Abstract
Minimal residual disease (MRD) is of the most important factor for predicting prognosis and guiding treatment of acute lymphoblastic leukemia (ALL). In this study, we investigated the prognostic significance of leukemia-associated immunophenotypes (LAIPs) as assessment of index of MRD in 125 adult B-lineage ALL (B-ALL) patients by eight-color flow cytometry. The LAIPs could be identified in 96% and 81.6% of patients with the sensitivity of 10−4 and 10−5, respectively. MRD-negative status could clearly predict a favorable 2-year relapse-free survival (RFS) and overall survival (OS) at the end of induction of complete remission and one cycle of consolidation treatment. Moreover, we identified a group of cases with MRD of 0.001% to <0.01%, which showed significantly higher 2-year relapse rate than those with undetectable one. In multivariate analysis, MRD status was associated with RFS or OS independently. Furthermore, MRD assessed by LAIPs and RQ-PCR assay for patients with BCR-ABL fusion gene yielded concordant results in 89.7% of cases. In conclusion, MRD evaluated by eight-color flow cytometry could provide an important tool to assess treatment response and prognosis precisely in adult B-ALL.
Keywords: B-acute lymphoblastic leukemia, prognosis, minimal residual disease, leukemia-associated immunophenotypes, eight-color flow cytometry
Introduction
It is well known that acute lymphoblastic leukemia (ALL) is a group of heterogeneous diseases in terms of chromosome translocations or molecular genetic abnormalities, which have an important role in the leukemogenesis and risk stratification.1, 2, 3 However, a great proportion of ALL patients lack these typical genetic abnormalities, and more importantly, minimal residual disease (MRD) has an essential role in predicting relapse and even overall survival (OS). Currently, MRD assessment is increasingly applied in clinical practice for monitoring ALL that may provide physicians enough information to intervene the planned treatment of a patient earlier with intensification of chemotherapy or allogeneic stem cell transplantation.4, 5, 6
Quantification of clonal rearrangements of immunoglobulin/T-cell receptor (IG/TCR) genes or fusion genes by PCR7, 8, 9, 10 and leukemia-associated immunophenotypes (LAIPs) by multiparameter flow cytometry (MFC)11, 12, 13, 14, 15, 16 are the most commonly used methods for MRD assessment. PCR assay has been highly standardized by several study groups and considered as the gold standard for MRD monitoring in most European trials.8, 17, 18, 19 Recently, with the development of multi-color flow cytometry and new markers, MFC method for MRD evaluation based on LAIPs is increasingly used in the management of ALL with high applicability, sensitivity and specificity and has been regarded as an important counterpart of PCR detection.4, 12, 13, 20, 21, 22 Eight-color flow cytometric assay in assessing MRD allows to explore the expression of more cellular antigens in one combination associating a larger number of monoclonal antibodies. In addition, as compared with the classic one (3–4 color assay), it can dramatically save samples and reagents and can also offer the possibility of increasing accuracy in population identification.23 Furthermore, this method has recently been well standardized by the Euro Flow Consortium, which provides researchers with a practicable guideline.24, 25
Although early MRD evaluation in induction period has been introduced to guide the treatment in most major childhood ALL protocols,11, 26, 27, 28, 29 the value of MRD in adult ALL is not evaluated so widely as pediatric patients.30, 31, 32, 33, 34, 35, 36 In this study, we attempted to utilize a sensitive and reliable assay for monitoring MRD by eight-color flow cytometry. Furthermore, we attempted to address the prognostic value of MRD status using LAIPs in adult B-ALL at different time points, such as the end of induction of complete remission (CR) and one consolidation therapy.
Materials and methods
Patients and samples
A total of 125 patients with de novo B-ALL were enrolled in this study from October 2008 to August 2011 in our center. The diagnostic and immunological classification of ALL was established according to the WHO 2008 criteria. All the patients were treated in a schedule of Shanghai Institute of Hematology-based regimen.3 All of them gave informed consent according to the Declaration of Helsinki.
At diagnosis, 120 out of 125 patients (96%) had at least one suitable LAIPs with 0.01% sensitivity for MRD measurement by eight-color flow cytometry. With the exclusion of 14 patients from the above 120 cases who could not achieve a CR, finally a cohort of 106 adult B-ALL patients was formed for further prognostic analysis. MRD evaluation were performed in 712 follow-up samples from the 106 cases that were obtained at the end of induction of CR and after one cycle of consolidation and then were followed up every 1–2 months if the white blood cells (WBCs) was >2 × 109/l within the first year. All of the 106 patients underwent MRD detection at the end of induction of CR and one cycle of consolidation. Then the patients were followed up if the samples were available at the time points of 1 (57 cases), 2 (25), 3 (52), 4 (58), 5 (54), 6 (38), 7 (58), 8 (56), 9 (51) and 10 (51) months after one consolidation. After CR was achieved, 33 cases were consolidated with stem cell transplantation and other 73 patients were treated with chemotherapy consolidation. The detailed baseline clinical, immunophenotypic and cytogenetic characteristics of 106 patients are shown in Table 1.
Table 1. Baseline clinical characteristics of 106 B-ALL patients enrolled for survival analysis.
| Characteristics | Number of cases/total number (%) |
|---|---|
| Gender | |
| Male | 53/106 (50.0) |
| Female | 53/106 (50.0) |
| Median age, in years (range) | 34 (18–60) |
| Median WBC count, × 109/l (range) | 9.5 (1.0–379.0) |
| >30 × 109/l | 20/106 (18.9) |
| ⩽30 × 109/l | 86/106 (81.1) |
| Median platelets count, × 109/l (range) | 41.0 (2–467) |
| <30 × 109/l | 42/106 (39.6) |
| ⩾30 × 109/l | 64/106 (60.4) |
| Median Hb level, g/l (range) | 93.0 (40–778) |
| <75 g/l | 32/106 (30.2) |
| ⩾75 g/l | 74/106 (69.8) |
| Median BM blasts, % (range) | 80.4 (8.6–97.9) |
| Median number of LAIPs per patient (range) | 4 (1–8) |
| B-ALL subtypes | |
| pro-B ALL | 23/106 (21.7) |
| pre-B ALL | 13/106 (12.3) |
| common-B ALL | 66/106 (62.3) |
| mature-B ALL | 4/106 (3.8) |
| Late CRa | 42/106 (39.6) |
| Cytogenetic normal | 28/95 (29.5) |
| Cytogenetic quantity abnormalities | |
| Hyperdipoidy | 2/95 (2.1) |
| Hypodipoidy | 7/95 (7.4) |
| Non-translocation structural changes | 17/95 (17.9) |
| Karyotype, fusion geneb | |
| t(9;22), BCR-ABL | 30/95 (31.6) |
| t(1;11), MLL-EPS15 | 1/95 (1.1) |
| t(4;11), MLL-AFF1 | 2/95 (2.1) |
| t(11;19), MLL-MLLT1 | 1/95 (1.1) |
| t(1;19), TCF3-PBX1 | 3/95 (3.2) |
| t(12;21), TEL-AML1 | 3/95 (3.2) |
| t(9;22) and t(1;19), BCR-ABL and TCF3-PBX1c | 1/95 (1.1) |
| CRLF2 overexpression | 5/65 (7.7) |
| IK6 variant of IKZF1 gene | 15/65 (23.1) |
Abbreviations: B-ALL, B-lineage acute lymphoblastic leukemia; BM, bone marrow; CR, complete remission; Hb, hemoglobin; LAIP, leukemia-associated immunophenotype; WBC, white blood cell.
