Abstract
Two compounds, 1,7-bis(4-hydroxyphenyl)-1,4,6-heptatrien-3-one (BHPHTO) and bisdemethoxycurcumin (BDMC) they have been isolated from the rhizomes of Alpinia galangal, and the structures of both pure constituents were determined using spectroscopic analyses. The study examined the bioeffectivenesses of the two compounds on the human melanoma A2058 and showed that significantly inhibited the proliferation of melanoma cells in the cell viability assay. This research was also taken on the tests to B16-F10 cell line and showed minor inhibitory consequences of cellular tyrosinase activities and melanin contents. Our results revealed the anticancer effects of A. galangal compounds, and therefore, the target compounds could be potentially applied in the therapeutic application and the food industry.
1. Introduction
Alpinia galangal belongs to the Zingiberaceae family, and the herb grows mainly in South East Asia. It is now cultivated throughout tropical and subtropical Asia, such as India, Egypt, Thailand, Malaysia, Indonesia, and China. The herbs are usually not only used for seasoning but also for traditional medicine. The rhizomes contain essential oil. Many essential oils were obtained by hydrodistillation. The yield of essential oils ranged from 1.32 to 0.143% [1–3]. The wide range of major volatile compounds was identified and characterized by GC and GC/MS as endo-fenchyl acetate, zerumbone, 1,8-cineole and myrcene. For A. galangal rhizome chemical studies, a group of related phenylpropanoids was identified [4, 5]. Among these phenylpropanoids, 1′S-1′-acetoxychavicol acetate (galangal acetate) was the most studied and reported to possess various activities, such as antioxidative, antifungal, antitumor, and anti-inflammatory activities. However, only some studies for A. galangal grown in Taiwan were published [6]. Human skin is normally contacted with damage stress, which is produced by external and intrinsic sources, such as ultraviolet (UV) radiation, free radicals, and reactive oxygen species [7]. There are many studies about the skin exposed to oxidative stress or UV radiation and are responsible for aging or tumorigenesis [8]. Melanoma, a malignant tumor of epidermal melanocytes, is one of the most deadly skin cancers. Within the past several decades, the occurrences of cutaneous malignant melanoma have increased because it has a strong propensity to metastasize and, therefore, is one of the most aggressive skin cancers. Unlike other cancers, malignant melanoma is not easy to treat with surgery, radiotherapy, or chemotherapy. A good chemotherapeutic agent will be a naturally occurring agent and can induce cytotoxicity in cancer cells.
In mammals, skin, hair, and eyes, darkening is determined by the synthesis and distribution of melanin. In skin, it is a mixture of pigmented biopolymers that is synthesized in a unique organelle, the melanosome of melanocytes. Excessive biosynthesis of melanin induces various related pigment disorders, such as senile lentigo, melasma, freckles, and pigmented acne scars, that are of particular concern to women as well as men. Their treatment usually involves the use of medicines or medicinal cosmetics containing depigmenting or skin-whitening components. Safe and effective regulators that act to minimize skin pigmentation abnormalities include natural and synthetic depigmenting agents. However, only a few are used as therapeutic agents, primarily because of various safety concerns and low whitening bioactivity. In melanogenesis, L-tyrosine is hydroxylated to dihydroxyphenylalanine (L-DOPA), and then L-DOPA is oxidized to DOPA-quinone with two initial steps [9]. Pigment coloring hair, skin, and eyes because of the key protein, tyrosinase, is recognized to be the first two and rate-limiting enzyme in the biosynthesis of melanins [10]. Recently, much attention has been drawn to the application of tyrosinase inhibitors to medical treatments and cosmetic businesses. Therefore, in clinical usage, tyrosinase inhibitors are being taken for dermatological disorder treatments related to melanin hyperaccumulation and are thus fundamental in cosmetics for depigmentation [11].
2. Materials and Methods
2.1. Reagents and Materials
All solvents were at analytical grade. Lipophilic Sephadex (LH-20) resin was purchased from Sigma-Aldrich Inc., (St. Louis, MO, USA). Reverse phase (C18; 25–40 μm) sorbent was purchased from Merck Chemical Co. (Darmstadt, Germany).
