Abstract
Healthy children are high transmitters of influenza and can experience poor influenza outcomes. Many questions remain about the efficacy and impect of preventive measures because most existing studies report imprecise proxies of influenza incidence, do not follow subjects throughout the entire influenza season and across multiple influenza seasons, or do not control for important factors such as timing of implementation and social contact patterns. Modeling and simulation are key methodologies to answer questions regarding influenza prevention. While vaccination may be the most efficacious existing intervention, variations in circulating strains and children’s immune systems keep current vaccines from being fully protective, necessitating further clinical and economic studies and technology improvements. Hand hygiene appears to be an important adjunct but improving compliance, standardizing regimens and quantifying its impact remain challenging. Future studies should help better define the specific indications and circumstances for antiviral use and the role of nutritional supplements and nonpharmaceutical interventions.
Keywords: antiviral medications, healthy children, influenza, influenza prevention, nonpharmaceutical interventions, vaccination
Each year, influenza infection among children is a significant worldwide problem. Although older adults have the highest influenza-related mortality, children experience substantial morbidity. Annually, influenza attack rates (i.e., percentage of people infected with influenza each year) are higher among children (i.e., <18 years of age) than any other age group. Each year, children contracting influenza results in missed school days (leading to considerable cost [1] ), missed working days for parents and other caregivers for the children while sick [2], and excess healthcare costs (antibiotics, doctors’ visits and hospitalizations [3]). Influenza can also lead to complications such as otitis media, pneumonia and, in rare instances, death [4] in not only children with underlying chronic medical conditions but also those otherwise healthy. In 2003–2004, 67% of the 149 children in the USA who died from influenza were previously healthy. In 2003–2005, 51% of the pediatric influenza deaths in California and 40% of intensive care unit admissions occurred in healthy children [5]. Additionally, children can be key spreaders in a population since they are in frequent contact with each other as well as family members, are more susceptible to influenza infection than adults, and can shed greater amounts of virus for longer periods of time [6,7]. In fact, the best predictor for influenza occurring in a household is the presence of a child [4].
Therefore, better influenza control among children could benefit the overall population. Prevention strategies for healthy children fall into two general categories: pharmaceutical interventions, that is, substances delivered to a person’s body to alter the virus or that person’s immune system (e.g., vaccines, antivirals and nutritional supplements); and nonpharmaceutical interventions (NPIs), that is, strategies or physical objects that prevent the virus from entering the human body (e.g., limiting social contact, improving hand hygiene and wearing face masks).
While each of these interventions have received attention during past influenza seasons and the 2009 H1N1 influenza pandemic, decision makers including children, parents, physicians, school officials, public health officials, research scientists, funders and policy makers may not be fully aware of the available evidence behind each.
Pharmaceutical interventions: vaccination
Types of vaccines
Currently, two general types of annual vaccines are available for children: inactivated influenza vaccine, which contains killed virus, is US FDA-approved for children 6 months and older, and is injected intramuscularly; and live attenuated influenza vaccine (LAIV), which contains a live but weakened virus, is FDA-approved for children 2 years and older, and is delivered as an intranasal spray [5].
Until recently, the vaccine has consisted of three strains of influenza, two A strains and one B strain, to comprise a trivalent influenza vaccine (TIV). However, in 2012, the FDA approved a quadrivalent influenza vaccine (QIV), a four-strain vaccine that includes an additional B strain, for use in people aged 2–49 years [8,9]. Since the circulating influenza viral strains tend to change each influenza season, vaccine scientists, manufacturers and policy makers attempt to anticipate these strains prior to the influenza season and incorporate them into the vaccine. Predicting the dominant strain(s) can be difficult, so the vaccine’s strains do not always match the circulating ones. Consequently, annual vaccine protection tends to vary and persist only through a single influenza season/year.
Therefore, scientists have been attempting to develop a vaccine that protects over multiple years, regardless of shifting circulating strains and antigenic drift [10]. Such a vaccine would target a ‘universal’ or conserved portion of the influenza virus – that is, one that persists across different strains. A multiyear vaccine would also circumvent the need for yearly immunization (and the annual compliance needed) and protect the population should a novel strain, such as the 2009 H1N1 pandemic strain, emerge. Currently, the universal vaccine is in preclinical development, as testing of vaccines targeting the influenza A M2 protein has been carried out in mouse models [10].
Since younger children (especially those less than 2 years old) may not have fully developed immune systems, the use of adjuvanted vaccines has been under study. Adjuvants are chemical compounds added to the vaccine to boost the host’s immune response to the vaccine antigen, enhancing protection [10–12]. Only the TIV is approved for children younger than 2 years, and a 2008 Cochrane review, using data from the only randomized controlled trial (RCT) in this age group, could not confirm its enhanced efficacy [13]. Among the most extensively studied adjuvants are oil-based ones such as ASO3 and MF59, and RCTs in young children have shown promising high seroconversion rates. Waddington et al. compared ASO3(B)-adjuvanted split virion influenza A/H1N1 vaccine to a nonadjuvanted whole virion influenza A/H1N1 vaccine in 937 children aged 6 months to 12 years [14].Among children younger than 3 years, seroconversion was 98.2% for the ASO3(B) vaccine versus only 80.1% for the nonadjuvanted vaccine. Vesikari et al. compared an MF59-adjuvanted trivalent subunit influenza vaccine to a nonadjuvanted trivalent split influenza vaccine in 222 children aged 6–36 months old [15]. Seroprotection was higher in the adjuvanted group against influenza A/H1N1 (100 vs 86%) and influenza B (99 vs 33%), and similarly high in both groups against influenza A/H3N2 (100 vs 99%). Unfortunately, neither of these trials generated efficacy data, and it is unclear whether higher seroconversion with adjuvant would translate to higher clinical efficacy against influenza. Side effect rates from adjuvanted vaccines, while acceptable in both studies, still exceeded those from unadjuvanted vaccines (e.g., ASO3(B) was associated with severe local reactions, increased irritability, decreased feeding and decreased activity, and MF59 with injection site swelling and pain) [15].
Efficacy & safety of vaccination
Despite years of research, much uncertainty remains about vaccine efficacy since conducting an ideal study is very difficult. Many of the problems stem from the challenges in properly diagnosing influenza cases, which requires detecting the virus in respiratory fluids via techniques such as direct antigen detection tests, cell culture, or real-time reverse-transcriptase PCR (rRT-PCR), while the child is actively shedding the virus. The tests may miss a case if administered too early (e.g., during the incubation period) or too late (e.g., after the infectious period) in the disease course. Since many influenza cases are asymptomatic, identifying every case would necessitate serially screening the entire study population throughout the influenza season, which currently would be prohibitively expensive and operationally challenging. As a result, many studies have utilized more indirect outcomes such as vaccine effectiveness, which measures prevention of influenza-like illness (ILI) cases – that is, any respiratory disease that resembles influenza in symptomatology (e.g., fever, cough and myalgias), regardless of the etiologic agent (e.g., parainfluenzavirus, coronavirus and rhinovirus). Although ILI cases may be easier to identify (i.e., from a clinical constellation of systems) than influenza cases, influenza vaccine effectiveness not only includes many noninfluenza cases but also misses asymptomatic influenza cases that still can transmit the virus to others. Even when true efficacy is measured via counting the cases of culture-confirmed influenza (CCI) prevented (efficacy), the duration and intensity of follow-up can significantly affect the results. Another major obstacle is matching the influenza strains included in the vaccine with the circulating influenza strain(s) for that season. A good match can yield high efficacy whereas a poor match can lead to low efficacy. Elucidating the relationship between strain similarity (when the vaccine and circulating strains do not match perfectly) and vaccine efficacy is a continuing conundrum.
Nevertheless, a 2008 Cochrane review attempted to calculate the efficacies of LAIV and TIV among children from available clinical studies. The review measured LAIV’s efficacy to be 82% (95% CI: 71–89%) and TIV’s efficacy to be 59% (95% CI: 41–71%) in children aged 6 months to 16 years [13]. This extensive systematic review, which included 16 RCTs, also highlighted the shortcomings of existing studies: more RCTs and cohort studies focus on ILI than on CCI, a majority screen only for symptomatic cases, and few comment on the match between the vaccine and circulating influenza. Additionally, the new QIV, with the additional B strain, may prove more efficacious than the TIV, particularly in years in which the TIV influenza B strain would have not matched the circulating B strain [8].
Controversy remains over whether TIV/QIV or LAIV is more efficacious. While the Cochrane review suggests that LAIV may be superior, this conclusion was based on pooled data from many influenza seasons with few direct comparisons between TIV and LAIV during the same influenza season. Pooling data across seasons neglects variations in season-to-season matches between the vaccine and circulating strains. A review by Ambrose et al. included three RCTs that directly compared TIV to LAIV during the same season for children aged from 6 months to 18 years [16]. All three showed 35–53% fewer cases of influenza in the LAIV arm compared with the TIV arm. All three studies were relatively recent (conducted between 2002 and 2005), had comparatively large study populations (2187–8352 participants), and used CCI, not ILI, as an end point. However, only one of these studies enrolled predominantly healthy children whereas the other two enrolled children with previous diagnoses of asthma or histories of respiratory infections, raising the possibility of differences between these populations and healthy children.
Influenza is a problem for healthy children throughout the world in high-, middle- and low-income countries. There are comparatively fewer intervention studies for middle- and lower-income countries (vs higher-income countries) in the published literature and most focus on TIV vaccination [17–19]. These studies support the efficacy of vaccination but it is not clear whether several possible factors (e.g., less consistency in getting vaccinated each year, nutritional deficiencies, suboptimal dosing due to vaccine heat exposure or incorrect administration, or different timing of influenza season) may reduce the efficacy in lower-income compared to higher-income countries. Despite the continuing problem of influenza, many middle- and lower-income countries do not currently routinely administer an influenza vaccine to children. The addition of influenza to routine immunization programs can strain existing vaccine distribution systems (i.e., supply chains) [20,21].
