Abstract
Self-control is defined as foregoing an immediate reward to gain a larger delayed reward. Methods used to test self-control comparatively include intertemporal choice tasks, delay of gratification tasks, and accumulation tasks. To date, capuchin monkeys have shown different levels of self-control across tasks. This study introduced a new task that could be used comparatively to measure self-control in an intuitive context that involved responses that required no explicit training. Capuchin monkeys (Cebus apella) were given a choice between two food items that were presented on a mechanized, revolving tray that moved those foods sequentially towards the monkeys. A monkey could grab the first item or wait for the second, but was only allowed one item. Most monkeys in the study waited for a more highly preferred food item or a larger amount of the same food item when those came later, and they inhibited the prepotent response to grab food by not reaching out to take less preferred foods or smaller amounts of food that passed directly in front of them first. These data confirm that the mechanisms necessary for self-control are present in capuchin monkeys, and indicate that the methodology can be useful for broader comparative assessments of self-control.
Keywords: Capuchin monkeys, Cebus apella, Self-Control, Delay of Gratification
Introduction
Self-control is defined as foregoing an immediate reward for a better, delayed reward (Rachlin and Green 1972). This is a complex behavioral process that requires an individual to endure a delay to obtain a larger reward, and perhaps to make a decision based on whether or not the larger reward is worth the temporal cost. In humans, self-control is a necessary and beneficial skill in many situations such as when one tries to make sound financial decisions (e.g., saving money for retirement instead of spending it now), when one engages in certain kinds of social interactions (e.g., not saying something in the heat of the moment but instead waiting for a more opportune time), and when one makes certain kinds of health related decisions (e.g., opting for healthful food instead of food devoid of nutrition so as to reach a healthy weight).
Self-control is also evident in the behavior of some nonhuman animals (hereafter animals), and it has been tested in a variety of ways (e.g., Ainslie 1974; Evans and Westergaard, 2006; Grosch and Neuringer 1981; Logue 1988; Tobin, Chelonis and Logue 1993; Tobin, Logue, Chelonis, Ackerman and May 1996; van Haaren, van Hest and van de Poll 1988). With nonhuman primates, self-control oftenis investigated using delay choice and delay maintenance paradigms. Delay choice tasks require participants to choose between a smaller or less preferred amount of a food item that is more immediately available (often referred to as smaller sooner or SS reward), or a larger or more preferred amount of food that becomes available after a delay (larger later or LL reward; e.g. Addessi, Paglieri and Focaroli 2011; Amici, Aurelli and Call 2008). Delay choice tasks usually do not allow subjects to reevaluate their decision once a choice has been made because there are no additional opportunities to alter their response. In contrast, delay maintenance tasks, which will be described in more detail below, do allow for the decision to be altered throughout the test. However, some versions of the delay choice task present the actual food rewards to animals as choice options (rather than presenting choice links or icons), and this can be problematic for interpreting performance. Because many animals exhibit a prepotent tendency to point to the larger of two arrays (e.g. Anderson, Awazu and Fujita 2000; Boysen and Berntson 1995; Genty, Palmier and Roeder 2004) this may introduce difficulty in determining a nonhuman animal’s understanding of the task, because what may appear to be a choice for the LL reward could really be a prepotent response to more food without any understanding of the role that delay plays in the test. This confounding of self-control responses and prepotent responses makes it difficult to accurately assess the level of self-control shown by an individual or a species.
In the visible-foods version of the delay choice task, primates often perform in a way that is consistent with a self-control interpretation, and apes and monkeys performed similarly on this type of task. For instance, tufted capuchins (Cebus apella) pointed to either two immediately available pieces of peanut or six pieces available after a variable delay (Addessi et al. 2011; see also Amici et al. 2008). The capuchins selected the LL option most of the time, and their indifference points did not differ significantly from those of chimpanzees (Pan troglodytes) and bonobos (Pan paniscus) from an earlier study (Rosati, Stevens, Hare and Hauser 2007). However, it is unclear if the capuchins understood that the larger quantity required a delay or if they merelypointed to the more appetitive option, which was then scored as a self-control response. In fact, research in reverse-reward contingency paradigms, which require subjects to choose a smaller quantity in order to receive the larger one (e.g. Boysen and Berntson 1995; Boysen, Berntson, Hannan and Cacioppo 1996; Kralik 2005) showed that capuchins did not choose a smaller reward when a larger was also offered, even though selecting the smaller amount would have yielded the most food (Anderson, Hattori and Fujita 2008). Corrective procedures did not alter performance in half of the monkeys that were tested, indicating that pointing to the larger of two food arrays may be a strong prepotent response for capuchins, and this is cause for concern with regard to interpreting the data from some previous delay of gratification tests that require monkeys to point to food (see Addessi and Rossi 2011 for a procedure that reduced the prepotency problem in capuchins).