Complete remission is achieved after 35 days after initiation of the treatment.
Two or one of them are positive.
This patient has two kinds of abnormalities.
Immunophenotypic investigation of MRD by LAIPs
Fresh heparinized whole-bone marrow (BM) samples were processed on a standard NH4Cl whole-blood lysing technique for immunophenotyping at diagnosis and MRD monitoring during follow-up. Briefly, the BM sample containing up to 3 × 106 WBCs were incubated with a titered reagent cocktail and incubated in the dark at room temperature for 15 min, then about 2.0 ml of buffered NH4Cl containing 0.25% ultrapure formaldehyde (Polysciences, Warrington, PA, USA) was added and incubated at room temperature in the dark for 15 min followed by a single wash with phosphate-buffered saline containing 0.3% bovine serum albumin. If >200 μl of BM were needed for collecting up to 3 × 106 WBCs, the lysing procedure followed by a single wash would be performed before staining process. For samples where TdT and cytoplasmic (Cy) CD79a and IgM (cμ) were assessed, the BM were processed using the Fix-and-Perm kit according to the manufacturer's guidelines. The information of monoclonal antibodies (mAbs) and reagents used at diagnosis are shown in Supplementary Table S1. At least 1 × 105 blast cells identified by a low expression of CD45 and low side scatter (SSC) properties were obtained, and antigenic expression on blast population was systematically analyzed by eight-color flow cytometry (LSR-II, Becton Dickinson, San José, CA, USA) at diagnosis. Subtypes of B-ALL were classified into four groups as pro-B, common-B, pre-B and mature-B ALL. LAIP was identified as a cell population that could be separated completely from its counterpart at specific stage of maturation in either normal or regenerating marrow by the patterns of antigenic expression. Four main types of aberrant phenotypes in B-ALL were defined at diagnosis for LAIPs as follows: (1) cross-lineage antigen expression, (2) asynchronous antigen expression, (3) antigen dim/strong expression, and (4) ectopic phenotypes.
Although the median number of LAIPs for each case were 4 (1–7), only the aberrant antigens expressed on majority (>90%) of leukemic blasts in certain case were chosen for MRD detection. Table 2 shows the mAb combinations utilized for MRD measurement in 120 B-lineage ALL cases by single-tube panel. Dead cells and debris were excluded by forward scatter (FSC)/SSC and CD45/SSC dot plots. Doublets were excluded on FSC-A/FSC-H dot plots. All B-lineage cells were identified by expression of CD19 with low-to-intermediate SSC. To identify LAIPs as specific as possible, we used the ‘and' logistic gating strategy by FACSDiva software (Becton Dickinson, San Jose, CA, USA) to define final MRD population to display co-existence of multiple aberrant antigen expression if leukemic population was homogeneous. The ‘or' logistic gating strategy was applied to include the highest quantity of MRD when the leukemic blasts were heterogeneous containing ⩾2 subpopulations with absolutely different aberrant phenotypes. MRD was defined as an accumulation of 10 clustered events showing lymphoid-scattering properties and LAIP characteristics. When identified, MRD was quantified as a percentage of the total WBCs. To reach a theoretical maximum sensitivity of 0.001%, we needed 2.0 × 106 WBCs for each sample. Within 712 total follow-up samples, 14 and 6 at the end of induction of CR and after one consolidation failed to reach this level (only 5.0 × 105 WBCs were attainable), respectively. In these 20 samples, 19 contained MRD >0.01% and one sample had MRD of 0.006%. For the remaining 201 and 491 samples, at least 1.0 × 106 and 2.0 × 106 cells could be obtained, respectively. Consequently, 97.2% (692/712) of follow-up BM samples with >1.0 × 106 WBCs available could reach a theoretical sensitivity of 1.0 × 10−5. We have shown in Supplementary Figure S1 the process of LAIPs identified at diagnosis and MRD assessed at different treatment time points of a B-ALL case in detail.
Table 2. The mAb combinations utilized for MRD follow-up in 120 B-ALL patients.
| Seven fixed mAbs | Alternative mAb | Number (%) |
|---|---|---|
| Including CD58-FITC, | CD66C-PE | 28 (23.3) |
| CD38-perCP-cy5.5, | CD13-PE | 4 (3.3) |
| CD34-PE-Cy7, CD10-APC, | CD33-PE | 8 (6.7) |
| CD20-APC-eFluor780, | CD13-PE+CD33-PE | 12 (10.0) |
| CD19-Brilliant Violet 421, | CD66C-PE+CD33-PE | 13 (10.8) |
| CD45-Pacific Orange | CD66C-PE+CD13-PE+CD33-PE | 1 (0.8) |
| 7.1-PE | 3 (2.5) | |
| CD15-PE | 1 (0.8) | |
| CD15-PE+7.1-PE | 5 (4.2) | |
| None | 45 (37.5) |
Abbreviations: APC, allophycocyanin; B-ALL, B-lineage acute lymphoblastic leukemia; FITC, fluorescein isothiocyanate; mAb, monoclonal antibody; MRD, minimal residual disease; PE, phycoerythrin; perCP, peridinin chlorophyl protein.
MAb clone of CD58, CD66C, CD10, CD13, CD33, 7.1 and CD15 are AICD58, KORSA-3544, ALB1, SJ1D1, D3HL60.251, 7.1 and 80H5, respectively, from Immunotech (Marseille, France); MAb clone of CD38 and CD34 are HIT2 and 581, respectively, from Becton Dickinson; MAb clone of CD20 is B9E9 from eBioscience (San Diego, CA, USA); MAb clone of CD19 is HIB19 from Biolegend (San Diego, CA, USA); MAb clone of CD45 is HI30 from Invitrogen (Camarillo, CA, USA).
MRD was considered negative when leukemic cells were <0.01%, and, for the patients with positive MRD results, three groups of low (0.01%⩽MRD<0.1%), intermediate (0.1%⩽MRD<1.0%) and high (MRD⩾1.0%) levels were classified.
