Abstract
The antioxidant activities based on the free radical scavenging, reducing power, and bleaching inhibition were investigated for the three commonly used honeys in Malaysia, namely, tualang, gelam, and acacia honey. The antioxidant capacity of the honey samples was correlated with their biochemical constituents such as total phenol, total flavonoid content, and total water-soluble vitamins (vitamin B1, B2, B3, B9, B12, and vitamin C). The total flavonoid content of honey samples was strongly correlated with the three antioxidative processes (r = 0.9276–0.9910). In contrast, the total water-soluble vitamins was found to be well correlated with the free radical scavenging activity (r = 0.8226). Vitamin B3 was likely to be in the highest concentration, which covered for 69–80% of the total vitamin content. A number of five phenolic acids, three flavonoids, and two organic acids had also been detected from the honey samples using UPLC-MS/MS, without sugar-removal procedure.
1. Introduction
Honey is well known as a natural dietary antioxidant. The components responsible for the redox properties of honey are likely to be phenolic acids, flavonoids, vitamins, and enzymes, as well as a small amount of mineral content, particularly copper and iron [1, 2]. However, little is known about the antioxidant capacity and the mechanism involved by each biochemical component either through reducing power or radical scavenging activity of honey from tropical countries. It might also be attributed to the combined activity of these minor components through synergistic effects [3, 4].
Numerous studies have reported that most chronic diseases such as cancer, coronary, and neurological degeneration are a consequence of oxidative damage. It is also proven that the therapeutic potential of honey is always associated with antioxidant capacity against reactive oxygen species [5]. Therefore, in recent years, studies have been focused on the composition of honeys and their biological properties such as antioxidant [6], anti-inflammatory [7], and antimicrobial activities [8] in wound healing [9], as well as in the treatment of skin ulcers [10] and gastrointestinal disorders [11].
To our knowledge, there is no official method available for the determination of antioxidant activity in honey samples [12]. The commonly used antioxidant assays include DPPH (free radical scavenging activity), FRAP (ferric reducing/antioxidant power), β-carotene bleaching assay, ORAC (oxygen radical absorbance capacity), ascorbic acid antioxidant content (AEAC), and Trolox equivalent antioxidant activity (TEAC). Each assay has its advantages and disadvantages. DPPH free radicals are reported to be unaffected by certain side reactions such as metal ion chelation and enzyme inhibition [13]. Furthermore, honey contains abundant free radical scavengers, which are able to reduce the imbalance between free radical production and antioxidant level [14]. The high amount of reducing sugars (>65%) such as glucose and fructose in honey could contribute to higher reducing antioxidant power in the FRAP method, which would lead to positive error in the determination of antioxidant activity [5].
Polyphenols are currently of particular interest to medical and food nutrition research, mainly because of their functional properties. Besides being radical scavenger, polyphenol could be an effective immune modulator and hormone action inhibitor [15]. Polyphenols are believed to be potent scavengers of peroxyl radicals, mainly because of the presence of high mobility of hydrogens in their molecular structures [6]. Of the polyphenols, phenolic acids are likely to be the major group in honey. They have also been reported to affect the flavor [16] and physical appearance of honey, particularly honey colour [17, 18]. Interestingly, they have been given considerable attention to be an eligible parameter for honey quality assessment [6], as well as for honey marker identification, with the help of the advancement of liquid chromatography and mass spectrometry technology nowadays.
Because of the great variation in honey composition, the biological activities exhibited by the honey samples varied according to the geographical and botanical origin of honey, while processing and storage condition might affect the biological activities only to a minor degree [3]. Therefore, this study has investigated the comparative antioxidant property of the three commonly used honeys in Malaysia in relation with their biochemical components. Since honey is an aqoueus-based foodstuff, only water-soluble vitamins were determined in this study.
2. Experimental
2.1. Chemicals and Reagents
Formic acid, acetic acid, phosphoric acid, sodium carbonate, iron (III) chloride, USA and aluminium chloride were obtained from Fisher Scientific (Pittsburgh, PA, USA). Coomassie Brilliant Blue G-250 (CBB), butylated hydroxytoluene, and HPLC-grade of methanol (MeOH), hexane, acetone, chloroform and acetonitrile (CH3CN) were obtained from Merck (Darmstadt, Germany). 18.2 MΩ·cm water was produced from a Barnstead NANOpure Diamond water purification system (Thermo, Waltham, MA, USA). Folin-Ciocalteau reagent, 1,1-diphenyl-2-picrylhydrazyl (DPPH), vitamin B1 (≥99%), vitamin B2 (≥98%), vitamin B3 (≥99.5%), vitamin B9 (≥97%), vitamin B12 (≥98.5%), vitamin C (≥99%), β-carotene (≥93%), linoleic acid (≥99%), Trolox (97%), Tween 20, and 2,4,6-tripyridyl-s-triazine (≥98%) were sourced from Sigma-Aldrich (St. Louis, MO, USA). The standard gallic acid (98%) and rutin (97%) were obtained from Acros Organics (Pittsburgh, PA, USA). Bradford reagent and bovine serum albumin (BSA) standard were obtained from Bio-Rad Laboratories, Hercules, USA. All reagents were of analytical grade unless otherwise stated.
