized as a salient feature of α-synucleinopathies such as PD, where 3-nitrotyrosine proteins such as nitrosylated neurofilaments and α-synuclein (αSyn) are detected in brain aggregates (5). However, levels of 3-nitrotyrosine proteins or free 3-nitrotyrosine in serum and cerebrospinal fluid (CSF) of patients with PD have not been studied, and the presence of free 3-nitrotyrosine deserves investigation, because it is known to be neurotoxic for striatal neurons (7).
Regarding αSyn, this protein represents a main component of Lewy bodies, the hallmarks of PD, and mutations in αSyn have been linked to familial PD. This protein contains 140 amino acids with four tyrosine residues (Tyr39 at amine terminus, and Tyr125, Tyr133, and Tyr136 at carboxyl terminus), which are readily accessible for modification by nitrating agents. These residues can be differentially nitrosylated leading to different functional effects (4). Although parkinsonian nitrosylation of αSyn is suspected to occur in brain tissue, this protein can also be detected in CSF and blood (2). The presence of αSyn in CSF is already known, but αSyn nitrosylation has not been studied. In contrast, nitro-α-synuclein (N-αSyn) has been detected in blood mononuclear cells of patients with PD (9), although serum levels are not known. The objectives of this study were (i) to determine in serum and CSF of patients with PD and control subjects the presence of 3-nitrotyrosine proteins and free 3-nitrotyrosine, markers for protein and amine nitrosylation, and (ii) to discern the effects of nitrosylation on serum and CSF αSyn.
Innovation.
We report for the first time the presence of selective nitrosylation stress in serum and cerebrospinal fluid of patients with early Parkinson's disease (PD), characterized by excess of 3-nitrotyrosine proteins other than nitroalbumin, without free 3-nitrotyrosine. We also detected nitrosative changes in serum nitro-α-synuclein (N-αSyn), characterized by an altered profile of tyrosine (Tyr) nitrosylation. Thus, the intensity of nitrosylation of Tyr125 136 residues is enhanced, and that of the Tyr39 site is reduced, and the ratio between both parameters is higher in patients with early PD relative to controls. Our observations lead to the hypothesis that evaluating nitrosative stress through enhanced levels of 3-nitrotyrosine proteins other than nitroalbumin, together with the profile of tyrosine nitrosylation of serum N-αSyn, could serve for the diagnosis of sporadic PD.
Results and Discussion
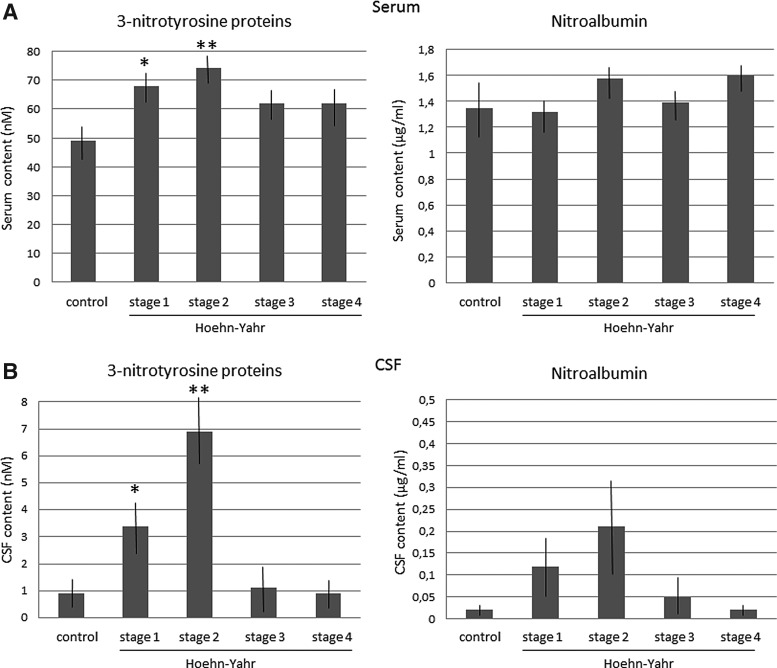
First, we verified that the main clinical characteristics were similar between the PD and control groups, except for hypertension, which was more frequent in patients (p<0.01). Then, we evaluated the presence of nitrosative stress in blood and CSF of patients with PD, and we quantified the serum levels of 3-nitrotyrosine proteins through an enzyme-linked immunosorbent assay (ELISA). Regarding serum, 3-nitrotyrosine protein levels were found to be significantly enhanced in serum of patients relative to control subjects (PD patients=70.6±4.7 nM; controls=48.3±6.8 nM; t=2.67, p<0.01). Taking into account the Hoehn-Yahr stages of the disease, 3-nitrotyrosine protein levels were found to be enhanced in all stages, but significant differences were only found in early or Hoehn-Yahr-stage-1 and 2 patients (Fig. 1). Nitroalbumin was also quantified, and differences were not found (PD patients=1.6±0.1 μg/ml; controls=1.4±0.4 μg/ml).
