Abstract
Significance: Antioxidant protein 1 (Atox1 in human cells) is a copper chaperone for the copper export pathway with an essential role in cellular copper distribution. In vitro, Atox1 binds and transfers copper to the copper-transporting ATPases, stimulating their catalytic activity. Inactivation of Atox1 in cells inhibits maturation of secreted cuproenzymes as well as copper export from cells. Recent Advances: Accumulating data suggest that cellular functions of Atox1 are not limited to its copper-trafficking role and may include storage of labile copper, modulation of transcription, and antioxidant defense. The conserved metal binding site of Atox1, CxGC, differs from the metal-binding sites of copper-transporting ATPases and has a physiologically relevant redox potential that equilibrates with the GSH:GSSG pair. Critical Issues: Tight relationship appears to exist between intracellular copper levels and glutathione (GSH) homeostasis. The biochemical properties of Atox1 place it at the intersection of cellular networks that regulate copper distribution and cellular redox balance. Mechanisms through which Atox1 facilitates copper export and contributes to oxidative defense are not fully understood. Future Directions: The current picture of cellular redox homeostasis and copper physiology will be enhanced by further mechanistic studies of functional interactions between the GSH:GSSG pair and copper-trafficking machinery. Antioxid. Redox Signal. 19, 945–957.
Introduction
In biological systems, the redox potential of copper ions (Cu+↔Cu2+) is effectively utilized by the electron transfer enzymes, including various oxidoreductases. Copper deficiency decreases the activity of cuproenzymes (48, 75–77) and causes cellular pathologies, many manifestations of which can be linked, at least in part, to defects in antioxidant defense mechanisms (82). Cells respond to copper deficiency by adjusting their glutathione (GSH) levels (1) and upregulating genes involved in GSH synthesis (16). Although copper deficiency is deleterious, excess copper is also harmful. Due to its inherent redox activity, copper can trigger nonspecific oxidation of proteins and lipids, alter cellular redox balance by changing the ratio of reduced:oxidized GSH, and induce radical mediated damage to DNA (28). Cells and tissues avoid Cu toxicity using tightly controlled Cu distribution mechanisms, in which copper ions are escorted to their destinations by small copper-binding molecules called copper chaperones (19, 70).
The discovery of copper chaperones in baker's yeast (57) was especially significant, because in addition to identifying a new class of proteins, the study highlighted the tight link between copper homeostasis and oxidative state of a cell. Specifically, the first copper chaperone was identified in a genetic screen for proteins able to functionally complement the loss of a cytosolic Cu, Zn-dependent superoxide dismutase (SOD1), the key protein involved in cellular defenses against reactive oxygen species. The newly discovered protein, antioxidant protein 1 (Atx1), required copper for its antioxidant function and had a well-defined copper-binding site, MxCxxC (57). Through the subsequent studies in yeast (58), insects (90), mice (29), and other species (59, 94), it has been firmly established that Atx1 and its orthologs (Atox1 in human cells) play an important role in copper distribution and transfer of copper to the secretory pathway. In addition, the protective role of the recombinant Atox1 against oxidants in mammalian neuronal cells was reported (46). The identification and characterization of other copper chaperones, such as copper chaperone for superoxide dismutase 1 (CCS) for activation of SOD1 in a cytosol (17) and SCOI/II (22, 33, 49, 68), COX11 (15), and COX17 (32) (for functional assembly of cytochrome c oxidase in mitochondria) further highlighted the ability of these proteins to deliver copper cofactor along with performing thiol-based redox reactions (54, 55).
Extensive biochemical studies followed these original observations. As a result, the structure and the copper-binding properties of Atox1 have been described in detail (4, 78), and the hypotheses on how Atox1 mediates copper transfer to Cu-ATPases in the secretory pathway have been developed (97). In stark contrast, only few studies have attempted to elucidate the basis and significance of Atox1 antioxidant activity. Yet, accumulating data indicate that the role of Atox1 in cell physiology is complex and cannot be fully explained by its function as a dedicated metal shuttle for the copper-transporting ATPases. Data obtained in several different organisms indicate that, although important, Atox1 is not obligatory for the activity of the copper-exporting systems, and may only be required for copper export via secretory pathway when copper is limiting. Mice and flies with the genetically deleted Atox1 are born and develop into adulthood, whereas this does not happen if they lack active copper transporters (34, 72). New data have emerged showing a protective effect of Atox1 on cells' growth in low GSH (30) along with the studies illustrating the dual role of Atox1 in maintaining the mRNA levels for the secreted Cu/Zn-dependent superoxide dismutase 3, SOD3 along with transferring copper to SOD3 during biosynthesis (42). Altogether, these observations suggest that Atox1 may play a key role at the intersection of several metabolic pathways by contributing to both copper homeostasis and cellular redox balance. Could specific activity of Atox1 be determined by the metabolic state of cells, including copper levels and cellular redox environment? This review summarizes available data on the structure, function, and physiology of Atox1; it also highlights issues that remain to be addressed in order to fully understand the physiologic role of this small yet extremely intriguing protein.
Discovery of Dual Function of Atox1
The Atx1 was first described in yeast Saccharomyces cerevisiae by Lin, Culotta, and colleagues, who found that the overexpression of a previously uncharacterized 8-kDa cytosolic protein suppressed the Lys/Met auxotrophy phenotype of a strain lacking Cu/Zn-dependent superoxide dismutase SOD1 (57). Reciprocally, a 10-fold increase in ATX1 levels increased cell resistance to dioxygen and paraquat even in the absence of SOD1 and SOD2, suggesting that this newly characterized protein had anti-oxidant properties. The name reflected this fact, and it was adopted for most of the Atx1 orthologs in other species (Atox1 in human cells). It was also noticed that the antioxidant function of Atx1 required copper, since no protection of the ΔSOD1 phenotype by overexpressed Atx1 was observed when copper was depleted either by copper chelation or by genetic inactivation of the copper uptake transporter Ctr1 (58).
Subsequent studies revealed that Atx1 was an important component of the pathway for the high affinity iron uptake (58). Atx1 was found to facilitate the delivery of copper to the Golgi lumen, where copper was used for biosynthetic maturation of ferroxidase FET3, an enzyme essential for the uptake of iron under iron-limiting conditions (58). Because high copper complemented the loss of Atx1 in yeast and because Atx1 bound copper in vitro, it was suggested that Atx1 acted as copper carriers or “copper chaperones” that ensured the delivery of copper to the secretory pathway (Fig. 1). These pioneering studies were subsequently replicated in a mammalian system, where genetic inactivation of a mouse Atx1 ortholog (Atox1) was found to greatly impair copper delivery from placenta to fetus resulting in copper deficiency and high mortality of newborn pups (29). More recent studies using Atox1−/− mice with a different genetic background found less copper deficiency and mortality compared to the earlier report (72). However, this latter study revealed a significant inhibitory effect of Atox1 inactivation on copper delivery (via Cu-ATPase ATP7A) to SOD3, an important antioxidant molecule at the cell surface. In addition, downregulation of SOD3 mRNA and protein in response to Atox1 inactivation was observed, making connection between copper metabolism and anti-oxidant defense even more intertwined (72).
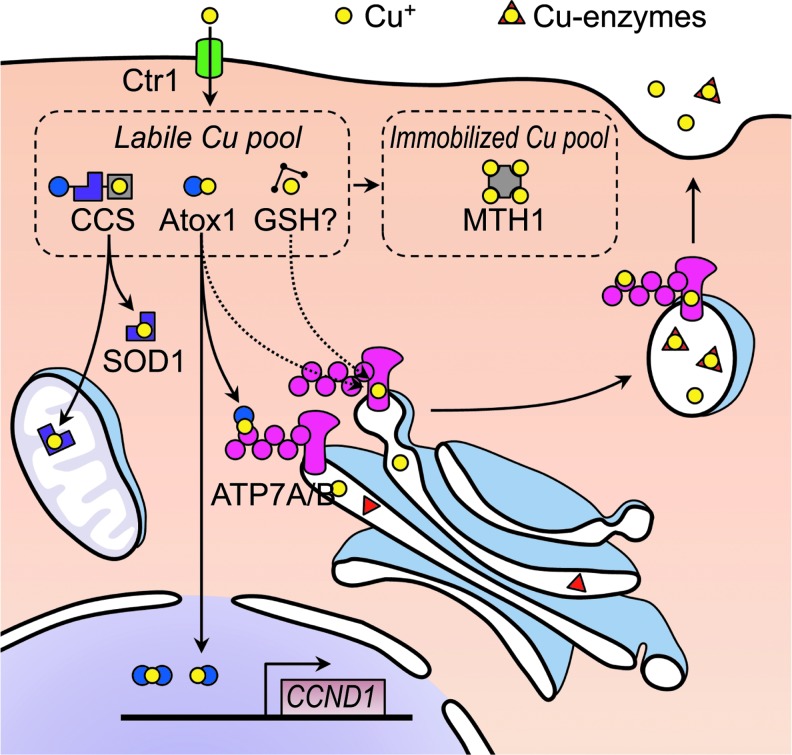
FIG. 1.
Atox1 role in cellular copper trafficking pathways. Copper enters the cell through the high-affinity copper transporter Ctr1 and binds to cytosolic copper chaperones (Atox1 and CCS) and/or small thiol molecules such as glutathione for further intracellular distribution (“Labile” Cu pool). Excess copper is sequestered by metallothioneins to avoid build-up to toxic levels (“Immobilized” copper pool). The arrows indicate either Cu or membrane trafficking pathways. Dashed arrows are hypothetical routes of the Atox1-mediated Cu transfer. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Does Atox1 Act as a Dedicated Copper Shuttle, a Storage Molecule for Exchangeable Copper, or a Regulator of Copper Pumps?