Since studies to date have not found any compelling links between influenza vaccines and severe adverse events in children, the available literature supports the relative safety of influenza vaccination for children [22,23]. Although there have been some reports of local skin reactions, gastritis/duodenitis, narcolepsy [24,25], febrile seizures [26] and rare occurrences of Guillain-Barre syndrome, no consistent trend or association has emerged. LAIV’s association with increased wheezing in children with a history of asthma (or wheezing in general) and those <2 years old con-traindicates its use in these groups, but evidence supports the continued use of TIV even in children with severe asthma [5,27]. However, despite the reassurances that existing studies have provided, there is a continuing need for better-designed safety studies, especially as new influenza vaccine candidates emerge. As the authors of a 2008 Cochrane review of the safety and efficacy of influenza vaccines have emphasized, inconsistency in the collection, presentation and availability of safety data has plagued a number of existing studies [28,29].
Economic value of vaccination
In addition to knowing the clinical value of a vaccine, delineating its economic value can be particularly important in guiding adoption [30,31]. Health system decision-makers must balance limited resources and competing health priorities. Cost–effectiveness is a measure of the trade-off between the relative costs and outcomes (health effects) of two or more alternative strategies or interventions. The health effects can include reductions in influenza cases, clinic visits, emergency room visits, hospitalizations, disability-adjusted life-years and deaths, or savings in life-years or quality-adjusted life-years (QALYs). The incremental cost effective ratio (ICER) is the ratio of the difference in costs and the difference in health effects between two strategies (in this case vaccine vs no vaccine):
Historically, many have considered an ICER of less than $50,000 per QALY to imply that a vaccine is cost effective, but controversy remains over this threshold (e.g., should this threshold be higher due to inflation over the years?). Studies have found influenza immunization to be very cost effective – that is, well below $50,000 per QALY saved (and in some cases cost-saving) for children [32,33].However, influenza vaccination in children is not a single monolithic intervention; cost–effectiveness may vary by vaccine type. Although TIV is less expensive, approximately $8–14 compared with $15–20 for LAIV [201], one cost–effectiveness analysis suggests that LAIV may save $45.80 more per child aged 24–59 months compared with TIV [34].
Furthermore, the value of the vaccine may depend heavily on when during the season a child receives the vaccine. Immunizing children earlier (i.e., by the end of October) could protect children for a greater proportion of the influenza season and in turn reap substantial savings for society and third-party payers in both financial cost (range: US$4.1–9.6 million) and QALYs (range: 647–942 QALYs) compared with when children currently receive the vaccine [35]. The ICER for October vaccination compared with later vaccination fell in the range of US$27,901–39,940 per QALY for TIV and US$36,538–48,282 per QALY for LAIV, indicating that earlier vaccination is cost-effective. Achieving earlier immunization may benefit not only vaccinated children by protecting them for a greater portion of the influenza season but also unvaccinated children (and adults) because rapidly attaining herd immunity (i.e., a large enough proportion of the population is protected to inhibit further spread) can curtail overall transmission in the population. Earlier immunization seems feasible as typically the manufacturers have already delivered over 70% of vaccines by October. Delays are likely to result from immunization location hours and availability, education about the benefits of earlier vaccination, procrastination and competing priorities. Another study showed that vaccines administered through the Vaccines For Children (VFC) program (which provides free vaccines to children who are uninsured or eligible for Medicaid) are given approximately 1 month later than non-VFC vaccines due to later arrival of VFC from distributors [36].
The cost–effectiveness of the Advisory Committee on Immunization Practices’ (ACIP’s) recommendation for immunizing the entire age range of healthy children (i.e., ages 6 months to 18 years) is also not clearly established. A 2011 review by Nichol included 20 cost–effectiveness analyses of influenza vaccination in healthy children [37]. Although the majority of these studies found influenza vaccination to be cost-effective or even cost-saving, only two studies included a broad enough range of ages to be applicable to the ACIP recommendations, and they yielded conflicting findings. The Weycker et al. study found immunization to be cost-saving among children aged 6 months to 18 years old [38], but the Prosser et al. study, which included children aged 6 months to 17 years old, found vaccination to be cost-effective only for high-risk children and children <4 years old [39]. The ICER for children aged 6 months to 4 years ranged from US$9,000 to US$25,000 per QALY gained, but the ICER for children aged 5–17 years ranged from US$72,000 to US$109,000, above the US$50,000 per QALY gained threshold for cost–effectiveness.
An economic modeling study suggested that administering a vaccine with immunity lasting multiple years to children could be extremely cost-effective [40]. The benefits of a multiyear vaccine are derived from reducing the number of immunizations necessary, eliminating the need for annual compliance with vaccination and improving the vaccine to match the circulating strain each year. Administering a vaccine that could protect children (5–18 year olds) for 5 years instead of annual immunization could save US$15 million–billion over their lifetimes. Increasing the duration of protection would of course increase these savings; protection for 10 years could increase these savings to US$5–9 billion. A vaccine that protected for at least 5 years dominated (i.e., was less costly and more effective than) the annual vaccine (regardless of compliance with the annual vaccine) when the multiyear vaccine cost ≤US$100/dose and had an efficacy ≥75%. A vaccine that protected for at least 10 years was also dominant when efficacy was ≥50%. A US$200 multiyear vaccine was only cost-effective when ≥75% efficacious for a 5-year duration when compliance with the annual vaccine was ≤25% and for a 10-year duration for all annual compliance rates. A universal vaccine was not cost effective when its cost was ≥US$200 and efficacy was ≤50%.
Obstacles to vaccination
Despite evidence supporting the benefits of influenza vaccination and the ACIP recommendation to vaccinate all children, vaccine coverage (i.e., the percentage of the population vaccinated) remains far below 100%, based on one study, languishing at only 49.0% [41]. The most commonly cited reasons for not getting vaccinated have been fear of side effects, inconvenience, lack of access to immunization locations, pain from the needles used for immunization, religious or philosophic opposition to vaccination, or beliefs that their children are not at risk for infection or that an infection would be mild [42–45]. Measures to overcome these obstacles have included education and public awareness campaigns to tout the benefits of vaccination and assure parents about safety, the advent of thimerasol-free forms of the vaccine for those with concerns about the preservative, and school-based vaccination to increase convenience for both children and parents [46–48]. There are some challenges to implementing school-based vaccination programs such as the cost, insurance reimbursement and the collection of parental consent forms [45].
Pharmaceutical interventions: antivirals
Types of antivirals
Antivirals can prevent influenza among children by the following two methods: postexposure prophylaxis (PEP), that is, administering antivirals to children who may have been exposed to the influenza virus before full-blown infections can develop; and treatment, that is, administering antivirals to already infected children to reduce their infectivity (the amount and duration of viral shedding).
Antivirals work by inhibiting replication of the virus in the human host, which initially occurs in pulmonary tissue. Therefore, administration must occur early in the virus’ time in the human host (i.e., during the incubation period) before substantial replication has occurred. Antivirals could be an important prevention measure since vaccine coverage is far less than 100% and mismatches between the vaccine and circulating strains frequently occur, especially when novel strains such as the 2009 H1N1 strain emerge. There are currently four licensed antivirals: two neuraminidase inhibitors (oral oseltamivir and inhaled zanamivir) effective against both influenza A and B and two adamantanes (amantadine and rimantadine) effective only against influenza A. Oseltamivir is approved for treatment and prophylaxis in children >1 year old and zanamivir for prophylaxis in children >5 years old and treatment in children >7 years old. Circulating influenza A strains tend to have high resistance to admantanes, limiting their use [49].
Efficacy & safety of antivirals
Measuring the true efficacy of antiviral PEP has been challenging. Several factors can greatly affect the efficacy of PEP: how soon and accurately the initial infectious case was identified, how much contact others have had with the infectious case before they received PEP, how soon they received PEP and what other infectious individuals they may have contacted before and during PEP. The earlier PEP is administered after initial exposure to influenza, the more efficacious it should be. Additionally, the efficacy of PEP can depend on the outcome of interest, for example, symptomatic influenza or both symptomatic and asymptomatic influenza. Finally, emerging viral resistance to antivirals can substantially dampen PEP efficacy, and resistance among the virus population can increase with increasing PEP use.
All of these factors have led to very wide ranges in PEP efficacy [50]. Several RCTs have explored PEP in household settings. According to data pooled from two household-based RCTs, oseltamivir administered to people in the same household as an index influenza case was 68–89% efficacious in preventing laboratory-confirmed influenza among individuals and 58.5–84% efficacious in households [51,52]. Using data from another two household-based RCTs, zanamivir demonstrated 80% efficacy in individuals and 72–79% efficacy in households (with one trial reporting household data only [53,54]). The strengths of these trials include large study population (277–487 households), rapid dissemination of antivirals for PEP (within 48 h of index case symptoms), twice-daily monitoring for influenza symptoms, and influenza confirmation through laboratory testing. Future RCTs should explore school settings as well and directly compare oseltamivir to zanamivir in household and school settings.
Measuring the efficacy of treatment in preventing further cases is fraught with even more challenges. As with PEP, treatment efficacy depends heavily on how early in the disease course the antiviral was administered. Once the incubation period has passed and the virus has ventured beyond the host’s respiratory tract, the ability of antivirals to mitigate shedding is greatly diminished. Measuring shedding is difficult and labor intensive and following the treated child’s contacts requires close monitoring. Moreover, a child’s contact rates may wax as symptoms decrease (e.g., with treatment) and wane as symptoms increase. Individuals may not take the entire course of antivirals, stopping once symptoms alleviate. Finally, efficacy is tied to the age and contact patterns of the treated child. Treatment of any age group can benefit children; when any adult or child household member develops influenza, children have the highest secondary attack rates compared with other age groups [55–58]. Unfortunately, to date, no RCTs have specifically addressed the efficacy of treatment in preventing influenza among children. Some published studies have analyzed this as a secondary outcome but have not convincingly demonstrated benefits [55,59].