FIG. 1.
Levels of 3-nitrotyrosine and nitroalbumin in PD fluids. Levels of 3-nitrotyrosine proteins and nitroalbumin in serum (A) and CSF (B) of patients with PD at the four Hoehn-Yahr stages, and control subjects. Mean±SEM, *p<0.05, **p<0.01 versus controls (serum, stage 1, t=2.2, p<0.05, stage 2, t=2.6, p<0.01; CSF, stage 1, t=2.39, p<0.02, stage 2, t=2.9, p<0.008; Student's t-test). CSF, cerebrospinal fluid; PD, Parkinson's disease.
Regarding CSF, 3-nitrotyrosine protein levels were found to be significantly enhanced in CSF of patients relative to controls (PD patients=4.6±1.1 nM; controls=0.9±0.4 nM; t=3.1, p<0.004). 3-nitrotyrosine protein levels were found to be significantly enhanced in the Hoehn-Yahr-stage-1 and 2 patients relative to controls and advanced patients (one-way analysis of variance [ANOVA] group effect, F3, 54=3.24, p<0.029; stage 1, p<0.05; stage 2, p<0.01 vs. controls; Fig. 1). Regarding nitroalbumin, the differences were not found to be significant (PD patients=0.09±0.02 μg/ml; controls=0.02±0.01 μg/ml). The Tibbling-Link index was lower than 0.7 in every patient with PD, discarding that the increase in CSF 3-nitrotyrosine proteins was caused by enhanced blood levels.
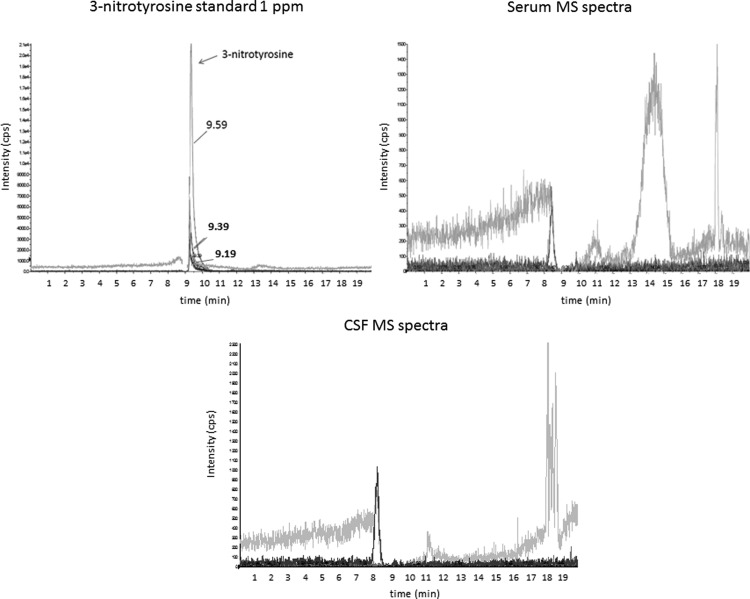
Since nitrosylative stress can also yield free nitrosylated amines such as 3-nitrotyrosine (7), we evaluated its presence in serum and CSF with mass spectrometry. 3-nitrotyrosine (retention time=9.19 to 9.59 min) was not detected in any patient, and only several unknown compounds with different retention times were observed in the MS spectra (Fig. 2).