In contrast to the demonstrated success of capuchins on delay choice tasks, performance is more variable on delay maintenance tasks. In accumulation delay maintenance tasks, animals are presented with a reward that grows as time passes, allowing them to take it at any point during a trial (which ends the accumulation) or wait until the accrual is complete (e.g. Anderson, Kuroshima and Fujita 2010; Beran 2002; Beran and Evans 2006; Evans and Beran 2007; Evans, Beran, Paglieri, and Addessi, in press; Pelé, Dufour, Michiletta, and Thierry 2011). In one accumulation-based delay maintenance task, Anderson et al. (2010) found that most tested capuchins and squirrel monkeys (Saimiri sciureus) failed to show self-control. This study also incorporated a second measure that accumulated increasingly larger food pieces throughout the trial. These items were visible before the experimenter placed them in the monkey’s reach, and this manipulation appeared to improve the performance of some monkeys. Of particular interest was that when retested on the first delay maintenance task without increasing item size, some monkeys that initially failed succeeded after experience with the ascending size task. This may indicate that the increasingly appetitive nature of ascending sizes helped unsuccessful monkeys understand the nature of the task. More specifically, adding an intuitive component like visibly increasing reward size could have added to the monkeys’ understanding of the task because the monkeys saw that a larger reward was coming compared to what was presently available. Seeing the larger reward and knowing that it would be placed in the food reservoir may have helped the unsuccessful monkeys to comprehend that waiting was required to receive the best reward (see also Evans and Westergaard, 2006, for another self-control test with qualitatively different food types that also produced positive results with capuchins).
Capuchin monkeys do not always, however, succeed on delay maintenance tasks. For example, the “cookie exchange” task requires subjects to hold and then exchange a smaller reward for a larger, delayed reward (Dufour, Pelé, Sterck and Thierry 2007; Pelé et al. 2011; Pelé, Michiletta, Uhlrich, Thierry and Dufour 2010). In these studies, primates are handed a small piece of cookie that they must hold until a delay has passed, after which they can exchange that piece for a substantially larger piece of cookie that remains visible during the delay. Chimpanzees, long-tailed macaques (Macaca fascicularis), and tonkean macaques (Macaca tonkeana) demonstrated the ability to delay gratification in these tasks contingent upon the length of the delay and the ratio of the smaller to the larger reward (Dufour et al. 2007; Pelé et al. 2010). Brown capuchin monkeys also appeared to delay gratification, but their performance did not improve as predicted when larger cookie pieces were used in conjunction with longer delays. Pelé et al. (2011) suggested that this difference in performance was a result of the capuchins’ consistent impulsivity and their significantly lower delay tolerance compared with other tested speciesin this task (see also Ramseyer, Pelé, Dufour, Chauvin and Thierry 2006). This impulsivity may explain capuchins’ apparent successful performance on visible-food delay choice tasks, in which it is likely that they pointed to the larger option regardless of the delay required. However, the partial success that was shown might be similar to that shown in Anderson et al.’s (2010) ascending sizes task, wherein the capuchin’s ability to see and understand that a better reward was present and eventually available could have functioned as incentive to wait for the better reward.
A different testing method may be useful for assessing delay of gratification in more impulsivespecies such as capuchin monkeys. A design that incorporates an intuitive understanding of the task’s nature might better reflect the self-control ability of capuchins and other animals. To develop such a method, we must first assume that the organisms being tested are capable of making informed decisions about SS and LL options. More specifically, they must be able to recognize that one option is more rewarding than the other, and have the capacity to try to obtain the more desired reward. One must also ensure that if there are prepotent responses (such as reaching toward food items) that those do not render the measure of self-control invalid if they are invoked. For example, if a monkey will not eat pumpkin but will eat grapes, it is not a demonstration of self-control if the monkey waits for grapes when pumpkin is presented as the SS option. However, if a monkey will wait for a large piece of banana as opposed to a small one, then one can more confidently conclude that self-control is evident.