2.2. Honey Samples
The three most commonly used honeys in Malaysia, tualang, gelam, and acacia honey, were harvested by local suppliers between April and June 2011. The results were expressed as mean of the data collected in triplicate from different months for each honey. Tualang and gelam honeys were obtained from Federal Agriculture Marketing Authority (FAMA), Kedah, whereas acacia honey was supplied by MB An-Nur Apiary, Johor.
2.3. Total Phenolic Content
The total phenolic content (TPC) of honey samples was analysed by using Folin-Ciocalteu reagent, based on the method described by Singleton et al. (1998) [19] with some modification. Honey solution (0.5 mL) was mixed with 2.5 mL of Folin-Ciocalteau reagent (2N) and incubated for 5 min. Subsequently, 2 mL of sodium carbonate solution (75 g/L) was added into the honey solution and incubated for another 2 h at 25°C. After incubation, the absorbance of the solution was measured at 765 nm by using a UV-Visible spectrophotometer (Perkin-Elmer Lambda 25, Waltham, MA, USA). Gallic acid (0–1000 mg/L) was used as a standard chemical for calibration curve preparation. The TPC was reported as mean value of triplicate assays and expressed as milligram of gallic acid equivalent (GAE) in gram of honey.
2.4. Total Flavonoid Content
The total flavonoid content (TFC) of honey samples was determined based on the method of Isla et al. (2011) [18], with some modification. A 5 mL of honey solution (0.1 g/mL) was mixed with 5 mL of 2% aluminium chloride (AlCl3). A flavonoid-aluminium complex was formed after 10 min of incubation time at 25°C. The formation of the complex was measured at 415 nm by using an UV-Visible spectrophotometer. Rutin (0–100 mg/L) was used as a standard chemical for calibration curve preparation. The TFC was reported as mean value of triplicate assays and expressed as milligram of rutin equivalent (RE) in gram of honey.
2.5. Free Radical Scavenging Activity
The free radical scavenging activity of honey samples was determined by the 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay as described by Isla et al. (2011) [18], with minor modification. The DPPH∙ solution (20 mg/L) was prepared by dissolving 2 mg of DPPH∙ in methanol (100 mL). A 0.75 mL of methanolic honey solution at different concentrations, ranging from 20 to 40 mg/mL, was added to 1.5 mL of DPPH∙ solution. The absorbance was measured at 517 nm after 15 min of incubation at 25°C. Ascorbic acid was used as positive control. The ability to scavenge the DPPH∙ was calculated using (1), where A control and A sample are the absorbances of control and sample, respectively. The concentration of honey sample required to scavenge 50% of DPPH∙ (EC50) was determined based on the ascorbic acid calibration curve (0–10 mg/L) [20]. The experiment was performed in triplicate:
| (1) |
2.6. Ferric Reducing/Antioxidant Power Assay
The reducing power of honey samples was determined based on the method described by Benzie and Strain (1996) [21] with minor modification. The principle of this method is based on the reduction of a ferric 2,4,6-tripyridyl-s-triazine complex (Fe3+-TPTZ) to its ferrous, coloured form (Fe2+-TPTZ) in the presence of antioxidants. The FRAP reagent was prepared by mixing 2.5 mL of a 10 mM TPTZ (2,4,6-tripyridyl-s-triazine) solution in 40 mM HCl, 2.5 mL of 20 mM FeCl3, and 25 mL of 0.3 M acetate buffer at the pH of 3.6. It was prepared daily and was warmed to 37°C before use. Honey (1 g) was well dissolved in 10 mL of n-hexane-acetone mixture (6 : 4), and the honey solution was filtered through Whatman number 4 filter paper. An aliquot of 200 μL of honey solution was mixed with 1.8 mL of FRAP reagent, and the absorbance of the reagent mixture was measured spectrophotometrically at 593 nm after incubation for 10 min. Trolox was used for the calibration curve, and the results were expressed as milligram of Trolox equivalent (TE) per 100 gram of honey.
