Abstract
In this study, we compared the clinicopathologic characteristics between the bilateral breast cancer (BiBC) and unilateral breast cancer (UBC) and investigated the role of CXC chemokine receptor type 4 (CXCR4) in BiBC. 48 BiBC and 1650 UBC were studied. We found BiBC patients were associated with family history of cancer, invasive lobular histology in the first tumor and an advanced nodal status as compared with UBC patients with. Survival analysis indicated that BiBC was not associated with impaired survival. The time interval between the development of first breast cancer and the contralateral cancer did not correlate with the prognosis. Patients with BiBC were more likely to have bone metastasis (P = 0.011) and visceral metastasis (P < 0.001) than those with UBC. However, CXCR4 was not found in any association with poor clinical outcome and increasing visceral metastasis in BiBC patients.
Patients with primary breast cancer have an increased risk of developing contralateral breast cancer1. When cancer is detected in the opposite breast, will the newly developed tumor impact the prognosis of the primary breast cancer? Bilateral breast cancer (BiBC) can be categorized as synchronous and metachronous based on the time window between the first and secondary breast cancer development. As to which type of BiBC is associated with the worse outcome is still debatable and yet to be conclusively determined. Some studies have indicated that there is no difference in survival between the unilateral versus bilateral breast cancer patient groups while other studies claim that a second primary carcinoma significantly reduces survival.
The CXC chemokine receptor type 4 (CXCR4) has been reported to be significantly overexpressed in a variety of human malignancies including breast cancer. CXCR4 was found to mediate cancer migration to visceral organs like liver and lung, which are rich in CXCR4 ligand such as SDF-1. Thus CXCR4 has been considered a poor prognostic factor for cancer2. However, breast cancer is a heterogeneous disease and whether or not CXCR4 also overexpress in bilateral breast cancer has not been previously reported.
The purpose of this study is to review the characteristics of patients with BiBC bilateral breast cancer and evaluate the role of CXCR4 overexpression in BiBC. The results presented here will help us to further understand the BiBC in Chinese patients and provide a new insight on developing novel therapeutic strategies for BiBC.
Results
Comparison of demographic and clinicopathological characteristics in the first tumor in bilateral cancer and unilateral cancer
Among the 2813 patients included in this study, 48 (1.7%) were diagnosed with BiBC. As shown in Table 1, the median age when BiBC is first diagnosed was similar to that of UBC (47.5 vs. 48 years. P = 0.266). There were also similar distributions on the ER, PR and HER-2 status between BiBC and UBC. However, patients with BiBC were more likely to have positive family cancer history than UBC patients (P = 0.022), suggesting family history of breast cancer is a risk factor for BiBC. Moreover, the percentage of infiltrating lobular carcinoma was higher in bilateral group (P = 0.003) approximately 13.5% of the first tumors in BiBC were infiltrating lobular carcinoma, compared with only 4.7% in the unilateral group.