Sensitivity evaluation of eight-color flow cytometric assay
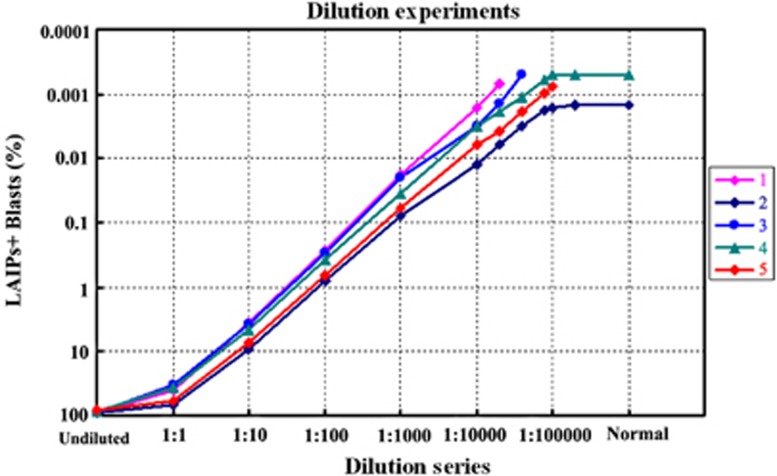
To examine the sensitivity of eight-color flow cytometric assay in MRD detection in B-ALL, dilution experiments were performed by adding LAIPs-positive (LAIPs+) cells obtained from B-ALL patient to regenerating BM sample from age-matched patients of AML with undetectable MRD after chemotherapy, in which the dilution factor ranged from 1:1, 1:10, 1:100, 1:1000, 1:10000, 1:20 000, 1:40 000, 1:80 000, 1:100 000 to 1:200 000 by volume. The BM samples from five B-ALL patients with main types of LAIPs, including major cross-lineage antigen expression and antigen dim/st expression, underwent this test. At least 2.0 × 106 WBCs were acquired in dilution series of 1:10 000 to 1:200 000. The same analytical strategies as that for leukemic blasts was applied in each dilution.
Normal BM cells from 20 non-hematological malignant patients and 30 regenerating BM samples after chemotherapy were used as control to establish normal antigenic expression patterns and evaluate the background expression of LAIP combinations.
Real-time quantitative (RQ)-PCR amplification of BCR-ABL fusion gene
Total RNA was extracted from mononuclear cells using TRIzol reagent (Life Technologies, Grand Island, NY, USA), and RQ-PCR was performed using 1 μg RNA with the BCR-ABL p210/p190 KIT (Yuanqi Bio-pharmaceutical CO., LTD, Shanghai, China) according to the manufacturer's recommendations in triplicate on ABI 7500 Real Time PCR system (Life Technologies). The standardization of RQ-PCR assay was performed as in previous studies.37, 38 The quantity of BCR-ABL transcript was calculated as a ratio of BCR-ABL copy number relative to ABL copies.
Statistical analysis
CR was defined by <5% blast cells in a regenerated BM aspirate, lack of extramedullary leukemia and peripheral blood platelet and neutrophil counts of >100 × 109/l and 1.5 × 109/l, respectively. OS was calculated from the date of disease diagnosis to death (failure) or alive at last follow-up (censored). Relapse-free survival (RFS) was defined as the time of achieving CR to treatment failure such as relapse, death or alive in CR at last follow-up (censored). The final visiting was censored on October 2012 with a median follow-up time of 18 (4–53) months, and 65 cases (61.3%) have already relapsed. Kaplan–Meier analysis was used to calculate the distribution of OS and RFS. Log rank comparison was performed to compare the difference of survivals. Two-sided P-values <0.05 were considered statistically significant. Cox model was used for the multivariate analysis of association of potential independent prognostic factors with OS and RFS. A limited backward selection procedure was used to exclude redundant variates. All the above statistical procedures were performed with the SPSS statistical software package, version 16.0 (IBM, Newyork, NY, USA).
Results
Sensitivity of eight-color flow cytometric assay in B-ALL
Linear correlation was shown between the percentage of LAIP+ cells and different titers of dilution from five BM samples of B-ALL diluted into regenerating BM. As shown in Figure 1, if enough WBCs (1.0–2.0 × 106) were attainable, the sensitivity of eight-color flow cytometric analysis of LAIPs for MRD detection could reach 10−5 with good linearity in four of the five samples; the other one could not reach this level because of the background expression in normal BM. The detailed analytical matrix by MFC for one sample is shown in Supplementary Figure S2. The quantification of LAIP+ blasts of each dilution in five cases are shown in Supplementary Table S3.
Figure 1.
Linear correlation was shown between the percentage of LAIPs+ blasts and different titers of dilution from five B-ALL samples with different LAIPs features. The LAIP combinations of samples from numbers 1 to 5 were shown as: CD10-CD34+/58st/38dim, CD10-CD34+, CD10+CD34+CD33+/CD38dim/45dim, CD10+CD34+CD45dim/CD19st/CD66c+ and CD10+CD34+58st/38dim/CD13+. ‘Undiluted' and ‘Normal' on the X-axis mean the original B-ALL BM sample and regenerating BM sample, respectively. The minimum MRD quantity by eight-color flow cytometric assay according to samples from numbers 1 to 5 were 0.0007, 0.0015, 0.0005, 0.0005 and 0.0008%, respectively. The data were not shown on the Figure when the number of LAIP+ blasts were <10 within 2 × 106 WBCs (<0.0005%).
In this work, using eight-color flow cytometry as compared with 3–4-color panels, different mAb combinations could represent several types of aberrant immunophenotypes in one tube. At diagnosis, 120 (96%) and 102 (81.6%) out of 125 patients could reach a sensitivity of 0.01% and 0.001% for MRD measurement, respectively, when compared with normal BM cells by eight-color flow cytometry.39, 40, 41, 42 The distribution of LAIP combinations in B-ALL samples and their background expression in normal BM are shown in Supplementary Table S2.
Frequencies and distribution of LAIPs and BCR-ABL and IK6 variants in B-ALL
The incidence and distribution of LAIP and molecular characteristics in 106 B-ALL patients are shown in Table 3 in detail. For comparison of LAIP and molecular abnormalities, we observed that an expression of CD66c (P=0.002), coexpression of CD66c and CD33 (P=0.001), dim expression of CD34 (P=0.021) and CD38 (P<0.001) were associated with a genotype of BCR-ABL+ ALL; a coexpression of CD66c and CD33 (P=0.024) and strong expression of CD34 (P=0.028) were related to IK6 variant of IKZF1 gene.
Table 3. Incidence and distribution of LAIP and BCR-ABL and IK6 variants in 106 B-ALL patients.