2.7. β-Carotene Bleaching Assay
The antioxidant activity of honey was evaluated based on the β-carotene linoleate model system as described by Ferreira et al. (2009) [5] with modification. 2 mL of β-carotene (0.2 g/L) in chloroform, 0.02 mL of linoleic acid, and 0.2 mL of Tween 20 were transferred into a 100 mL round bottom flask. A 0.2 mL honey solution was added into the mixture. After evaporation to dryness under vacuum at room temperature, 50 mL of distilled water was added into the flask. The mixture was agitated vigorously to form an emulsion. A 2 mL aliquot of the emulsion was transferred to another test tube and immediately placed in water bath at 50°C. The absorbance of the sample was measured every 20 minutes for 2 hours at 470 nm using a UV-Visible spectrophotometer. Butylated hydroxytoluene (200 mg/mL) was used as standard chemical for the construction of calibration curve. The results were expressed as mean of triplicate assay. The β-carotene bleaching activity (CBI) was calculated as inhibition relative to control using (2) where B control and B sample were the bleaching rates of β-carotene in control and sample, respectively [22]:
| (2) |
2.8. Water-Soluble Vitamins Analysis
Water-soluble vitamins in honey samples were determined by using the method reported by Ciulu et al. (2011) [23], with some minor modification. A total of six water-soluble vitamins such as vitamin B1 (thiamin), B2 (riboflavin), B3 (niacinamide), B9 (folic acid), B12 (cobalamin), and vitamin C (ascorbic acid) were quantified using a reversed phase HPLC (Waters 600, MA, USA) connected with a PDA detector (Waters 996, MA, USA). The stock solution of the vitamin standard mixture was prepared by dissolving 2.5 mg of vitamin B1, B2, B9, B12 and 20 mg of vitamin C into 50 mL of phosphate buffer (1 M, pH 5.5) and 4 mL of sodium hydroxide (2 mol/L) in a 100 mL volumetric flask. Only the stock solution of standard vitamin B3 (5 mg) was individually prepared. The stock solutions were then topped up to the mark with ultrapure water and kept at 4°C. A serial concentration of the standard solutions (0–100 mg/L) was prepared freshly for calibration curve construction.
A 2 g of homogenised honey sample was weighted and dissolved in 2 mL of ultrapure water. Then, 0.2 mL of sodium hydroxide and 2.5 mL of phosphate buffer were added and topped up to 5 mL. The honey solution was filtered with nylon filter (0.45 μm, 13 mm) before injection. A Synergy C18 column (5 μm, 250 × 4.6 mm) from Phenomenex (CA, USA) was used for the separation with the mobile phases of trifluoroacetic acid (0.025% v/v) aqueous solution as solution A and acetonitrile (100% v/v) as solution B at a constant flow rate of 1.0 mL/min. The gradient of the mobile phases was as follow: 100–75% A in 11 min at 254 nm; 75–55% A in 8 min at 210 nm; 55–100% A in 3 min at 210 nm, and then equilibrate the column in 100% A for 5 min. The total run time was 27 min with the flow rate 1 mL/min. Sample injection volume was 10 μL.
2.9. Polyphenols Determination by UPLC-MS/MS
An ultraperformance liquid chromatography (UPLC), Waters Acquity (Milford, MA, USA), system was coupled with a triple-quadrupole-linear ion trap tandem mass spectrometer (Applied Biosystems 4000 Q TRAP; Life Technologies Corporation, Carlsbad, CA, USA) with an electrospray ionisation (ESI) source. A C18 reversed phase Acquity column (1.7 μm, 150 mm × 4.6 mm) protected by a guard column was used throughout this study.
The mobile phase was a binary solvent system consisting of solvent A (water with 0.1% formic acid) and solvent B (CH3CN). The UPLC gradient for mass screening was 0–5 min, 90% A; 5–15 min, 90–10% A; 15–20 min, 10% A; 20–25 min, 10–90% A; 25–30 min, 90% A for final washing and equilibration of the column for the next run. The UPLC gradient for the detection of target mass was similar to mass screening profile, except that the total run time was shortened to 15 min. The flow rate was 0.25 mL/min, and the injection volume was 5 μL. All samples were filtered with 0.2 μm nylon membrane filter prior to injection.
The mass spectra were acquired from m/z 100–1000 with a 20 ms ion accumulation time. All mass spectrometric data were acquired in positive ionisation mode. The capillary and voltage of the ESI source were maintained at 400°C and −4.5 kV, respectively. All other parameters were as follows: nitrogen was used as ion source gas for nebulisation, 40 psi; for drying solvent, 40 psi; curtain gas, 10 psi; collision gas, high; declustering potential, −40 V; and collision exit energy, −10 V. The scan rate was 1000 amu/s. Data acquisition and data processing were performed using Analyst 1.4.2. MS Fragmenter 12.0 (Advanced Chemistry Development, Toronto, Canada) was used to predict compound fragmentation.
3. Results and Discussion
3.1. TPC and TFC
TPC is considered as a fast and simple method to measure the total phenol in complex matrix like honey. Al et al. (2009) [24] reported that this method was sensitive enough for total phenol estimation in honey samples. In the present study, the TPC of honey samples was in the narrow range from 110.394 to 196.500 mg GAE/100 g honey (Table 1). The TPC results were higher than commercial Indian honeys (47–98 mg GAE/100 g honey) [25] and Argentinean northwest honeys (18.730–107.213 mg GAE/100 g honey) [18], as well as Burkina Fasan honeys (32.59–114.75 mg GAE/100 g honey) [2].