Table 1. Comparison of clinicopathological characteristics (unilateral versus first tumor of BiBC).
| Characteristics | Unilateral (n = 1650) | First tumor of BiBC (n = 48) | P value |
|---|---|---|---|
| Median age (years) (range) | 48 (21 ~ 89) | 47.5 (27 ~ 69) | 0.266 |
| Nodal status | |||
| N0 | 678 (41.1%) | 12 (25.0%) | 0.000 |
| N1 | 477 (28.9%) | 12 (25.0%) | |
| N2 | 379 (23.0%) | 11 (22.9%) | |
| N3 | 116 (7.0%) | 13 (27.1%) | |
| TNM stage | |||
| I | 107 (6.5%) | 3 (6.3%) | 0.082 |
| II | 757 (45.9%) | 14 (29.2%) | |
| III | 786 (47.6%) | 31 (64.5%) | |
| Histologic type | |||
| Infiltrating Duct Carcinoma | 1089 (66.0%) | 35 (73.0%) | 0.003 |
| Infiltrating Lobular Carcinoma | 78 (4.7%) | 5 (10.4%) | |
| Others | 483 (29.3%) | 8 (16.7%) | |
| ER status | |||
| Positive | 1077 (65.3%) | 28 (58.6%) | 0.457 |
| Negative | 573 (34.7%) | 20 (41.4%) | |
| PR status | |||
| Positive | 945 (57.3%) | 25 (52.0%) | 0.548 |
| Negative | 705 (42.7%) | 23 (47.9%) | |
| HER-2 status | |||
| Positive | 470 (28.5%) | 15 (31.3%) | 0.818 |
| Negative | 1180 (71.5%) | 33 (68.8%) | |
| Family history of breast cancer | |||
| Positive | 104 (6.3%) | 7 (14.6%) | 0.022 |
| Negative | 1546 (93.7%) | 41 (85.4%) | |
| Bone metastasis | |||
| Positive | 181 (11.0%) | 11 (22.9%) | 0.011 |
| Negative | 1469 (89.0%) | 37 (77.1%) | |
| Visceral metastasis | |||
| Positive | 219 (13.3%) | 17 (35.4%) | 0.000 |
| Negative | 1431 (86.7%) | 31 (64.6%) |
The overall survival between BiBC and UBC was then evaluated with median length of follow-up of 44.5 months (range from 3 to 228 months) in BiBC and 52 months in UBC. As shown in Table 1, although patients with BiBC cancer were associated with higher incidence of bone metastasis (P = 0.011) and visceral metastasis (lung, liver or brain) (P < 0.001) than those with UBC, the difference in overall survival (OS) was not significant between the BiBC and UBC (5-year OS rates of 70.5% vs. 69.1%, respectively, P = 0.714).
Comparison of demographic and clinicopathological characteristics in the first and second tumor in bilateral cancer
Moreover, we evaluated the difference of demographic and clinicopathological characteristics between the first and second tumor in BiBC, hoping to find correlations between the two. The median time intervals between the first and the subsequent breast cancer diagnosis were 9.5 months (range from 0 to 226 months). As shown in Table 2, we did not find any significantly difference on the nodular status, TNM stage, histologic type, ER,PR and HER-2 status between the first and second breast cancer of the BiBC.
Table 2. Comparison of clinicopathological characteristics between first tumor and second tumor of the BiBC group.
| Characteristics | First tumor of BiBC | Second tumor of BiBC | P value |
|---|---|---|---|
| Median Age (years) (range) | 47.5 (27 ~ 69) | 50 (29 ~ 70) | 0.311 |
| Nodal status | |||
| N0 | 12 (25.0%) | 23 (47.9%) | 0.179 |
| N1 | 12 (25.0%) | 8 (16.7%) | |
| N2 | 11 (22.9%) | 5 (10.4%) | |
| N3 | 13 (27.1%) | 12 (25.0%) | |
| TNM stage | |||
| I | 3 (6.3%) | 10 (20.8%) | 0.114 |
| II | 14 (29.2%) | 15 (31.3%) | |
| III | 31 (64.5%) | 23 (47.9%) | |
| Histologic type | |||
| Infiltrating Duct Carcinoma | 35 (73.0%) | 33 (68.8%) | 0.887 |
| Infiltrating Lobular Carcinoma | 5 (10.4%) | 8 (16.7%) | |