These findings opened an avenue for intensive investigations of biochemical properties of Atox1 and the mechanism of Atox1-mediated copper delivery to the secretory pathway. Over the years, great progress has been made in these areas overshadowing several intriguing observations, which remain largely unexplained by the current model of Atox1 action. For example, many tissues show little correlation between the expression levels of Atox1 and Cu-ATPases. In the brain, Atox1 has been found predominantly in neurons (66, 92), whereas Cu-ATPases are expressed in neurons and astrocytes (18, 92). The large-scale analysis of human transcriptome showed highest expression of Atox1 in the adult kidney, liver, and spleen (GO dataset: GDS181). The levels of Atox1 in the pituitary gland and trachea were low compared to other tissues. In contrast, expression of Cu-ATPases ATP7A (in pituitary) and ATP7B (in trachea) analyzed in the same study were high compared to most other tissues. The discrepancy can be explained by the fact that mRNA levels do not necessarily reflect protein levels. Yet, this lack of a direct correlation between Atox1 and Cu-ATPases is not consistent with Atox1 serving solely as a copper delivery vehicle for Cu-ATPases, and this discrepancy has also been observed in other systems.
In Caenorhabditis elegans, intestinal cells expressed orthologs of both Atox1 (CUC-1) and Cu-ATPase (CUA-1), whereas cells of pharyngeal muscle have only Cu-ATPase but lack the Atox1 ortholog (94). Similarly, some prokaryotes express Atox1 orthologs, whereas others do not. For example, the genomes of Escherichia coli and Legionella pneumophila do not encode for the Atox1-like copper chaperone, despite the presence of functionally characterized copper pumps. It is also significant that even in eukaryotic cells in which Atox1 and Cu-ATPases are co-expressed, the copper export ability of Cu-ATPases is only partially lost when Atox1 is inactivated (83). In the study of copper efflux from mammalian cells, the Atox1+/+ cells were shown to retain 33%±8% of the initial load of radioactive copper, whereas the Atox1−/− cells retained 53%±8% (p<0.03)(83). The lack of tight functional dependence is also true in cyanobacteria, where the Atox1 ortholog, Atx1, is not required for copper delivery to thylakoids, even though copper uptake into this compartment requires Cu-ATPases (93). In yeast, increasing extracellular copper overcomes the lack of the chaperone and allows copper delivery to the secretory pathway.
Clearly, while Atox1 is an important facilitator of copper transport, it is not essential for the ability of Cu-ATPases to bind and transfer copper across membranes. In other words, the delivery of copper to the transporters can be accomplished by other molecules. Biochemical data provide illustration of how Atox1 function can be complemented. The Cu-ATPase CopA from Archaeoglobus fulgidus is currently the best characterized copper transport system, from a biochemical point of view. CopA is thought to receive copper from a copper chaperone CopZ, which has an Atox1-like domain (23). When expressed as a recombinant protein, this domain increases the Vmax of CopA ATPase activity by two-fold (to 5.5 μmol/mg/h) compared to “free” copper (23, 101). CopA also requires 10–20 mM cysteine for its activity (60). In the absence of chaperone, cysteine increases the CopA Vmax to a comparable or even higher level (9.2 μmol/mg/h) (101), demonstrating that in vitro abundant thiol-containing molecules fully substitute for the lack of a copper chaperone. In fact, current data in eukaryotes do not exclude the possibility that copper is delivered to Cu-ATPases by small molecules (like GSH) whereas Atox1 regulates the ATPase turnover and stimulates transport activity by ensuring that the regulatory sites are loaded with copper.
It has been suggested (as well as demonstrated in cyanobacteria) that the primary role of the Atox1-like copper chaperone is to prevent copper binding to nonspecific targets (93). In mammalian system, such role is also likely. In Atox1−/− mouse embryonic fibroblasts that lack Atox1, copper redistributes toward nuclei, where it is normally (in Atox1+/+ cells) present at a relatively low level (62). In the Atox1−/− nuclei, the accumulating copper inhibits transcription of cyclin D1 and decreases cell proliferation rate (40). Thus, it could be that the role of Atox1 in transcription is to prevent the entry and inhibitory effects of copper in the nucleus, although direct interaction of recombinant Atox1 with DNA has been observed in several independent assays, suggesting a more complex mechanism (41, 62).
In mammalian cells, inactivation of Atox1 has different consequences for cellular copper content depending whether or not cells express metallothioneins, small metal chelating proteins (63). In regular fibroblasts, the Atox1 knockdown is associated with a slight increase in copper concentration, perhaps due to slower copper export by Cu-ATPases in the absence of Atox1. Cells in which both Atox1 and metallothioneins are downregulated accumulate considerably more copper, which is sequestered in cytosolic vesicles (63). A possible explanation for this phenomenon is that in the absence of copper buffering/sequestering molecules, copper forms complexes with GSH, which are then transported by the GSH-recognizing ABC-transporters (such as multidrug-resistance associated proteins) into intracellular vesicles without proper copper export. This scenario suggests that one of the functions of Atox1 could be to prevent copper from binding to reduced GSH and to restrict futile export of Cu-GSH complexes. In addition, restriction of Cu binding to GSH is beneficial, because such binding would decrease amounts of reduced GSH in favor of Cu(GSH)2 complex, which cells may perceive as an alteration in the glutathione:glutathione disulfide (GSH:GSSG) ratio.
Atox1 has higher affinity for copper than GSH, but lower affinity than metallothioneins (10, 100). Thus, it is well suited to serve as a storage molecule for a labile cytosolic copper pool. Buffering copper and preventing copper entry into the nuclei would also preclude upregulation of expression of metallothioneins, which have extremely high affinity for copper and cannot easily release copper to copper-utilizing pathways (10). In the buffering capacity, Atox1 can also act as a copper sensor, stimulating copper export machinery when cytosolic copper is high (by transferring copper to the regulatory sites of copper pumps, see below) and inhibiting the transporter activity to its basal level when copper is low (96). The regulatory/sensory role of Atox1 is fully consistent with its role as a facilitator of copper transport; it also allows for existence of other other/non Cu-transport functions of Atox1.
Antioxidant Function of Atox1
The ability of overexpressed Atox1 to protect cells against oxidative stress induced by dioxygen or paraquat is a very intriguing but poorly understood activity of this protein. Functional measurements revealed that yeast Atx1 can consume superoxide, but compared to SOD1 the dismutase activity of Atox1 is very low (74). Therefore, under normal conditions, Atx1 contribution to cellular anti-oxidant defenses via this mechanism is small, if any. Experiments in yeast also suggest that the antioxidant function of Atx1 although dependent on copper does not involve the delivery of copper to the secretory pathway, that is, the chaperone and antioxidant functions can be uncoupled (74). Given significant structural similarity between Atx1 and Atox1, the same is probably true for mammalian cells.
Recent data indicate that Atox1 redox-equilibrates with the GSH/GSSG pair in vitro and in cell (see below for details) and that genetic ablation of Atox1 sensitized cells to the decrease in GSH levels (30). The wild-type mouse embryonic fibroblasts are resistant to treatment with 1 mM buthioninesulfoximine (BSO, an inhibitor of GSH synthesis), whereas Atox1−/− fibroblasts are much more sensitive as evident by their retarded growth and death in the presence of drug (IC50 42 μM). In mammalian cells, redox buffering of the cytosol is maintained by various thiol-containing enzymes and metabolites, including GSH. The increased sensitivity of Atox1−/−cells to GSH depletion likely reflects the loss of Atox1 contribution to cell redox buffering capacity. The Atox1−/− cells are sensitive to GSH depletion in either high or low copper, further illustrating that the antioxidant function of Atox1 may not be directly linked to its copper chaperone function.
These observations raise several questions. Does Atox1 work at the intersection of metal and redox homeostases and contribute significantly to maintaining a necessary pool of reduced GSH upon fluctuating metal levels? Is it possible that in addition to Cu-ATPases, Atox1 binds to and regulates/stimulates activity of proteins in other cellular pathways? Interactions between Atox1 and the peptidyl-prolyl-isomerase domain of immunophilin FKBP52 were reported along with the stimulatory effect of FKBP52 on copper efflux (84, 85). The reverse experiment testing the effect of Atox1 on FKBP52 function would also be interesting, especially given recent findings that FKBP52 deficiency reduces the protein levels of peroxiredoxin 6 and promotes peroxide-induced cell death (31).
In a series of articles, Fukai and colleagues reported that in the presence of 100 μM CuCl2, the amount of SOD3 mRNA in Atox1−/− cells was markedly decreased, but the decrease can be reversed by expressing the recombinant Atox1 (42). Co-changes in the SOD3 and Atox1 levels were also observed in THP-1 macrophages in response to treatment with iron and ascorbate (61). This latter treatment increased the ratio of oxidized GSH to total GSH, thus shifting cellular redox balance, and was accompanied by a two-fold decrease in the mRNA levels for both SOD3 and Atox1(61). This observation suggests that Atox1 activity (and protein levels) is an integral part of the cell response either to GSH oxidation or/and availability of reduced GSH.
Recent studies of genes induced in the GSH-depleted yeast cells (conditions that generate an iron starvation phenotype) revealed that both Atx1 and Cu-ATPase CCC2 were induced [2.34 and 6.36-fold, respectively (50)], linking copper, iron, and GSH metabolism. Also in yeast, the expression of Atx1 is upregulated by oxygen and by iron levels (57). The same iron-sensing trans-activator, Aft1p, which regulates CCC2 and FET3, also upregulates expression of Atx1 (58), in agreement with an important role of Atx1 in the iron acquisition/utilization pathway. It should be noted that there is a strong agreement between various published reports that the levels of Atx1/Atox1 are not regulated by copper, except in an unusual case of marine polychaetes, which inhabit copper-polluted waters and have significant increase in the Atox1 protein and mRNA (67). Therefore, changes in Atox1 mRNA in response to iron (or GSH:GSSG ratio) are significant. They suggest that in addition to (or instead of) directly regulating the GSH:GSSG balance, Atox1 may mediate its antioxidant effect indirectly by contributing to iron homeostasis. Upregulation of Atox1 was also seen in the colon-derived HCT116 cells treated with another redox-influencing molecule, selenomethionine, although the magnitude of effect was very different depending on the method of analysis (26).