Table 1.
Total phenols and total flavonoids in honey samples.
| Honey | aTPC (mg GAE /100 g honey) | bTFC (mg RE /100 g honey) | TFC/TPC | ||
|---|---|---|---|---|---|
| Mean | cSD | Mean | cSD | ||
| Tualang | 110.394 | 3.829 | 18.511 | 2.803 | 0.168 |
| Gelam | 159.743 | 9.004 | 32.886 | 0.780 | 0.206 |
| Acacia | 196.500 | 6.323 | 30.741 | 2.886 | 0.156 |
aTotal phenolic content in milligram of gallic acid equivalent per 100 gram of honey.
bTotal flavonoid content in milligram of rutin equivalent per 100 gram of honey.
cStandard deviation.
Total phenol was also compared to the TPC value reported for the similar type of honey from different countries. It was found that acacia honey from Malaysia showed higher TPC value (196.500 mg GAE/100 g honey) than acacia honeys from Germany (4.6 mg/100 g honey) [3], from Romania (2.00–39.00 mg GAE/100 g honey) [24], from Burkina Faso (93.43 mg GAE/100 g honey) [2], and from Slovenia (4.48 mg GAE/100 g honey) [12].
To the best of our knowledge, there are two widely used spectrophotometric methods to determine total flavonoid in honey samples. Both methods measure the formation of coloured complex substances quantitatively, after reacting flavonoids with aluminium ion (III) or 2,4-dinitrophenylhydrazine (DNP). Since DNP method has been reported as less reliable [26], the TFC of honey samples was determined based on the method of aluminium chloride (AlCl3), which was specific for flavones and flavonols [17]. Furthermore, Iurlina et al. (2009) [27] reported that the predominant flavonoid in honey samples was from the group of flavonols (45% quercetin).
The TFC assay found that the honey samples in this study exhibited higher TFC values (18.511–32.866 mgRE/100 g honey) than those values reported for Portuguese heater honey (0.06–0.50 mg/100 g honey) and Spanish rosemary honey (0.50–2.00 mg/100 g honey) [28]. TFC of this study was approximately covered for 15–20% of the total phenols (Table 1).
3.2. Water-Soluble Vitamins in Honey
In addition to polyphenols, the antioxidant activity of honey might be also attributed to the presence of several water-soluble vitamins such as vitamin B1 (thiamine), B2 (riboflavin), B3 (nicotinic acid), B9 (folic acid), B12 (cyanocobalamin), and vitamin C as presented in Table 2. The RP-HPLC method proved the presence of these vitamins in the honey samples with vitamin B3 as the highest content, approximately covered for 62–80% of the total water-soluble vitamin content. The validation data, namely, sensitivity in terms of limit of detection (LOD) and limit of quantification (LOQ), and linearity within the range of 0–250 mg/kg with correlation coefficients (r 2) more than 0.998 were determined (Table 2). Significantly, the vitamin content (B2, B3, B9, and C) in acacia honey was higher than the results reported for Italian acacia honey by Ciulu et al. (2011) [23]. The vitamin C content in all honey samples was also higher than the usual level of vitamin C concentration, 50 mg/kg honey [29]. Somehow, vitamins in honey are sensitive to processing and storage. Gheldof et al. (2002) [3] reported that the loss of vitamin C in the selected commercial honey most likely was due to the processing and storage.
Table 2.
Water-soluble vitamins in honey samples.
| Water-soluble vitamins | aRt | Sensitivity (mg/kg) | Linearity (mg/kg) | Tualang | Gelam | Acacia | ||
|---|---|---|---|---|---|---|---|---|
| (min) | bLOD | cLOQ | Concentration | d R 2 | Mean ± eSD (mg/kg honey) | |||
| Thiamine (vitamin B1) | 3.10 | 3.51 | 10.62 | 0–75 | 0.999 | <LOQ | 13.85 ± 0.24 | <LOQ |
| Riboflavin (vitamin B2) | 14.69 | 1.80 | 5.44 | 0–75 | 0.999 | <LOD | <LOQ | 11.85 ± 3.90 |
| Nicotinic acid (vitamin B3) | 1.83 | 4.76 | 14.42 | 0–250 | 0.999 | 170.38 ± 9.60 | 355.38 ± 56.11 | 134.67 ± 3.53 |
| Folic acid (vitamin B9) | 13.74 | 4.35 | 13.17 | 0–75 | 0.998 | <LOQ | <LOD | <LOD |
| Cyanocobalamin (vitamin B12) | 14.03 | 1.02 | 3.10 | 0–75 | 0.999 | <LOD | <LOQ | <LOD |
| Ascorbic acid (vitamin C) | 3.95 | 10.13 | 30.71 | 0–200 | 1.000 | 52.20 ± 3.29 | 67.36 ± 10.48 | 62.80 ± 8.60 |
aRetention time in minute.
bLimit of detection (3.3 standard deviation/slope of the calibration curve).
cLimit of quantitation (10 standard deviation/slope of the calibration curve).
dCorrelation coefficient.
eStandard deviation.