The current study involved a novel method to test delay of gratification in capuchin monkeys by presenting a rotating disk that allowed a monkey to clearly see when the first and second food choices would be available as each of those items moved within reach of the monkey. This eliminated the concern that animals would simply select the larger of two options without understanding the delay involved in the choice. It also enhanced the likelihood that the monkeys understood the nature of the task. Because the monkeys could clearly see when either of the food items would be within their reach, they could make an informed decision about whether or not to grab the first food item that passed. Additionally, the monkeys could take the food directly from the tray as opposed to pointing or pressing a button, giving them more intuitive control over the process and thus providing less potential confusion about the task demands. The idea behind the task was that the rotating disk provided subjects with a clear view of the entire task at once. There was little uncertainty involved in watching both food items move steadily towards the monkey, making this method ideal for testing self-control in animals.
We tested capuchins using this novel method and varied delay and reward magnitude to further explore the capacity in this species for delay of gratification. We predicted that highly preferred foods would be selected over less preferred foods, and that larger amounts of highly preferred foods would be selected over smaller amounts of the same food. We also predicted that, similar to previous studies (e.g. Anderson et al. 2010; Pelé et al. 2011; Rosati, Stevens and Hauser 2006), increased delays and decreased differences between reward magnitudes might decrease delay of gratification. For the purposes of the present study we were more concerned with determining whether capuchin monkeys would allow some foods to move past them so that other (better) foods would come within reach, rather than trying to determine the temporal duration at which they showed no preference or to otherwise assess points of indifference as in other kinds of tests (e.g., Addessi et al. 2011; Rosati et al. 2007).
Methods
Participants
Eight brown tufted capuchin monkeys at the Language Research Center at Georgia State University participated in this study (3 monkeys were females and 5 monkeys were males). Subjects’ ages ranged from 5 to 30 years. Each subject voluntarily entered an individual test box attached to his/her enclosure for testing. Test boxes measured 33 × 46 × 61 cm stainless steel mesh, and were suspended from a stainless steel mesh wall approximately 1 m above the floor. Visual access to the remaining colony members was maintained at all times. Four test boxes were attached to each colony’s indoor enclosure and were positioned 0.5 m apart. Rectangular openings allowed monkeys to enter the test boxes directly from their enclosure, and vertically sliding doors allowed experimenters to secure the monkeys in the mesh boxes for testing. The sliding doors were made from solid Lexan except for a 4 cm diameter circle cut from the middle to allow monkeys to reach out and toward the apparatus. Water was available ad libitum, and the subjects were otherwise maintained on their normal diet of fruits, vegetables, and protein sources independent of their performance on this task.
Apparatus
The apparatus used was a 38 cm diameter elevated revolving disk affixed to a rolling cart (Figure 1). The disk was mounted on top of a rotation device that moved at 3 revolutions per minute. Attached to the disk were two 10 cm × 2.5 cm metal bars onto which food items were placed at the onset of a trial. The bars could be moved to different positions on the diskto increase or decrease the delayuntil those bars reachedthe monkey. The starting position of the disk could be adjusted as well. A Lexan barrier with a 20 cm × 10 cm rectangular opening was attached to the front of the cart so that subjects were prevented from reaching around the side of the text box to take the food before it was in front of them.
Figure 1.
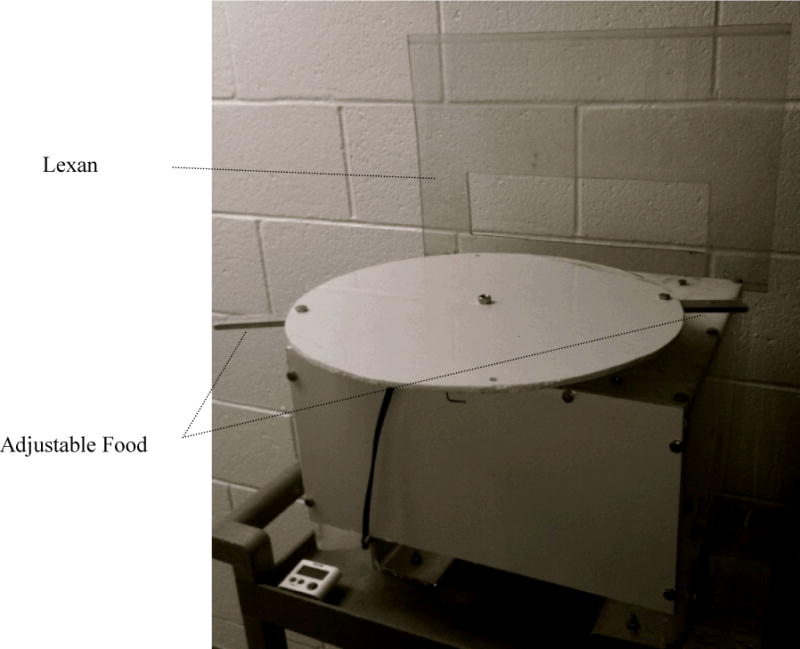
The rotating reward tray.