| LAIPs | Number of patients (%) |
B-ALL subtypes (n=106) |
BA fusion gene (n=95) |
IK6 variant of IKZF1 gene (n=65) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pro-B ALL | Common-B ALL | Pre-B ALL | Mature-B ALL | BA+a (n=31) | BA− (n=64) | P-value | IK6+b (n=15) | IK6− (n=50) | P-value | ||
| Cross-lineage antigen expression | |||||||||||
| CD13+ | 26/106 (24.5) | 6 | 18 | 2 | 0 | 8 | 14 | 0.670 | 2 | 12 | 0.601 |
| CD33+ | 42/106 (39.6) | 10 | 32 | 0 | 0 | 17 | 22 | 0.057 | 8 | 20 | 0.360 |
| CD15 or CD65+ | 16/106 (15.1) | 10 | 4 | 2 | 0 | 2 | 12 | 0.202 | 2 | 3 | 0.325 |
| CD66c+ | 53/106 (50.0) | 6 | 43 | 3 | 1 | 23 | 26 | 0.002 | 12 | 24 | 0.059 |
| CD13+CD33+ | 19/106 (17.9) | 5 | 14 | 0 | 0 | 6 | 11 | 0.796 | 1 | 10 | 0.415 |
| CD13+CD66c+ | 11/106 (10.4) | 3 | 8 | 0 | 0 | 4 | 5 | 0.674 | 2 | 5 | 1.000 |
| CD33+CD66c+ | 25/106 (23.6) | 3 | 22 | 0 | 0 | 14 | 9 | 0.001 | 7 | 9 | 0.024 |
| CD13+CD33+CD66c+ | 7/106 (6.6) | 2 | 5 | 0 | 0 | 3 | 3 | 0.388 | 1 | 3 | 1.000 |
| Asynchronous antigen expression | |||||||||||
| CD20+CD34+ | 16/106 (15.1) | 4 | 9 | 2 | 1 | 4 | 11 | 0.813 | 3 | 7 | 0.875 |
| CD20+CD10+ | 8/106 (7.5) | 1 | 5 | 0 | 2 | 3 | 5 | 1.000 | 1 | 4 | 1.000 |
| Antigen dim/st expression | |||||||||||
| CD38dimc | 66/106 (62.3) | 13 | 48 | 5 | 0 | 29 | 30 | <0.001 | 12 | 28 | 0.170 |
| CD19dim | 5/106 (4.7) | 2 | 1 | 1 | 1 | 0 | 4 | 0.300 | 1 | 1 | 0.411 |
| CD34dim | 13/106 (12.3) | 3 | 10 | 0 | 0 | 7 | 3 | 0.021 | 2 | 7 | 1.000 |
| CD10dim | 24/106 (22.6) | 5 | 16 | 3 | 0 | 6 | 14 | 0.778 | 4 | 10 | 0.847 |
| CD45dim | 45/106 (42.5) | 5 | 35 | 5 | 0 | 16 | 25 | 0.247 | 8 | 20 | 0.360 |
| CD58std | 54/106 (50.9) | 11 | 32 | 9 | 2 | 13 | 35 | 0.244 | 6 | 28 | 0.277 |
| CD19st | 40/106 (37.7) | 12 | 25 | 2 | 1 | 15 | 22 | 0.189 | 8 | 17 | 0.177 |
| CD34st | 20/106 (18.9) | 6 | 13 | 1 | 0 | 5 | 14 | 0.512 | 5 | 5 | 0.028 |
| CD10st | 16/106 (15.1) | 0 | 14 | 1 | 1 | 5 | 9 | 0.790 | 3 | 7 | 0.875 |
| Ectopic phenotype | |||||||||||
| NG2+ e | 11/106 (10.4) | 10 | 1 | 0 | 0 | 0 | 8 | 0.050 | 1 | 1 | 0.411 |
Abbreviations: BA, BCR-ABL; B-ALL, B-lineage acute lymphoblastic leukemia; LAIP, leukemia-associated immunophenotype.
BCR-ABL positive.
IK6 variant of IKZF1 gene.
Dim expression.
Strong expression.
Human homologue of the rat chondroitin sulfate proteoglycan NG2.
Comparison of MRD quantification by RQ-PCR and flow cytometry in BCR-ABL-positive patients
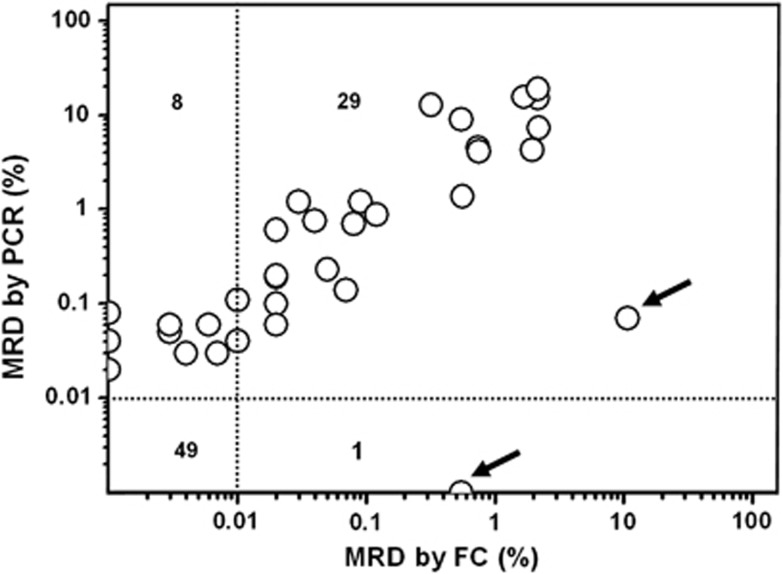
As shown in Figure 2, we assessed MRD in 87 samples obtained from 28 B-ALL patients with BCR-ABL fusion gene after CR by RQ-PCR and by flow cytometric assessment of LAIPs simultaneously. Two methods showed concordant results in 78 out of 87 (89.7%) samples studied when 0.01% was used as threshold to define MRD positivity, among which 29 were MRD positive and 49 were MRD negative according to the criteria by the two methods. The proportion of leukemic cells in the 29 MRD-positive (⩾0.01%) samples ranged from 0.01% to 10.8% of nucleated cells (median, 0.08%) by flow cytometry and from 0.04% to 19.0% (median, 0.7%) of mononuclear cells by PCR. In other eight samples, MRD was positive by PCR but negative by flow cytometry, of which five samples had detectable leukemic cells between 0.001% and 0.01% by flow cytometric assay. Conversely, in two samples from the same patient, MRD were 10.8% and 0.55% according to flow cytometry but 0.07% and <0.001% by PCR, respectively (as indicated by arrows in Figure 2).
Figure 2.
Comparison of MRD quantification by RQ-PCR and flow cytometry (FC) in BCR-ABL-positive patients. Circles indicate the percentage of MRD⩾0.01% obtained by the two methods in each sample. Two samples indicated by arrows were obtained from the same patient at different time points.