FIG. 2.
Mass spectrometry (MS) spectra of 3-nitrotyrosine standard 1ppm, and representative MS spectra of serum and CSF of a patient with PD. The transitions for nitrotyrosine are 227.1/181.0, 227.1/133.1, and 227.1/116.9, with retention times from 9.19 to 9.59 min.
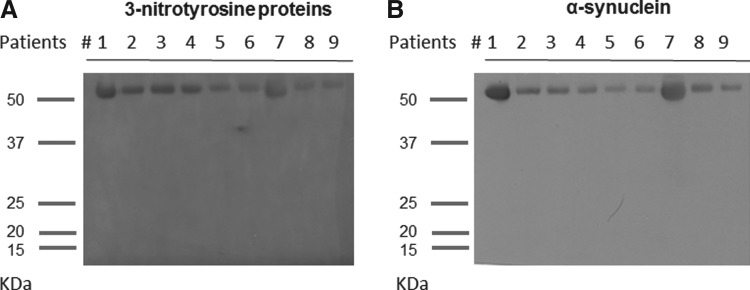
The findings suggested that nitrosylation was quite selective, affecting 3-nitrotyrosine proteins other than albumin. Then, we decided to evaluate which proteins were nitrosylated. For this purpose, immunoblots of serum needs previous depletion of serum albumin or immunoprecipitation of proteins of interest, and since usual techniques are denaturalizing and distort the physiologically oligomeric structure of many proteins, we used a low-denaturalizing method based on one-step filtration. This method allowed obtaining a filtrate containing proteins lower than 60 kDa. Surprisingly, it led to the detection of a single protein band at ∼56 kDa in serum from patients and controls, after anti-3-nitrotyrosine (Fig. 3A). Of note is that a band around 56 kDa is characteristic of tetrameric αSyn, the physiological form of endogenous αSyn (1). Hence, we also tested an anti-αSyn monoclonal antibody, and similar ∼56-kDa blot bands were observed in the same patients (Fig. 3B). Hence, the immunoblot signal of 3-nitrotyrosine proteins (<60 kDa) seems to correspond to N-αSyn. In fact, if we used more denaturalizing methods such as immunoprecipitation of 3-nytrotyrosine proteins, which breaks cross-linking of Tyr residues, immunoblots yielded not only ∼56-kDa bands but also ∼28- and ∼14-kDa bands in most subjects (Fig. 4). However, when we measured the levels of total αSyn in serum through ELISA, no differences were found (PD patients=327±45 pg/ml, controls=425.8±65 pg/ml). ELISA levels of 3-nitrotyrosine proteins in filtered serum were similar in patients and controls (PD patients=2.3±0.7 nM, controls=3.1±1.1 nM). Besides, after measuring the intensity of bands of 3-nitrotyrosine and αSyn in blots of filtered serum, no differences were observed between patients with PD and controls (data not shown). All these findings indicate that serum nitrosative stress in PD increases the levels of 3-nitrotyrosine proteins (>60 kDa) other than N-αSyn and nitroalbumin. These proteins are under study in our laboratory.
FIG. 3.
Representative immunoblots of 3-nitrotyrosine proteins and α-synuclein of PD patients. Immunoblot analysis of serum 3-nitrotyrosine proteins (A) and serum α-synuclein (B) in the same patients with PD (n=9). Similar blot signals were obtained, with bands located to ∼56 kDa of molecular mass. These blots were done after serum filtration and removal of proteins higher than 60 kDa, including serum albumin.
FIG. 4.