Structure of Atox1
Atox1 is a small polypeptide (68 amino acid residue, 7.4 kDa for human Atox1) with one copper-binding site formed by a pair of Cys thiols (Fig. 2) in the CxxC site. Atox1 has a ferredoxin-like βαββαβ-fold (81) in which two α-helices (α1 and α2) are located on one side of the anti-parallel β sheet (β1–4). Analyses of the Atox1 orthologs have identified two sequence motifs, MxCxxC and KTGK, which together form a conserved cluster on the protein surface and are crucial for antioxidant function of Atox1 in yeast (36, 58). Mutation of second lysine in this motif has only minor effects on protein ability to bind and transfer copper, but it impairs the antioxidant function (36).
FIG. 2.
Structure of human Atox1. (A) Ribbon representation of Cu-Atox1 atomic model [PDB code 1TL4 (3)]. The Cys residues in the Cu-binding MTCXGC motif and basic residues in the α1 and α2 helices are shown. (B) Topology diagram of human Atox1. Strands are shown as arrows and labeled from β1 to β4. The conserved MTCXGC and KTGK motifs are indicated. Bound copper ion is represented by a green sphere; thiols in the MTCXGC motif are in yellow; nitrogen of the invariant Lys is in blue. Lys/Arg-rich regions around the C-terminal halves of α-helices are indicated. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The M10xC12xxC15 site is situated in the first solvent-exposed loop (β1-α1). In this site, copper ligation is mediated by sulfur atoms from Cys12 and Cys15 located at the distance of 3.9–4.0Å from each other [PDB 1FEE(97)]. One copper ion binds with an association constant Ka of 5.6×1017 M−1(7) in either linear or trigonal environment; in the latter case, the third ligand may come from the solvent thiols like GSH (78). The conserved Met does not contribute to copper coordination and is not essential for copper transport or antioxidant function (36). Solution structures of the apo and holo-Atox1 demonstrated that, upon copper binding, the β1-α1 coil becomes more rigid, the solvent accessibility of Cys thiols is reduced, and Cys15 is wound into the α2-helix (6). With the exception of these effects near the copper-binding site, no significant conformational change was observed in the nuclear magnetic resonance (NMR) studies of Atox1. Recent molecular dynamics simulation studies suggest that subtle effects of copper binding may extend throughout the protein (79). The CXXC motif is also implicated in binding of cisplatin, a platinum-based antitumor drug. Crystal structures of cisplatin-Atox1 adducts revealed platinum coordination environment (13) and offered biophysical explanation to cisplatin-resistance mediated by Atox1 (45). Direct interaction of Atox1 and cisplatin in solution was further confirmed by NMR (5, 73).
The K57TGK60 motif of Atox1 has its last two residues as a part of another loop (α2-β4), situated near the M10xC12xxC15 site. In this motif, Lys60 is important for antioxidant activity and interactions with Cu-ATPase (38), it also modulates affinity of the CxxC site for copper (37). However, mutation of this residue is not detrimental for copper delivery to the secretory pathway (36). Recent mechanistic studies have yielded insights into a specific role of Lys60. The Nɛ atom of Lys60 was shown to form a hydrogen bond with Cys15 and influence cysteine deprotonation, whereby shifting its pKa by 1.5 unit (from 7.0 to 5.5). Low pKa was proposed to reduce nucleophilicity of Cys15 and help to replace a copper-Cys15 bond with a more favorable interaction between copper and Cys in an acceptor protein, which is more nucleophilic compared to Cys15 in Atox1 (7). The Nɛ atom of Lys has also been implicated in electrostatic neutralization of a thiol-rich environment around the MxCxxC site (6) (Fig. 2). It seems likely that the effect of Lys on Cys nucleophilicity may contribute to a marked difference in the reducing potentials between Atox1 and the metal-binding domains of Cu-ATPase ATP7B (30), although this remains to be formally tested.
While the Gly59Lys60 residues are strictly conserved, Lys57 is often substituted with Arg, indicating that the positive charge, rather than the size of a side chain, is important in this position. In addition to electrostatic effects, the K57TGK60 motif has been implicated in translocation of Atox1 into the nucleus (40). A typical nuclear localization signal (NLS) consists of Lys/Arg-rich repeats; consequently, a possible NLS function for the K57TGK60 motif was suggested and tested (65). Although deletion of this sequence was accompanied by the loss of Atox1 movement to the nucleus, some uncertainties remain. Both monopartile and bipartile NLSs are typically observed in a coil or loop structures (outside the secondary structure elements). This common property makes the NLS function less likely for the KTGK site and an adjacent positive-patch of Atox1, in which basic residues belong mainly to α-helices except for Lys60 in the α2-β4 loop. Thus, the loss of nuclear localization upon deletion of the KTGK sequence may represent a negative effect of the deletion on overall protein folding.
Atox1-Like Proteins
In human genome, three other proteins contain the Atox1-like domains: the copper chaperone for cytosolic superoxide dismutase SOD1 (CCS) and two Golgi-localized copper-transporting ATPases (ATP7A and ATP7B). CCS has three distinct domains (domains I-III), among which domain I is strikingly similar to Atox1 (51). It has the same ferredoxin fold as Atox1 as well as one MxCxxC site. The SOD1-like domain II of CCS is thought to facilitate specific copper transfer through heterodimerization with SOD1 (87), whereas the role of the Atox1-like domain I is not entirely clear. In yeast, domain I is not required for SOD1 activation by CCS except under copper-limiting conditions, when it becomes essential, suggesting its role as a high-affinity copper acceptor (88). Structural similarities between Atox1 and domain I of CCS suggest that these proteins may compete for the same source of copper. Competition between the copper chaperone, if occurs, would serve as a negotiation point between the two distinct copper trafficking pathways (to SOD1 and the Golgi lumen). The in-cell data provide support to such functional competition between the two pathways. Whereas Atox1 mRNA and protein levels are not regulated by copper, the CCS levels are highly sensitive to copper fluctuations (52, 53), indicating that the Atox1:CCS ratio (and presumably copper distribution between the two pathways) is copper-dependent. Indeed, in macrophages, when oxygen limitation increases copper influx, the delivery of copper to the ATP7A/secretory pathway (presumably via Atox1) is enhanced, whereas the activity of the CCS target SOD1 under the same conditions is markedly reduced (98).
The Cu-ATPases ATP7A and ATP7B belong to the second group of proteins containing the Atox1-like domains. The Cu-ATPases act downstream of Atox1 and transport copper from the cytosol into the lumen on intracellular compartments, Golgi and vesicles (Fig. 1). These large membrane proteins form the copper translocation pathway within the membrane, where two copper ions are thought to bind transiently during the translocation process (24). The N-termini of human ATP7A/B contain 6 metal-binding domains (MBD1–6), each with a very similar fold to Atox1. It has been proposed that Atox1 delivers copper to MBD via heterodimerization (13, 97). Numerous in vitro data support this view, although recent structural studies of Cu-ATPase LCopA offered an alternative model for Atox1 docking to Cu-ATPase and direct delivery of copper to the intra-membrane binding sites (Fig. 3) (23, 27).
FIG. 3.

Proposed direct and indirect routes for Atox1-mediated delivery of copper to human Cu-ATPases. The two proposed pathways of Cu transfer from Atox1 to Cu-ATPase are indicated by solid arrow (to the N-terminal metal binding domain) and dashed arrow (direct delivery to the trans-membrane sites).The spatial organization of domain shown in the figure is a schematic representation generated through molecular modeling and docking studies to assist discussion in the text and does not represent an experimentally determined structure. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Biochemically, structural similarity often implies competition for the substrate. In the case of Atox1 and MBDs such common substrate could be copper or another domain of Cu-ATPase, with which MBD and Atox1 may interact. Several reports indicated that MBDs have an auto-inhibitory function in Cu-ATPases(11). Atox1 may both transfer copper to MBDs and displace them thus disrupting the autoinhibitory contacts and stimulating transport activity. The Atox1-mediated transfer of copper to MBDs is reversible, further suggesting the role of Atox1 in regulating metal occupancy of MBDs (96). Although copper binding characteristics of Atox1 and MBS are similar (7) the redox properties of their CxxC motif are very different, in agreement with more diverse cellular functions for Atox1 compared to MBDs.
Sequence analyses identified Atox1 homologs and related proteins in all phyla from bacteria to human (Fig. 4). The first atomic models were determined for yeast Atx1 (81) and an Atox1-like bacterial copper chaperone CopZ (99); these were followed by numerous structures, including apo and holo Atox1 (6, 97), Atox1-MBD complexes (8), bacterial homologs of Atox1, and Atox1 adducts with drugs (2, 13). This structural information, along with the homology models, enables mechanistic classification of the Atox1-like proteins and helps to identify essential amino acid residues and their role in various activities of Atox1.
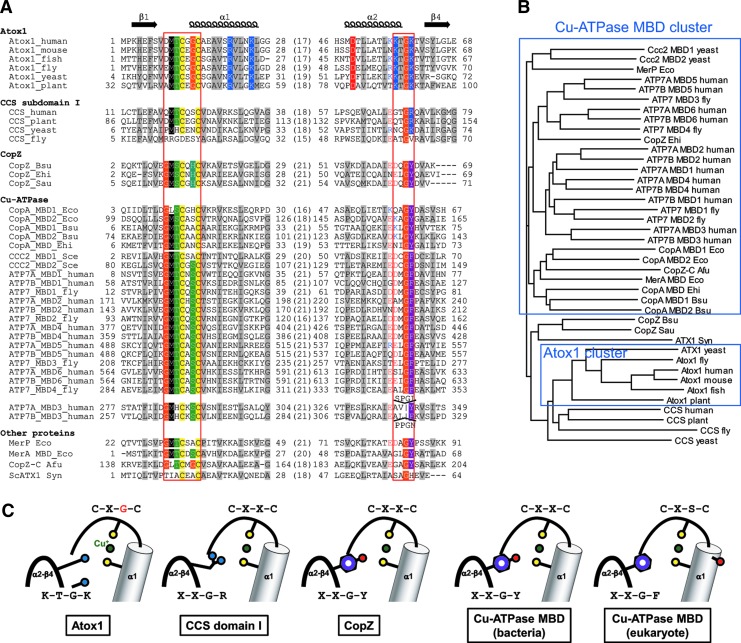
FIG. 4.