Studies to date have found some minor adverse events such as vomiting but no major adverse events [50]. However, like vaccines, many currently available studies did not collect or present safety data in a clear, consistent manner.
Economic value of antivirals
The dearth of consensus on PEP and treatment efficacy hinders assessments of the economic value of antivirals. As with vaccination, antiviral use is not a single monolithic intervention with a single economic value. The only currently available published cost–effectiveness analysis of PEP is a decision analytic model by Talbird et al., which found oseltamivir PEP in children (1–12 years old) to have an ICER of US$41,452 compared with no intervention, assuming that no children in the population had been immunized against influenza [60]. While this study is helpful in showing that PEP could be cost-effective (i.e., ICER <$50,000 per QALY) under certain circumstances, further investigation is needed. Future studies should examine how the economic value of antivirals vary with factors such as administration strategy (PEP vs treatment), timing of influenza diagnosis, timing of administration, type of person receiving antivirals, severity of influenza season, degree of resistance among circulating strains, general health of those receiving PEP or treatment and vaccination status. The last factor is especially important since it is unlikely that PEP will replace vaccination as a primary prevention measure, except in years in which the vaccine is either unavailable or ineffective.
Obstacles to antiviral use
In addition to the shortage of consistent, definitive evidence, the biggest obstacles to antiviral use are viral resistance, uncertain recommendations for use, and difficulty in timing administration appropriately. Growing viral resistance has neutralized the use of adamantanes and threatens oseltamivir. Zanamivir-resistant influenza strains remain relatively rare [49,61]. Selection pressure from increasing antiviral use could increase the resistant strain prevalence, which further complicates selecting the target populations to receive PEP and treatment. The more antivirals are used for prevention (hence increasing resistance prevalence), the less efficacious they may be in treating life-threatening severe cases who have no other recourse. Therefore, recommendations should be a balance between resistance concerns and the need for preventative measures beyond immunizations. How this would translate to specific recommendations (e.g., when a child develops influenza, whom should receive PEP? Just household contacts, the child’s close friends at school, or the child’s entire school class?) Some observational school-based studies that combine antiviral treatment with school closure to mitigate outbreaks suggest that antivirals may help but are not enough to establish clear recommendations [62–64]. Finally, initiating PEP within 48 h of exposure and treatment within 48 h of symptoms requires rapid index case diagnosis (which may not be consistently possible with currently available diagnostics) and antiviral dissemination within a very narrow timeframe [65].
Pharmaceutical intervention: nutritional supplements
Types of supplements
There is continued interest in the role of nutritional supplements in influenza prevention. Vitamin D has received attention since influenza is more common in winter months when diminished sun exposure results in low levels of vitamin D, although the mechanism behind influenza seasonality has not been clearly established [66]. Vitamin D has several immunomodulatory functions, including upregulation of antiviral peptides that are part of human innate immunity and can inactivate influenza virus [66,67]. Other nutritional supplements including Echinacea root, green tea capsules, panax quinquefolium (ginseng extract), oscillococcium (duck liver and heart derivatives) and Gan Mao Jiao Nan (chrysanthemum and mulberry tablet) have also drawn interest [68–71].
Efficacy & safety of supplements
There is a paucity of well-designed clinical studies supporting the use of nutritional supplements to prevent influenza in children. Linday et al. explored the impact of cod liver oil (which contains both vitamin A and D) and multivitamin supplements on pediatric visits for upper respiratory infections in children aged 6 months to 5 years [72]. The children randomized to the intervention group took the oil and supplements (together containing 600–700 IU vitamin D) for approximately 6 months while the control group received neither vitamins nor supplements. This study found no statistically significant difference in the number of pediatric visits between the two groups, but had several important limitations. Confirmed influenza was not the study’s primary end point; pediatric visits can result from numerous reasons; the study did not fully account for compliance with regimen and nutritional intake among the control group or check serum vitamin D levels; and the vitamin D doses may have been too low to have benefits. Another trial by Urashima et al. compared the effects of vitamin D supplementation (1200 IU of vitamin D daily for 4 months) versus placebo on influenza A incidence in 6–15 year olds [73]. The trial found a statistically significant decrease in influenza A incidence in the intervention group (10 out of 167 [10.8%] of the intervention group compared with 18 out of 167 [18.6%] of the placebo group for a 41.9% protective efficacy). Although the study had limitations (e.g., baseline vitamin D levels were not assessed), these results should motivate follow-up studies to determine their reproducibility. Evidence for other nutritional supplements is even scarcer. Since supplements often do not face the same regulation as medical products, supplements may have poorer quality control and include contaminants that may pose safety concerns. The safety and efficacy of supplements are important areas for future investigation.
Economic value of supplements
Without an adequate body of clinical studies, the efficacy and hence the cost–effectiveness of nutritional supplements remain unclear. In addition to the challenge of drawing the link between nutritional supplements and influenza risk and outcomes, the body of literature assessing the cost–effectiveness of nutritional supplements is relatively small. Many existing cost–effectiveness studies of supplements focus on their impact on long-term chronic outcomes (e.g., disease or conditions such as heart disease that affect life expectancy and quality of life over many years) after years of taking the supplements and hence employ a long time horizon [74,75]. Relatively fewer economic studies analyze the use of nutritional supplements to prevent acute and more self-limited events that are more directly tied to malnutrition (e.g., anemia or hip fractures from osteoporosis) than influenza [76–78]. The time horizon for an economic study of nutritional supplements for influenza can be complicated. How long and consistently should you assume the child takes the supplements (to determine the cost of the intervention) and how many influenza seasons should the study encompass?
Obstacles to supplements
Despite the lack of evidence, anecdotal reports suggest that nutritional supplements are being used for influenza prevention, but the frequency and extent of their use remain a mystery. The design and execution of adequate clinical studies face multiple hurdles. First, funding for this line of inquiry is currently limited. Second, considerable controversy exists over the dosing of these supplements and how much of these nutritional elements can come from standard diets. A study must be able to control for variations in diet and compliance with supplement regimens. Moreover, many children may take supplements for reasons other than influenza prevention.
Nonpharmaceutical interventions: social distancing measures
Types of social distancing measures
Regardless of future development in vaccines, antivirals and nutritional supplements, NPIs should continue to play important roles since vaccines and antivirals are not always available or adequate, particularly in resource-poor settings or years when the vaccine poorly matches circulating strains or antiviral resistance is high. NPIs physically separate susceptible children from the virus and infectious individuals. Social distancing measures remove susceptible children from the vicinity of infectious individuals. Isolation involves restricting the contacts of an individual who is known to be infectious. Quarantine entails restricting the contacts of individuals who have been exposed to an infectious individual. Other social distancing measures include closing locations of high mixing, such as schools or daycare centers.
Efficacy & safety of social distancing measures
Measuring the efficacy of social distancing measures is difficult. In theory, perfectly executed social distancing measures should be nearly 100% efficacious. If infectious individuals have no chance of contacting susceptible individuals, there should be no chance of transmission and spread (one caveat is that transmission can occur via surfaces and objects such as tabletops and doorknobs). In reality, social distancing measures are rarely perfectly executed. Typically, there are delays in identifying infectious cases and their contacts and implementing measures. Social distancing measures often are only partially implemented. For isolation and quarantine, completely eliminating contact with a child is practically impossible. Young children need to be fed and bathed; older children may chafe at being kept from contacting others; and closing high mixing locations such as schools may simply shift mixing locations to movie theaters, malls, playgrounds, parties and so on.
As conducting a RCT of social distancing measures is an ambitious task, most of the available evidence comes from observational studies during outbreaks and epidemics and modeling and simulation studies. School closures have been the most extensively studied social distancing measure. Wu et al. followed transmission of the 2009 pandemic influenza A/H1N1 in schoolchildren in Hong Kong, where primary schools closed for 4 weeks due to the pandemic, followed by summer vacation in primary and secondary schools [79]. During the periods of school closure, there was an estimated 70% reduction in intra-age group transmission. The estimated population-wide reproductive rate (R0) – that is, the number of additional cases generated by an index infectious case – was 1.7 before school closure and declined to 1.1 by the end of summer vacation. While school closure may abate an outbreak, if the strain remains in the general population, the outbreak may resume once schools reopen. Wheeler et al. analyzed Arizona children over 4 consecutive school years and found that influenza incidence among the school-age population remained constant during winter breaks (when schools closed), but increased significantly after the winter break when schools reopened [80]. Similarly, in their study of Mexican schoolchildren during the 2009 pandemic [81], Chowell et al. built a transmission model and found that during spring school closure, the overall population influenza spread decreased by approximately 29.6%. The ratio of student cases to nonstudent cases remained low during summer vacation but climbed once schools reopened.
As with other influenza interventions, all school closures are not the same monolithic intervention. Timing of school closures (both when school closure is initiated and concluded) seems essential to its efficacy; closure must occur early enough to prevent cases. As Cowling et al. found in Hong Kong [82], school closures that occurred after influenza attack rates had already peaked had no significant effect on transmission in children. Similarly, in their study of school closure in rural Pennsylvania, Cauchemez et al. [83] determined that school closure was initiated after 27% of schoolchildren already had influenza symptoms and did not reduce influenza transmission among children. Several other models have attempted to identify thresholds of ill children at which schools should be closed to prevent an influenza outbreak but assumed indefinite school closure [84,85]. Lee et al. showed the importance of school closure duration and that short closure durations could actually worsen an influenza outbreak [86]. School closure keeps a large segment of potential high mixers susceptible to influenza. If schools are reopened while the outbreak persists in the community, the sudden remixing of susceptible children with infectious individuals could simply refuel the outbreak, causing a new surge in cases. Therefore, once the decision to close schools is made, it may be important to maintain school closures until the outbreak has subsided, which in the case of a large epidemic can be over 8 weeks. This has tremendous implications from an economic standpoint, as will be detailed later.