| Others | 8 (16.7%) | 7 (14.6%) | |
| ER status | |||
| Positive | 28 (58.6%) | 32 (66.7%) | 0.453 |
| Negative | 20 (41.4%) | 16 (33.3%) | |
| PR status | |||
| Positive | 25 (52.0%) | 21 (43.8%) | 0.559 |
| Negative | 23 (47.9%) | 27 (56.3%) | |
| HER-2 status | |||
| Positive | 15 (31.3%) | 18 (37.5%) | 0.548 |
| Negative | 33 (68.8%) | 30 (62.5%) |
Comparison of overall survival in patients with synchronous and metachronous bilateral breast cancer
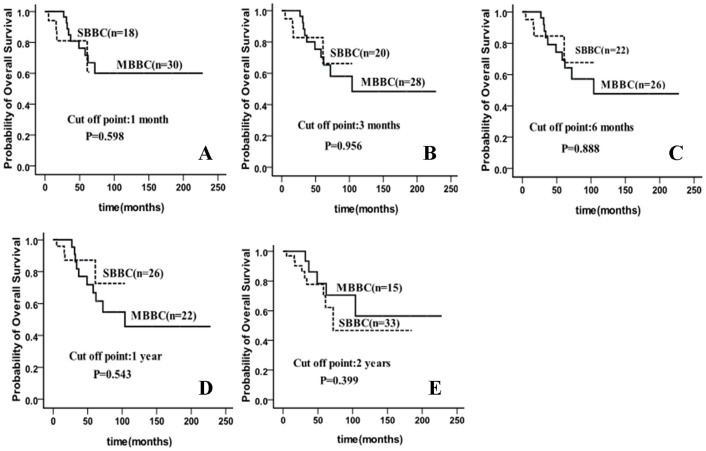
According to the literatures5,6, bilateral breast cancer is often categorized as synchronous and metachronous BiBC. However, the inconsistency in defining the time interval to differentiate breast cancer as either synchronous or metachronous resulted in outcomes that are varied from study to study. In this clinical cohort study, we examined whether the time interval between first and second breast cancer impact the survival of patients with BiBC. We used different cut off points ranging from 0 to 24 months to define synchronous and metachronous BiBC. In our study, we did not find any differences in outcome between synchronous and metachronous BiBC regardless of the time interval. For instance, if the cut off point x was 1 month, then 37.5% were synchronous and there was no difference in terms of overall survival (P = 0.598). If the cutoff point is set to 3 months, then 41.7% were synchronous and without significant difference was observed in overall survival (P = 0.956). We then adjusted the cut off point x to 6 months, 12 months, as well as 24 months, respectively and we also failed to demonstrate significant difference in overall survival between the synchronous and metachronous BiBC. (Figure 1).
Figure 1. No differences in overall survival (OS) between synchronous and metachronous BiBC regardless different cut off point by Kaplan-Meier method and log-rank test.
(A) cut off point = 1 month, (B) cut off point = 3 month, (C) cut off point = 6 month, (D) cut off point = 12 month, (E) cut off point = 24 month.
The prognostic significance of CXCR4 expression in bilateral cancer
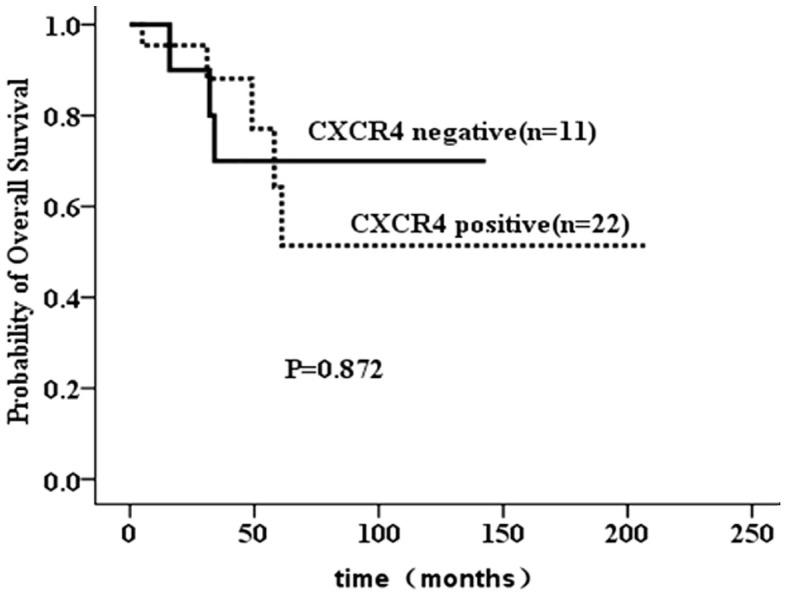
As our data clearly indicated higher incidence of visceral metastasis in BiBC compared to UBC, we further analyzed the expression of CXCR4 in BiBC because of CXCR4's role in increasing visceral metastasis in cancers, especially in breast cancer7. The expression of CXCR4 was primarily detected in the cytoplasm of tumor cells using a semi-quantitative scoring system as previously described7 (Figure 2). As shown in Table 3, 22 cases out of the available 33 first cancer of BiBC analyzed (66.7%) had revealed positive CXCR4 protein expression. On the other hand, CXCR4 expression was detected in 95 cases (54.0%) in 176 consecutive UBC. The difference of CXCR4 expression levels between the first tumor of BiBC and that of UBC wasn't statistically significant (P = 0.178). In addition, we evaluated the differences in visceral metastasis (lung, liver or brain) using the chi-square statistic according to the CXCR4 status in BiBC and did not find a difference in the visceral metastasis rates between CXCR4 positive and CXCR4 negative patients with BiBC, as reported in Table 3. Finally, there were no significant differences in the survival curves between CXCR4 positive or negative subgroups (Figure 3) in BiBC.