2.2. Extraction and Purification of BHPHTO and BDMC
The rhizomes of A. galangal were collected from the Native Plant Ecological Garden in National Chiayi University. The air-dried rhizomes (300 g) of A. galangal were ground into a fine powder and extracted successively with 95% ethanol at room temperature. The crude ethanol extract (CEE) was filtered and evaporated to slurry using a rotary evaporator. The slurry was then suspended with water and partitioned successively three times with hexane (×3) and ethyl acetate (×3). The hexane and ethyl acetate extracts were separately dried using a rotary evaporator at 40°C, followed by freeze-drying for 48 h to give dried hexane and ethyl acetate extract (AG-EtOAC), respectively. The AG-EtOAC fraction was first subjected to passage over LH-20, using 95% ethanol. The eluted solution was collected by test tubes. Only the tubes that contained major components were concentrated and followed by eluting isocratically in the self-pack C18 with 80% methanol solution to obtain BHPHTO and BDMC. The identities of 1,7-bis(4-hydroxyphenyl)-1,4,6-heptatrien-3-one (BHPHTO) and bisdemethoxycurcumin (BDMC) (Figure 1) were confirmed by comparison of their NMR and MS spectral data with those available in the literature [12].
Figure 1.
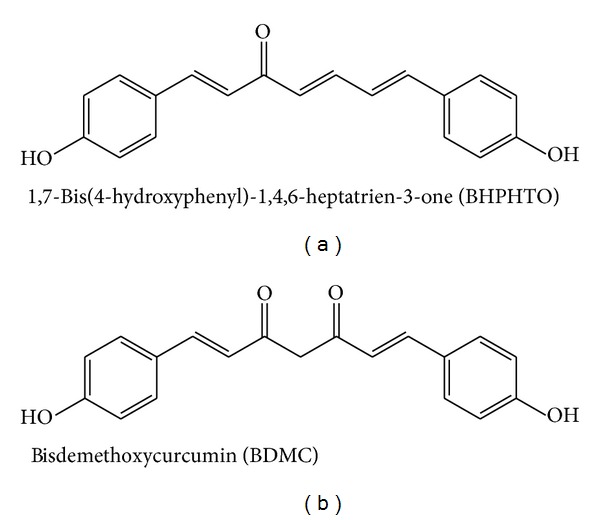
The structure of 1,7-bis(4-hydroxyphenyl)-1,4,6-heptatrien-3-one (BHPHTO) and bisdemethoxycurcumin (BDMC).
2.3. Human Melanoma A2058 Cell and Mouse B16-F10 Melanoma Cell Cultures
The human skin cancer A2058 cell lines were derived and purchased from the Bioresource Collection and Research Center (BCRC number: 60240, Hsinchu, Taiwan, ROC) the American Type Culture Collection (ATCC number: CRL-11147, Manassas, VA, USA). The human metastatic melanoma A2058 cell line is derived from 43-year-old male [13].
And the melanoma B16-F10 cells (BCRC 60031) were obtained from ATCC (Manassas, VA, USA), cultured in DMEM (13.4 mg/mL Dulbecco's modified Eagle's medium, 10 mM HEPES, 143 U/mL benzylpenicillin potassium, 100 mg/mL streptomycin sulfate, and 24 mM NaHCO3, pH 7.1) containing 10% FBS, 1% P/S, and incubated at 37°C under 5% CO2 atmosphere [14].
2.4. Cell Proliferation Assay
The effects of compounds on cell growths were according to the MTT assay procedures [15]. The method is based on the ability of a mitochondrial dehydrogenase from viable cells to cleave the tetrazolium rings of the pale yellow MTT and form impermeable crystals of a dark-blue formazan, thus resulting in accumulation within healthy cells. Briefly, cells were seeded in 96-well plates at a density of 8 × 103 cells/well. The medium was then changed, and cells were maintained in either solvent alone (control cells) or in the presence of the indicated each sample in a final volume of 100 μL in 10% FBS culture medium. Each sample was added to a microplate and incubated under the same conditions as above for 24 h. After 24 h of incubation, the medium was replaced with 100 μL of fresh medium including 0.5 mg/mL MTT. The plate was cultured in a 37°C incubator filled with 5% CO2 for 2 h. Each precipitate in a specific dish was dissolved in 100 μL of DMSO to dissolve the purple formazan crystals. After the dishes were gently shaken for 10 min in the dark to ensure maximal dissolution of formazan crystals, the absorbance (A) values of the supernatant were measured at 595 nm (UV_vis, BioTek, Winooski, VT). Cell growth was calculated as
| (1) |
2.5. Measurement of B16-F10 Cellular Tyrosinase Activity
Testing cell tyrosinase activity in vitro, B16-F10 melanoma cells (105 cells per well) were placed in 24-well plates in 300 μL of medium containing various concentrations of testing samples and incubated for 2 days [16]. The sample-treated cells were washed with phosphate-buffered saline (PBS) and lysed with 1% Triton X-100/PBS. The enzyme extract of cellular lysate was added to 50 μL of 2 mM L-tyrosine. This reaction was then incubated at 37°C for 3 h in a dark environment, and the absorbance at 490 nm was measured on a spectrophotometer.