Unless no other efficacious interventions are available, school closure may not be the only measure employed in an influenza outbreak. Vaccination, antiviral use and other social distancing measures may be concurrently in place. Modeling studies have attempted to determine the impact of combining such measures but it can be difficult to isolate the relative contribution of each measure and characterize the synergistic effects of interventions [87–89].
Studies of other social distancing measures such as isolation and quarantine have not focused on children and typically have included other interventions (e.g., vaccination and antiviral administration [90–98]). One would expect the efficacy of isolation and quarantine to depend heavily on the speed and accuracy at which cases and potential contacts are detected and the compliance with isolation and quarantine. The limited number of published studies are either observational studies, which would bear a variety of confounders, or mathematical and computational models.
Social distancing measures are not without potential ‘safety’ concerns, which have not been well studied. Extended school closures could lead to problems in nutrition from missing school lunches and development from missing classes and facing unstructured time that may lead to disciplinary problems. Impeding human contact via isolation and quarantine can have emotional and psychological effects on children early in their development. It may even affect them physically if isolation or quarantine hinders treatment administration for urgent medical problems. These areas call for further exploration.
Economic value of social distancing measures
Most of the existing economic studies of social distancing measures have focused on school closure. Implementation costs are a big limitation of school closure and other social distancing measures. As Lempel et al. calculated, closing all US schools for 4 weeks would cost US $10–47 billion or 0.1–0.3% of the US gross domestic product [99]. In addition to teaching children, schools employ teachers and other staff, care for students during the day while their parents are at work, and provide meals and other activities. Shutting down a school interferes with teacher, parent and other caregiver productivity. As Brown et al. demonstrated, the tremendous cost associated with school closure would dwarf the cost-savings from preventing influenza-associated medical costs under 2009 H1N1 pandemic circumstances; an 8-week school closure would cost a median US $14,000–25,000 per influenza case averted, far higher than vaccination (< US $100 per influenza case averted) [1]. This suggests that long-term school closure to quell a large-scale epidemic would not be cost-effective and merited unless morbidity and mortality were much higher than in the 2009 pandemic. The cost–effectiveness of short-term school closure as a means to stem a much smaller outbreak remains an open question.
Obstacles to social distancing measures
Social distancing measures are not easy to implement, potentially disrupting lives, limiting productivity, generating resentment among those who do not understand or agree with their purpose, and requiring difficult-to-achieve compliance. The negative effects of school closures can be differentially devastating to those with a lower income or tenuous job security [100–103]. Closing schools by no means guarantees decreased contact among children. Various surveys found that at least 30–40% of students engaged in social activities (e.g., attending church, visiting family and friends and attending parties or sleepovers) during school closure regardless of public health recommendations [101,102,104].
Nonpharmaceutical interventions: physical barriers to protect against virus transmission
Types of physical barrier protections
Another category of NPIs is barrier interventions, measures that prevent the virus from reaching susceptible children. These include steps to remove the virus from common vehicles of transmission, such as hand hygiene and environmental cleaning [105,106] and accoutrements that block the virus from contacting a child’s mucous membranes, such as gowns, gloves and face masks.
Efficacy & safety of physical barrier protections
While a number of RCTs have studied the efficacy of hand hygiene, study outcomes have typically been reduction in ILIs or respiratory illnesses, rather than CCI. One recent large RCT enrolled 20,882 elementary school students and compared an intensive hand hygiene campaign to standard practice in elementary schools in Egypt using both ILIs and CCIs as end points [107]. In the intervention group, ILI-attributable absences decreased by 40% (p < 0.0001) and laboratory-confirmed influenza rates fell by 47% (p < 0.0001) [107]. These promising results may underestimate the benefits of hand washing since in the subset of students who were absent due to ILIs, fewer students in the control group (12%) underwent influenza testing compared with the intervention group (22%), potentially leading to case undercounting in the control group. By contrast, a 2011 cluster RCT by Stebbins et al. enrolled 3360 children to examine the impact of an intensive hand hygiene intervention on influenza A and influenza B acquisition in elementary school students in Pittsburgh (PA, USA) [104]. The study found no significant difference in total CCI cases between the intervention and control groups, a reduction of influenza A cases by 52% (p < 0.02) in the intervention group, and no reduction in influenza B cases [104].
Several studies evaluated the use of facemasks on influenza prevention. MacIntyre et al. identifiad pediatric patients with ILIs and randomized these patients’ household members to wearing no masks, surgical masks, or P2 masks. Although the study found no overall differences in subsequent household member ILI incidence, the small subset of patients (30) who report good compliance (wearing mask often or always) experienced 60–80% lower ILI incidence than those who did not wear masks [108]. Canini et al. randomized patients with confirmed influenza to wearing either a surgical mask or no mask and tracked how many household members developed ILIs. Despite overall relatively high compliance with facemask use (over 60% of patients wore masks >80% of the time), they found no significant differences between the mask and no-mask groups [103].
Two groups have examined the effects of combined barrier interventions. Cowling et al. performed a pilot study and follow-up RCT in Hong Kong [109,110]. The pilot study randomized households of index influenza patients to receive no intervention, surgical facemasks or hand hygiene and revealed no significant difference in secondary attack rate among the three groups; the follow-up study randomized households to no intervention, hand hygiene or facemask plus hand hygiene groups. Applying the hand hygiene plus facemask intervention within 36 h of symptom onset in the index case reduced influenza transmission (adjusted odds ratio: 0.33; 95% CI: 0.13–0.87). However, hand hygiene appeared to have no significant effect on transmission, regardless of timing. Larson et al. found no differences in ILI/influenza secondary attack rates when randomizing US households with a sick individual to one of three groups: education only, education plus hand sanitizer, or education plus hand sanitizer plus surgical facemasks [111]. However, a potential confounder was overall increased hand sanitizer use in the community due to a concomitant methicillin-resistant staphylococcus aureus scare. Assuming their proper use, no substantial safety concerns have emerged for barrier interventions.
Economic value of barrier interventions
Currently, no studies have explored the economic value of hand hygiene or barrier interventions. Studies examining the economic value of barrier interventions in the control of healthcare-associated infections may serve as a model for future studies of barrier interventions for influenza. These include both models and clinical studies introducing protective equipment such as gloves and gowns to limit the spread of pathogens such as MRSA, norovirus and Acinteobacter [112–115].
Obstacles to physical barrier protections
The primary impediments to barrier protections in both study and nonstudy settings have been proper execution and compliance. Even under observation, children do not always wash their hands properly. Protective accoutrements may be uncomfortable and hinder communication and eating. Both measuring and enforcing compliance grows more difficult the younger the child and the more peers are present (e.g., in a school classroom). These problems may confound the results of clinical studies, potentially contributing to the dearth of clear evidence supporting some barrier interventions.
Access to barrier interventions is another issue. Hand hygiene stations (e.g., soap dispensers, faucets and sinks) need to be easily accessible for children, which can be particularly challenging given their high degree of interaction. The frequency of hand washing must keep pace with their frequency of interaction. Installing and maintaining hand hygiene stations incurs cost and occupies space.
Expert commentary & five-year view
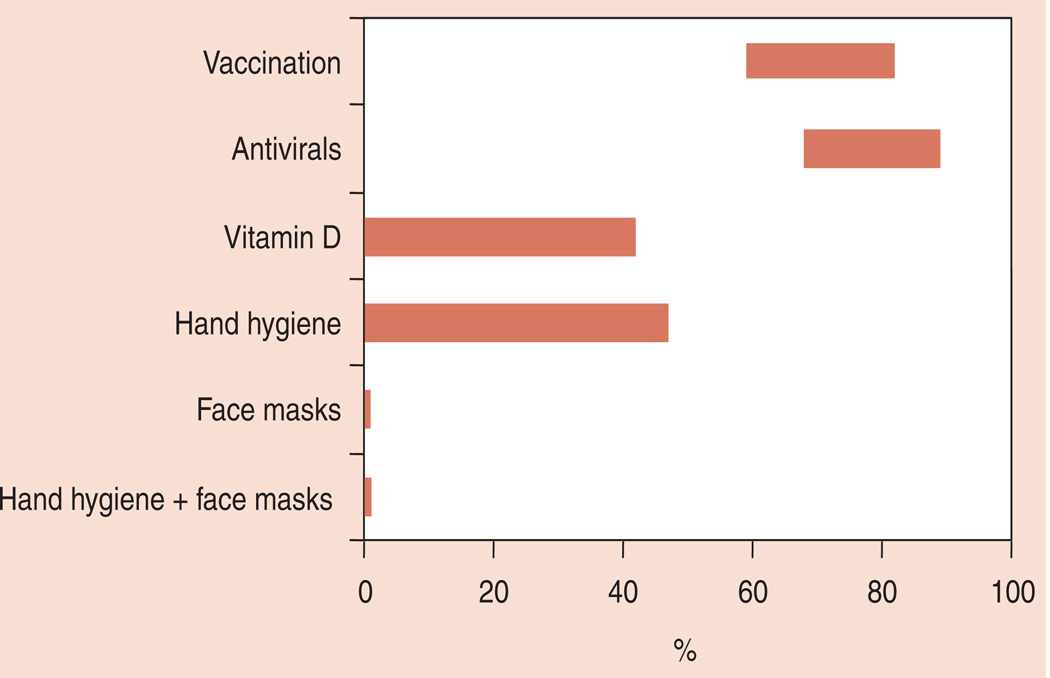
Since preventing influenza among children is key to controlling its spread, there is a continuing need to better understand the efficacy of, economic value of and obstacles in implementing existing and potential control measures. As Figure 1 demonstrates, there is still substantial variation in the measured efficacy of currently available control measures. Part of this variation is due to variation in influenza seasons (including circulating strains and severity) but part is also due to limitations in study design. While studies to date have provided useful information, there remains much to learn. Specifically, over the next 5 years, it will be important to:
Conduct more studies that directly measure the efficacy of influenza interventions throughout the entire influenza season and over multiple influenza seasons. Rather than relying solely on proxies such as ILI, studies should endeavor to directly measure the presence or absence of influenza infection for the duration of an influenza season and over multiple seasons. Studies that cover only a fraction of the entire influenza season may not paint an accurate picture of the intervention’s efficacy and value. Limiting a study to just one influenza season will not reveal how robust an intervention remains over different circulating strains. While designing such studies may be more costly and challenging logistically, the generated information would be of tremendous value.