Design and Procedure
At the beginning of each trial, the cart was pushed to within 8–12 cm of the test box depending on the reach length of the subject. The monkeywas shown two food items. The first items was presented for 2 seconds and then was placed on the nearest bar, and the second item shown was shown for 2 seconds and then placed on the farthest bar. The motor of the apparatus was turned on, and food items began to rotate in a counter clockwise direction towards a monkey. Simultaneously, a 60-second timer was activated. The monkeys could grab the first item or wait for the second, but were only allowed one item. As soon as a monkey selected an item, the cart was pulled out of their reach and the disk continued to revolve for the remainder of the 60 seconds. The timer was activated again for a 60-second inter-trial interval. Thus, total trial duration was equated no matter which food item a monkey selected. The number of trials in a session varied based on the phase of the study, and subjects completed approximately 4 sessions per week.
To ensure that the monkeys could not reach the food until the entire delay had passed, a timeout period of 30 seconds was imposed if a monkey pulled the disk towards them at any point during a trial. After 30 seconds, the trial was restarted from the beginning. Responses were defined as any touching of a food bar regardless of whether or not a monkey took the food. To ensure that the monkeys waited through the entire delay period, a 30 second timeout period was also imposed during the first phase if a monkey touched the bar or grabbed food before the bar was directly in front of the center of the 8” × 4” opening. Subsequent phases also included a second experimenter who stood several feet away, made no eye contact with the subjects, and verbally indicated when a response was made. When the second experimenter was present, the first experimenter baited the food bars and then faced in the opposite direction until the subject responded, and its choice was verbally announced by the second experimenter. This was done to prevent any cueing from the first experimenter, and this eliminated the possibility that this experimenter in any way contributed to inhibition in reaching on the part of the monkeys.
The study was conducted in six phases. Before the study began, monkeys were given 10 forced trials with each food item. In the carrot forced trials, a piece of carrot was placed on the first (nearer) food bar, and in the banana forced trials, a piece of banana was placed on the first food bar. In both types of forced trials nothing was placed on the second (farther) food bar. These forced trials were conducted to ensure that the monkeys would consume both foods, and a criterion of 90% consumption was required for a monkey to be eligible for the study.
From this point forward, to move from one phase to the next, monkeys were required to wait for the food on the second bar, effectively exhibiting self-control, for the two test trials included in each session in at least four out of five consecutive sessions. This indicated a statistically significant preference over this number of trials (p < 0.05, binomial test). Thus, the minimum number of trials that a monkey could be given in a phase was eight trials because perfect performance in the first four sessions would negate the need for a fifth session (although some monkeys received more trials than that even if they showed perfect selection of the LL reward because of experimenter error). Subjects were discontinued in the study if they failed to wait for the food on the second bar during at least one test trial in each of 10 consecutive sessions.
Phase 1: Carrot – 5 s delay; Banana – 10 s delay
On trial 1 of each session, 2.8 g of carrot was presented on the first food bar with nothing on the second bar as a forced trial, followed by a forced trial of 11.2 g of banana on the second food bar with nothing on the first. Two test trials were subsequently conducted in which the carrot was placed on the first bar and the banana on the second. The first bar took 5 s to move to the center of the opening, and the second food bar took 10 s to move to the center.
Phase 2: Carrot – 5 s delay; Banana – 15 s delay
The second phase was identically structured to Phase 1except that the second food bar was moved farther from the first so that monkeys had to wait 5 s for the carrot and 15 s for the banana.