Univariate analysis of relationship between MRD level and survival
Association between MRD level and prognosis at the end of induction of CR
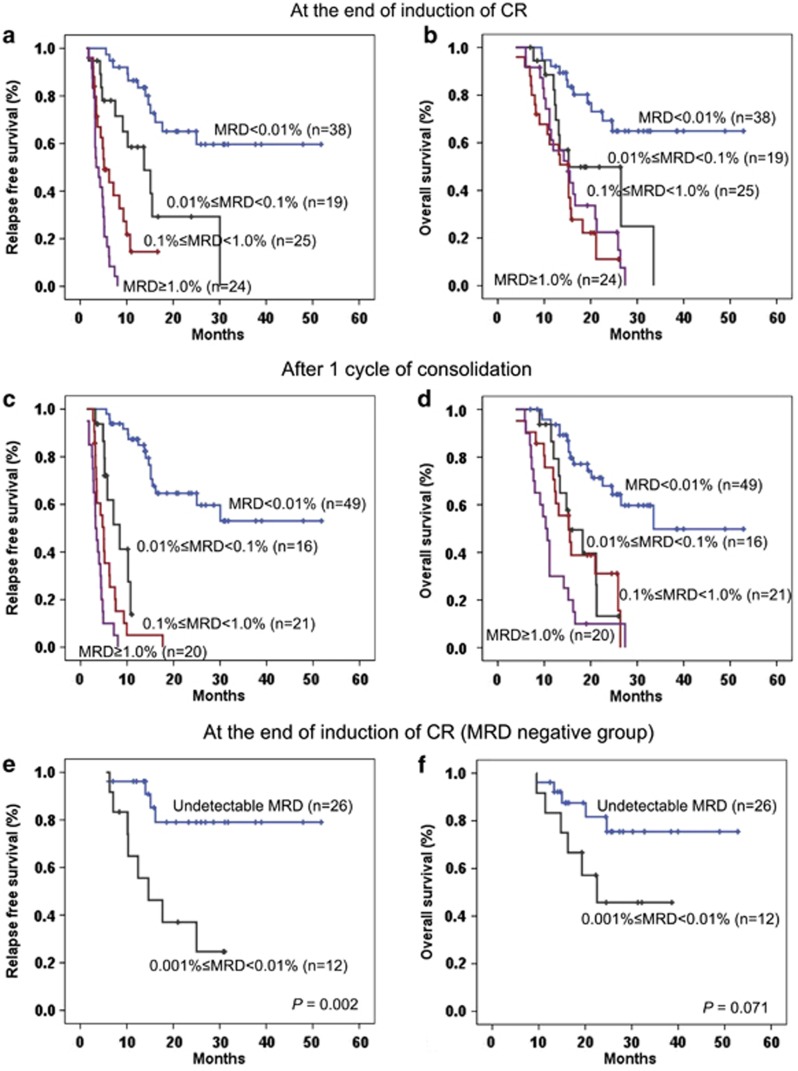
Immunophenotypic study of MRD was investigated at the end of induction therapy of CR, and 38 patients (35.8%) achieved MRD negative, in contrast to 68 patients (64.2%) who remained MRD positive. Among the patients who were MRD positive, low (0.01%⩽MRD<0.1%), intermediate (0.1%⩽MRD<1.0%) and high (MRD⩾1.0%) levels of MRD were presented in 19 (27.9%), 25 (36.8%) and 24 (35.3%) patients, respectively. Statistical significance was reached when comparing the 2-year RFS rate (P<0.001) and estimated 2-year OS rate (P<0.001) between the patients with MRD negative and MRD positive. Similarly, 2-year RFS was statistically different among the three groups of patients at different MRD levels (low vs intermediate, P=0.014; intermediate vs high, P=0.004). However, no statistical significance was reached in terms of OS (low vs intermediate, P=0.070; intermediate vs high, P=0.411). The detailed data are shown in Figures 3a and b.
Figure 3.
The 2-year RFS and OS in 106 adult patients with B-ALL according to immunophenotypic MRD level at the end of induction of CR and after one consolidation. (a) The RFS of the patients with negative and positive MRD status at the end of induction of CR were 65.1±8.7% and 12.3±5.1%, respectively (P<0.001). In parallel, the RFS of the patients with low and intermediate burden of MRD were 29.3±13.4% and 14.5±8.6% however, the patients with high burden of MRD all relapsed (low vs intermediate, P=0.014; and intermediate vs high, P=0.004). (b) The estimated 2-year OS of the patients with negative and positive MRD status at the end of induction of CR were 69.2±8.3% and 25.0±6.2%, respectively (P<0.001). No statistical significance was observed in the three groups with a 2-year estimated OS rate of 49.8±12.7, 11.0±9.0 and 22.4±9.3%, respectively (low vs intermediate, P=0.070; and intermediate vs high, P=0.411). (c) The 2-year RFS of the patients with negative MRD status after one consolidation were 64.6±7.8%, and no patients with positive MRD status were relapse-free (P<0.001). (d) The estimated 2-year OS of the patients with negative and positive MRD status after one consolidation were 67.9±7.5% and 18.9±6.2%, respectively (P<0.001). There was no significance between the three groups in MRD-positive status: Low, 13.2±11.7% Intermediate, 31.1±11.3% and High, 10.0±6.7% (low vs intermediate, P=0.859; and intermediate vs high, P=0.056). (e) The 2-year RFS of the patients with MRD 0.001% to <0.01% and those with undetectable MRD at the end of induction of CR (n=38) were 37.0±14.6% and 79.1±9.5%, respectively (P=0.002). (f) Patients with undetectable MRD had a better 2-year OS than those with MRD 0.001% to <0.01% at the end of induction of CR (81.7±8.5% and 45.7±15.5%, respectively, P=0.071), but no statistical significance was observed.
Association between MRD level and prognosis after one cycle of consolidation
After one course of consolidation was completed, 49 patients (46.2%) showed MRD negative while 57 patients (53.8%) were MRD positive. And low, intermediate and high levels of MRD were distributed in 16 (28.1%), 21 (36.8%) and 20 (35.1%) patients, respectively. Identically, there was statistical significance of 2-year RFS rate (P<0.001) and estimated 2-year OS rate (P<0.001) between the MRD negative and MRD positive groups. An improved 2-year OS rate between the patients with intermediate and with high MRD level was observed after one consolidation (P=0.056), but all the patients with positive MRD experienced relapse, which are displayed in Figures 3c and d in detail.
The potential prognostic value of MRD between 0.001% and 0.01% in the MRD-negative group
Of the 38 patients who achieved MRD negative (<0.01%) at the end of induction of CR, 12 had an MRD of 0.001% to <0.01%, and for another 26 patients, LAIPs were undetectable. Most of the patients (n=37) could reach enough WBCs of 1.0–2.0 × 106, with the exception of one patient with MRD of 0.006% (only 5.0 × 105 WBCs were attainable). Patients with this very low but detectable levels of leukemic blasts experienced an inferior 2-year RFS (P=0.002) and OS (P=0.071) rate to those with undetectable MRD (shown in Figures 3e and f). No further analysis for different MRD levels in the negative group was performed after one consolidation therapy, as LAIPs were not detectable in nearly all the patients (48/49, 98.0%).
Association between dynamic MRD change and prognosis
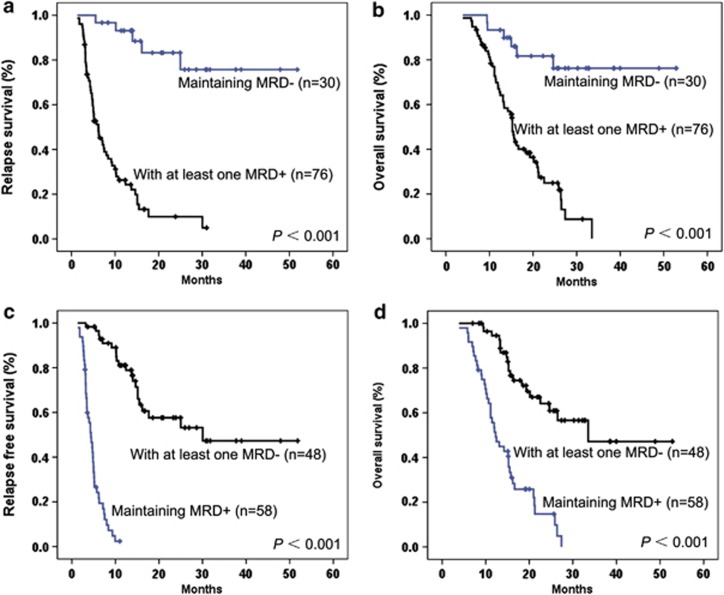
Among the 106 patients who achieved CR, 76 patients (71.7%) presented at least one MRD-positive result during the follow-up within the first year of treatment, and 30 patients (28.3%) sustained in MRD-negative status. Statistical significances were observed in these two groups of patients when the 2-year RFS rate (P<0.001) and OS rate (P<0.001) were compared as seen in Figures 4a and b.