Representative immunoblots of 3-nitrotyrosine proteins in 2 controls and 2 patients with PD, after immunoprecipitation (PureProteome™ Protein G Magnetic Bead System; Millipore). Three bands of proteins lower than 60 kDa of molecular mass can be observed, at ∼56, ∼28, and ∼14 kDa. The denaturalizing treatment of samples is known to break cross-linking between tyrosine residues of proteins. If the ∼56-kDa band was observed to contain tetramers of αSyn, it seems that ∼28- and ∼14-kDa bands would contain dimers and monomers of αSyn, respectively. αSyn, α-synuclein.
Regarding CSF, blots of filtered CSF did not reveal the presence of 3-nitrotyrosine proteins, indicating that CSF αSyn was not nitrosylated. When we measured the levels of total αSyn in CSF through ELISA, the levels were found to be significantly lower in patients (25.5±3 pg/ml) relative to controls (33.1±2.3 pg/ml, p<0.05), in accordance with others (8). Hence, αSyn is present in CSF of patients with PD at lower values than normal, but its nitrosylated form is not detected.
To further characterize the effects of nitrosative stress on serum N-αSyn, and taking into account that the physiologically tetrameric structure of N-αSyn was very well preserved with our method of albumin removal, and Tyr residues of N-αSyn can be differentially nitrosylated leading to different functional effects (9), we evaluated the sites of Tyr nitrosylation of the molecule. Two monoclonal antibodies (mAbs) were used for detecting nitrosylated αSyn at Tyr residues of the carboxyl terminus (α-synuclein with nitrosylated tyrosines 125 and 136 [N-αSyn-Tyr125/136] or nSyn12 mAb), and of the amine terminus (α-synuclein with nitrosylated tyrosine 39 [N-αSyn-Tyr39] or nSyn14 mAb). The intensity of blot bands (Scion units) was measured at each Hoehn-Yahr stage, and two-way ANOVA revealed a significant interaction effect (p<0.0001, Fig. 5). The intensity of nitrosylation at Tyr125/136 was enhanced in the Hoehn-Yahr-stage-1 and 2 patients relative to advanced patients (p<0.01; Newman-Keuls) as well as controls (stage 1, t=2.48, p<0.02; stage 2, t=2.6, p<0.01) as shown in Figure 5. The intensity of the N-αSyn-Tyr39 band was found to be significantly reduced in stage-1 patients relative to controls (t=2.1, p<0.05; Fig. 5). Besides, after comparing the two types of immunoblot bands at each stage, the intensity of the N-αSyn-Tyr125/136 band was significantly higher than that of N-αSyn-Tyr39 in patients with early disease (stage 1, t=4.5, p<0.001; stage 2, t=3.18, p<0.01). The data also indicate that disease progression leads to recovery of the control intensity of the N-αSyn-Tyr125/136 and N-αSyn-Tyr39 bands.
FIG. 5.
Immunoblot analysis of serum of patients and controls after using two monoclonal antibodies (mAbs) against nitrosylated αSyn with nitrosylation of Tyr residues at the carboxyl terminus (N-αSyn-Tyr125/136 mAb) or the amine terminus (N-αSyn-Tyr39 mAb). (A) Representative blot bands of Hoehn-Yahr-stage-1, 2, and 3 patients and controls, after N-αSyn-Tyr125/136 mAb and N-αSyn-Tyr39 mAb. (B) Intensity values (Scion units) of blot bands in controls and patients (Hoehn-Yahr stage 1 to 4). Two-way ANOVA indicated a significant interaction effect on the intensity of bands after N-αSyn-Tyr125/136 mAb and N-αSyn-Tyr39 mAb at the four Hoehn-Yahr stages (F3, 79=8.78, p<0.0001, n=10 per group). Mean±SEM, *p<0.05, **p<0.01 versus corresponding band in controls; ##p<0.01 versus N-αSyn-Tyr39 band (Student's t-test). ANOVA, analysis of variance; mAbs, monoclonal antibodies; N-αSyn, nitro-α-synuclein; N-αSyn-Tyr125/136, α-synuclein with nitrosylated tyrosines 125 and 136; N-αSyn-Tyr39, α-synuclein with nitrosylated tyrosine 39; Tyr, tyrosine.