Multiple sequence alignment of Atox1-like proteins. (A) The alignment shows two conserved regions corresponding to Cu binding site of Atox1 (MTCXGC and KTGK in Atox1). For clarity, intervening sequences are replaced with numbers denoting the residues in these excised regions. The top line of the alignment represents the secondary structure of human Atox1 (PDB ID 1TL4). Widely conserved pattern of hydrophobic residues (A, C, F, I, L, M, V, W and Y) contribute to protein folding and are shaded in gray. In the regions corresponding to MTCXGC and KTGK motifs of Atox1, conserved residues are shaded in various colors; cysteines in yellow, hydroxyl residues (S and T) in green, glycine in orange, basic residues (K and R) in blue, acidic residues (D and E) in red, aromatic residues (F, W and Y) in purple, histidines in steel-blue. To highlight electrostatic nature of C-terminal half of the α2 helix, acidic and basic residues in the corresponding region are also colored but not shaded. Sequences are identified by the protein name followed by abbreviated species name. Species names are as follows: human, Homo sapiens; mouse, Musmusculus; fish, Danio rerio; fly, Drosophila melanogaster; plant, Arabidopsis thaliana; yeast, Saccharomyces cerevisiae; Syn, Synechocystis; Eco, Escherichia coli; Ehi, Enterococcus hirae; Bsu, Bacillus subtilis; Afu, Archaeoglobus fulgidus. Alignment was produced using CLUSTALW(91). (B) Phyrogenic clusters for Atox1-like proteins. The phylogram was generated based on the similarity among sequences shown in A. Blue squares represent the major two clusters. (C) Schematic representation Cu binding sites in various Atox1-like proteins. Bound copper ion is represented by a green sphere; thiols from CXXC motif in yellow; nitrogen from the conserved Lys/Argin blue; aromatic rings in purple. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Sequence analysis suggests that the Atox1-like proteins can be divided into two groups based on the amino-acid patterns within their two functional motifs: MxCxxC and KTGK. The MxCxxC motif is present in all Atox1-like proteins including bacterial homologs (CopZ), MBDs of copper transporters, and domain I of CCS. The in vitro and in-cell data indicate that these proteins all bind copper. However, the identity of residues located between two invariant cysteines correlates with the distinct redox properties of these proteins. The MBDs of human Cu-ATPases ATP7A/ATP7B show the canonical pattern MT/HCxSC (Fig. 4). Recent data demonstrate that these sites have an average redox potential of approximately −180 mV and therefore are likely to remain reduced under a variety of intracellular conditions (30). In Atox1, X1 varies, whereas X2 is invariably Gly; that is, the consensus pattern of Atox1 site is MTCxGC (Fig. 4). This latter pattern resembles the redox site of oxidoreductases, such as ferredoxins, thioredoxins, and glutaredoxins. The redox site of these proteins is formed by the CxxC motif, which is similarly to Atox1 positioned at the end of the loop connected to the α-helix. The intervening dipeptide sequence (X1 and X2 in CX1X2C) is the primary determinant of the redox properties of the site. In thioredoxins and glutaredoxins, the X1X2 sequences are invariantly Gly-Pro and Pro-Tyr, respectively. The presence of helix-breaking proline and glycine resembles the CxGC site of Atox1. Coincidentally, the redox potential of CxxC site of Atox1 is similar to that of glutaredoxin and can be influenced by changes in the GSH:GSSG balance (30).
The clear structural distinction between MBDs and Atox1 suggests a potential functional specialization such as binding of copper (for MBD) and a dual role in copper binding and redox chemistry (for Atox1). The bacterial CopZ and copper transporters share the sequence pattern MSCxH/SC with MBDs; the presence of Gly in the X2 position is rare in CopZ and copper transporters. We speculate that these structural features may reflect the main role for these proteins in copper handling. Similarly, Doman I of CCS has a sequence pattern more similar to MBDs, suggestive of its role as a copper acceptor.
The sequence pattern of the region corresponding to the conserved KTGK motif of Atox1 is also unique for each group of the Atox1-like proteins (Fig. 4). Within the tetrapeptide sequence, only Gly is invariant in all these proteins. Glycine is a general helix-breaker (71), and Gly59in Atox1 defines a transition from the α2helix to a loop. The lysine residue that follows glycine (Lys60) is characteristic of Atox1 and is absent in the MBD/CopZ group. As described above, this lysine lowers the pKa of the second thiol in the CxxC motif and is essential for the antioxidant function of Atox1 (36). In the MBDs of copper-transporting ATPases and bacterial CopZ, the corresponding positions are taken by Tyr or Phe; in CCS, it is Arg. The difference in nucleophilicity of thiols was suggested to underlie a lower copper affinity of Atox1 compared to MBDs (more nucleophilicthiolate) and favorable copper transfer from Atox1 to MBDs. However, the lower pKa of Cys in Atox1 is a trade-off since it stabilizes thiol in an active deprotonated state and increases its reactivity toward various electrophillic targets as observed for thioredoxin (21). Furthermore, stabilization of thiol by a lysine residue enables regulation of redox properties of the CxxC site, because the lysine residue can be displaced or become involved in other ion-paring interactions upon Atox1 docking to the target proteins.
Atox1 and its orthologs also differ from the rest of Atox1-like proteins in the electrostatic pattern on the α helical side (α-side) of the molecule. The α-side of Atox1 has a positive patch next to the MxCxxC/KTGK cluster (Fig. 5). This is mainly due to presence of Lys/Arg on the C-terminal halves of the α1 and α2 helices and only infrequent occurrence of acidic residues in the region (Figs. 2 and 4). The electronegative environment of the CxxC motif is neutralized by Lys60 (the electrostatic surface model with Lys60>Ala shows a significantly negative pattern around the CXXC motif). Corresponding positive patch is not observed in CopZ and other Atox1-like proteins, which typically show neutral or fairly negatively charged surfaces.
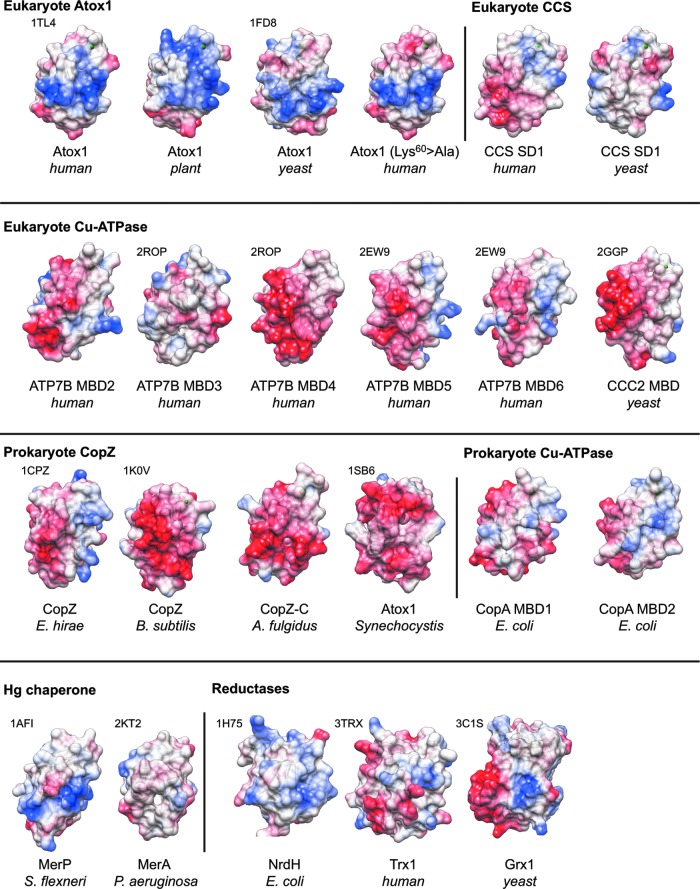
FIG. 5.
Surface electrostatic patterns of Atox1-like proteins. Blue (positive) and red (negative) colors illustrate the electrostatic surface potentials of Atox1-like proteins. Protein backbone (ribbon and arrows) and key residues corresponding to Cys12, Cys15, Lys60 in human Atox1 are also shown. For comparison, models of representative reductases, NrdH, Trx1, and Grx1, are included. PDB code is indicated above each model. The structures for human ATP7B MBD3–6 were derived from two models (2ROP and 2EW9) and represented separately. For proteins without PDB codes, the model was generated based on homology using human Cu-Atox1 (PDB code 1TL4) as a template structure. Protein and species names are represented in the same way as in Figure 4; P. aeruginosa, Pseudomonas aeruginosa; S. flexneri, Shigella flexneri. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
It has been proposed that complimentary electrostatic surfaces of the α sides of Atox1 (positive) and MBDs (negative) facilitate their interaction and hence copper transfer (35). However, this model does not work well for the bacterial CopA-CopZ pair both of which have either neutral or negative surfaces. Such charge similarity may point toward competitive rather than complementary interactions. The Atox1-specific positive patch suggests more unique, Atox1-specific, interactions. Interestingly, a conserved positive patch near the CxxC motif and a neutralized environment around the catalytically active Cys are also observed for a thioredoxin-like reductase NrdH and glutaredoxin 1 (44). In these redox enzymes, the CxxC motif sits at the edge of a hydrophobic pocket surrounded by the positively charged rims, which form platforms for thioredoxin-reductase and GSH, respectively. It is tempting to speculate that the unique electrostatic surface pattern of Atox1 may facilitate its redox reactions given that GSH has a fairly negative surface charge. The unique positive surface of Atox1 may also be involved in the recently demonstrated Atox1-DNA interactions (40).