Figure 4.
The 2-year RFS and OS in 106 adult patients with B-ALL according to dynamic MRD. (a, b) The 2-year RFS and estimated 2-year OS rate of the patients with at least a MRD-positive result or sustaining negative during the first year were 9.9±4.5% and 83.2±7.8% (P<0.001), respectively, and 24.9±5.9% and 81.7±7.5% (P<0.001), respectively. (c, d) The 2-year RFS and estimated 2-year OS rate of the patients with at least a MRD-negative result or never achieving this status during the first year were 57.6±7.7% and 2.4±2.4% (P<0.001), respectively, and 64.2±7.2% and 14.8±6.1% (P<0.001), respectively.
In a similar way, 58 patients (54.7%) obtained at least one MRD-negative result in contrast to 48 (45.3%) patients never reached during the treatment of the first year. Figures 4c and d shows the statistical difference of 2-year RFS (P<0.001) and OS rate (P<0.001) between these two groups.
Multivariate analysis of MRD status and clinical factors with survival
In order to explore whether the status of MRD was an independent prognostic factor for RFS and OS among adult patients with ALL, a multivariate analysis was performed with the variants, including clinical factors such as gender, age, WBC count, Hb level, platelet count, consolidation therapy, blasts percentage in BM and time to achieving CR, BCR-ABL-positive, number of LAIPs, B-ALL subtype and levels of MRD at the end of induction of CR and completion of one consolidation therapy. Univariate analysis indicated that WBC count, platelet count, late CR, consolidation therapy and MRD status at CR1 and after consolidation had association with RFS (P<0.05); MRD status at two time points had association with OS (P<0.05). Multivariate analysis showed that positive MRD status (MRD >0.01%) after induction (P=0.002) and one consolidation (P<0.001) were associated with an increased risk of relapse independently. However, only MRD-positive status after one consolidation (P<0.001) suggested an inferior OS independently. The detailed data are shown in Table 4.
Table 4. Univariate and multivariate analysis for clinical and MRD variables of RFS and OS.
| Variables |
RFS |
OS |
||||
|
Univariate analysis |
Multivariate analysis |
Univariate analysis |
Multivariate analysis |
|||
| |
P-value |
P-value |
OR (95% CI) |
P-value |
P-value |
OR (95% CI) |
| Gender | 0.440 | NS | — | 0.668 | NS | — |
| Age | 0.187 | NS | — | 0.791 | NS | — |
| WBC count | 0.003 | NS | — | 0.280 | NS | — |
| Hb level | 0.552 | NS | — | 0.442 | NS | — |
| Platelets count | 0.016 | NS | — | 0.431 | NS | — |
| BM blasts | 0.842 | NS | — | 0.099 | NS | — |
| Late CR | <0.001 | NS | — | 0.138 | NS | — |
| B-ALL subtype | 0.552 | NS | — | 0.692 | NS | — |
| Consolidation therapya | 0.009 | NS | — | 0.224 | NS | — |
| BCR-ABL-positive | 0.273 | NS | — | 0.681 | NS | — |
| Number of LAIPs | 0.440 | NS | — | 0.612 | NS | — |
| MRD positiveb at the end of induction | <0.001 | 0.002 | 4.427 (1.750–11.197) | <0.001 | NS | — |
| MRD positive after one consolidation | <0.001 | <0.001 | 9.832 (4.545–21.268) | <0.001 | <0.001 | 5.652 (2.739–11.662) |
| Undetectable MRD at the end of induction | <0.001 | NS | — | <0.001 | NS | — |
Abbreviations: B-ALL, B-lineage acute lymphoblastic leukemia; BM, bone marrow; CI, confidence interval; CR, complete remission; Hb, hemoglobin; LAIP, leukemia-associated immunophenotype; MRD, minimal residual disease; NS, not significant; OR, odds ratio; OS, overall survival; RFS, relapse-free survival; WBC, white blood cell.
Allogeneic stem cell transplantation or chemotherapy.
MRD⩾0.01%.
Discussion
MRD status quantified by flow cytometric analysis has been integrated as an essential part of the algorithm of the treatment guidelines in evaluating the early treatment response and predicting outcome in pediatric patients.43, 44 However, the correlation between MRD and prognosis in adult ALL needs further exploration. Therefore, we performed this study to establish the role of MRD status in predicting the prognosis of adult B-ALL by eight-color flow cytometric method.
In our study, linear correlation analysis showed the stability and sensitivity of this method in quantification of the leukemic cells in different dilution levels. If enough WBCs (2.0 × 106) could be reached, the sensitivity of methodology of eight-color flow cytometry could be as high as close to 1.0 × 10−5. As shown in Figure 1, the background clusters were <0.001% in regeneration BM according to the aberrant phenotypic features of B-ALL samples except for one patient (1.5 × 10−5). These very low levels of noisy signal ensured the high specificity of methodology of eight-color MFC. Also, we observed that eight-color evaluation procedure could identify LAIPs in most of the patients by single-tube panel with a high sensitivity (96.0% for 10−4 and 81.6% for 10−5, respectively) in comparison to normal BM. As shown in one clinical application sample in Supplementary Figure S1, LAIPs could be monitored precisely during treatment at 0.005% level even with shifted immunophenotypes by eight-color flow cytometry.
Four main types of LAIPs were identified at diagnosis (Table 3). We also observed that coexpression of CD66c and CD33 was associated with BCR-ABL fusion gene and IK6 variant of IKZF1 gene, which suggested potential genetic associations between these two molecular abnormalities, as previously reported from our group by Chen et al.2
Many studies proved that MRD evaluated by PCR targeting to IG/TCR rearrangement and MFC for LAIPs yielded concordant results in the vast majority of patients.7, 45, 46 A recent study by Garand et al.20 proved that QPCR and MFC can therefore be comparable if properly standardized and are highly complementary. More recently, Denys et al.12 reported that six-color MFC could significantly improve the concordance with PCR-based MRD data (88 versus 96%) and particularly improve the specificity of the MRD analysis as compared with 4-color MFC. In our study, we attempted to test the reliability and accuracy of MFC assay in MRD measurement in comparison to RQ-PCR method targeting BCR-ABL fusion gene transcripts. PCR evaluation of fusion gene transcripts reported an about 10-fold higher sensitivity (10−5–10−6) than MFC, but when a cutoff level of 0.01% was used to define MRD positivity, our results showed remarkable concordance between both the methods in MRD status. In the present study, mononuclear cell by Ficoll centrifugation and whole BM by lysing procedure were performed during sample preparation of PCR and MFC, which may lead to systematically higher MRD data of RQ-PCR as compared with the flow cytometry. Interestingly, as shown in Figure 2, two samples from the same patient indicated by arrows showed higher MRD level by flow cytometry (10.8% and 0.55%, respectively) as compared with RQ-PCR (0.07% and <0.001%, respectively), which could be explained by the fact that the leukemic cells may contain more than one genetic abnormality or this patient has complicated chromosomal translocations, as the quantification of BCR-ABL fusion gene transcripts of this case was as low as 0.18% at diagnosis while LAIPs+ blasts were 95.9%.