Figure 2. Immunostaining for CXCR4 expression in bilateral breast cancer.
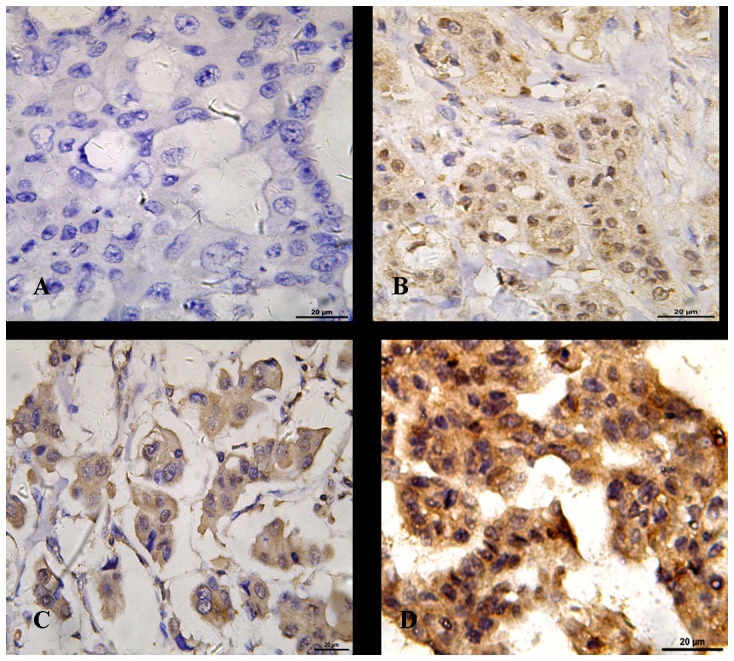
(A) Negative expression. (B) Weak cytoplasmic expression. (C) Moderate cytoplasmic expression. (D) Strong cytoplasmic expression. Combined with the percentages of positive tumor cells, the final scores were given. Original magnifications: × 400. Scale bars, 20 μm.
Table 3. Relationship between CXCR4 expression and visceral metastasis in bilateral breast cancer and unilateral breast cancer groups.
| CXCR4 | ||||
|---|---|---|---|---|
| group | Visceral metastasis | Negative | Positive | P value |
| BiBC | negative | 8 | 11 | 0.278 |
| positive | 3 | 11 | ||
| UBC | negative | 75 | 77 | 0.026 |
| positive | 6 | 18 | ||
Figure 3. Overall survival according to CXCR4 expression of the BiBC by Kaplan-Meier method and log-rank test.
Discussion
BiBC has posed a great challenge for oncologists because many questions still yet to be answered. Whether or not decrease in overall survival is linked to BiBC still remains controversial. Women with breast cancer are at risk of developing contralateral breast cancer. Among women with operable breast cancer, the occurrence of contralateral breast cancer ranged from 6% to 8.9%8. Some authors have suggested that primary breast cancer diagnosed at younger age is associated with increased susceptibility to bilateral breast cancer. Bilateral breast cancer is more frequently seen in women under the age of 50 as opposed to a mean age of 63.5 years when unilateral breast cancer is diagnosed9,10. In our study, patients with BiBC were also younger than 50 years of age when it was first diagnosed. However, we did not find any difference between the median age of UBC and BiBC patients at first diagnosis. The median age for the entire group with BiBC was 47.5 years of age, comparing to 48 years of age in UBC. This discrepancy may be explained by race-dependent epidemiology of breast cancer around the world. In Asian countries, the peak age for breast cancer in Asia was 40–50 years, whereas it was 60–70 years in the Western countries11. Some data has indicated that family history of breast cancer is an additional factor that increases the risk of bilateral breast cancer12,13, whereas others did not find the correlation between family history of breast cancer and risk of contralateral breast cancer14. In our study, significant correlation between family history of breast cancer and risk of bilateral breast cancer was found. However, BiBC patients with family history of breast cancer showed similar survival time when compared with BiBC patients without family history of breast cancer. It is also important to note that in our study, women with bilateral breast cancer had higher proportion of having invasive lobular cancer than those with unilateral breast cancer. Our study is in accordance with others' study which show women with lobular histology develop second primary breast cancer more frequently than ductal cells1,15, suggesting a more frequent follow-up with diagnostic procedures and surveillance is necessary for early contralateral breast cancer detection in patients with invasive lobular breast cancer. Furthermore, ER/PR negativity tumors are reported to be a risk factor for contralateral breast cancer16 because lack of ER renders drug Tamoxifen ineffective. Tamoxifen typically reduce the risk of contralateral breast cancer in ER-positive breast cancer whereas it has no effect on the risk for ER-negative disease17. HER-2 positivity was also regarded as a risk factor for developing bilateral disease as previously reported14. HER-2 was a potent oncogene and a strong predictor of prognosis. However, we did not find any significant differences in ER/PR/HER-2 status between BiBC and UBC in this study. This finding may be due to insufficient power to detect the association caused by a small number of bilateral cases as further investigation is needed.