Although the Atox1-like proteins/domains have almost identical backbones and substantial sequence similarity to true Atox1 molecules, features specific to Atox1 point to different biochemical properties/functions of the latter. In fact, in a phylogenetic tree, the Atox1 orthologs form a cluster distinct from the Cu-ATPase MBDs (Fig. 4), even when only MBDs (and not the entire Cu-ATPases) are used for alignments. CopZ is more often observed in the cluster of Cu-ATPase MBDs, perhaps reflecting its predominantly copper-binding/buffering function. Compared to MBDs and CopZ, CCS is more closely related to Atox1 although the possible relation between CCS and Atox1 has not been explored. The grouping places CopZ of A. fulgidus in a unique position; it has the CxGC motif as seen in all Atox1 molecules and Phe instead of Lys in the α2-β4 loop like the MBDs of eukaryotic Cu-ATPases. These structural features suggest that while the Atox1-like domain of A. fulgidus CopZ binds copper but may lack redox activity. Therefore, it is particularly interesting that in A. fulgidus CopZ, the Atox1-like domain is fused to the N-terminal domain containing an Fe-S cluster (a well-known redox moiety). Altogether, the A. fulgidus CopZ may play a highly specialized role, which could be expected in this sulfate-reducing anaerobe that grows optimally at 76°C(12).
Atox1-Mediated Copper Delivery to Cu-ATPases: The Target Sites and the Role of Redox
Experiments in Drosophila melanogaster indicate that Atox1 becomes more important for the organism survival under conditions of copper limitations (34). One explanation for this observation is that the chaperone is needed under these conditions to increase affinity of the transporter for copper. However, this does not seem to be the case. Strong in vitro evidence exists for the chaperone-mediated activation of the copper transporting ATPases (in the case of CopA and human copper ATPase); in neither case chaperone significantly increases the affinity of transporter for copper compared to free copper. However, these in vitro data could be misleading since the experiments are performed in the absence of other copper-binding proteins, which may markedly decrease copper availability for the copper-export machinery.
Currently, the biochemical mechanism of Atox1-dependent copper transfer to the secretory pathway is not well understood. Two models have been proposed. One model suggests that Atox1 docks at the positively charged surface near the first and second trans-membrane segments (TM1, 2) of copper ATPase and directly delivers copper to the transport site (Fig. 3). Another model suggests that Atox1 transfers copper to the MBDs in the regulatory N-terminal domain, which in turn indirectly (through transferring copper to other metal-binding sites) or directly (through causing opening access to the trans-membrane portion) facilitate copper entry to the transport site (Fig. 3). Evidence exists for both models (25, 39, 64, 95), but neither model provides clear explanation of how the MBD sites and the intramembrane sites communicate with each other and whether/how Atox1 prioritizes copper delivery to these structurally different sites. It is possible that the role of Atox1 in stimulating copper transport resembles the role of calmodulin in activating calcium export systems. The transport activity of the plasma membrane Ca2+-ATPase is markedly upregulated by Ca2+-calmodulin. Upon Ca2+ binding, calmodulin disrupts the autoinhibitory inter-domain interactions within the Ca2+-ATPase (14) allowing ATPase to bind Ca2+ at the transport sites and undergo conformation transitions required for calcium export. Similarly, Atox1 may combine copper delivery role with the disruption of autoinhibitory interactions between MBDs and other domains of Cu-ATPases and stimulate transport activity of Cu-ATPases when copper is elevated.
The role of the cellular redox-maintaining machinery in the copper transfer/transport mechanism is an important and only partially characterized issue. Recent study suggests that the MBDs of Cu-ATPases can be glutathionylated (89). It has been proposed that upon copper elevation glutaredoxin(s) remove the GSH units from the CxxC motif making it available for copper binding (89). The model is logical, and the dependence of copper efflux on the presence of glutaredoxins (Grx1 and Grx2) in a cell has been clearly demonstrated. Nevertheless, the mechanism through which copper stimulates interactions between MBDs and Grx1 needs further study. Specifically, it was previously shown that intact cysteines in the MBDs were necessary for copper-dependent interactions with Grx1 (56). Since both proteins have the CxxC motif, copper dependence of interactions is explained by the formation of the complex between the MBD and Grx1 bridged by copper (as was shown for the MBD-Atox1 pairs). Glutathionylation of cysteines at steady state before copper addition (89) would prevent copper binding to MBDs and disrupt rather than facilitate interactions with Grx. Thus, how copper stimulates the Grx1-Cu-ATPase interactions and facilitate the removal of GSH from the CxxC site remains unclear. Are there other molecules that regulate Grx1 in a copper-dependent manner? Could that be Atox1? Quantitative analyses of glutathionylation (and more detailed study of the Grx1 partners) may be useful in resolving this conundrum. Recent data on labeling of Cys residues indicate that in cell Atox1 and MBDs of ATP7B are largely reduced (see below), which means that glutathionylation of Cu-ATPase is likely to be partial. Markedly different redox properties of Atox1 and MBDs also suggest that Atox1 is better suited to respond to fluctuations in the GSH:GSSG ratio and that the effect of Grx1 downregulation on copper efflux could be controlled through Atox1.
Redox Properties of Atox1
To better understand the link between Atox1 function and cellular redox homeostasis, we recently characterized the redox properties of the MxCxxC site in Atox1 (30). As observed for most of the thiol-based enzymes, Atox1 is susceptible to aerobic oxidation. In vitro, a freshly purified Atox1 binds copper readily; upon storage, the copper binding ability is lost, but can be recovered by treatment with exogenous reductants. This phenomenon is due to reversible oxidation of the MxCxxC site with the formation of an intra-molecular disulfide. Similar disulfide formation is observed in the CXXC-containing oxidoreductases including thioredoxin and glutaredoxin. Comparison of the standard redox potentials identified Atox1 as a protein particularly susceptible to reversible Cys oxidation under physiologic conditions. Furthermore, the standard redox potential of Atox1 and Grx1 are close, −229 and −220 mV, respectively, even though the overall fold of these proteins is not the same. In contrast, MBDs of ATP7B have average potential of −178 mV despite having overall structure very similar to that of Atox1. As described above, Atox1 is characterized by the conserved Gly in the X2 position and Lys60, which is likely to contribute to the relatively low standard redox potential of Atox1 Cys residues. MBDs of ATP7B have Ser in the X2 position and Phe instead of Lys. These features may explain marked differences in redox potentials of Atox1 and MBDs, although this hypothesis still needs to be tested.
The unique properties of the Atox1 CxxC site beg the question about physiologic significance of a relatively low standard redox potential of this protein. If the CXXC motif of Atox1 is involved only in copper binding, susceptibility to oxidation would be detrimental to such function. However, if Atox1 acts as a reductase or if the function of Atox1 is regulated by cellular redox environment, then reversible oxidation of its metal-binding site could be very significant. The intersection between copper binding and redox reactivity has been observed for other metallochaperone systems. Mammalian enzyme SOD1 is inactive even in the presence of its chaperone CCS when GSH is depleted (43). This observation suggests functional interactions between cellular GSH and chaperone in generating copper-bound SOD1, an important antioxidant molecule. It is not unusual for proteins to have a dual role as a metal carrier and a reductase. For example, glutaredoxins are well-characterized cytosolic thiol reductases and, at the same time, some glutaredoxins (e.g., Grx3) participate in the formation and transporting of Fe-S clusters (80). Furthermore, some proteins can couple metal trafficking with redox reactions. In recent study, a mitochondrial copper carrier Cox17 was shown to pass copper ion and electron at the same time to the downstream acceptor protein Sco1 (9). It is tempting to speculate that Atox1 may also be involved in such dual or coupled reactions.
Low redox potential of Atox1 also raises questions about the mechanism of redox maintenance of the CxxC site. We have previously found that, in solution, GSH effectively reduces Atox1 either directly or in a glutaredoxin-dependent manner, suggesting GSH involvement in the redox maintenance of Atox1 (30). Physiologic relevance of this observation was tested in cultured cells by inhibiting GSH reductase activity. Inhibition of GSH reductase caused significant oxidation of Atox1, consistent with redox equilibration between cytosolic GSH and Atox1. Oxidation of Atox1, in turn, resulted in a limited supply of copper to the downstream copper-dependent enzyme (ceruloplasmin) in the secretory pathway (30). In proliferative conditions, the redox potential of cytosolic GSH/GSSG ranges from −258 to −220 mV (HT29, fibroblasts, HL-60, murine hybridoma cells (47)), which is lower than the standard redox potential of Atox1. Indeed, in proliferating stage, Atox1 is present mostly in the reduced form (30). However, the GSH/GSSG-based redox potential may change, particularly during cell differentiation and polarization (69), serum depletion (86), or apoptosis (20) from −258 up to −165 mV (47). Such conditions would favor Atox1 oxidation and may significantly affect copper trafficking routes in cells. Further studies are needed to test these predictions.
Perspective
The discovery and subsequent studies of Atox1 demonstrated the important role of this small cytosolic protein in copper export from the cytosol and laid solid foundation for understanding of intracellular copper trafficking. Accumulating data point to properties and functions of Atox1 that go beyond an established role as a specialized copper carrier. Recent findings of the physiologically relevant redox properties of the Atox1 CxxC site highlight the need to revisit the original role of Atox1 as an antioxidant protein. Exploring this property of Atox1 in more detail is likely to yield new insights into the link between metal homeostasis and a cellular redox state.
Abbreviations Used:
- ABC transporters
ATP-binding cassette transporters
- Atx1, Atox1
antioxidant protein 1
- BSO
buthioninesulfoximine
- CCS
copper chaperone for superoxide dismutase 1 (CCS)
- Grx
glutaredoxin
- GSH
glutathione
- GSSG
glutathione disulfide
- MBD
metal binding domain
- NLS
nuclear localization signal
- NMR
nuclear magnetic resonance
- SOD
superoxide dismutase
- TM
transmembrane segment
- Trx
thioredoxin
Acknowledgment
This work was supported by National Institutes of Health grant R01 DK071865 to S.L.