2.6. Determination of B16-F10 Cellular Melanin Contents
Briefly, we followed the previous method with minor modifications [17]. Cell pellets were dissolved in 2.0 N NaOH containing 10% DMSO and heated at 90°C for 1 h, and suspensions were clarified by centrifugation for 10 min at 10,000 ×g. The amount of melanin was determined spectrophotometrically based on the absorbance at 475 nm [18].
2.7. Statistical Analysis
The data were expressed as the mean value obtained in three experiments. Statistical comparisons were performed by Student's t-test for paired values.
3. Results and Discussion
3.1. Cellular Proliferative Depressing Effects of BHPHTO and BDMC on Melanoma Cells
The main reason of the death of a patient is due to the tumor proliferation and metastasis [12]. Therefore, it is urgent to find valuable and significant novel biomedicinal components for anticancer therapies. MTT assay illustrated the anticell proliferation of two kinds of human skin melanoma cells, A2058 cells for 24 h treatments. In Figure 2, to evaluate the effects of several ginger (BHPHTO and BDMC) extracts on cancer cytotoxicity, cells were treated with various concentrations from 0 to 100 μM. We demonstrated the cytotoxic abilities on human skin melanoma A2058 cells of the extracts, and cellular proliferations were inhibited in dose-dependent manner when cells were exposed to a high dose of all extracts, BHPHTO and BDMC (50 μM), and cell viabilities exhibited less than 25% after 24 h of treatment.
Figure 2.
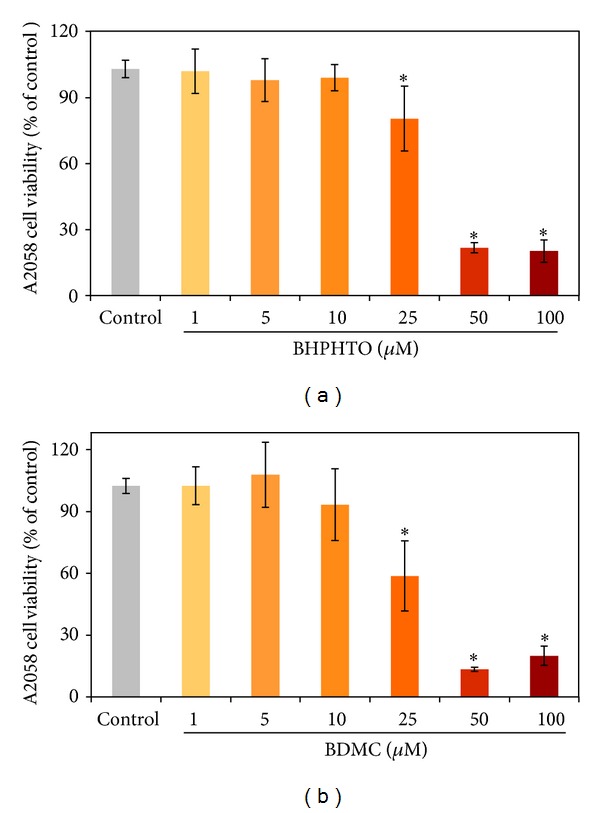
The effects of ginger extracts (BHPHTO and BDMC) on A2058 cell growths measured by MTT assay. The data represented the mean value ± SD of triplicate values for three independent experiments. Comparisons were subjected to Student's t-test. Significantly different at *P < 0.05.
3.2. Cytotoxicity of BHPHTO and BDMC in B16-F10 Cells
3-(4,5-Dimetylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay was investigated to study the cytotoxicities of BHPHTO and BDMC in B16F10 cells. The samples were treated with various concentrations, from 1 μM to 100 μM, and the vehicle control group had no testing agents with 0.5% DMSO. As shown in Figure 3. With 25 μM BHPHTO, about 55% B16-F10 cells remained, and with 25 μM BDMC, still 65% B16-F10 cells remained.
Figure 3.