3.3. Polyphenols and Antioxidant Activity of Honey
A number of five phenolic acids had been detected from the honey samples by using multiple reaction monitoring (MRM) and enhanced product ion (EPI) scan modes of UPLC-MS/MS method (Table 3). The phenolic acids were detected at the positive mode of mass screening and then confirmed by enhanced product ion scan. Besides phenolic acids, the other detected flavonoids include flavone (quercetin) and flavanone (pinobanksin-3-O-butyrate and pinobanksin-3-O-propionate). It is important to highlight that pinobanksin has been demonstrated to be a potent antioxidant [30]. This hybrid method offers obvious advantages, in terms of sensitivity and selectivity, without the need for standard chemical, sugar separation, and clean-up procedures. The compounds were identified based on the characteristic ions as structural information from the fragmentation pattern. The positive mode of mass spectra for the detected phenolic acids is presented in Figure 1.
Table 3.
Phenolic acids, flavonoids, and organic acids detected from honey samples.
| Compound | aRt (min) | [M + H]+ | bHoney | Fragment ions |
|---|---|---|---|---|
| Phenolic acids | ||||
| Coumaric acid | 1.39 | 165 | T, G, A | 165/147(–H2O)/137(–CO)/123(–CO–CH2)/119(–H2O–CO)/109/95/91/78 |
| Caffeic acid | 1.40 | 181 | T, G, A | 181/163(–H2O)/153(–CO)/139(–CO–CH2)/125(–CO–2CH2)/111(–CO–3CH2)/99/79 |
| Ferulic acid | 1.50 | 195 | T, G, A | 195/177(–H2O)/167(–CO)/149(–H2O–CO)/135(–CO–CH3OH)/107(–2CO–CH3OH)/95 |
| Cinnamic acid | 1.51 | 149 | T, G, A | 149/131(–H2O)/121(–CO)/112/87 |
| Chlorogenic acid | 8.09 | 355 | T, G, A | 355/338(–OH)/337(–H2O)/313(–CO–CH2)/272/245(–4H2O–C3H2)/149 |
| Flavonoids | ||||
| Pinobanksin-3-O-propionate | 7.20 | 329 | T, G | 329/311(–H2O)/293(–2H2O)/273/265(–CO)/245/179/167 |
| Pinobanksin-3-O-butyrate | 7.47 | 343 | T | 343/325(–H2O)/301/273/240 |
| Quercetin | 8.98 | 303 | T, G, A | 303/285(–H2O)/261(–C2H2O)/220/180(–1,2A)/152(–1,3A)/114/96 |
| Organic acids | ||||
| Fumaric acid | 0.90 | 117 | T, G, A | 117/116(–H)/99(–H2O)/89(–CO)/85/75/73(–CO2) |
| Gluconic acid | 1.10 | 197 | T, G, A | 197/179(–H2O)/161(–2H2O)/151(–H2O–CO)/136(–H2O–CO–CH3)/119(–H2O–CO–CH3–OH)/105/95 |
aRetention time in minute.
bT: tualang; G: gelam; A: acacia.
Figure 1.
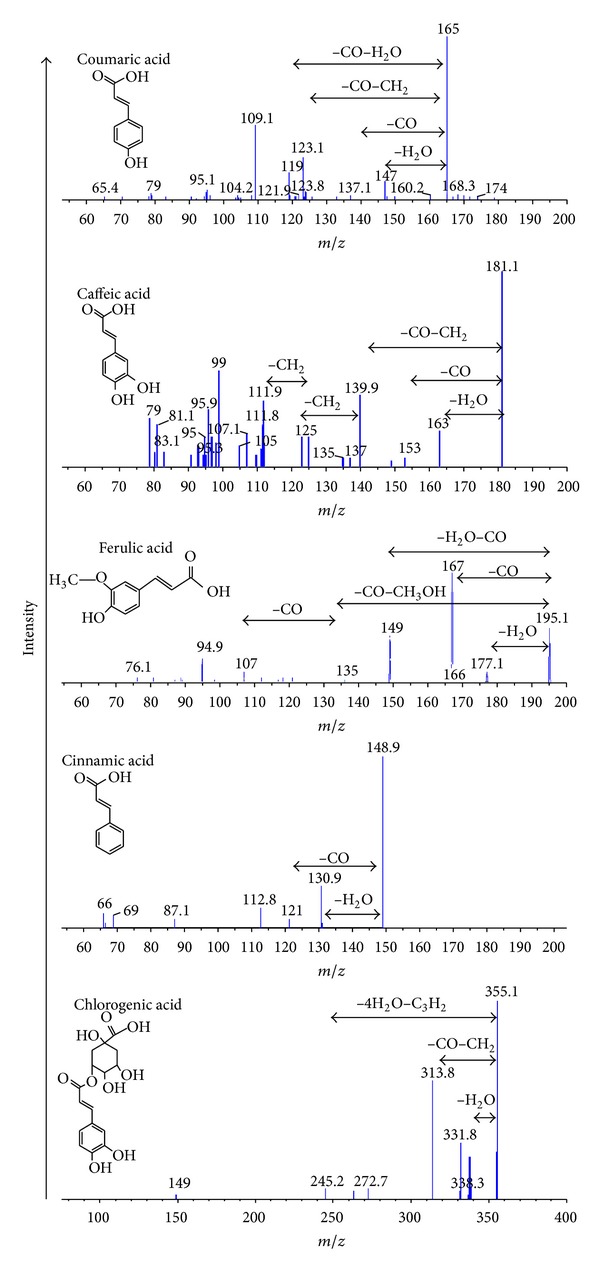
Mass spectra of phenolic acids at the positive mode of enhanced product ion scan.