Phase 3: 1:4 Banana ratio – 5 s and 10 s delay
In the third phase, the foods changed to 11.2 g of banana for the first food bar, which reached a monkey at 5 s, followed by 44.8 g of banana for the second bar, which reached a monkey at 10 s. There was one forced trial at the beginning of each session using the larger banana on the second bar only, followed by two test trials.
Phase 4: 1:4 Banana ratio – 5 s and 10 s delay, no forced trial
To ensure that satiation was not a factor due to the large quantity of banana used in the forced trial in Phase 3, the fourth phase was conducted without the forced trial. More specifically, we wanted to eliminate the possibility that the monkeys waited for the larger piece of banana on test trials because they were already satiated from the amount they received in the forced trial.
Phase 5: 1:2 Banana ratio – 5 s and 15 s delay
In the fifth phase, the difference in the size of the bananas was decreased, and the delay was increased. At 5 s, the monkeys could retrieve 11.2 g of banana, or they could wait for 15 s and take 22.4 g of banana. No forced trials were used in this phase. However, a randomly ordered control trial that presented the larger banana first was included in each session to demonstrate that the monkeys’ choices were based on portion size and not a learned response to wait for the second item no matter what it was.
Phase 6: 1:2 Banana ratio – 2.5 s and 12.5 s delay
The sixth phase utilized the same food portions and control trial as Phase 5, but the first food bar started within the monkeys’ reach. The item did not reach the center of the armhole until 2.5 s, however the item could be reached immediately (i.e., before the disk began revolving). The second bar reached the center of the opening at 12.5 s. This phase was critical because all previous phases required that a monkey had to wait at least some period of time before anything could be grabbed, because nothing was in reach at the start of the trial. Thus, necessarily having to wait some short period of time at the outset of the trial might have been critical to the success that the monkeys showed to this point. In this phase, food was available as soon as the trial started, and so inhibition was required right from the start of the trial. Thus, we were able to test the monkeys’ ability to inhibit right from the trial’s beginning, similar to accumulation tasks where the first item to be presented was available right at the start of the trial. Again, no forced trials were conducted.
Results
All eight monkeys met the pre-testing criterion and were considered eligible for the experiment. The overall performance of each monkey is presented in Table 1, which shows the total number of trials at each phase for which the LL reward was selected. These performance levels were each compared to chance using a binomial distribution probability test (alpha = .05), so that the overall performance for each monkey in each phase is shown. On three occasions, subjects significantly differed from chance toward impulsivity in lieu of self-control. Each occurrence resulted in the removal of the subject from further study per our previously outlined criterion. All other statistically significant probabilities indicate self-control.
Table 1.
Overall Performance of Each Monkey for Each Phase
| Monkey (gender, age) |
Phase 1 | Phase 2 | Phase 3 | Phase 4 | Phase 5 | Phase 6 |
|---|---|---|---|---|---|---|
| Drella (male, 21) |
12/16 | 10/14 | 0/20*a | N/A | N/A | N/A |
| Gabe (male, 13) |
9/16 | 8/8* | 0/20*a | N/A | N/A | N/A |
| Griffin (male, 14) |
14/24 | 8/12 | 15/22 | 10/10* | 10/10* | 10/10* |
| Liam (male, 8) |
9/10* | 10/10* | 9/12 | 10/10* | 8/10 | 11/12* |
| Lily (female, 14) |
10/14 | 9/12 | 8/10 | 10/10* | 19/22* | 8/10 |
| Logan (male, 6) |
8/10 | 9/12 | 11/12* | 10/10* | 8/8* | 9/10* |
| Nala (female, 9) |
8/8* | 12/12* | 9/10* | 10/10* | 0/20*a | N/A |
| Wren (female, 9) |
13/14* | 12/14* | 9/10* | 8/8* | 14/14* | 10/10* |
Performance significantly different from chance indicating self-control, p < 0.05
Performance significantly different from chance indicating impulsivity, p < 0.05.
N/A indicates that a monkey did not perform in these phases because it was discontinued in the study for choosing the SS reward at least once per session for 10 consecutive sessions.