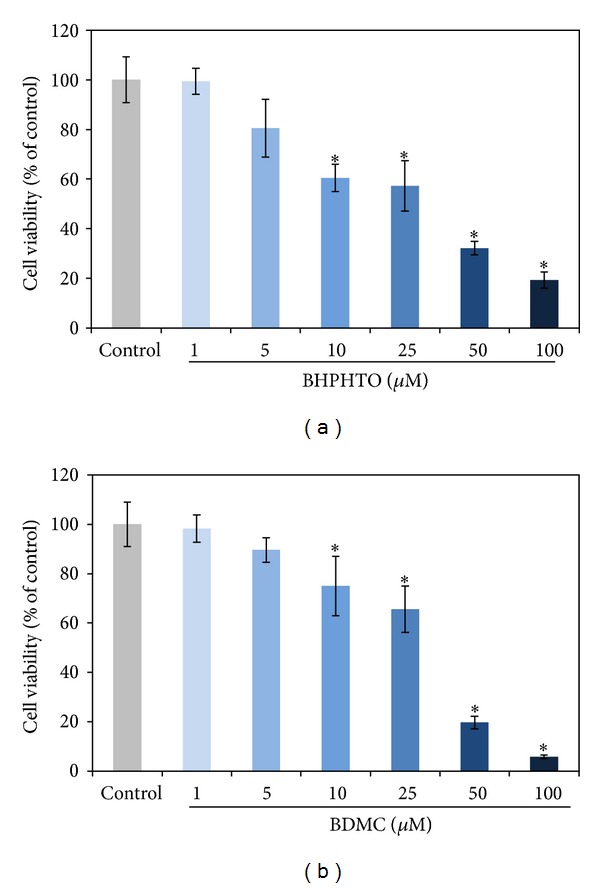
The impact of BHPHTO and BDMC to B16-F10 melanoma cell viabilities. The data represented the mean value ± SD of triplicate values for three independent experiments. Comparisons were subjected to Student's t-test. Significantly different at *P < 0.05.
3.3. B16-F10 Cellular Tyrosinase Activity and Melanin Content of BHPHTO and BDMC
Melanin is a vitally important factor in determining the skin color of human. The melanogenesis pathway consists of the enzymatic L-tyrosine hydroxylation and the oxidation of L-dopa to its corresponding dopaquinone [9]. After the two tyrosinase-catalyzed steps, additional multiple biosynthesis steps followed and yielded melanin [10]. We tried to investigate if BHPHTO and BDMC have mouse B16F10 cellular tyrosinase-inhibiting abilities (Figure 4) and melanin content (Figure 5) decreasing power [11]. Although, the tyrosinase activity of B16-F10 results showed that with 5 or 10 μM of BHPHTO and BDMC there is no obvious variation and with concentrations higher than 25 μM tyrosinase activity decreased for low cell viability. But interestingly, even the cell viability is low, the tyrosinase activity is completely high. Melanin content, however, did not show evident reduction and on the contrary, with highest concentrations of BHPHTO and BDMC at 25 μM, the melanin content decreased for low cell viability. As the tendency of tyrosinase activity, melanin content is completely higher than cell viability.
Figure 4.
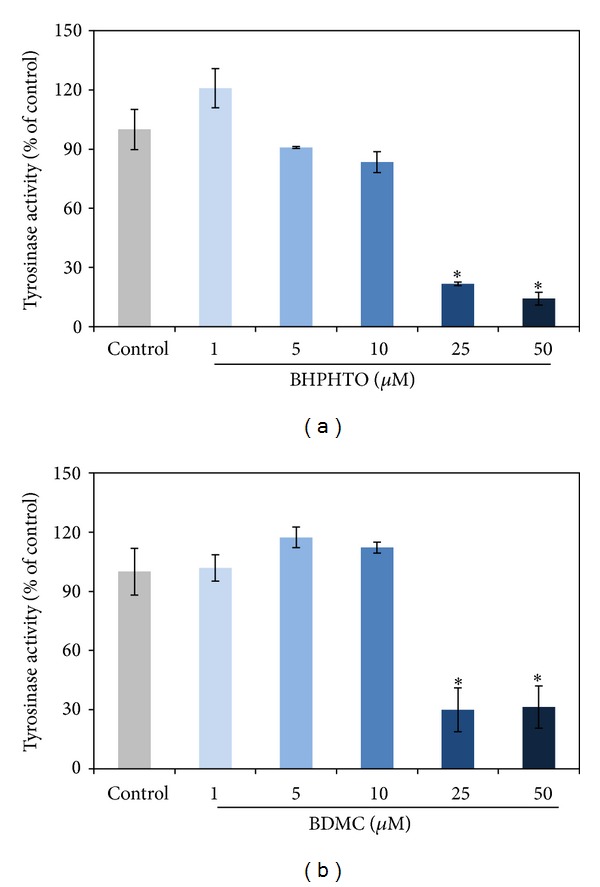
The tyrosinase activity of B16-F10 cells treated with various concentrations of BHPHTO and BDMC. The data represented the mean value ± SD of triplicate values for three independent experiments. Comparisons were subjected to Student's t-test. Significantly different at *P < 0.05.