Establish consistency in study design & execution & outcome measurement & reporting. Until clearly established consensus guidelines emerge, the great variability in studies makes it difficult to compare, interpret and pool results. These guidelines could include suggestions on how to measure intervention efficacy (e.g., preventing symptomatic and asymptomatic influenza), identify cases (e.g., test-confirmed influenza), implement interventions, measure compliance, follow patients and describe studies. Funding bodies and journals may want to encourage adherence to such guidelines.
Determine the symptomatic rate for influenza among children. Knowing the proportion of infected children who exhibit clinically apparent symptoms will help better estimate influenza’s true burden, transmission patterns and intervention impact. Infectious yet asymptomatic children play a large role in spreading the disease. Moreover, the split between symptomatic and asymptomatic infectious children can affect the efficacy of antiviral prophylaxis and social distancing measures. Establishing this proportion would require following a cohort of children closely and testing for influenza extensively.
Develop better rapid diagnostics & testing strategies. Improved diagnostics will help design and execute studies, target preventative interventions, and elucidate the symptomatic–asymptomatic ratio. This includes shortening turnaround time (without sacrificing accuracy) to identify cases before they can transmit to others. Also, new diagnostics may alter testing strategy. Who should be tested (e.g., just the symptomatic or those exposed to a symptomatic case) and how often? Is mass screening ever justifiad?
Consider timing of vaccination & encourage earlier vaccination. Studies should capture and report when during the influenza season its subjects were immunized as this timing can greatly influence the vaccine’s impact. Also, it may not be enough to simply promote immunization without also expounding on the value of getting immunized earlier.
Reduce barriers to implementing interventions. Continuing suboptimal implementation of preventative measures (e.g., low vaccination coverage) calls for new strategies to overcome potential barriers. This includes making interventions more readily available (e.g., better access to vaccines and hand washing stations) and improving education, awareness and knowledge dissemination. Policy makers may consider using information dissemination techniques traditionally used by marketers and businesses, including new social media strategies.
Develop new vaccine technologies. Vaccine development is proceeding in two general directions: improving vaccine efficacy and administration. Strategies for augmenting vaccine efficacy include those to enhance strain matching (e.g., adding more strains to the vaccine and developing a vaccine that includes a conserved portion of the virus) and the immune response (e.g., adjuvanted vaccines). Improved vaccine administration may occur via jet injectors, dermal patches or even oral formulations. Alternative administration methods can confer multiple benefits: improving compliance via avoiding painful injections, reducing reliance on trained healthcare workers, expanding immunization locations and easing vaccine storage requirements.
Establish clearer guidelines for antiviral use. The uncertainty over when and how to use antivirals calls for more RCTs that measure the timing of antiviral administration in relation to exposure and account for social contact patterns.
More studies on nutritional supplements & other pharmaceutical interventions. The dearth of data on nutritional supplements prevents specific recommendations. Conducting adequate RCTs requires overcoming many challenges, such as controlling for immunization status, regular diet, compliance, duration of supplement ingestion, bioavailability of active substances, and influenza exposure.
Better characterizing social contact patterns & transmission dynamics. The value of NPIs (and pharmaceutical interventions) depends on the way children interact and spread influenza. Better characterizing these patterns can also help identify new NPIs. This entails following children closely and carefully, and integrating collected data into mathematical models and computational simulations.
Economic studies of interventions. There is a need for more economic studies of both current and future interventions. These studies should orient their outcome measures to be relevant to the appropriate decision-makers (e.g., insurers will be interested in direct medical costs, school system will follow their direct costs and so on). Performing economic studies early in an intervention’s development can help guide the development of that intervention [116].
Figure 1.
How the influenza prevention efficacy reported by studies in the literature ranges for each type of intervention.
Conclusion
Although influenza has been long known to be a problem among children, many questions remain about the efficacy and impact of preventive options because of the limitations of many existing studies. In the future, more studies should directly measure the efficacy of influenza interventions throughout the entire influenza season and over multiple influenza seasons. It would be helpful to establish more consistency in study design, execution, outcome measurement and reporting. Knowing the proportion of infected children who exhibit clinically apparent symptoms will help better estimate influenza’s true burden, transmission patterns and intervention impact. Improved diagnostics will help design and execute studies, target preventative interventions and elucidate the symptomatic–asymptomatic split. Studies should capture and report when during the influenza season its subjects were immunized as this timing can greatly influence the vaccine’s impact. Also, it may not be enough to simply promote immunization without also expounding on the value of getting immunized earlier. Continuing suboptimal implementation of preventative measures (e.g., low vaccination coverage) calls for new strategies to overcome potential barriers. Vaccine development is proceeding in two general directions: improving vaccine efficacy and administration. The uncertainty over when and how to use antivirals calls for more RCTs that measure the timing of antiviral administration in relation to exposure and account for social contact patterns. The dearth of data on nutritional supplements prevents specific recommendations. Better characterizing social contact patterns and transmission dynamics can help better assess the value of current and developing interventions. There is a need for more economic studies of both current and future interventions. Performing economic studies early in an intervention’s development can help guide the development of that intervention.
Key issues.
Healthy children are high transmitters of influenza and can experience poor influenza outcomes.
Many questions remain about the efficacy and impact of preventive measure because many existing studies report imprecise proxies of influenza incidence (e.g., influenza-like illness incidence), do not follow subjects throughout the entire influenza season and across multiple influenza seasons, and do not control for many important factors such as timing of vaccination and social contact patterns.
The limitations of clinical studies make modeling and simulation key methodologies to answer questions regarding influenza prevention.
While vaccination may be the most efficacious existing intervention, variations in circulating strains and children’s immune systems keep current vaccines from being fully protective, necessitating further clinical and economic studies and improvements in vaccine technology.
Evidence suggests that live attenuated influenza vaccine may be more efficacious among young children (with less pre-existing immunity) than trivalent influenza vaccine, which may translate to being either comparable (due to higher pricing) or slightly more cost effective than trivalent influenza vaccine.
Hand hygiene appears to be an important adjunct but improving compliance, standardizing regimens and quantifying its impact has been challenging. Additional evidence could help guide its implementation.
The efficacy of antivirals in preventing influenza depends heavily on its early administration and resistance among circulating strains. Therefore, future studies need to help define the specific indications and circumstances for their use.
More studies could better define the role of nutritional supplements and nonpharmaceutical interventions such as school closure, isolation, quarantine and wearing protective garb.
Better characterization of children’s social contact patterns and influenza transmission dynamics can assist in addressing all of the above issues.
Acknowledgements
The authors would like to acknowledge the assistance of Sarah M Bartsch, MPH, Coordinator and Senior Analyst of the Public Health Computational Operations Research (PHICOR) group, and Michelle Lee, Assistant Analyst in PHICOR.