The main result of interest, however, is how far each monkey progressed with the successively harder phases of the experiment, and this required meeting the above outlined criterion. Of the eight monkeys tested, all monkeys met criterion on Phase 1 and Phase 2. Six of the eight met criterion on Phase 3 and Phase 4, and five monkeys met criterion on Phase 5 and Phase 6. Thus, the majority of the monkeys successfully passed every phase in this experiment, and in many cases they did so with perfect or near perfect selection of the LL reward within a phase (Table 1). It is important to note that by the end of the experiment, monkeys were not simply waiting on the second reward even if it was of lesser value. For the control trials given in Phase 5 and Phase 6, in which the better reward passeda monkey first, monkeys chose that reward on 68 of 68 such trials (100%). This clearly indicated that the monkeys were not simply avoiding the first thing to pass by them on every trial.
Discussion
The purpose of the present study was to devise and test a new method for assessing self-control in nonhuman animals. Specifically, we sought to develop an intuitive, simple task that would allow the animals to understand the nature of the test from their earliest exposure to it. Some previous tests of self-control required either extensive training to get positive results (e.g., the reverse-reward test), or many sessions to establish a point of indifference between a SS and LL reward (e.g., inter-temporal choice tests). We wanted a test for which the criterion level establishing preference (for either SS or LL rewards) could occur quickly, and for which it was easy to vary the relative value of the SS and LL rewards as well as the delay. Our task accomplished these goals and, upon use with capuchin monkeys, showed that many animals gave clear behavioral signals of self-control and delay of gratification.
Most monkeys in the study waited for a more preferred food type or larger food item in lieu of a less-preferred or smaller food item across all phases of the experiment. Perhaps more importantly, many monkeys reached criterion to move to successive phases of the study in the minimum number of trials or with perfect performance, which suggests that they spontaneously exhibited self-control. These results also suggest that the ease of understanding the task allowed the monkeys to exhibit good performance, and they did so almost from the outset. Further, this new method helps avoid some of the potential confounds of tasks such as the visible-foods delay choice test that could reflect a predisposition to simply point to the larger or better option.
Although the delay times were relatively short in the present experiment, the goal was to confirm that capuchin monkeys would inhibit reaching for an appetitive reward that passed right in front of them, and wait instead for a second reward that came later. This allowed for an intuitive test that now can be used to assess other aspects of behavioral inhibition and self-control, and also that can easily be adapted for use with any other species that can make responses by taking food from a tray within reach.
Despite the overall success of this group of monkeys, there were individual differences in performance. Two monkeys, Drella and Gabe, did not inhibit their response to take the SS option when both foods were highly preferred bananas, and therefore did not progress past Phase 3. Because pre-study preference testing was conducted in which all of the monkeys ate the available carrot when it was the only option offered, we can assume that this change in self-control performance was not based on an aversion to the SS option in the first two phases. However, when the choice became more difficult – a small versus a large piece of banana – performance deteriorated. Perhaps these two monkeys were less astute at telling apart the relative difference in amount of banana, but this seems unlikely given the ratio of difference in Phase 3, and the previous experience of these monkeys in making quantity judgments of close similarity (e.g., Beran, Evans, Leighty, Harris and Rice 2008; Evans, Beran, Harris and Rice 2009). One other monkey, Nala, showed self-control in all phases until the difference in banana piece sizes was halved and the delay was doubled in Phase 5, when she exhibited statistically significant impulsivity. Prior to this phase, her selection of the LL option was consistently high, indicating that she clearly understood the task requirements necessary to receive the more appetitive reward. Because of this potential demonstration of self-control in previous phases, it is possible that the increased delay and decreased size of the LL reward created a point at which this individual was no longer motivated to wait for the LL reward. However, because both the size of the LL reward was decreased and the delay was increased, we do not know which factor contributed to her performance shift. To discern which factor more readily elicits performance change, future studies should individually modify either size or delay when designing successive phases.
The remaining five monkeys in the study progressed through every phase of the study, seemingly without difficulty given the small number of sessions needed to meet criterion. Indeed, each of these five monkeys chose the LL option more often than predicted by chance in each of the last three phases. When presented with an immediately available and preferred food item, they did not take it, but instead waited for the larger piece of the same food type to reach them.
Phase 6 performance deserves special mention, because the success of the five monkeys here indicated that, even when there was no delay for the first appetitive reward, these monkeys exercised behavioral inhibition. This pattern of performance clearly shows that self-control in these monkeys is not based on some requirement that at trial outset there is no SS reward available, which was true for the earlier phases, but instead suggests the monkeys’ performance is a true demonstration of inhibiting the response to grab the first food that reached them.