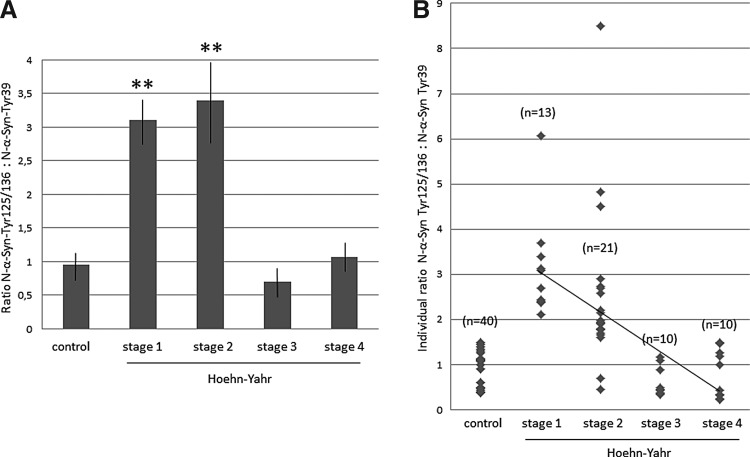
The ratio defined as the intensity of the N-αSyn-Tyr125/136 band between the intensity of the N-αSyn-Tyr39 band was also quantified as another index of nitrosylation. This ratio was significantly elevated in patients with early PD versus advanced patients and controls (p<0.001; Fig. 6A). All patients at stage 1, and 18 out of 21 patients at stage 2 (90%), showed a ratio higher than 1.6, whereas this ratio was ever observed to be lower than 1.6 in control subjects and advanced patients (Fig. 6B). This value can be considered as a limit between patients with early PD and controls.
FIG. 6.
Values of ratios of nitrosylation at Tyr125/136 residues between nitrosylation at Tyr39 site of α-synuclein. (A) Values of the N-αSyn-Tyr125/136: N-αSyn-Tyr39 ratio in controls and patients (Hoehn-Yahr stages 1 to 4). One-way ANOVA revealed a significant stage effect (F3, 53=4.14, p<0.011). Mean±SEM. **p<0.01 versus ratio in controls (stage 1, t=6.7, p<0.0001; stage 2, t=2.73, p<0.01). (B) Individual N-αSyn-Tyr125/136:N-αSyn-Tyr39 ratios in controls and patients. All patients at stage 1 and 90% (18/21) of stage-2 patients showed a ratio higher than 1.6, while this ratio was ever observed to be lower than 1.6 in controls and advanced patients. The regression line of patients with PD is represented.
Finally, to further analyze N-αSyn properties, simple linear regression analyses between the intensity of the N-αSyn-Tyr125/136 or N-αSyn-Tyr39 bands and clinical characteristics were carried out. No significant regressions were observed in controls, and the intensity of the N-αSyn-Tyr125/136 or N-αSyn-Tyr39 bands were not affected by any clinical feature. However, in patients with PD, the N-αSyn-Tyr125/136 intensity and N-αSyn-Tyr125/136:N-αSyn-Tyr39 ratio were found to be related with Hoehn-Yahr stage (N-αSyn-Tyr125/136, R2=0.258, T=2.3, p<0.05; ratio, R2=0.13, T=2.89, p<0.004). Linear regression of all individual N-αSyn-Tyr125/136:N-αSyn-Tyr39 ratios of patients with PD are shown in Figure 5B. It is worth noting that antiparkinsonian medication (levodopa, dopaminergic agonists, and rasagiline), use of statins or aspirin, or vitamin A/E supplements were devoid of effects on these progressive changes.