Figure 5.
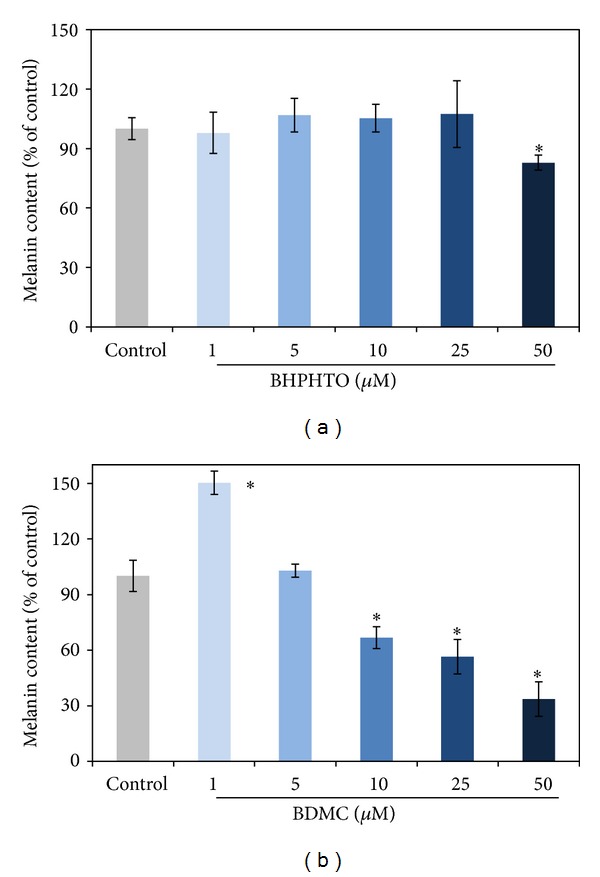
The melanin content of B16-F10 cells treated with various concentrations of BHPHTO and BDMC. The data represented the mean value ± SD of triplicate values for three independent experiments. Comparisons were subjected to Student's t-test. Significantly different at *P < 0.05.
Conflict of Interests
No contributing author has a conflict of interests in the publication of this study.
Authors' Contribution
Chih-Yu Lo and Po-Len Liu contributed equally to this work.
Acknowledgment
The work was supported by the National Science Council under the Grant nos. NSC 99-2313-B-415-006-MY3 and NSC 99-2221-E-037-006-MY3.
References
- 1.Saeio K, Chaiyana W, Okonogi S. Antityrosinase and antioxidant activities of essential oils of edible Thai plants. Drug Discoveries and Therapeutics. 2011;5(3):144–149. doi: 10.5582/ddt.2011.v5.3.144. [DOI] [PubMed] [Google Scholar]
- 2.Yusoff MM, Ibrahim H, Hamid NA. Chemical characterization and antimicrobial activity of rhizome essential oils of very closely allied zingiberaceae species endemic to borneo: Alpinia ligulata K. SCHUM. and Alpinia nieuwenhuizii VAL. Chemistry and Biodiversity. 2011;8(5):916–923. doi: 10.1002/cbdv.201000270. [DOI] [PubMed] [Google Scholar]
- 3.Padalia RC, Verma RS, Sundaresan V, Chanotiya CS. Chemical diversity in the genus Alpinia (Zingiberaceae): comparative composition of cour Alpinia species grown in Northern India. Chemistry and Biodiversity. 2010;7(8):2076–2087. doi: 10.1002/cbdv.201000013. [DOI] [PubMed] [Google Scholar]
- 4.Matsuda H, Pongpiriyadacha Y, Morikawa T, Ochi M, Yoshikawa M. Gastroprotective effects of phenylpropanoids from the rhizomes of Alpinia galanga in rats: structural requirements and mode of action. European Journal of Pharmacology. 2003;471(1):59–67. doi: 10.1016/s0014-2999(03)01785-0. [DOI] [PubMed] [Google Scholar]
- 5.Morikawa T, Ando S, Matsuda H, Kataoka S, Muraoka O, Yoshikawa M. Inhibitors of nitric oxide production from the rhizomes of Alpinia galanga: structures of new 8-9′ linked neolignans and sesquineolignan. Chemical and Pharmaceutical Bulletin. 2005;53(6):625–630. doi: 10.1248/cpb.53.625. [DOI] [PubMed] [Google Scholar]
- 6.Noro T, Sekiya T, Katoh M, et al. Inhibitors of xanthine oxidase from Alpinia galanga . Chemical and Pharmaceutical Bulletin. 1988;36(1):244–248. [Google Scholar]
- 7.Wang H-M, Chen C-Y, Wen Z-H. Identifying melanogenesis inhibitors from Cinnamomum subavenium with in vitro and in vivo screening systems by targeting the human tyrosinase. Experimental Dermatology. 2011;20(3):242–248. doi: 10.1111/j.1600-0625.2010.01161.x. [DOI] [PubMed] [Google Scholar]
- 8.Briganti S, Picardo M. Antioxidant activity, lipid peroxidation and skin diseases: what’s new. Journal of the European Academy of Dermatology and Venereology. 2003;17(6):663–669. doi: 10.1046/j.1468-3083.2003.00751.x. [DOI] [PubMed] [Google Scholar]