Another two commonly found organic acids in honey such as fumaric and gluconic acids were also detected in this study. Even though organic acids are just covered for less than 0.5% of honey composition, the presence of organic acids might affect the honey flavor and stability against microorganisms. These organic acids might also contribute to the antioxidant capacity of honey by chelating with metals, thus synergistically enhancing the antioxidative action of phenolics [31]. According to Cherchi et al. (1994) [32], gluconic acid is the predominant organic acid in honey which could achieve up to 50-fold higher concentration than other acids.
The scavenging activity of honey samples had been measured by using DPPH assay, and ascorbic acid was used as positive control. The unpaired electron of DPPH forms a pair with a hydrogen donated by free radical scavenging antioxidant from honey and thus converting the purple coloured odd electron DPPH to its reduced form in yellow. The degree of decolourization would be measured by UV-Visible spectrophotometer in order to determine the scavenging activity of honey. It was found that the EC50 of ascorbic acid was about a few thousands lower than all honey samples. The lower the EC50 value the higher the scavenging capacity of honey, because it requires lesser amount of radical scavenger from the honey to reduce DPPH. Table 4 indicates that gelam honey has the highest scavenging activity, followed by acacia and tualang honey samples.
Table 4.
Antioxidant activity of honey samples.
| Honey |
aDPPH (mg AAE/100 g honey) |
bEC50 |
cFRAP (mg TE/100 g honey) |
dCBI (%) | Kinetic equation of CBI | r 2 (Kinetic equation) | ||
|---|---|---|---|---|---|---|---|---|
| Mean | eSD | (mg/mL) | Mean | eSD | ||||
| Tualang | 9.650 | 0.570 | 48.896 | 52.386 | 5.192 | 35.81 | y = −8.94 × 10−5 x + 1.31 × 10−2 | 0.868 |
| Gelam | 50.169 | 5.541 | 15.681 | 82.529 | 5.032 | 67.41 | y = −4.54 × 10−5 x + 1.23 × 10−2 | 0.858 |
| Acacia | 29.983 | 6.064 | 29.846 | 82.386 | 5.930 | 74.66 | y = −3.53 × 10−5 x + 8.20 × 10−3 | 0.812 |
a1,1-diphenyl-2-picrylhydrazyl scavenging activity in milligram of ascorbic acid equivalent in a 100 g of honey.
b50% scavenging activity of ascorbic acid was 8.611 µg/mL.
cFerric reducing/antioxidant power in milligram of Trolox equivalent in a 100 g of honey.
d β-carotene bleaching inhibition of butylated hydroxytoluene was 81.54%.
eStandard deviation of triplicate assay.
The findings were in contradiction with the previous study conducted by Kishore et al. (2011) [14]. They reported that tualang honey (EC50 = 5.8 mg/mL) had the highest scavenging activity compared to other honey samples, including gelam honey (EC50 = 6.68 mg/mL). However, the scavenging activity of gelam honey was found to be higher than tualang honey in this study. Hence, gelam honey was about three times more efficient than tualang honey as free radical scavenger. The contradiction was also observed in the results of TPC and TFC, even though the honey samples were collected from the similar supplier, FAMA, Kedah in the study of Kishore et al. (2011) [14]. Most probably, the variance was contributed by the difference in honey harvesting time.
In term of reducing power, tualang honey has also expressed the lowest content of reductant against oxidative damage compared to gelam and acacia honey (Table 4). The antioxidant capacity of tualang honey based on the inhibition of β-carotene bleaching activity had also the lowest value (35.81%) as exhibited in the assay of DPPH and FRAP. This bleaching assay describes the ability of antioxidants in honey to reduce the chromophore degradation of β-carotene throughout the duration of the assay [20]. However, the bleaching inhibition of acacia honey (74.66%) was higher than gelam honey (67.41%). It means that gelam honey showed higher antioxidant activity in term of radical scavenging and ferric-reducing mechanism than acacia honey but lower in term of β-carotene bleaching activity.