The prognosis of synchronous and metachronous BiBC still remains uncertain. Some reported that synchronous BiBC has a worse prognosis, whereas others showed a prognosis similar to that of metachronous BiBC1. The inconsistency in these results may be caused by a lack of a consensus in diagnostic criteria, which could potentially bias the results. In order to determine if the length of time interval between primary first breast cancer and contralateral breast cancer influence the outcome of BiBC, different cutoff points, 1 months, 3 months, 6 months, 1 year and 2 year are used to define synchronous and metachronous. In our study, we did not find any differences in outcome between synchronous and metachronous BiBC regardless of the time interval. This finding could be contributed by the genetic component observed in different races. In addition, definition of OS may also influence the results. Some define survival from the time of the first cancer18, whereas others define survival based on the second cancer13,19. In this study, patients with BiBC taking the date of diagnosis of the first tumor as reference date. Nevertheless, our study was supported by Diaz et.al19, who also observed patients with synchronous bilateral breast cancers show similar survival with those with metachronous breast cancer. Furthermore, we found no statistically significant difference between BiBC and UBC, which was consistent with the majority of previous studies, which did not indicate the survival of BiBC patients was markedly worse than unilateral diseases patients5,20. We strongly believe that the studied population, adjuvant therapy after first and/or second surgery and treatments after recurrence maybe the reasons responsible for differences between our finding from the others. Furthermore, BiBC developed distant metastasis (bone, visceral organ) significantly more frequently than UBC [28 of 48 (58.3%) versus 400 of 1650 (24.2%)] in our study. The reasons for a higher proportion of bone metastasis and visceral metastasis occurred in patients with BiBC are not clear. We first analyzed the clinical characteristics of both BiBC patients and the UBC patients in our study. We found that BiBC patients was more likely to have an advanced N stage at diagnosis than UBC patients. It may be the reason that more BiBC patients presented with bone metastasis and visceral metastasis than that in UBC patient. In addition, it had been demonstrated that CXC chemokine receptor type 4 (CXCR4) played an important role in cancer progression and mediating metastasis homing to particular organs, such as bone and visceral organ. Thus, we investigate the relationship between CXCR4 expression and overall survival to evaluate whether CXCR4 could provide any valuable information for BiBC patients' clinical characteristic and outcome. Data revealed that, for BiBC, it was the biology of the first tumor to determine the prognosis, whereas a newly developed contralateral breast cancer did not appear to worsen the prognosis. Therefore, we assessed CXCR4 level in the first tumor of available 33 specimens of BiBC and found CXCR4 positive expression was detected in 22 cases (66.7%). In our previous study, we had analyzed CXCR4 expression in 176 cases of UBC, which showed 95 cases (54%) with positive CXCR4 protein expression3. Making a comparison with our previous study, we do not observe patients with BiBC express higher levels of CXCR4 than that in UBC. CXCR4 positive BiBC patients did not show significantly higher rate of distant metastasis. The expression level of CXCR4 was not significantly related to shorter overall survival either. This suggests that CXCL12/CXCR4 axis may not play as an important role in the prognosis of BiBC as that in UBC. However, this was probably due to the fact that our database on BiBC numbers is still small for CXCR4 evaluation in our study. Further analyses of different molecular mechanism between BiBC and UBC are strongly needed.