Although there were different cutoff values of MRD,11, 47 we used a more popular cutoff value of <0.01% as the criteria of MRD negative. In this study, we proved that statistical significance could be reached when comparing the 2-year RFS rate (P<0.001 and P<0.001, respectively) and estimated OS rate (P<0.001 and P<0.001, respectively) between the patients with MRD-negative and -positive status at the end of induction and after one cycle of consolidation. In a hierarchical analysis of the prognosis of patients with positive MRD result, a decreased RFS and OS was observed in the patients with low, intermediate and high levels of leukemic blasts at two time points, respectively. Furthermore, in dynamic analysis of MRD change and prognosis, we had proved that maintenance of a MRD-negative status was important to the prolonging of the RFS and OS, as the patients with persistent MRD-negative results in the 1 year of treatment had a superior RFS (P<0.001) and OS (P<0.001) to those without.
Interestingly, in examining the few patients with MRD-negative status (<0.01%) who suffered from short relapse with poor OS, we found that most of such patients had a detectable low level of MRD of 0.001% to <0.01% at the end of induction. Although our results showed that patients with MRD of 0.001 to 0.01% experienced significantly higher cumulative risk of relapse and a tendency of inferior 2-year OS rate than those with undetectable MRD, more patients were needed to be enrolled to testify this supposition.
In multivariate prognostic analysis in this series of patients, we proved that MRD-positive status at the end of induction and after one consolidation were independently associated with an increased risk of relapse; on the other hand, MRD of >0.01% after one consolidation was the only factor related with OS independently. The prognostic value of BCR-ABL fusion gene in adult ALL was not obvious in our analysis, which might be caused by the wide application of tailored tyrosine kinase inhibitor treatment.
In the treatment of adult ALL, no standard guideline has been established until now, and the patients who should receive more intensive chemotherapy or early allogenetic stem cell transplantation are still uncertain, with a few exception for tailored tyrosine kinase inhibitor treatment for patients with Ph chromosome.1, 48, 49 Due to the poor prognosis of adult ALL when compared with pediatric counterparts, a care of more patient-specific way should be considered. MRD assessing by flow cytometric analysis may provide an important parameter for guiding the next treatment as indicated in this study. Patients with <0.01% leukemic cells at the end of remission induction and after one cycle of consolidation are likely to have an excellent treatment outcome, especially for those with undetectable MRD; otherwise, early intervention with high-dose chemotherapy or other strategies should be considered.
In conclusion, our study suggests that MRD status at the end of induction and one cycle of consolidation by eight-color flow cytometry could be potentially taken as a routine index in the evaluation of the treatment response for adult patients with B-ALL. Standardization of MRD assessing method in order to compare the treatment outcome of adult ALL using different treatment protocols is warranted by further multi-centered study.
Acknowledgments
This work was supported, in part, by a Special Research Fund of the Ministry of Health (201202003), Chinese National Key Basic Research Project (973: 2013CB966800, 2010CB529200), Mega-projects of Science Research for the 12th Five-Year Plan (2013ZX09303302), the National High Tech Program for Biotechnology (863: 2012AA02A505), the National Natural Science Foundation of China (81123005), the Shanghai Municipal Commission for Science and Technology (10411965600), the Shanghai Rising Star Program (11QA1404300) and the Samuel Waxman Cancer Research Foundation Co-PI Program.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Blood Cancer Journal website (http://www.nature.com/bcj)
Supplementary Material
References
- Fielding AK. Current treatment of Philadelphia chromosome-positive acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2011. pp. 231–237. [DOI] [PubMed]
- Chen B, Wang YY, Shen Y, Zhang WN, He HY, Zhu YM, et al. Newly diagnosed acute lymphoblastic leukemia in China (I): abnormal genetic patterns in 1346 childhood and adult cases and their comparison with the reports from Western countries. Leukemia. 2012;26:1608–1616. doi: 10.1038/leu.2012.26. [DOI] [PubMed] [Google Scholar]
- Mi JQ, Wang X, Yao Y, Lu HJ, Jiang XX, Zhou JF, et al. Newly diagnosed acute lymphoblastic leukemia in China (II): prognosis related to genetic abnormalities in a series of 1091 cases. Leukemia. 2012;26:1507–1516. doi: 10.1038/leu.2012.23. [DOI] [PubMed] [Google Scholar]
- Campana D.Role of minimal residual disease monitoring in adult and pediatric acute lymphoblastic leukemia Hematol Oncol Clin North Am 2009231083–1098.vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campana D. Minimal residual disease in acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2010. pp. 7–12. [DOI] [PubMed]
- Campana D, Coustan-Smith E. Detection of minimal residual disease in acute leukemia by flow cytometry. Cytometry. 1999;38:139–152. doi: 10.1002/(sici)1097-0320(19990815)38:4<139::aid-cyto1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Kerst G, Kreyenberg H, Roth C, Well C, Dietz K, Coustan-Smith E, et al. Concurrent detection of minimal residual disease (MRD) in childhood acute lymphoblastic leukaemia by flow cytometry and real-time PCR. Br J Haematol. 2005;128:774–782. doi: 10.1111/j.1365-2141.2005.05401.x. [DOI] [PubMed] [Google Scholar]
- Pongers-Willemse MJ, Seriu T, Stolz F, d'Aniello E, Gameiro P, Pisa P, et al. Primers and protocols for standardized detection of minimal residual disease in acute lymphoblastic leukemia using immunoglobulin and T cell receptor gene rearrangements and TAL1 deletions as PCR targets: report of the BIOMED-1 CONCERTED ACTION: investigation of minimal residual disease in acute leukemia. Leukemia. 1999;13:110–118. doi: 10.1038/sj.leu.2401245. [DOI] [PubMed] [Google Scholar]
- Szczepanski T, Willemse MJ, van Wering ER, van Weerden JF, Kamps WA, van Dongen JJ. Precursor-B-ALL with D(H)-J(H) gene rearrangements have an immature immunogenotype with a high frequency of oligoclonality and hyperdiploidy of chromosome 14. Leukemia. 2001;15:1415–1423. doi: 10.1038/sj.leu.2402206. [DOI] [PubMed] [Google Scholar]