3.4. Correlation between Antioxidant Activity and Its Biochemical Component
Statistical tool can be considered as a useful complimentary approach to investigate the relationship between the antioxidant activities of honey and its biochemical composition. From the table of correlation matrix (Table 5), TPC was strongly correlated with FRAP and β-carotene bleaching activity, but not for DPPH assay. However, TFC was more likely well correlated with all the three antioxidant assays with different mechanisms. In contrast, the total water-soluble vitamin content of honey samples in this study was only correlated with the scavenging activity. The water-soluble vitamins in honey might act as radical scavenger to minimize oxidation process. The scavenging activity of the water-soluble vitamins was likely to be higher than phenolic compounds but lower than flavonoids in the honey samples.
Table 5.
Correlation matrix between antioxidant activity and biochemical component.
| aTPC | bTFC | cTWSV | dDPPH | eFRAP | fCBI | |
|---|---|---|---|---|---|---|
| aTPC | 1 | |||||
| bTFC | 0.8375* | 1 | ||||
| cTWSV | 0.0052* | 0.5508 | 1 | |||
| dDPPH | 0.5728* | 0.9276 | 0.8226 | 1 | ||
| eFRAP | 0.9033 | 0.9910* | 0.4338* | 0.8691 | 1 | |
| fCBI | 0.9656* | 0.9508 | 0.2649 | 0.7662 | 0.9838 | 1 |
*Significant difference at P < 0.05 (two-tail t-test).
aTotal phenolic content.
bTotal flavonoid content.
cTotal water-soluble vitamins.
d1,1-diphenyl-2-picrylhydrazyl scavenging activity.
eFerric-reducing antioxidant power.
f β-carotene bleaching inhibition.
4. Conclusions
The total flavonoid content of honey samples was strongly correlated with the antioxidant activities such as DPPH free radical scavenging activity (r = 0.9276), ferric reducing antioxidant power (r = 0.9910), and β-carotene bleaching inhibition (r = 0.9508). However, the total phenol was only strongly correlated with ferric-reducing antioxidant power (r = 0.9033) and β-carotene bleaching inhibition (r = 0.9656). In contradiction with the correlation of total phenol, the total water-soluble vitamins was found to be well correlated with the free radical scavenging activity (r = 0.8226). From the fast screening method using UPLC-MS/MS, a few phenolic acids were detected from honey samples, in addition to flavone (quercetin) and flavanone (pinobanksin-3-O-butyrate and pinobanksin-3-O-propionate), as well as organic acids. Besides vitamins, these compounds were most probably the key constituents contributing to the antioxidant capacity of honey samples.
References
- 1.Erlund I. Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutrition Research. 2004;24(10):851–874. [Google Scholar]
- 2.Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chemistry. 2005;91(3):571–577. [Google Scholar]
- 3.Gheldof N, Wang X, Engeseth NJ. Identification and quantification of antioxidant components of honeys from various floral sources. Journal of Agricultural and Food Chemistry. 2002;50(21):5870–5877. doi: 10.1021/jf0256135. [DOI] [PubMed] [Google Scholar]
- 4.Erejuwa OO, Sulaiman SA, Wahab MS. Honey: a novel antioxidant. Molecules. 2012;17(4):4400–4423. doi: 10.3390/molecules17044400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferreira ICFR, Aires E, Barreira JCM, Estevinho LM. Antioxidant activity of Portuguese honey samples: different contributions of the entire honey and phenolic extract. Food Chemistry. 2009;114(4):1438–1443. [Google Scholar]
- 6.Al-Mamary M, Al-Meeri A, Al-Habori M. Antioxidant activities and total phenolics of different types of honey. Nutrition Research. 2002;22(9):1041–1047. [Google Scholar]
- 7.Tonks AJ, Cooper RA, Jones KP, Blair S, Parton J, Tonks A. Honey stimulates inflammatory cytokine production from monocytes. Cytokine. 2003;21(5):242–247. doi: 10.1016/s1043-4666(03)00092-9. [DOI] [PubMed] [Google Scholar]
- 8.Brudzynski K, Kim L. Storage-induced chemical changes in active components of honey de-regulate its antibacterial activity. Food Chemistry. 2011;126(3):1155–1163. [Google Scholar]
- 9.Nasir NM, Halim AS, Singh KB, Dorai AA, Haneef MM. Antibacterial properties of tualang honey and its effect in burn wound management: a comparative study. BMC Complementary and Alternative Medicine. 2010;10, article 31 doi: 10.1186/1472-6882-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subrahmanyam M. Topical application of honey in treatment of burns. British Journal of Surgery. 1991;78(4):497–498. doi: 10.1002/bjs.1800780435. [DOI] [PubMed] [Google Scholar]