References
- 1.Allen KG. Arthur JR. Morrice PC. Nicol F. Mills CF. Copper deficiency and tissue glutathione concentration in the rat. Proc Soc Exp Biol Med. 1988;187:38–43. doi: 10.3181/00379727-187-42634. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez HM. Xue Y. Robinson CD. Canalizo-Hernandez MA. Marvin RG. Kelly RA. Mondragon A. Penner-Hahn JE. O'Halloran TV. Tetrathiomolybdate inhibits copper trafficking proteins through metal cluster formation. Science. 2010;327:331–334. doi: 10.1126/science.1179907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anastassopoulou I. Banci L. Bertini I. Cantini F. Katsari E. Rosato A. Solution structure of the apo and copper(I)-loaded human metallochaperone HAH1. Biochemistry. 2004;43:13046–13053. doi: 10.1021/bi0487591. [DOI] [PubMed] [Google Scholar]
- 4.Arnesano F. Banci L. Bertini I. Cantini F. Ciofi-Baffoni S. Huffman DL. O'Halloran TV. Characterization of the binding interface between the copper chaperone Atx1 and the first cytosolic domain of Ccc2 ATPase. J Biol Chem. 2001;276:41365–41376. doi: 10.1074/jbc.M104807200. [DOI] [PubMed] [Google Scholar]
- 5.Arnesano F. Banci L. Bertini I. Felli IC. Losacco M. Natile G. Probing the interaction of cisplatin with the human copper chaperone Atox1 by solution and in-cell NMR spectroscopy. J Am Chem Soc. 2011;133:18361–18369. doi: 10.1021/ja207346p. [DOI] [PubMed] [Google Scholar]
- 6.Arnesano F. Banci L. Bertini I. Huffman DL. O'Halloran TV. Solution structure of the Cu(I) and apo forms of the yeast metallochaperone, Atx1. Biochemistry. 2001;40:1528–1539. doi: 10.1021/bi0014711. [DOI] [PubMed] [Google Scholar]
- 7.Badarau A. Dennison C. Copper trafficking mechanism of CXXC-containing domains: insight from the pH-dependence of their Cu(I) affinities. J Am Chem Soc. 2011;133:2983–2988. doi: 10.1021/ja1091547. [DOI] [PubMed] [Google Scholar]
- 8.Banci L. Bertini I. Cantini F. Felli IC. Gonnelli L. Hadjiliadis N. Pierattelli R. Rosato A. Voulgaris P. The Atx1-Ccc2 complex is a metal-mediated protein-protein interaction. Nat Chem Biol. 2006;2:367–368. doi: 10.1038/nchembio797. [DOI] [PubMed] [Google Scholar]
- 9.Banci L. Bertini I. Ciofi-Baffoni S. Hadjiloi T. Martinelli M. Palumaa P. Mitochondrial copper(I) transfer from Cox17 to Sco1 is coupled to electron transfer. Proc Natl Acad Sci U S A. 2008;105:6803–6808. doi: 10.1073/pnas.0800019105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banci L. Bertini I. Ciofi-Baffoni S. Kozyreva T. Zovo K. Palumaa P. Affinity gradients drive copper to cellular destinations. Nature. 2010;465:645–648. doi: 10.1038/nature09018. [DOI] [PubMed] [Google Scholar]
- 11.Barry AN. Otoikhian A. Bhatt S. Shinde U. Tsivkovskii R. Blackburn NJ. Lutsenko S. The lumenal loop Met672-Pro707 of copper-transporting ATPase ATP7A binds metals and facilitates copper release from the intramembrane sites. J Biol Chem. 2011;286:26585–26594. doi: 10.1074/jbc.M111.229039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beeder J. Nilsen RK. Rosnes JT. Torsvik T. Lien T. Archaeoglobus fulgidus isolated from hot north sea oil field waters. Appl Environ Microbiol. 1994;60:1227–1231. doi: 10.1128/aem.60.4.1227-1231.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boal AK. Rosenzweig AC. Crystal structures of cisplatin bound to a human copper chaperone. J Am Chem Soc. 2009;131:14196–14197. doi: 10.1021/ja906363t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carafoli E. Garcia-Martin E. Guerini D. The plasma membrane calcium pump: recent developments and future perspectives. Experientia. 1996;52:1091–1100. doi: 10.1007/BF01952107. [DOI] [PubMed] [Google Scholar]
- 15.Carr HS. George GN. Winge DR. Yeast Cox11, a protein essential for cytochrome c oxidase assembly, is a Cu(I)-binding protein. J Biol Chem. 2002;277:31237–31242. doi: 10.1074/jbc.M204854200. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y. Saari JT. Kang YJ. Expression of gamma-glutamylcysteine synthetase in the liver of copper-deficient rats. Proc Soc for Exp Biol Med Soc Exp Biol Med. 1995;210:102–106. doi: 10.3181/00379727-210-43928. [DOI] [PubMed] [Google Scholar]
- 17.Culotta VC. Klomp LW. Strain J. Casareno RL. Krems B. Gitlin JD. The copper chaperone for superoxide dismutase. J Biol Chem. 1997;272:23469–23472. doi: 10.1074/jbc.272.38.23469. [DOI] [PubMed] [Google Scholar]
- 18.El Meskini R. Cline LB. Eipper BA. Ronnett GV. The developmentally regulated expression of Menkes protein ATP7A suggests a role in axon extension and synaptogenesis. Dev Neurosci. 2005;27:333–348. doi: 10.1159/000086713. [DOI] [PubMed] [Google Scholar]
- 19.Field LS. Luk E. Culotta VC. Copper chaperones: personal escorts for metal ions. J Bioenerg Biomembr. 2002;34:373–379. doi: 10.1023/a:1021202119942. [DOI] [PubMed] [Google Scholar]
- 20.Filomeni G. Ciriolo MR. Redox control of apoptosis: an update. Antioxid Redox Signal. 2006;8:2187–2192. doi: 10.1089/ars.2006.8.2187. [DOI] [PubMed] [Google Scholar]
- 21.Forman-Kay JD. Clore GM. Gronenborn AM. Relationship between electrostatics and redox function in human thioredoxin: characterization of pH titration shifts using two-dimensional homo- and heteronuclear NMR. Biochemistry. 1992;31:3442–3452. doi: 10.1021/bi00128a019. [DOI] [PubMed] [Google Scholar]
- 22.Glerum DM. Shtanko A. Tzagoloff A. SCO1 and SCO2 act as high copy suppressors of a mitochondrial copper recruitment defect in Saccharomyces cerevisiae. J Biol Chem. 1996;271:20531–20535. doi: 10.1074/jbc.271.34.20531. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Guerrero M. Arguello JM. Mechanism of Cu+-transporting ATPases: soluble Cu+ chaperones directly transfer Cu+ to transmembrane transport sites. Proc Natl Acad Sci U S A. 2008;105:5992–5997. doi: 10.1073/pnas.0711446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez-Guerrero M. Eren E. Rawat S. Stemmler TL. Arguello JM. Structure of the two transmembrane Cu+ transport sites of the Cu+-ATPases. J Biol Chem. 2008;283:29753–29759. doi: 10.1074/jbc.M803248200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez-Guerrero M. Hong D. Arguello JM. Chaperone-mediated Cu+ delivery to Cu+ transport ATPases: requirement of nucleotide binding. J Biol Chem. 2009;284:20804–20811. doi: 10.1074/jbc.M109.016329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goulet AC. Watts G. Lord JL. Nelson MA. Profiling of selenomethionine responsive genes in colon cancer by microarray analysis. Cancer Biol Ther. 2007;6:494–503. doi: 10.4161/cbt.6.4.3813. [DOI] [PubMed] [Google Scholar]
- 27.Gourdon P. Liu XY. Skjorringe T. Morth JP. Moller LB. Pedersen BP. Nissen P. Crystal structure of a copper-transporting PIB-type ATPase. Nature. 2011;475:59–64. doi: 10.1038/nature10191. [DOI] [PubMed] [Google Scholar]
- 28.Halliwell B. Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 29.Hamza I. Faisst A. Prohaska J. Chen J. Gruss P. Gitlin JD. The metallochaperone Atox1 plays a critical role in perinatal copper homeostasis. Proc Natl Acad Sci U S A. 2001;98:6848–6852. doi: 10.1073/pnas.111058498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hatori Y. Clasen S. Hasan NM. Barry AN. Lutsenko S. Functional partnership of the copper export machinery and glutathione balance in human cells. J Biol Chem. 2012 doi: 10.1074/jbc.M112.381178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirota Y. Acar N. Tranguch S. Burnum KE. Xie H. Kodama A. Osuga Y. Ustunel I. Friedman DB. Caprioli RM. Daikoku T. Dey SK. Uterine FK506-binding protein 52 (FKBP52)-peroxiredoxin-6 (PRDX6) signaling protects pregnancy from overt oxidative stress. Proc Natl Acad Sci U S A. 2010;107:15577–15582. doi: 10.1073/pnas.1009324107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horng YC. Cobine PA. Maxfield AB. Carr HS. Winge DR. Specific copper transfer from the Cox17 metallochaperone to both Sco1 and Cox11 in the assembly of yeast cytochrome C oxidase. J Biol Chem. 2004;279:35334–35340. doi: 10.1074/jbc.M404747200. [DOI] [PubMed] [Google Scholar]
- 33.Horng YC. Leary SC. Cobine PA. Young FB. George GN. Shoubridge EA. Winge DR. Human Sco1 and Sco2 function as copper-binding proteins. J Biol Chem. 2005;280:34113–34122. doi: 10.1074/jbc.M506801200. [DOI] [PubMed] [Google Scholar]
- 34.Hua H. Gunther V. Georgiev O. Schaffner W. Distorted copper homeostasis with decreased sensitivity to cisplatin upon chaperone Atox1 deletion in Drosophila. Biometals. 2011;24:445–453. doi: 10.1007/s10534-011-9438-1. [DOI] [PubMed] [Google Scholar]
- 35.Huffman DL. O'Halloran TV. Energetics of copper trafficking between the Atx1 metallochaperone and the intracellular copper transporter, Ccc2. J Biol Chem. 2000;275:18611–18614. doi: 10.1074/jbc.C000172200. [DOI] [PubMed] [Google Scholar]