In summary, BiBC is a rare presentation of breast cancer. Our results suggest no difference in overall survival between patients with bilateral breast cancers and unilateral breast cancer. UBC patient with a family history of cancer are at higher risk for developing contralateral breast cancer, particularly if the first primary is of lobular histology and in advanced nodal status. Consideration of more surveillances and intensive therapy should be given in this subgroup for early detection of contralateral breast. However, our study has some limitations. The follow-up is not long enough and the number of BiBC patients involved is not large. Additional studies are needed for a deeper understanding of the mechanisms underlying the behavior of bilateral breast cancer, which will aid in improving prognosis.
Methods
Patients
Among the 2813 patients with primary breast carcinoma who underwent surgical therapies at Cancer Hospital of Shantou University Medical College, Southern China between Sep 2000 and Jan 2008, 48 patients were diagnosed with BiBC without any systemic lesions. 1650 unilateral breast caner (UBC) female patients had sufficient details data were included to be a matched group in this study. For the use of these clinical materials for research purposes, prior consents from the patients and approval from the Ethics Committees of the hospitals were obtained. All patients were female with a median age of 47.5 years at the time of first breast cancer diagnosis (range, 27–69 years). The clinical data and follow-ups were complete and include age, pathological type, tumor size, lymph node status, disease stage, treatment, menopausal status, the time and the location of metastases and survival outcomes. For the purpose of the current study, a positive family history was defined as any first or second-degree relative with a history of breast cancer. Synchronous (metachronous) is defined as cancer diagnosed in both breasts within (after) a period of x months of diagnosis of the first tumor (x = cut off point). X ranges from 0 to 24 months.
All cases were tested for ER, PR and HER2/neu expression by immunohistochemistry. ER and PR positive expression referred to ≥ 10% tumor cell staining, and Her-2 positive expression referred to ≥ 30% of tumor cells staining membrane completely. The histological grade was determined according to the Nottingham combined histological grade that is also known as the Nottingham grading system. Tumors were staged when initially diagnosed according to the American Joint Committee on Cancer (AJCC) pathologic tumor-node-metastasis (TNM) classification, sixth edition (2002). Exclusion criteria included neoadjuvant chemotherapy, prior malignancies, and stage IV disease. Standardized treatment and surveillance protocols were followed. Patients were followed in the clinic every 3 months for the first 3 years and subsequently every 6 months to year 5, and annually thereafter. At follow-ups, surveillance consists of a complete physical examination, chest x-ray, mammogram, complete blood workup and liver function tests annually. Biopsies of suspicions lesions and additional imaging were directed upon abnormal history and/or physical findings.
Immunohistochemistry for CXCR4 expression
33 first tumor of BiBC paraffin section were obtained and cut at 4-micron thickness. Immunohistochemical stains for CXCR4 were performed using the streptavidin-peroxidase method as previously described3. Anti-CXCR4 antibody (dilution 1:500, mouse monoclonal to CXCR4, clone ab58176, Abcam Corp. Cambridge, United Kingdom) was incubated overnight at 4°C. After rinsing in phosphate-buffered saline (PBS), the sections were incubated with the biotinylated secondary antibody (GTVision I, Anti-Mouse/Rabbit Detection System, Gene Tech Company, Shanghai, China) for 30 minutes at 37°C. Staining was visualized with 3,3-diamino-benzi-dine (DAB) and counterstained with hematoxylin.
CXCR4 expression was evaluated based on the percentage of positive tumor cells and staining intensity. According to Hao's method4, the percentages of positive tumor cells were scored as follows: 0 (<5% positive cells), 1 (6–25% positive cells), 2 (26–50% positive cells), 3 (51–75% positive cells) and 4 (>75% positive cells). The intensity of cytoplasmic staining was also assessed on a scale as the following: 0 (negative), 1(weakly positive), 2 (moderately positive) and 3 (strongly positive)(Fig. 1). The final expression level was calculated by multiplying the two scores and was assigned to the following: 0 (negative), + (1–4), ++ (5–8), and +++ (9–12).
Statistical methods
Statistical analysis was performed using SPSS 17.0 software(for Windows, Chicago, IL). The relationships among various characteristics and different groups of breast cancer patients were analyzed using chi-square tests. Overall survival was measured from the date of first breast cancer diagnosis to the date of death or last follow-up. The Kaplan–Meier method was used to estimate overall survival and the log rank statistics was used to test the difference among each subgroups. A two-tailed P < 0.05 was considered statistically significant.