This task offers an intuitive measure for better understanding the delay of gratification capacity of many species. Here, we validated its utility with capuchin monkeys, a species with a mixed history of performance on various other self-control and delay of gratification tasks (e.g., Addessi et al. 2011; Anderson et al. 2010). Capuchin monkeys showed clearly that they would let food pass by them if the later outcome was better, and many monkeys did this almost immediately, and without any extensive training. These data indicate that the mechanisms necessary for self-control are present in capuchin monkeys even in tasks in which a choice to wait must be maintained in the face of continual temptation from the available SS reward. Future studies of this kind should include comparative data from animals other than primates, and these data should be compared to performance in other self-control tests. Gaining a broader understanding of these abilities both within capuchin monkeys and across species will allow researchers to continue to create a clearly defined phylogenetic map of self-control abilities, and perhaps elucidate the evolutionary origins of these abilities.
Acknowledgments
This research was supported by Grant HD-060563 from the National Institute of Child Health and Human Development and by Grant BCS-0924811 from the National Science Foundation. The authors thank Betty Chan and Emilie Menzel for their assistance with this project. All applicable institutional rules and regulations regarding animal care and use were followed in the care and testing of the monkeys and the experiment complied with all laws of the United States of America.
References
- Addessi E, Paglieri F, Focaroli V. The ecological rationality of delay tolerance: Insights from capuchin monkeys. Cognition. 2011;119:142–147. doi: 10.1016/j.cognition.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Addessi E, Rossi S. Tokens improve capuchin performance in the reverse-reward contingency task. Proc Biol Sci. 2011;278:849–54. doi: 10.1098/rspb.2010.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainslie GW. Impulse control in pigeons. J Exp Anal Behav. 1974;21:485–489. doi: 10.1901/jeab.1974.21-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amici F, Aurelli F, Call J. Fission-fusion dynamics, behavioral flexibility, and inhibitory control in primates. Curr Biol. 2008;18:1415–1419. doi: 10.1016/j.cub.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Anderson JR, Awazu S, Fujita K. Can squirrel monkeys (Saimiri sciureus) learn self-control: A study using food array selection tests and reverse-reward contingency. J Exp Psychol Anim Behav Proc. 2000;26:87–97. doi: 10.1037//0097-7403.26.1.87. [DOI] [PubMed] [Google Scholar]
- Anderson JR, Kuroshima H, Fujita K. Delay of gratification in capuchin monkeys (Cebus apella) and squirrel monkeys (Saimiri sciureus) J Comp Psychol. 2010;124:205–210. doi: 10.1037/a0018240. [DOI] [PubMed] [Google Scholar]
- Anderson JR, Hattori Y, Fujita K. Quality before quantity: rapid learning of reverse-reward contingency by capuchin monkeys (Cebus apella) J Comp Psychol. 2008;122:445–448. doi: 10.1037/a0012624. [DOI] [PubMed] [Google Scholar]
- Beran MJ. Maintenance of self-imposed delay of gratification by four chimpanzees (Pan troglodytes) and an orangutan (Pongo pygmaeus) J Gen Psychol. 2002;129:49–66. doi: 10.1080/00221300209602032. [DOI] [PubMed] [Google Scholar]
- Beran MJ, Evans TA. Maintenance of delay of gratification by four chimpanzees (Pan troglodytes): The effects of delayed reward visibility, experimenter presence, and extended delay intervals. Behav Proc. 2006;73:315–324. doi: 10.1016/j.beproc.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Beran MJ, Evans TA, Leighty KA, Harris EH, Rice D. Summation and quantity judgments of sequentially presented sets by capuchin monkeys (Cebus apella) Am JPrimatol. 2008;70:191–194. doi: 10.1002/ajp.20474. [DOI] [PubMed] [Google Scholar]