- 36.Hung IH. Casareno RL. Labesse G. Mathews FS. Gitlin JD. HAH1 is a copper-binding protein with distinct amino acid residues mediating copper homeostasis and antioxidant defense. J Biol Chem. 1998;273:1749–1754. doi: 10.1074/jbc.273.3.1749. [DOI] [PubMed] [Google Scholar]
- 37.Hussain F. Olson JS. Wittung-Stafshede P. Conserved residues modulate copper release in human copper chaperone Atox1. Proc Natl Acad Sci U S A. 2008;105:11158–11163. doi: 10.1073/pnas.0802928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hussain F. Rodriguez-Granillo A. Wittung-Stafshede P. Lysine-60 in copper chaperone Atox1 plays an essential role in adduct formation with a target Wilson disease domain. J Am Chem Soc. 2009;131:16371–16373. doi: 10.1021/ja9058266. [DOI] [PubMed] [Google Scholar]
- 39.Huster D. Lutsenko S. The distinct roles of the N-terminal copper-binding sites in regulation of catalytic activity of the Wilson's disease protein. J Biol Chem. 2003;278:32212–32218. doi: 10.1074/jbc.M305408200. [DOI] [PubMed] [Google Scholar]
- 40.Itoh S. Kim HW. Nakagawa O. Ozumi K. Lessner SM. Aoki H. Akram K. McKinney RD. Ushio-Fukai M. Fukai T. Novel role of antioxidant-1 (Atox1) as a copper-dependent transcription factor involved in cell proliferation. J Biol Chem. 2008;283:9157–9167. doi: 10.1074/jbc.M709463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Itoh S. Ozumi K. Kim HW. Nakagawa O. McKinney RD. Folz RJ. Zelko IN. Ushio-Fukai M. Fukai T. Novel mechanism for regulation of extracellular SOD transcription and activity by copper: role of antioxidant-1. Free Radic Biol Med. 2009;46:95–104. doi: 10.1016/j.freeradbiomed.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jeney V. Itoh S. Wendt M. Gradek Q. Ushio-Fukai M. Harrison DG. Fukai T. Role of antioxidant-1 in extracellular superoxide dismutase function and expression. Circ Res. 2005;96:723–729. doi: 10.1161/01.RES.0000162001.57896.66. [DOI] [PubMed] [Google Scholar]
- 43.Jensen LT. Culotta VC. Activation of CuZn superoxide dismutases from Caenorhabditis elegans does not require the copper chaperone CCS. J Biol Chem. 2005;280:41373–41379. doi: 10.1074/jbc.M509142200. [DOI] [PubMed] [Google Scholar]
- 44.Jordan A. Aslund F. Pontis E. Reichard P. Holmgren A. Characterization of Escherichia coli NrdH. A glutaredoxin-like protein with a thioredoxin-like activity profile. J Biol Chem. 1997;272:18044–18050. doi: 10.1074/jbc.272.29.18044. [DOI] [PubMed] [Google Scholar]
- 45.Katano K. Kondo A. Safaei R. Holzer A. Samimi G. Mishima M. Kuo YM. Rochdi M. Howell SB. Acquisition of resistance to cisplatin is accompanied by changes in the cellular pharmacology of copper. Cancer Res. 2002;62:6559–6565. [PubMed] [Google Scholar]
- 46.Kelner GS. Lee M. Clark ME. Maciejewski D. McGrath D. Rabizadeh S. Lyons T. Bredesen D. Jenner P. Maki RA. The copper transport protein Atox1 promotes neuronal survival. J Biol Chem. 2000;275:580–584. doi: 10.1074/jbc.275.1.580. [DOI] [PubMed] [Google Scholar]
- 47.Kirlin WG. Cai J. Thompson SA. Diaz D. Kavanagh TJ. Jones DP. Glutathione redox potential in response to differentiation and enzyme inducers. Free Radic Biol Med. 1999;27:1208–1218. doi: 10.1016/s0891-5849(99)00145-8. [DOI] [PubMed] [Google Scholar]
- 48.Korte JJ. Prohaska JR. Dietary copper deficiency alters protein and lipid composition of murine lymphocyte plasma membranes. J Nutr. 1987;117:1076–1084. doi: 10.1093/jn/117.6.1076. [DOI] [PubMed] [Google Scholar]
- 49.Krummeck G. Rodel G. Yeast SCO1 protein is required for a post-translational step in the accumulation of mitochondrial cytochrome c oxidase subunits I and II. Curr Genet. 1990;18:13–15. doi: 10.1007/BF00321109. [DOI] [PubMed] [Google Scholar]
- 50.Kumar C. Igbaria A. D'Autreaux B. Planson AG. Junot C. Godat E. Bachhawat AK. Delaunay-Moisan A. Toledano MB. Glutathione revisited: a vital function in iron metabolism and ancillary role in thiol-redox control. EMBO J. 2011;30:2044–2056. doi: 10.1038/emboj.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lamb AL. Wernimont AK. Pufahl RA. Culotta VC. O'Halloran TV. Rosenzweig AC. Crystal structure of the copper chaperone for superoxide dismutase. Nat Struct Biol. 1999;6:724–729. doi: 10.1038/11489. [DOI] [PubMed] [Google Scholar]
- 52.Lassi KC. Prohaska JR. Erythrocyte copper chaperone for superoxide dismutase is increased following marginal copper deficiency in adult and postweanling mice. J Nutr. 2012;142:292–297. doi: 10.3945/jn.111.150755. [DOI] [PubMed] [Google Scholar]
- 53.Lassi KC. Prohaska JR. Rapid alteration in rat red blood cell copper chaperone for superoxide dismutase after marginal copper deficiency and repletion. Nutr Res. 2011;31:698–706. doi: 10.1016/j.nutres.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 54.Leary SC. Redox regulation of SCO protein function: controlling copper at a mitochondrial crossroad. Antioxid Redox Signal. 2010;13:1403–1416. doi: 10.1089/ars.2010.3116. [DOI] [PubMed] [Google Scholar]
- 55.Leitch JM. Jensen LT. Bouldin SD. Outten CE. Hart PJ. Culotta VC. Activation of Cu,Zn-superoxide dismutase in the absence of oxygen and the copper chaperone CCS. J Biol Chem. 2009;284:21863–21871. doi: 10.1074/jbc.M109.000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lim CM. Cater MA. Mercer JF. La Fontaine S. Copper-dependent interaction of glutaredoxin with the N termini of the copper-ATPases (ATP7A and ATP7B) defective in Menkes and Wilson diseases. Biochem Biophys Res Commun. 2006;348:428–436. doi: 10.1016/j.bbrc.2006.07.067. [DOI] [PubMed] [Google Scholar]
- 57.Lin SJ. Culotta VC. The ATX1 gene of Saccharomyces cerevisiae encodes a small metal homeostasis factor that protects cells against reactive oxygen toxicity. Proc Natl Acad Sci U S A. 1995;92:3784–3788. doi: 10.1073/pnas.92.9.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin SJ. Pufahl RA. Dancis A. O'Halloran TV. Culotta VC. A role for the Saccharomyces cerevisiae ATX1 gene in copper trafficking and iron transport. J Biol Chem. 1997;272:9215–9220. [PubMed] [Google Scholar]
- 59.Lockhart PJ. Mercer JF. Identification of the copper chaperone SAH in Ovis aries: expression analysis and in vitro interaction of SAH with ATP7B. Biochim Biophys Acta. 2000;1490:11–20. doi: 10.1016/s0167-4781(99)00230-4. [DOI] [PubMed] [Google Scholar]
- 60.Mandal AK. Cheung WD. Arguello JM. Characterization of a thermophilic P-type Ag+/Cu+-ATPase from the extremophile Archaeoglobus fulgidus. J Biol Chem. 2002;277:7201–7208. doi: 10.1074/jbc.M109964200. [DOI] [PubMed] [Google Scholar]
- 61.Marcil V. Lavoie JC. Emonnot L. Seidman E. Levy E. Analysis of the effects of iron and vitamin C co-supplementation on oxidative damage, antioxidant response and inflammation in THP-1 macrophages. Clin Biochem. 2011;44:873–883. doi: 10.1016/j.clinbiochem.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 62.McRae R. Lai B. Fahrni CJ. Copper redistribution in Atox1-deficient mouse fibroblast cells. J Biol Inorg Chem. 2010;15:99–105. doi: 10.1007/s00775-009-0598-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miyayama T. Suzuki KT. Ogra Y. Copper accumulation and compartmentalization in mouse fibroblast lacking metallothionein and copper chaperone, Atox1. Toxicol Appl Pharmacol. 2009;237:205–213. doi: 10.1016/j.taap.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 64.Morin I. Gudin S. Mintz E. Cuillel M. Dissecting the role of the N-terminal metal-binding domains in activating the yeast copper ATPase in vivo. FEBS J. 2009;276:4483–4495. doi: 10.1111/j.1742-4658.2009.07155.x. [DOI] [PubMed] [Google Scholar]
- 65.Muller PA. Klomp LW. ATOX1: a novel copper-responsive transcription factor in mammals? Int J Biochem Cell Biol. 2009;41:1233–1236. doi: 10.1016/j.biocel.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 66.Naeve GS. Vana AM. Eggold JR. Kelner GS. Maki R. Desouza EB. Foster AC. Expression profile of the copper homeostasis gene, rAtox1, in the rat brain. Neuroscience. 1999;93:1179–1187. doi: 10.1016/s0306-4522(99)00175-x. [DOI] [PubMed] [Google Scholar]
- 67.Neave MJ. Streten-Joyce C. Nouwens AS. Glasby CJ. McGuinness KA. Parry DL. Gibb KS. The transcriptome and proteome are altered in marine polychaetes (Annelida) exposed to elevated metal levels. J Proteomics. 2012;75:2721–2735. doi: 10.1016/j.jprot.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 68.Nittis T. George GN. Winge DR. Yeast Sco1, a protein essential for cytochrome c oxidase function is a Cu(I)-binding protein. J Biol Chem. 2001;276:42520–42526. doi: 10.1074/jbc.M107077200. [DOI] [PubMed] [Google Scholar]