- 9.Liao WT, Huang TS, Chiu CC, et al. Biological properties of acidic cosmetic water from seawater. International Journal of Molecular Sciences. 2012;13(5):5952–5971. doi: 10.3390/ijms13055952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiu C-C, Chou H-L, Wu P-F, Chen H-L, Wang H-M, Chen C-Y. Bio-functional constituents from the stems of Liriodendron tulipifera . Molecules. 2012;17(4):4357–4372. doi: 10.3390/molecules17044357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolbe L, Mann T, Gerwat W, et al. 4-n-butylresorcinol, a highly effective tyrosinase inhibitor for the topical treatment of hyperpigmentation. Journal of the European Academy of Dermatology and Venereology. 2013;27(supplement 1):19–23. doi: 10.1111/jdv.12051. [DOI] [PubMed] [Google Scholar]
- 12.Mohamad H, Lajis NH, Abas F, et al. Antioxidative constituents of Etlingera elatior. Journal of Natural Products. 2005;68(2):285–288. doi: 10.1021/np040098l. [DOI] [PubMed] [Google Scholar]
- 13.Wang H-M, Chiu C-C, Wu P-F, Chen C-Y. Subamolide E from Cinnamomum subavenium induces sub-G1 cell-cycle arrest and caspase-dependent apoptosis and reduces the migration ability of human melanoma cells. Journal of Agricultural and Food Chemistry. 2011;59(15):8187–8192. doi: 10.1021/jf2018929. [DOI] [PubMed] [Google Scholar]
- 14.Wang H-M, Chen C-Y, Chen C-Y, et al. (-)-N-Formylanonaine from Michelia alba as a human tyrosinase inhibitor and antioxidant. Bioorganic and Medicinal Chemistry. 2010;18(14):5241–5247. doi: 10.1016/j.bmc.2010.05.045. [DOI] [PubMed] [Google Scholar]
- 15.Chen B-H, Chang H-W, Huang H-M, et al. (-)-Anonaine induces DNA damage and inhibits growth and migration of human lung carcinoma H1299 cells. Journal of Agricultural and Food Chemistry. 2011;59(6):2284–2290. doi: 10.1021/jf103488j. [DOI] [PubMed] [Google Scholar]
- 16.Wang H-M, Chou Y-T, Hong Z-L, et al. Bioconstituents from stems of Synsepalum dulcificum Daniell (Sapotaceae) inhibit human melanoma proliferation, reduce mushroom tyrosinase activity and have antioxidant properties. Journal of the Taiwan Institute of Chemical Engineers. 2011;42(2):204–211. [Google Scholar]
- 17.Lin Y-P, Hsu F-L, Chen C-S, Chern J-W, Lee M-H. Constituents from the Formosan apple reduce tyrosinase activity in human epidermal melanocytes. Phytochemistry. 2007;68(8):1189–1199. doi: 10.1016/j.phytochem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Jang JY, Lee JH, Kang BW, Chung KT, Chol YH, Choi BT. Dichloromethane fraction of Cimicifuga heracleifolia decreases the level of melanin synthesis by activating the ERK or AKT signaling pathway in B16F10 cells. Experimental Dermatology. 2009;18(3):232–237. doi: 10.1111/j.1600-0625.2008.00794.x. [DOI] [PubMed] [Google Scholar]