This manuscript was supported by the National Institute of General Medical Sciences Models of Infectious Disease Agent Study (MIDAS) grant 5U54GM088491-02 and by the CDC grant U01 IP000467. The funders had no role in the design, preparation, review or approval of the manuscript.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Brown ST, Tai JH, Bailey RR, et al. Would school closure for the 2009 H1N1 influenza epidemic have been worth the cost?: a computational simulation of Pennsylvania. BMC Public Health. 2011;11:353. doi: 10.1186/1471-2458-11-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iskander M, Booy R, Lambert S. The burden of influenza in children. Curr. Opin. Infect. Dis. 2007;20(3):259–263. doi: 10.1097/QCO.0b013e3280ad4687. [DOI] [PubMed] [Google Scholar]
- 3.Fairbrother G, Cassedy A, Ortega-Sanchez IR, et al. New Vaccine Surveillance Network (NVSN). High costs of influenza: Direct medical costs of influenza disease in young children. Vaccine. 2010;28(31):4913–4919. doi: 10.1016/j.vaccine.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 4.Principi N, Esposito S. Are we ready for universal influenza vaccination in paediatrics? Lancet Infect. Dis. 2004;4(2):75–83. doi: 10.1016/S1473-3099(04)00926-0. [DOI] [PubMed] [Google Scholar]
- 5.Fiore AE, Uyeki TM, Broder K, et al. Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm. Rep. 2010;59(RR-8):1–62. [PubMed] [Google Scholar]
- 6.Stevenson E, Barrios L, Cordell R, et al. Pandemic influenza planning: addressing the needs of children. Am. J. Public Health. 2009;99(Suppl. 2):S255–S260. doi: 10.2105/AJPH.2009.159970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bell DM. World Health Organization Writing Group. Non-pharmaceutical interventions for pandemic influenza, international measures. Emerging Infect. Dis. 2006;12(1):81–87. doi: 10.3201/eid1201.051370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine. 2012;30(11):1993–1998. doi: 10.1016/j.vaccine.2011.12.098. [DOI] [PubMed] [Google Scholar]
- 9.Della Cioppa G, Vesikari T, Sokal E, Lindert K, Nicolay U. Trivalent and quadrivalent MF59(®)-adjuvanted influenza vaccine in young children: a dose- and schedule-finding study. Vaccine. 2011;29(47):8696–8704. doi: 10.1016/j.vaccine.2011.08.111. [DOI] [PubMed] [Google Scholar]
- 10.Principi N, Esposito S, Marchisio P. Present and future of influenza prevention in pediatrics. Expert Opin. Biol. Ther. 2011;11(5):641–653. doi: 10.1517/14712598.2011.562495. [DOI] [PubMed] [Google Scholar]
- 11.Lee BY, Stalter RM, Bacon KM, et al. Cost–effectiveness of adjuvanted versus nonadjuvanted influenza vaccine in adult hemodialysis patients. Am. J. Kidney Dis. 2011;57(5):724–732. doi: 10.1053/j.ajkd.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee BY, Ercius AK, Smith KJ. A predictive model of the economic effects of an influenza vaccine adjuvant for the older adult (age 65 and over) population. Vaccine. 2009;27(16):2251–2257. doi: 10.1016/j.vaccine.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jefferson T, Rivetti A, Harnden A, Di PC, Demicheli V. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev. 2008;2:CD004879. doi: 10.1002/14651858.CD004879.pub3. [DOI] [PubMed] [Google Scholar]
- 14.Waddington CS, Walker WT, Oeser C, et al. Safety and immunogenicity of AS03B adjuvanted split virion versus nonadjuvanted whole virion H1N1 influenza vaccine in UK children aged 6 months-12 years: open label, randomised, parallel group, multicentre study. BMJ. 2010;340:c2649. doi: 10.1136/bmj.c2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vesikari T, Pellegrini M, Karvonen A, et al. Enhanced immunogenicity of seasonal influenza vaccines in young children using MF59 adjuvant. Pediatr. Infect. Dis. J. 2009;28(7):563–571. doi: 10.1097/INF.0b013e31819d6394. [DOI] [PubMed] [Google Scholar]
- 16.Ambrose CS, Levin MJ, Belshe RB. The relative efficacy of trivalent live attenuated and inactivated influenza vaccines in children and adults. Influenza Other Respi. Viruses. 2011;5(2):67–75. doi: 10.1111/j.1750-2659.2010.00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pedroza A, Huerta JG, Garcia Mde L, et al. The safety and immunogenicity of influenza vaccine in children with asthma in Mexico. Int. J. Infect. Dis. 2009;13(4):469–475. doi: 10.1016/j.ijid.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Sullender W, Fowler K, Krishnan A, et al. Design and initiation of a study to assess the direct and indirect effects of influenza vaccine given to children in rural India. Vaccine. 2012;30(35):5235–5239. doi: 10.1016/j.vaccine.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verma R, Khanna P. Hepatitis A vaccine should receive priority in National Immunization Schedule in India. Hum. Vaccin. Immunother. 2012;8(8) doi: 10.4161/hv.20475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Assi TM, Brown ST, Djibo A, et al. Impact of changing the measles vaccine vial size on Niger’s vaccine supply chain: a computational model. BMC Public Health. 2011;11:425. doi: 10.1186/1471-2458-11-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee BY, Assi TM, Rajgopal J, et al. Impact of introducing the pneumococcal and rotavirus vaccines into the routine immunization program in Niger. Am. J. Public Health. 2012;102(2):269–276. doi: 10.2105/AJPH.2011.300218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hambidge SJ, Glanz JM, France EK, et al. Vaccine Safety Datalink Team. Safety of trivalent inactivated influenza vaccine in children 6 to 23 months old. JAMA. 2006;296(16):1990–1997. doi: 10.1001/jama.296.16.1990. [DOI] [PubMed] [Google Scholar]
- 23.France EK, Glanz JM, Xu S, et al. Safety of the trivalent inactivated influenza vaccine among children: a population-based study. Arch. Pediatr. Adolesc. Med. 2004;158(11):1031–1036. doi: 10.1001/archpedi.158.11.1031. [DOI] [PubMed] [Google Scholar]
- 24.Nohynek H, Jokinen J, Partinen M, et al. AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PLoS ONE. 2012;7(3):e33536. doi: 10.1371/journal.pone.0033536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han F, Lin L, Warby SC, et al. Narcolepsy onset is seasonal and increased following the 2009 H1N1 pandemic in China. Ann. Neurol. 2011;70(3):410–417. doi: 10.1002/ana.22587. [DOI] [PubMed] [Google Scholar]
- 26.Armstrong PK, Dowse GK, Effler PV, et al. Epidemiological study of severe febrile reactions in young children in Western Australia caused by a 2010 trivalent inactivated influenza vaccine. BMJ Open. 2011;1(1):e000016. doi: 10.1136/bmjopen-2010-000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The American Lung Association Asthma Clinical Research Centers. The safety of inactivated influenza vaccine in adults and children with asthma. N. Engl. J. Med. 2001;345(21):1529–1536. doi: 10.1056/NEJMoa011961. [DOI] [PubMed] [Google Scholar]
- 28.Jefferson T, Rivetti A, Harnden A, Di PC, Demicheli V. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev. 2008;2:CD004879. doi: 10.1002/14651858.CD004879.pub3. [DOI] [PubMed] [Google Scholar]
- 29.Jefferson T, Smith S, Demicheli V, Harnden A, Rivetti A. Safety of influenza vaccines in children. Lancet. 2005;366(9488):803–804. doi: 10.1016/S0140-6736(05)67204-2. [DOI] [PubMed] [Google Scholar]
- 30.Lee BY, Biggerstaff BJ. Screening the United States blood supply for West Nile Virus: a question of blood, dollars, and sense. PLoS Med. 2006;3(2):e99. doi: 10.1371/journal.pmed.0030099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chin RF, Neville BG, Peckham C, Wade A, Bedford H, Scott RC. NLSTEPSS Collaborative Group. Socioeconomic deprivation independent of ethnicity increases status epilepticus risk. Epilepsia. 2009;50(5):1022–1029. doi: 10.1111/j.1528-1167.2008.01796.x. [DOI] [PubMed] [Google Scholar]
- 32.Lee BY, Tai JH, Bailey RR, Smith KJ, Nowalk AJ. Economics of influenza vaccine administration timing for children. Am. J. Manag. Care. 2010;16(3):e75–e85. [PMC free article] [PubMed] [Google Scholar]
- 33.Fleming DM, Elliot AJ. Health benefits, risks, and cost–effectiveness of influenza vaccination in children. Pediatr. Infect. Dis. J. 2008;27(Suppl. 11):S154–S158. doi: 10.1097/INF.0b013e31818a5443. [DOI] [PubMed] [Google Scholar]
- 34.Luce BR, Nichol KL, Belshe RB, et al. Cost–effectiveness of live attenuated influenza vaccine versus inactivated influenza vaccine among children aged 24–59 months in the United States. Vaccine. 2008;26(23):2841–2848. doi: 10.1016/j.vaccine.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 35.Lee BY, Tai JH, Bailey RR, Smith KJ, Nowalk AJ. Economics of influenza vaccine administration timing for children. Am J Manag Care. 2010;16(3):e75–e85. [PMC free article] [PubMed] [Google Scholar]
- 36.Bhatt P, Block SL, Toback SL, Ambrose CS. Timing of the availability and administration of influenza vaccine through the vaccines for children program. Pediatr. Infect. Dis. J. 2011;30(2):100–106. doi: 10.1097/INF.0b013e3181efff54. [DOI] [PubMed] [Google Scholar]
- 37.Nichol KL. Cost–effectiveness and socio-economic aspects of childhood influenza vaccination. Vaccine. 2011;29(43):7554–7558. doi: 10.1016/j.vaccine.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 38.Weycker D, Edelsberg J, Halloran ME, et al. Population-wide benefits of routine vaccination of children against influenza. Vaccine. 2005;23(10):1284–1293. doi: 10.1016/j.vaccine.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 39.Prosser LA, Bridges CB, Uyeki TM, et al. Health benefits, risks, and cost–effectiveness of influenza vaccination of children. Emerging Infect. Dis. 2006;12(10):1548–1558. doi: 10.3201/eid1210.051015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee BY, Tai JH, McGlone SM, et al. The potential economic value of a ‘universal’ (multi-year) influenza vaccine. Influenza Other Respi. Viruses. 2012;6(3):167–175. doi: 10.1111/j.1750-2659.2011.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention (CDC) Interim results: state-specific influenza vaccination coverage – United States, August 2010–February 2011. MMWR Morb. Mortal. Wkly Rep. 2011;60(22):737–743. [PubMed] [Google Scholar]