In summary, our results show for the first time that there are indicators of selective nitrosylation stress of proteins in serum and CSF of patients at early stages of PD, characterized by excess of 3-nitrotyrosine proteins (>60 kDa) other than nitroalbumin, and the absence of free 3-nitrotyrosine. Free 3-nitrotyrosine is toxic for striatal neurons in the experimental models of PD, but this nitrated amine seems not to participate on human PD. The findings also confirm that there is a physiological level of protein nitrosylation in serum (5). Among 3-nitrotyrosine proteins, N-αSyn was detected in serum, not CSF, of patients and controls. Of note is that N-αSyn is an abundant component of the Lewy bodies. The protein αSyn was detected in both serum and CSF, where levels were found to be lower in patients than controls (8), and as a tetramer (∼56 kDa), its actual physiological shape (1).
Serum N-αSyn levels were not modified in PD, but our study is the first one reporting a selective change in nitrosylation of the Tyr sites of serum αSyn, characterized by dominant nitrosylation of Tyr125/136 residues and low nitrosylation of the Tyr39 site. In other words, the carboxyl terminus tyrosines of the molecule showed higher nitrosylation than the amine terminus one in patients with early PD. The ratio between the blot intensity of both parameters (N-αSyn-Tyr125/136:N-αSyn-Tyr39 ratio) was significantly higher in patients with early PD relative to controls and advanced patients. Nitrosative effects are progressively reduced over time, and medication does not contribute to these changes.
Taken together, the findings suggest the presence of protein nitrosative stress in serum and CSF of Hoehn-Yahr-stage-1 and 2 patients, leading to the selective increase of 3-nitrotyrosine proteins other than nitroalbumin. We propose that evaluating nitrosative stress through enhanced levels of 3-nitrotyrosine proteins in serum and CSF without changes in nitroalbumin, together with the profile of Tyr nitrosylation of serum N-αSyn, could serve for the diagnosis of sporadic PD.
Notes
Study participants
Patients suffering from PD and clinically and underwent single-photon-emission computerized tomography (SPECT)-based diagnosis were included in the study. Patients were classified according to the Hoehn-Yahr stages, UPDR scales, and the duration of PD in years. The duration of PD was calculated on the basis of the year when first symptoms were reported by the patient. All participants were nonsmokers or nonalcohol drinkers. Control subjects were recruited from either patients' relatives or volunteers without any neurological disorder and subjected to intradural anesthesia for traumatologic surgery in Macarena Hospital. Individuals presenting with any of renal, liver, and cardiac dysfunction, malabsorption, autoimmune diseases, AIDS, diabetes mellitus, rheumatoid arthritis, and infectious conditions (oxidative stress markers in peripheral blood may be altered in such conditions) were excluded from both PD and control groups. Clinical information was gathered from each patient: age, sex, body weight, hypertension, dyslipidemia, fasting blood sugar, coffee drinking, smoking, taking of vitamin A/Vitamin E supplement, statins, and aspirins, daily levodopa dose, type and dose of dopamine agonists, and rasagiline (3).
Blood and CSF collection and biochemical measures
Five ml of blood was collected, after cephalic vein puncture, in gel-coated tubes to induce blood coagulation, and to obtain serum (BD Vacuotainer). Serum was centrifuged at 2500 rpm during 10 min, and then it was aliquoted, coded, and frozen at −80°C. CSF was collected by using lumbar puncture. Five ml of CSF was collected and stored in polypropylene tubes (Eurotube), and rapidly aliquoted, coded, and frozen at −80°C for further analyses. A 1-ml collection in a glass tube was employed to observe the absence of traumatic puncture and to quantify red cells before storing (3). CSF with excess of red cells was discarded (>500 red cells/μl). For measuring 3-nitrotyrosine proteins and αSyn, commercial kits were used (Oxiselect Nitrotyrosine kit; Cell Biolabs, Inc., Human αSyn Elisa Kit; BlueGene Biotech), following the manufacturers' instructions. Filtered serum and CSF were not diluted. Finally, the Tibbling-Link index was calculated as already reported (3).