- 11.Ladas SD, Haritos DN, Raptis SA. Honey may have a laxative effect on normal subjects because of incomplete fructose absorption. American Journal of Clinical Nutrition. 1995;62(6):1212–1215. doi: 10.1093/ajcn/62.6.1212. [DOI] [PubMed] [Google Scholar]
- 12.Bertoncelj J, Doberšek U, Jamnik M, Golob T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chemistry. 2007;105(2):822–828. [Google Scholar]
- 13.Amarowicz R, Pegg RB, Rahimi-Moghaddam P, Barl B, Weil JA. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chemistry. 2004;84(4):551–562. [Google Scholar]
- 14.Kishore RK, Halim AS, Syazana MSN, Sirajudeen KNS. Tualang honey has higher phenolic content and greater radical scavenging activity compared with other honey sources. Nutrition Research. 2011;31(4):322–325. doi: 10.1016/j.nutres.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacology and Therapeutics. 2002;96(2-3):67–202. doi: 10.1016/s0163-7258(02)00298-x. [DOI] [PubMed] [Google Scholar]
- 16.Steeg E, Montag A. Minor ingredients of honey with flavour relevance. II. Sensorially active decomposition products of carboxylic acids and glycosidally bonded aromatics. Deutsche Lebensmittel Rundschau. 1988;84:147–150. [Google Scholar]
- 17.Alvarez-Suarez JM, Tulipani S, Díaz D, et al. Antioxidant and antimicrobial capacity of several monofloral Cuban honeys and their correlation with color, polyphenol content and other chemical compounds. Food and Chemical Toxicology. 2010;48(8-9):2490–2499. doi: 10.1016/j.fct.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 18.Isla MI, Craig A, Ordoñez R, et al. Physico chemical and bioactive properties of honeys from Northwestern Argentina. Lebensmittel-Wissenschaft & Technologie. 2011;44(9):1922–1930. [Google Scholar]
- 19.Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods in Enzymology. 1998;299:152–178. [Google Scholar]
- 20.Barbosa-Pereira L, Angulo I, Paseiro-Losada P, Cruz JM. Phenolic profile and antioxidant properties of a crude extract obtained from a brewery waste stream. Food Research International. 2013;51:663–669. doi: 10.1155/2013/408491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Analytical Biochemistry. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 22.Al-Saikhan MS, Howard LR, Miller JC., Jr. Antioxidant activity and total phenolics in different genotypes of potato (Solanum tuberosum, L) Journal of Food Science. 1995;60:341–343. [Google Scholar]
- 23.Ciulu M, Solinas S, Floris I, et al. RP-HPLC determination of water-soluble vitamins in honey. Talanta. 2011;83(3):924–929. doi: 10.1016/j.talanta.2010.10.059. [DOI] [PubMed] [Google Scholar]
- 24.Al ML, Daniel D, Moise A, Bobis O, Laslo L, Bogdanov S. Physico-chemical and bioactive properties of different floral origin honeys from Romania. Food Chemistry. 2009;112(4):863–867. [Google Scholar]
- 25.Saxena S, Gautam S, Sharma A. Physical, biochemical and antioxidant properties of some Indian honeys. Food Chemistry. 2010;118(2):391–397. [Google Scholar]
- 26.Chang C, Yang M, Wen H, Chern J. Estimation of total flavonoid content in propolis by two complementary colometric methods. Journal of Food and Drug Analysis. 2002;10(3):178–182. [Google Scholar]
- 27.Iurlina MO, Saiz AI, Fritz R, Manrique GD. Major flavonoids of Argentinean honeys. Optimisation of the extraction method and analysis of their content in relationship to the geographical source of honeys. Food Chemistry. 2009;115(3):1141–1149. [Google Scholar]
- 28.Ferreres F, Andrade P, Tomás-Barberán FA. Flavonoids from Portuguese heather honey. Zeitschrift für Lebensmittel-Untersuchung und -Forschung. 1994;199(1):32–37. [Google Scholar]
- 29.Ondrias K, Stasko A, Hromadova M, Suchy V, Nagy M. Pinobanksin inhibits peroxidation of low density lipoprotein and it has electron donor properties reducing α-tocopherol radicals. Pharmazie. 1997;52(7):566–567. [PubMed] [Google Scholar]
- 30.Rajalakshmi D, Narasimhan S. Food antioxidants: sources and methods of evaluation. In: Madhavi DL, Deshpande SS, Salunkhe DK, editors. Food Antioxidants: Technological, Toxicological and Health Perspectives. New York, NY, USA: Marcel Dekker; 1996. pp. 65–158. [Google Scholar]
- 31.White JW. Composition of honey. In: Crane E, editor. Honey. A Comprehension Surveyed. New York, NY, USA: Crane, Russak & Company; 1975. pp. 157–206. [Google Scholar]
- 32.Cherchi A, Spanedda L, Tuberoso C, Cabras P. Solid-phase extraction and high-performance liquid chromatographic determination of organic acids in honey. Journal of Chromatography A. 1994;669(1-2):59–64. [Google Scholar]