- 42.Diekema DS. Improving childhood vaccination rates. N. Engl. J. Med. 2012;366(5):391–393. doi: 10.1056/NEJMp1113008. [DOI] [PubMed] [Google Scholar]
- 43.Centers for Disease Control and Prevention (CDC) Intent to receive influenza A (H1N1) 2009 monovalent and seasonal influenza vaccines – two counties, North Carolina, August 2009. MMWR Morb Mortal Wkly Rep. 2009;58(50):1401–1405. [PubMed] [Google Scholar]
- 44.Falagas ME, Zarkadoulia E. Factors associated with suboptimal compliance to vaccinations in children in developed countries: a systematic review. Curr. Med. Res. Opin. 2008;24(6):1719–1741. doi: 10.1185/03007990802085692. [DOI] [PubMed] [Google Scholar]
- 45.Cawley J, Hull HF, Rousculp MD. Strategies for implementing school-located influenza vaccination of children: a systematic literature review. J. Sch. Health. 2010;80(4):167–175. doi: 10.1111/j.1746-1561.2009.00482.x. [DOI] [PubMed] [Google Scholar]
- 46.Tran CH, McElrath J, Hughes P, et al. Implementing a community-supported school-based influenza immunization program. Biosecur. Bioterror. 2010;8(4):331–341. doi: 10.1089/bsp.2010.0029. [DOI] [PubMed] [Google Scholar]
- 47.Hull HF, Ambrose CS. The impact of school-located influenza vaccination programs on student absenteeism: a review of the U.S. literature. J. Sch. Nurs. 2011;27(1):34–42. doi: 10.1177/1059840510389182. [DOI] [PubMed] [Google Scholar]
- 48.Martin E. Improving influenza vaccination rates for pediatric asthmatics by use of an asthma educational tool and a patient electronic care system. Clin. Pediatr. (Phila.) 2008;47(6):588–592. doi: 10.1177/0009922808314902. [DOI] [PubMed] [Google Scholar]
- 49.Fiore AE, Fry A, Shay D, Gubareva L, Bresee JS, Uyeki TM. Centers for Disease Control and Prevention (CDC). Antiviral agents for the treatment and chemoprophylaxis of influenza – recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR. Recomm. Rep. 2011;60(1):1–24. [PubMed] [Google Scholar]
- 50.Wang K, Shun-Shin M, Gill P, Perera R, Harnden A. Neuraminidase inhibitors for preventing and treating influenza in children (published trials only) Cochrane Database Syst. Rev. 2012;4:CD002744. doi: 10.1002/14651858.CD002744.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hayden FG, Belshe R, Villanueva C, et al. Management of influenza in households: a prospective, randomized comparison of oseltamivir treatment with or without postexposure prophylaxis. J. Infect. Dis. 2004;189(3):440–449. doi: 10.1086/381128. [DOI] [PubMed] [Google Scholar]
- 52.Welliver R, Monto AS, Carewicz O, et al. Oseltamivir Post Exposure Prophylaxis Investigator Group. Effectiveness of oseltamivir in preventing influenza in household contacts: a randomized controlled trial. JAMA. 2001;285(6):748–754. doi: 10.1001/jama.285.6.748. [DOI] [PubMed] [Google Scholar]
- 53.Hayden FG, Gubareva LV, Monto AS, et al. Zanamivir Family Study Group. Inhaled zanamivir for the prevention of influenza in families. Zanamivir Family Study Group. N. Engl. J. Med. 2000;343(18):1282–1289. doi: 10.1056/NEJM200011023431801. [DOI] [PubMed] [Google Scholar]
- 54.Monto AS, Pichichero ME, Blanckenberg SJ, et al. Zanamivir prophylaxis: an effective strategy for the prevention of influenza types A and B within households. J. Infect. Dis. 2002;186(11):1582–1588. doi: 10.1086/345722. [DOI] [PubMed] [Google Scholar]
- 55.Goldstein E, Cowling BJ, O’Hagan JJ, et al. Oseltamivir for treatment and prevention of pandemic influenza A/H1N1 virus infection in households, Milwaukee, 2009. BMC Infect. Dis. 2010;10:211. doi: 10.1186/1471-2334-10-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee DH, Kim CW, Kim JH, et al. Risk factors for laboratory-confirmed household transmission of pandemic H1N1 2009 infection. Am. J. Infect. Control. 2010;38(10):e43–e45. doi: 10.1016/j.ajic.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 57.Komiya N, Gu Y, Kamiya H, et al. Household transmission of pandemic 2009 influenza A (H1N1) virus in Osaka, Japan in May 2009. J. Infect. 2010;61(4):284–288. doi: 10.1016/j.jinf.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 58.Leung YH, Li MP, Chuang SK. A school outbreak of pandemic (H1N1) 2009 infection: assessment of secondary household transmission and the protective role of oseltamivir. Epidemiol. Infect. 2011;139(1):41–44. doi: 10.1017/S0950268810001445. [DOI] [PubMed] [Google Scholar]
- 59.Ng S, Cowling BJ, Fang VJ, et al. Effects of oseltamivir treatment on duration of clinical illness and viral shedding and household transmission of influenza virus. Clin. Infect. Dis. 2010;50(5):707–714. doi: 10.1086/650458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Talbird SE, Brogan AJ, Winiarski AP. Oseltamivir for influenza postexposure prophylaxis: economic evaluation for children aged 1–12 years in the U.S. Am. J. Prev. Med. 2009;37(5):381–388. doi: 10.1016/j.amepre.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 61.Tamura D, Sugaya N, Ozawa M, et al. Frequency of drug-resistant viruses and virus shedding in pediatric influenza patients treated with neuraminidase inhibitors. Clin. Infect. Dis. 2011;52(4):432–437. doi: 10.1093/cid/ciq183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rajatonirina S, Heraud JM, Randrianasolo L, et al. Pandemic influenza A(H1N1) 2009 virus outbreak among boarding school pupils in Madagascar: compliance and adverse effects of prophylactic oseltamivir treatment. J. Infect. Dev. Ctries. 2011;5(3):156–162. doi: 10.3855/jidc.1318. [DOI] [PubMed] [Google Scholar]
- 63.Kitching A, Roche A, Balasegaram S, Heathcock R, Maguire H. Oseltamivir adherence and side effects among children in three London schools affected by influenza A(H1N1) May 2009 – an internet-based cross-sectional survey. Euro Surveill. 2009;14(30):19287. doi: 10.2807/ese.14.30.19287-en. [DOI] [PubMed] [Google Scholar]
- 64.Wallensten A, Oliver I, Lewis D, Harrison S. Compliance and side effects of prophylactic oseltamivir treatment in a school in South West England. Euro Surveill. 2009;14(30):19285. doi: 10.2807/ese.14.30.19285-en. [DOI] [PubMed] [Google Scholar]
- 65.Lee BY, McGlone SM, Bailey RR, et al. To test or to treat? An analysis of influenza testing and antiviral treatment strategies using economic computer modeling. PLoS ONE. 2010;5(6):e11284. doi: 10.1371/journal.pone.0011284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cannell JJ, Vieth R, Umhau JC, et al. Epidemic influenza and vitamin D. Epidemiol. Infect. 2006;134(6):1129–1140. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beard JA, Bearden A, Striker R. Vitamin D and the anti-viral state. J. Clin. Virol. 2011;50(3):194–200. doi: 10.1016/j.jcv.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guo R, Pittler MH, Ernst E. Complementary medicine for treating or preventing influenza or influenza-like illness. Am. J. Med. 2007;120(11):923–929. doi: 10.1016/j.amjmed.2007.06.031. e3. [DOI] [PubMed] [Google Scholar]
- 69.Matsumoto K, Yamada H, Takuma N, Niino H, Sagesaka YM. Effects of green tea catechins and theanine on preventing influenza infection among healthcare workers: a randomized controlled trial. BMC Complement. Altern. Med. 2011;11:15. doi: 10.1186/1472-6882-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Melchart D, Walther E, Linde K, Brandmaier R, Lersch C. Echinacea root extracts for the prevention of upper respiratory tract infections: a double-blind, placebo-controlled randomized trial. Arch. Fam. Med. 1998;7(6):541–545. doi: 10.1001/archfami.7.6.541. [DOI] [PubMed] [Google Scholar]
- 71.Cohen HA, Varsano I, Kahan E, Sarrell EM, Uziel Y. Effectiveness of an herbal preparation containing echinacea, propolis, and vitamin C in preventing respiratory tract infections in children: a randomized, double-blind, placebo-controlled, multicenter study. Arch. Pediatr. Adolesc. Med. 2004;158(3):217–221. doi: 10.1001/archpedi.158.3.217. [DOI] [PubMed] [Google Scholar]
- 72.Linday LA, Shindledecker RD, Tapia-Mendoza J, Dolitsky JN. Effect of daily cod liver oil and a multivitamin-mineral supplement with selenium on upper respiratory tract pediatric visits by young, inner-city, Latino children: randomized pediatric sites. Ann. Otol. Rhinol. Laryngol. 2004;113(11):891–901. doi: 10.1177/000348940411301108. [DOI] [PubMed] [Google Scholar]
- 73.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am. J. Clin. Nutr. 2010;91(5):1255–1260. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 74.Schmier JK, Rachman NJ, Halpern MT. The cost–effectiveness of omega-3 supplements for prevention of secondary coronary events. Manag. Care. 2006;15(4):43–50. [PubMed] [Google Scholar]
- 75.Tice JA, Ross E, Coxson PG, et al. Cost–effectiveness of vitamin therapy to lower plasma homocysteine levels for the prevention of coronary heart disease: effect of grain fortification and beyond. JAMA. 2001;286(8):936–943. doi: 10.1001/jama.286.8.936. [DOI] [PubMed] [Google Scholar]
- 76.Norman K, Pirlich M, Smoliner C, et al. Cost–effectiveness of a 3-month intervention with oral nutritional supplements in disease-related malnutrition: a randomised controlled pilot study. Eur. J. Clin. Nutr. 2011;65(6):735–742. doi: 10.1038/ejcn.2011.31. [DOI] [PubMed] [Google Scholar]
- 77.Jansen JP, Gaugris S, Bergman G, Sen SS. Cost–effectiveness of a fixed dose combination of alendronate and cholecalciferol in the treatment and prevention of osteoporosis in the United Kingdom and The Netherlands. Curr. Med. Res. Opin. 2008;24(3):671–684. doi: 10.1185/030079908X260998. [DOI] [PubMed] [Google Scholar]