Mass spectrometry
Chromatographic separation was performed using a PelkinElmer Series 200 high-performance liquid chromatography (HPLC) system coupled to an Applied Biosystems QTRAP LC/MS/MS system consisting of a hybrid triple-quadruple linear ion-trap (QqQlit) mass spectrometer equipped with an electrospray ion source. HPLC analyses were performed on a 100×2.1-mm Xselect HSS PFP reversed-phase column with a particle size of 2.5 μm (Waters). The flow rate was 0.2 ml min−1. Chromatographic separation was performed using a binary gradient consisting of (A) water and (B) methanol. Both components contained 0.05% trifluoroacetic acid (v/v). The elution profile was 10% B (1 min) and a linear gradient up to 90% B (9 min), 90% B (5 min), and followed by 5 min of re-equilibration of the column before the next run. The injection volume was 20 μl. Multiple Reaction Monitoring (MRM) experiment was applied where the precursor ions and fragment ions were monitored at Q1 and Q3, respectively. The transitions for nitrotyrosine are 227.1/181.0, 227.1/133.1, and 227.1/116.9. For HPLC-ESI-MS/MS analyses, the mass spectrometer was set to the following optimized tune parameters: curtain gas 20 psi, ion spray voltage 5500 V, and source temperature 350°C.
Albumin removal and western blotting
A low-denaturalizing method was used for removing albumin from serum and CSF samples, the most abundant protein, based on filtration with Amicon Ultra 50K (Millipore). We have observed that one-step filtration (following the manufacturer's instructions) allows obtaining a filtrate with molecules lower than 60 kDa, and hence albumin is depleted without the need of magnetic beads or immunoprecipitation. This method proved to be crucial, because the physiological tetrameric structure of serum αSyn was not distorted. Serum and CSF samples were then lysed in 10% glycerol, 137 mM NaCl, and 20 mM Tris–HCl, pH 7.5, containing peptidase inhibitors (1 μg/ml aproteinin and leupeptin, and 1 mM PMSF). The protein levels were quantified by using the Bradford method. Samples were boiled, and aliquots containing 15 μg of protein each were subjected to SDS/polyacrylamide gel electrophoresis. Proteins were transferred electrophoretically to PVDF membranes. Immunolabeling was conducted with primary antibodies for 3-nitrotyrosine (monoclonal antibody to nitrotyrosine; Hycult Biotech), α-synuclein (monoclonal anti-α-synuclein antibody Syn211; Sigma-Aldrich), nitrosylated αSyn at Tyr residues 125/136 (anti-nitro-α/β-synuclein antibody, nSYn12; Millipore), and nitrosylated αSyn at tyrosine 39 (anti-nitro-α/β-synuclein antibody Tyr39, nSYn14; Millipore). Primary antibodies were detected with peroxidase-linked secondary antibodies (Santa Cruz Biotechnology), with enhanced chemiluminescence (ECL; Amersham, GE HealthCare) and autoradiography. Band densities of the resulting autoradiograms were quantified by using the Scion Image program for PC (NIH). Values are given as the intensity of bands in arbitrary Scion Units. Finally, immunoprecipitation was used at the beginning of the study for albumin removal, based on the PureProteome™ Protein G Magnetic Bead System (Millipore), following the manufacturer's instructions. This method proved to be more denaturalizing than western blotting after filtration.
Statistical analysis and ethics
We enrolled 54 patients, comprising four Hoehn-Yahr stages (stage 1, n=13; stage 2, n=21; stage 3, n=10; stage 4, n=10), and 40 controls. Differences in the nitrosylation markers and clinical characteristics between the PD and control groups were analyzed by the χ2 test or Student's t-test (independent samples). Comparisons between clinical characteristics and nitrosylation markers within patients at different PD stages were analyzed by one-way ANOVA, followed by the Student's t-test. When two factors were studied, two-way ANOVA was used, followed by Newman–Keuls' test or Student's t-test when appropriate. Two groups were compared with the Student's t-test. If needed, normalization was verified with the Shapiro–Wilk test. In patients and controls, simple regression analysis was used for searching for correlations between nitrosylation markers and clinical factors. Informed consent forms under a protocol approved by the University of Seville and Macarena Hospital internal ethics and scientific boards were obtained from all the subjects, and the subjects' consent was obtained according to the Declaration of Helsinki (BMJ 1991; 302: 1194).