- 69.Nkabyo YS. Ziegler TR. Gu LH. Watson WH. Jones DP. Glutathione and thioredoxin redox during differentiation in human colon epithelial (Caco-2) cells. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1352–G1359. doi: 10.1152/ajpgi.00183.2002. [DOI] [PubMed] [Google Scholar]
- 70.O'Halloran TV. Culotta VC. Metallochaperones, an intracellular shuttle service for metal ions. J Biol Chem. 2000;275:25057–25060. doi: 10.1074/jbc.R000006200. [DOI] [PubMed] [Google Scholar]
- 71.O'Neil KT. DeGrado WF. A thermodynamic scale for the helix-forming tendencies of the commonly occurring amino acids. Science. 1990;250:646–651. doi: 10.1126/science.2237415. [DOI] [PubMed] [Google Scholar]
- 72.Ozumi K. Sudhahar V. Kim HW. Chen GF. Kohno T. Finney L. Vogt S. McKinney RD. Ushio-Fukai M. Fukai T. Role of copper transport protein antioxidant 1 in angiotensin ii-induced hypertension: a key regulator of extracellular superoxide dismutase. Hypertension. 2012;60:476–486. doi: 10.1161/HYPERTENSIONAHA.111.189571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Palm ME. Weise CF. Lundin C. Wingsle G. Nygren Y. Bjorn E. Naredi P. Wolf-Watz M. Wittung-Stafshede P. Cisplatin binds human copper chaperone Atox1 and promotes unfolding in vitro. Proc Natl Acad Sci U S A. 2011;108:6951–6956. doi: 10.1073/pnas.1012899108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Portnoy ME. Rosenzweig AC. Rae T. Huffman DL. O'Halloran TV. Culotta VC. Structure-function analyses of the ATX1 metallochaperone. J Biol Chem. 1999;274:15041–15045. doi: 10.1074/jbc.274.21.15041. [DOI] [PubMed] [Google Scholar]
- 75.Prohaska JR. Brokate B. Copper deficiency alters rat dopamine beta-monooxygenase mRNA and activity. J Nutr. 1999;129:2147–2153. doi: 10.1093/jn/129.12.2147. [DOI] [PubMed] [Google Scholar]
- 76.Prohaska JR. Brokate B. Dietary copper deficiency alters protein levels of rat dopamine beta-monooxygenase and tyrosine monooxygenase. Exp Biol Med (Maywood) 2001;226:199–207. doi: 10.1177/153537020122600307. [DOI] [PubMed] [Google Scholar]
- 77.Prohaska JR. Lukasewycz OA. Copper deficiency during perinatal development: effects on the immune response of mice. J Nutr. 1989;119:922–931. doi: 10.1093/jn/119.6.922. [DOI] [PubMed] [Google Scholar]
- 78.Ralle M. Lutsenko S. Blackburn NJ. X-ray absorption spectroscopy of the copper chaperone HAH1 reveals a linear two-coordinate Cu(I) center capable of adduct formation with exogenous thiols and phosphines. J Biol Chem. 2003;278:23163–23170. doi: 10.1074/jbc.M303474200. [DOI] [PubMed] [Google Scholar]
- 79.Rodriguez-Granillo A. Wittung-Stafshede P. Structure and dynamics of Cu(I) binding in copper chaperones Atox1 and CopZ: a computer simulation study. J Phys Chem B. 2008;112:4583–4593. doi: 10.1021/jp711787x. [DOI] [PubMed] [Google Scholar]
- 80.Rodriguez-Manzaneque MT. Tamarit J. Belli G. Ros J. Herrero E. Grx5 is a mitochondrial glutaredoxin required for the activity of iron/sulfur enzymes. Mol Biol Cell. 2002;13:1109–1121. doi: 10.1091/mbc.01-10-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rosenzweig AC. Huffman DL. Hou MY. Wernimont AK. Pufahl RA. O'Halloran TV. Crystal structure of the Atx1 metallochaperone protein at 1.02 A resolution. Structure. 1999;7:605–617. doi: 10.1016/s0969-2126(99)80082-3. [DOI] [PubMed] [Google Scholar]
- 82.Saari JT. Copper deficiency and cardiovascular disease: role of peroxidation, glycation, and nitration. Can J Physiol Pharmacol. 2000;78:848–855. doi: 10.1139/cjpp-78-10-848. [DOI] [PubMed] [Google Scholar]
- 83.Safaei R. Maktabi MH. Blair BG. Larson CA. Howell SB. Effects of the loss of Atox1 on the cellular pharmacology of cisplatin. J Inorg Biochem. 2009;103:333–341. doi: 10.1016/j.jinorgbio.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sanokawa-Akakura R. Cao W. Allan K. Patel K. Ganesh A. Heiman G. Burke R. Kemp FW. Bogden JD. Camakaris J. Birge RB. Konsolaki M. Control of Alzheimer's amyloid beta toxicity by the high molecular weight immunophilin FKBP52 and copper homeostasis in Drosophila. PLoS One. 2010;5:e8626. doi: 10.1371/journal.pone.0008626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sanokawa-Akakura R. Dai H. Akakura S. Weinstein D. Fajardo JE. Lang SE. Wadsworth S. Siekierka J. Birge RB. A novel role for the immunophilin FKBP52 in copper transport. J Biol Chem. 2004;279:27845–27848. doi: 10.1074/jbc.C400118200. [DOI] [PubMed] [Google Scholar]
- 86.Satoh T. Sakai N. Enokido Y. Uchiyama Y. Hatanaka H. Survival factor-insensitive generation of reactive oxygen species induced by serum deprivation in neuronal cells. Brain Res. 1996;733:9–14. doi: 10.1016/0006-8993(96)00527-6. [DOI] [PubMed] [Google Scholar]
- 87.Schmidt PJ. Kunst C. Culotta VC. Copper activation of superoxide dismutase 1 (SOD1) in vivo. Role for protein-protein interactions with the copper chaperone for SOD1. J Biol Chem. 2000;275:33771–33776. doi: 10.1074/jbc.M006254200. [DOI] [PubMed] [Google Scholar]
- 88.Schmidt PJ. Rae TD. Pufahl RA. Hamma T. Strain J. O'Halloran TV. Culotta VC. Multiple protein domains contribute to the action of the copper chaperone for superoxide dismutase. J Biol Chem. 1999;274:23719–23725. doi: 10.1074/jbc.274.34.23719. [DOI] [PubMed] [Google Scholar]
- 89.Singleton WC. McInnes KT. Cater MA. Winnall WR. McKirdy R. Yu Y. Taylor PE. Ke BX. Richardson DR. Mercer JF. La Fontaine S. Role of glutaredoxin1 and glutathione in regulating the activity of the copper-transporting P-type ATPases, ATP7A and ATP7B. J Biol Chem. 2010;285:27111–27121. doi: 10.1074/jbc.M110.154468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Southon A. Burke R. Norgate M. Batterham P. Camakaris J. Copper homoeostasis in Drosophila melanogaster S2 cells. Biochem J. 2004;383:303–309. doi: 10.1042/BJ20040745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thompson JD. Higgins DG. Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tiffany-Castiglioni E. Hong S. Qian Y. Copper handling by astrocytes: insights into neurodegenerative diseases. Int J Dev Neurosci. 2011;29:811–818. doi: 10.1016/j.ijdevneu.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 93.Tottey S. Patterson CJ. Banci L. Bertini I. Felli IC. Pavelkova A. Dainty SJ. Pernil R. Waldron KJ. Foster AW. Robinson NJ. Cyanobacterial metallochaperone inhibits deleterious side reactions of copper. Proc Natl Acad Sci U S A. 2012;109:95–100. doi: 10.1073/pnas.1117515109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wakabayashi T. Nakamura N. Sambongi Y. Wada Y. Oka T. Futai M. Identification of the copper chaperone, CUC-1, in Caenorhabditis elegans: tissue specific co-expression with the copper transporting ATPase, CUA-1. FEBS Lett. 1998;440:141–146. doi: 10.1016/s0014-5793(98)01431-8. [DOI] [PubMed] [Google Scholar]
- 95.Walker JM. Huster D. Ralle M. Morgan CT. Blackburn NJ. Lutsenko S. The N-terminal metal-binding site 2 of the Wilson's disease protein plays a key role in the transfer of copper from Atox1. J Biol Chem. 2004;279:15376–15384. doi: 10.1074/jbc.M400053200. [DOI] [PubMed] [Google Scholar]
- 96.Walker JM. Tsivkovskii R. Lutsenko S. Metallochaperone Atox1 transfers copper to the NH2-terminal domain of the Wilson's disease protein and regulates its catalytic activity. J Biol Chem. 2002;277:27953–27959. doi: 10.1074/jbc.M203845200. [DOI] [PubMed] [Google Scholar]
- 97.Wernimont AK. Huffman DL. Lamb AL. O'Halloran TV. Rosenzweig AC. Structural basis for copper transfer by the metallochaperone for the Menkes/Wilson disease proteins. Nat Struct Biol. 2000;7:766–771. doi: 10.1038/78999. [DOI] [PubMed] [Google Scholar]
- 98.White C. Kambe T. Fulcher YG. Sachdev SW. Bush AI. Fritsche K. Lee J. Quinn TP. Petris MJ. Copper transport into the secretory pathway is regulated by oxygen in macrophages. J Cell Sci. 2009;122:1315–1321. doi: 10.1242/jcs.043216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wimmer R. Herrmann T. Solioz M. Wuthrich K. NMR structure and metal interactions of the CopZ copper chaperone. J Biol Chem. 1999;274:22597–22603. doi: 10.1074/jbc.274.32.22597. [DOI] [PubMed] [Google Scholar]
- 100.Xiao Z. Brose J. Schimo S. Ackland SM. La Fontaine S. Wedd AG. Unification of the copper(I) binding affinities of the metallo-chaperones Atx1, Atox1, and related proteins: detection probes and affinity standards. J Biol Chem. 2011;286:11047–11055. doi: 10.1074/jbc.M110.213074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang Y. Mandal AK. Bredeston LM. Gonzalez-Flecha FL. Arguello JM. Activation of Archaeoglobus fulgidus Cu(+)-ATPase CopA by cysteine. Biochim Biophys Acta. 2007;1768:495–501. doi: 10.1016/j.bbamem.2006.09.013. [DOI] [PubMed] [Google Scholar]