- 78.Casey GJ, Sartori D, Horton SE, et al. Weekly iron–folic acid supplementation with regular deworming is cost–effective in preventing anaemia in women of reproductive age in Vietnam. PLoS ONE. 2011;6(9):e23723. doi: 10.1371/journal.pone.0023723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu JT, Cowling BJ, Lau EH, et al. School closure and mitigation of pandemic (H1N1) 2009, Hong Kong. Emerging Infect. Dis. 2010;16(3):538–541. doi: 10.3201/eid1603.091216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wheeler CC, Erhart LM, Jehn ML. Effect of school closure on the incidence of influenza among school-age children in Arizona. Public Health Rep. 2010;125(6):851–859. doi: 10.1177/003335491012500612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chowell G, Echevarría-Zuno S, Viboud C, et al. Characterizing the epidemiology of the 2009 influenza A/H1N1 pandemic in Mexico. PLoS Med. 2011;8(5):e1000436. doi: 10.1371/journal.pmed.1000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cowling BJ, Lau EH, Lam CL, et al. Effects of school closures, 2008 winter influenza season, Hong Kong. Emerging Infect. Dis. 2008;14(10):1660–1662. doi: 10.3201/eid1410.080646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cauchemez S, Bhattarai A, Marchbanks TL, et al. Pennsylvania H1N1 working group. Role of social networks in shaping disease transmission during a community outbreak of 2009 H1N1 pandemic influenza. Proc. Natl Acad. Sci. USA. 2011;108(7):2825–2830. doi: 10.1073/pnas.1008895108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Glass K, Barnes B. How much would closing schools reduce transmission during an influenza pandemic? Epidemiology. 2007;18(5):623–628. doi: 10.1097/EDE.0b013e31812713b4. [DOI] [PubMed] [Google Scholar]
- 85.Sasaki A, Hoen AG, Ozonoff A, et al. Evidence-based tool for triggering school closures during influenza outbreaks, Japan. Emerging Infect. Dis. 2009;15(11):1841–1843. doi: 10.3201/eid1511.090798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee BY, Brown ST, Cooley P, et al. Simulating school closure strategies to mitigate an influenza epidemic. J. Public Health Manag. Pract. 2010;16(3):252–261. doi: 10.1097/PHH.0b013e3181ce594e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Halloran ME, Ferguson NM, Eubank S, et al. Modeling targeted layered containment of an influenza pandemic in the United States. Proc. Natl Acad. Sci. USA. 2008;105(12):4639–4644. doi: 10.1073/pnas.0706849105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cauchemez S, Ferguson NM, Wachtel C, et al. Closure of schools during an influenza pandemic. Lancet Infect. Dis. 2009;9(8):473–481. doi: 10.1016/S1473-3099(09)70176-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ferguson NM, Cummings DA, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature. 2006;442(7101):448–452. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jefferson T, Del Mar C, Dooley L, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. BMJ. 2009;339:b3675. doi: 10.1136/bmj.b3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pang X, Yang P, Li S, et al. Pandemic (H1N1) 2009 among quarantined close contacts, Beijing, People’s Republic of China. Emerging Infect. Dis. 2011;17(10):1824–1830. doi: 10.3201/eid1710.101344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van Gemert C, Hellard M, McBryde ES, et al. Intrahousehold transmission of pandemic (H1N1) 2009 virus, Victoria, Australia. Emerging Infect. Dis. 2011;17(9):1599–1607. doi: 10.3201/eid1709.101948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jefferson T, Del Mar CB, Dooley L, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst. Rev. 2011;7:CD006207. doi: 10.1002/14651858.CD006207.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Halder N, Kelso JK, Milne GJ. Analysis of the effectiveness of interventions used during the 2009 A/H1N1 influenza pandemic. BMC Public Health. 2010;10:168. doi: 10.1186/1471-2458-10-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Effler PV, Carcione D, Giele C, Dowse GK, Goggin L, Mak DB. Household responses to pandemic (H1N1) 2009-related school closures, Perth, Western Australia. Emerging Infect. Dis. 2010;16(2):205–211. doi: 10.3201/eid1602.091372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Halloran ME, Longini IM, Cowart DM, Nizam A. Community interventions and the epidemic prevention potential. Vaccine. 2002;20(27–28):3254–3262. doi: 10.1016/s0264-410x(02)00316-x. [DOI] [PubMed] [Google Scholar]
- 97.Kelso JK, Milne GJ, Kelly H. Simulation suggests that rapid activation of social distancing can arrest epidemic development due to a novel strain of influenza. BMC Public Health. 2009;9:117. doi: 10.1186/1471-2458-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Longini IM, Jr, Nizam A, Xu S, et al. Containing pandemic influenza at the source. Science. 2005;309(5737):1083–1087. doi: 10.1126/science.1115717. [DOI] [PubMed] [Google Scholar]
- 99.Lempel H, Epstein JM, Hammond RA. Economic cost and health care workforce effects of school closures in the US. PLoS Curr. 2009;1:RRN1051. doi: 10.1371/currents.RRN1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Berkman BE. Mitigating pandemic influenza: the ethics of implementing a school closure policy. J. Public Health Manag. Pract. 2008;14(4):372–378. doi: 10.1097/01.PHH.0000324566.72533.0b. [DOI] [PubMed] [Google Scholar]
- 101.Impact of seasonal influenza-related school closures on families – southeastern Kentucky, February 2008. MMWR Morb. Mortal. Wkly Rep. 2009;58(50):1405–1409. [PubMed] [Google Scholar]
- 102.Johnson AJ, Moore ZS, Edelson PJ, et al. Household responses to school closure resulting from outbreak of influenza B, North Carolina. Emerging Infect. Dis. 2008;14(7):1024–1030. doi: 10.3201/eid1407.080096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Canini L, Andréoletti L, Ferrari P, et al. Surgical mask to prevent influenza transmission in households: a cluster randomized trial. PLoS ONE. 2010;5(11):e13998. doi: 10.1371/journal.pone.0013998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stebbins S, Stark JH, Vukotich CJ., Jr. Compliance with a multilayered nonpharmaceutical intervention in an urban elementary school setting. J. Public Health Manag. Pract. 2010;16(4):316–324. doi: 10.1097/PHH.0b013e3181cb4368. [DOI] [PubMed] [Google Scholar]
- 105.Guinan M, McGuckin M, Ali Y. The effect of a comprehensive handwashing program on absenteeism in elementary schools. Am. J. Infect. Control. 2002;30(4):217–220. doi: 10.1067/mic.2002.120366. [DOI] [PubMed] [Google Scholar]
- 106.Brienen NC, Timen A, Wallinga J, van Steenbergen JE, Teunis PF. The effect of mask use on the spread of influenza during a pandemic. Risk Anal. 2010;30(8):1210–1218. doi: 10.1111/j.1539-6924.2010.01428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Talaat M, Afifi S, Dueger E, et al. Effects of hand hygiene campaigns on incidence of laboratory-confirmed influenza and absenteeism in schoolchildren, Cairo, Egypt. Emerging Infect. Dis. 2011;17(4):619–625. doi: 10.3201/eid1704.101353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.MacIntyre CR, Cauchemez S, Dwyer DE, et al. Face mask use and control of respiratory virus transmission in households. Emerging Infect. Dis. 2009;15(2):233–241. doi: 10.3201/eid1502.081167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cowling BJ, fung RO, Cheng CK, et al. Preliminary findings of a randomized trial of non-pharmaceutical interventions to prevent influenza transmission in households. PLoS ONE. 2008;3(5):e2101. doi: 10.1371/journal.pone.0002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cowling BJ, Chan KH, Fang VJ, et al. Facemasks and hand hygiene to prevent influenza transmission in households: a cluster randomized trial. Ann. Intern. Med. 2009;151(7):437–446. doi: 10.7326/0003-4819-151-7-200910060-00142. [DOI] [PubMed] [Google Scholar]
- 111.Larson EL, Ferng YH, Wong-McLoughlin J, Wang S, Haber M, Morse SS. Impact of non-pharmaceutical interventions on URIs and influenza in crowded, urban households. Public Health Rep. 2010;125(2):178–191. doi: 10.1177/003335491012500206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee BY, Bailey RR, Smith KJ, et al. Universal methicillin-resistant Staphylococcus aureus (MRSA) surveillance for adults at hospital admission: an economic model and analysis. Infect. Control Hosp. Epidemiol. 2010;31(6):598–606. doi: 10.1086/652524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee BY, Wettstein ZS, McGlone SM, et al. Economic value of norovirus outbreak control measures in healthcare settings. Clin. Microbiol. Infect. 2011;17(4):640–646. doi: 10.1111/j.1469-0691.2010.03345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee BY, McGlone SM, Doi Y, Bailey RR, Harrison LH. Economic value of Acinetobacter baumannii screening in the intensive care unit (ICU) Clin Microbiol Infect. 2011;17(11):1691–1697. doi: 10.1111/j.1469-0691.2011.03491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Karchmer TB, Durbin LJ, Simonton BM, Farr BM. Cost–effectiveness of active surveillance cultures and contact/droplet precautions for control of methicillin-resistant Staphylococcus aureus. J. Hosp. Infect. 2002;51(2):126–132. doi: 10.1053/jhin.2002.1200. [DOI] [PubMed] [Google Scholar]
- 116.Lee BY, Burke DS. Constructing target product profiles (TPPs) to help vaccines overcome post-approval obstacles. Vaccine. 2010;28(16):2806–2809. doi: 10.1016/j.vaccine.2009.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Website
- 201.Centers for Disease Control and Prevention. Vaccines & Immunizations, Programs & Tools, CDC Vaccine Price List. Atlanta, GA, USA: [Accessed 24 April 2012]. www.cdc.gov/vaccines/programs/vfc/ cdc-vac-price-list.htm. [Google Scholar]