Abbreviations Used
- ANOVA
analysis of variance
- CSF
cerebrospinal fluid
- ELISA
enzyme-linked immunosorbent assay
- HPLC
high-performance liquid chromatography
- mAb
monoclonal antibodies
- MRM
Multiple Reaction Monitoring
- N-αSyn
nitro-α-synuclein
- N-αSyn-Tyr125/136
α-synuclein with nitrosylated tyrosines 125 and 136
- N-αSyn-Tyr39
α-synuclein with nitrosylated tyrosine 39
- PD
Parkinson's disease
- SPECT
single-photon-emission computerized tomography
- Tyr
tyrosine
- αSyn
α-synuclein
Acknowledgments
The authors thank Mara Guerra and Silvia Castellano (University of Seville) for their excellent technical assistance; Macarena Rus (Hospital Macarena) for her help with blood collection; Dr. Guillermo Izquierdo for allowing the use of the facilities of the Service of Neurology; Dr. Maria-Isabel Garcia and the Biobanco Hospitalario Macarena (National Biobank Network, Carlos III Health Institute RD09/0076/00080) for its help with Tibbling-Link measures and support in the sample collection procedure and storage; Dr. Cinta Calvo and the Service of Nuclear Medicine (Hospital Macarena, Seville) for SPECT analyses; Dr. Eugenia Soria and Servicio de Espectometria de Masas-CITIUS (University of Seville) for mass spectrometry analyses, and Lola Rivero for her support and help. The authors are most grateful to all patients and their partners as well as control subjects who participated in this study. Supported by the grants to EF by Junta de Andalucia (BIO127), and Spanish Ministerio de Sanidad (RETICS, RD06/001/002; RD06/010/1007; Instituto Carlos III, co-financing with FEDER, European Fund for Regional Development).
References
- 1.Bartels T. Choi JG. Selkoe DJ. α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477:107–110. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Agnaf OM, et al. Alpha-synuclein implicated in Parkinson's disease is present in extracellular biological fluids, including human plasma. FASEB J. 2003;17:1945–1947. doi: 10.1096/fj.03-0098fje. [DOI] [PubMed] [Google Scholar]
- 3.García-Moreno JM, et al. May serum levels of advanced oxidized protein products serve as a prognostic marker of disease duration in patients with sporadic Parkinson's disease? Antioxid Redox Signal. 2013;18:1296–1302. doi: 10.1089/ars.2012.5026. [DOI] [PubMed] [Google Scholar]
- 4.Giasson BI, et al. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitrosylation in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 5.Ischiropoulos H. Protein tyrosine nitrosylation—an update. Arch Biochem Biophys. 2009;484:117–121. doi: 10.1016/j.abb.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 6.Jenner P. Oxidative stress in Parkinson's disease. Ann Neurol. 2003;53(Suppl. 3):S26–S36. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- 7.Mihm MJ, et al. Free 3-nitrotyrosine causes striatal neurodegeneration in vivo. J Neurosci. 2001;21:RC149. doi: 10.1523/JNEUROSCI.21-11-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mollenhauer B, et al. Total CSF α-synuclein is lower in de novo Parkinson patients than in healthy subjects. Neurosci Lett. 2013;532:44–48. doi: 10.1016/j.neulet.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Prigione A, et al. Alpha-synuclein nitrosylation and autophagy response are induced in peripheral blood cells from patients with Parkinson disease. Neurosci Lett. 2010;477:6–10. doi: 10.1016/j.neulet.2010.04.022. [DOI] [PubMed] [Google Scholar]