Author Contributions
C.-S.F. contributed to the experiments and clinical data interpretation. C.-W.D. discussed the results and analysed the data and wrote the manuscript conceived and designed the experiments. P.Y. and H.-W.Z. contributed to the statistical analysis. M.K. contributed to polishing the English to improve the quality of this manuscript. G.-J.Z. supervised and directed the overall project. All the authors were involved in the discussions.
Acknowledgments
This work was supported by the funds from Major State Basic Research Development Program (No.2011CB707705), The Innovation Training Program for National College Students, National Natural Science Foundation of China(No. 30973377, No.31271068), Key Laboratory of Breast Cancer Prevention and Treatment of Guangdong Province, P.R. China.
References
- Schwentner L. et al. Survival of patients with bilateral versus unilateral breast cancer and impact of guideline adherent adjuvant treatment: a multi-centre cohort study of 5292 patients. Breast 21(2), 171 (2012). [DOI] [PubMed] [Google Scholar]
- Parker C. C., Kim R. H., Li B. D. & Chu Q. D. The chemokine receptor CXCR4 as a novel independent prognostic marker for node-positive breast cancer patients. J Surg Oncol 106(4), 393 (2012). [DOI] [PubMed] [Google Scholar]
- Chen H. W. et al. Cytoplasmic CXCR4 high-expression exhibits distinct poor clinicopathological characteristics and predicts poor prognosis in triple-negative breast cancer. Curr Mol Med (2013). [PubMed] [Google Scholar]
- Hao L. et al. Recombination of CXCR4, VEGF, and MMP-9 predicting lymph node metastasis in human breast cancer. Cancer Lett 253(1), 34 (2007). [DOI] [PubMed] [Google Scholar]
- Schmid S. M. et al. Prognosis of early-stage synchronous bilateral invasive breast cancer. Eur J Surg Oncol 37(7), 623 (2011). [DOI] [PubMed] [Google Scholar]
- Alkner S. et al. Prediction of outcome after diagnosis of metachronous contralateral breast cancer. Bmc Cancer 11, 114 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A. et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 410(6824), 50 (2001). [DOI] [PubMed] [Google Scholar]
- Fisher B. et al. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med 347(8), 567 (2002). [DOI] [PubMed] [Google Scholar]
- Shi Y. X. et al. Comparison of clinicopathological characteristics and prognoses between bilateral and unilateral breast cancer. J Cancer Res Clin Oncol 138(4), 705 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T. et al. The risk factors and prognosis of bilateral primary breast cancer: a comparative study with unilateral breast cancer. Oncol Res 19(3–4), 171 (2011). [DOI] [PubMed] [Google Scholar]
- Leong S. P. et al. Is breast cancer the same disease in Asian and Western countries? World J Surg 34(10), 2308 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe K. et al. Predictors of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. Br J Cancer 104(9), 1384 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman M. et al. Genetic implications of bilateral breast cancer: a population based cohort study. Lancet Oncol 6(6), 377 (2005). [DOI] [PubMed] [Google Scholar]
- Vuoto H. D. et al. Bilateral breast carcinoma: clinical characteristics and its impact on survival. Breast J 16(6), 625 (2010). [DOI] [PubMed] [Google Scholar]
- Beckmann K. R. et al. Clinical characteristics and outcomes of bilateral breast cancer in an Australian cohort. Breast 20(2), 158 (2011). [DOI] [PubMed] [Google Scholar]
- Kheirelseid E. A. et al. Bilateral breast cancer: analysis of incidence, outcome, survival and disease characteristics. Breast Cancer Res Treat 126(1), 131 (2011). [DOI] [PubMed] [Google Scholar]
- Fisher B. et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 90(18), 1371 (1998). [DOI] [PubMed] [Google Scholar]
- Verkooijen H. M. et al. Survival after bilateral breast cancer: results from a population-based study. Breast Cancer Res Treat 105(3), 347 (2007). [DOI] [PubMed] [Google Scholar]
- Diaz R. et al. Synchronous and metachronous bilateral breast cancer: a long-term single-institution experience. Med Oncol 29(1), 16 (2012). [DOI] [PubMed] [Google Scholar]
- Kheirelseid E. A. et al. Bilateral breast cancer: analysis of incidence, outcome, survival and disease characteristics. Breast Cancer Res Treat 126(1), 131 (2011). [DOI] [PubMed] [Google Scholar]