- Boysen ST, Berntson GG. Responses to quantity: Perceptual versus cognitive mechanisms in chimpanzees (Pan troglodytes) J Exp Psychol Anim Behav Proc. 1995;21:82–86. doi: 10.1037//0097-7403.21.1.82. [DOI] [PubMed] [Google Scholar]
- Boysen ST, Berntson GG, Hannan MB, Cacioppo JT. Quantity-based interference and symbolic representations in chimpanzees (Pan troglodytes) J Exp Psychol Anim Behav Proc. 1996;22:76–86. [PubMed] [Google Scholar]
- Dufour V, Pelé M, Sterck EHM, Thierry B. Chimpanzee (Pan troglodytes) anticipation of food return: Coping with waiting time in an exchange task. J Comp Psychol. 2007;121:145–155. doi: 10.1037/0735-7036.121.2.145. [DOI] [PubMed] [Google Scholar]
- Evans TA, Beran MJ. Delay of gratification and delay maintenance by rhesus macaques (Macaca mulatta) J Gen Psychol. 2007;134:199–216. doi: 10.3200/GENP.134.2.199-216. [DOI] [PubMed] [Google Scholar]
- Evans TA, Beran MJ, Harris EH, Rice D. Quantity judgments of sequentially presented food items by capuchin monkeys (Cebus apella) Anim Cogn. 2009;12:97–105. doi: 10.1007/s10071-008-0174-z. [DOI] [PubMed] [Google Scholar]
- Evans TA, Beran MJ, Paglieri F, Addessi E. Delaying gratification for food and tokens in capuchin monkeys (Cebus apella) and chimpanzees (Pan troglodytes): When quantity is salient, symbolic stimuli do not improve performance. Anim Cogn. doi: 10.1007/s10071-012-0482-1. (in press) [DOI] [PubMed] [Google Scholar]
- Evans TA, Westergaard GC. Self-control and tool use in tufted capuchin monkeys (Cebus apella) J Comp Psychol. 2006;120:163–166. doi: 10.1037/0735-7036.120.2.163. [DOI] [PubMed] [Google Scholar]
- Genty E, Palmier C, Roeder JJ. Learning to suppress responses to the larger of two rewards in two species of lemurs (Eulemur fulvus and E. macaco) Anim Behav. 2004;67:925–932. [Google Scholar]
- Grosch J, Neuringer A. Self-control in pigeons under the Mischel paradigm. J Exp Anal Behav. 1981;35:3–21. doi: 10.1901/jeab.1981.35-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralik JD. Inhibitory control and response selection in problem solving: How cotton-top tamarins (Saguinus oedipus) overcome a bias for selecting the larger quantity of food. J Comp Psychol. 2005;119:78–89. doi: 10.1037/0735-7036.119.1.78. [DOI] [PubMed] [Google Scholar]
- Logue AW. Research on self-control: An integrating framework. Behav Brain Sci. 1988;1:665–709. [Google Scholar]
- Pelé M, Dufour V, Micheletta J, Thierry B. Long-tailed macaques display unexpected waiting abilities in exchange tasks. Anim Cogn. 2010;13:263–271. doi: 10.1007/s10071-009-0264-6. [DOI] [PubMed] [Google Scholar]
- Pelé M, Micheletta J, Uhlrich P, Thierry B, Dufour V. Delay Maintenance in tonkean macaques and brown capuchin monkeys. Int J Primatol. 2011;32:149–166. [Google Scholar]
- Rachlin H, Green L. Commitment, choice, and self-control. J Exp Anal Behav. 1972;48:347–353. doi: 10.1901/jeab.1972.17-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramseyer A, Pele M, Dufour V, Chauvin C, Theirry B. Accepting loss: the temporal limits of reciprocity in brown capuchin monkeys. Proc Royal Soc London B. 2006;273:179–184. doi: 10.1098/rspb.2005.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati AG, Stevens JR, Hare B, Hauser MD. The evolutionary origins of human patience: Temporal preferences in chimpanzees, bonobos, and human adults. Curr Biol. 2007;17:1663–1668. doi: 10.1016/j.cub.2007.08.033. [DOI] [PubMed] [Google Scholar]
- Rosati AG, Stevens JR, Hauser MD. The effect of handling time on temporal discounting in two new world primates. Anim Behav. 2006;71:1379–1387. [Google Scholar]
- Tobin H, Chelonis JJ, Logue AW. Choice in self-control paradigms using rats. Psychol Rec. 1993;43:441–454. [Google Scholar]
- Tobin H, Logue AW, Chelonis JJ, Ackerman KT, May JG. Self-control in the monkey Macaca fascicularis. Anim Learn Behav. 1996;24:168–174. [Google Scholar]
- van Haaren F, van Hest A, Van de Poll NE. Self-control in male and female rats. J Exp Anal Behav. 1988;49:201–211. doi: 10.1901/jeab.1988.49-201. [DOI] [PMC free article] [PubMed] [Google Scholar]