- Bruggemann M, Droese J, Bolz I, Luth P, Pott C, von Neuhoff N, et al. Improved assessment of minimal residual disease in B cell malignancies using fluorogenic consensus probes for real-time quantitative PCR. Leukemia. 2000;14:1419–1425. doi: 10.1038/sj.leu.2401831. [DOI] [PubMed] [Google Scholar]
- Borowitz MJ, Devidas M, Hunger SP, Bowman WP, Carroll AJ, Carroll WL, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children's Oncology Group study. Blood. 2008;111:5477–5485. doi: 10.1182/blood-2008-01-132837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denys B, van der Sluijs-Gelling AJ, Homburg C, van der Schoot CE, de Haas V, Philippe J, et al. Improved flow cytometric detection of minimal residual disease in childhood acute lymphoblastic leukemia. Leukemia. 2012;27:635–641. doi: 10.1038/leu.2012.231. [DOI] [PubMed] [Google Scholar]
- Bjorklund E, Mazur J, Soderhall S, Porwit-MacDonald A. Flow cytometric follow-up of minimal residual disease in bone marrow gives prognostic information in children with acute lymphoblastic leukemia. Leukemia. 2003;17:138–148. doi: 10.1038/sj.leu.2402736. [DOI] [PubMed] [Google Scholar]
- Dworzak MN, Gaipa G, Ratei R, Veltroni M, Schumich A, Maglia O, et al. Standardization of flow cytometric minimal residual disease evaluation in acute lymphoblastic leukemia: multicentric assessment is feasible. Cytometry B Clin Cytom. 2008;74:331–340. doi: 10.1002/cyto.b.20430. [DOI] [PubMed] [Google Scholar]
- Lucio P, Gaipa G, van Lochem EG, van Wering ER, Porwit-MacDonald A, Faria T, et al. BIOMED-I concerted action report: flow cytometric immunophenotyping of precursor B-ALL with standardized triple-stainings. BIOMED-1 concerted action investigation of minimal residual disease in cute leukemia: International Standardization and Clinical Evaluation. Leukemia. 2001;15:1185–1192. doi: 10.1038/sj.leu.2402150. [DOI] [PubMed] [Google Scholar]
- Coustan-Smith E, Sancho J, Behm FG, Hancock ML, Razzouk BI, Ribeiro RC, et al. Prognostic importance of measuring early clearance of leukemic cells by flow cytometry in childhood acute lymphoblastic leukemia. Blood. 2002;100:52–58. doi: 10.1182/blood-2002-01-0006. [DOI] [PubMed] [Google Scholar]
- van Dongen JJ, Langerak AW, Bruggemann M, Evans PA, Hummel M, Lavender FL, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003;17:2257–2317. doi: 10.1038/sj.leu.2403202. [DOI] [PubMed] [Google Scholar]
- van der Velden VH, Cazzaniga G, Schrauder A, Hancock J, Bader P, Panzer-Grumayer ER, et al. Analysis of minimal residual disease by Ig/TCR gene rearrangements: guidelines for interpretation of real-time quantitative PCR data. Leukemia. 2007;21:604–611. doi: 10.1038/sj.leu.2404586. [DOI] [PubMed] [Google Scholar]
- Bruggemann M, Schrauder A, Raff T, Pfeifer H, Dworzak M, Ottmann OG, et al. Standardized MRD quantification in European ALL trials: proceedings of the Second International Symposium on MRD assessment in Kiel, Germany, 18–20 September 2008. Leukemia. 2010;24:521–535. doi: 10.1038/leu.2009.268. [DOI] [PubMed] [Google Scholar]
- Garand R, Beldjord K, Cave H, Fossat C, Arnoux I, Asnafi V, et al. Flow cytometry and IG/TCR quantitative PCR for minimal residual disease quantitation in acute lymphoblastic leukemia: a French multicenter prospective study on behalf of the FRALLE, EORTC and GRAALL. Leukemia. 2012;27:370–376. doi: 10.1038/leu.2012.234. [DOI] [PubMed] [Google Scholar]
- Solly F, Angelot F, Garand R, Ferrand C, Seilles E, Schillinger F, et al. CD304 is preferentially expressed on a subset of B-lineage acute lymphoblastic leukemia and represents a novel marker for minimal residual disease detection by flow cytometry. Cytometry A. 2012;81:17–24. doi: 10.1002/cyto.a.21162. [DOI] [PubMed] [Google Scholar]
- Coustan-Smith E, Song G, Clark C, Key L, Liu P, Mehrpooya M, et al. New markers for minimal residual disease detection in acute lymphoblastic leukemia. Blood. 2011;117:6267–6276. doi: 10.1182/blood-2010-12-324004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood B. 9-color and 10-color flow cytometry in the clinical laboratory. Arch Pathol Lab Med. 2006;130:680–690. doi: 10.5858/2006-130-680-CACFCI. [DOI] [PubMed] [Google Scholar]
- Kalina T, Flores-Montero J, van der Velden VH, Martin-Ayuso M, Bottcher S, Ritgen M, et al. EuroFlow standardization of flow cytometer instrument settings and immunophenotyping protocols. Leukemia. 2012;26:1986–2010. doi: 10.1038/leu.2012.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen JJ, Lhermitte L, Bottcher S, Almeida J, van der Velden VH, Flores-Montero J, et al. EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia. 2012;26:1908–1975. doi: 10.1038/leu.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen JJ, Seriu T, Panzer-Grumayer ER, Biondi A, Pongers-Willemse MJ, Corral L, et al. Prognostic value of minimal residual disease in acute lymphoblastic leukaemia in childhood. Lancet. 1998;352:1731–1738. doi: 10.1016/S0140-6736(98)04058-6. [DOI] [PubMed] [Google Scholar]
- Coustan-Smith E, Sancho J, Hancock ML, Boyett JM, Behm FG, Raimondi SC, et al. Clinical importance of minimal residual disease in childhood acute lymphoblastic leukemia. Blood. 2000;96:2691–2696. [PubMed] [Google Scholar]
- Conter V, Bartram CR, Valsecchi MG, Schrauder A, Panzer-Grumayer R, Moricke A, et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010;115:3206–3214. doi: 10.1182/blood-2009-10-248146. [DOI] [PubMed] [Google Scholar]
- Schrappe M, Valsecchi MG, Bartram CR, Schrauder A, Panzer-Grumayer R, Moricke A, et al. Late MRD response determines relapse risk overall and in subsets of childhood T-cell ALL: results of the AIEOP-BFM-ALL 2000 study. Blood. 2011;118:2077–2084. doi: 10.1182/blood-2011-03-338707. [DOI] [PubMed] [Google Scholar]
- Vidriales MB, Perez JJ, Lopez-Berges MC, Gutierrez N, Ciudad J, Lucio P, et al. Minimal residual disease in adolescent (older than 14 years) and adult acute lymphoblastic leukemias: early immunophenotypic evaluation has high clinical value. Blood. 2003;101:4695–4700. doi: 10.1182/blood-2002-08-2613. [DOI] [PubMed] [Google Scholar]
- Holowiecki J, Krawczyk-Kulis M, Giebel S, Jagoda K, Stella-Holowiecka B, Piatkowska-Jakubas B, et al. Status of minimal residual disease after induction predicts outcome in both standard and high-risk Ph-negative adult acute lymphoblastic leukaemia. The Polish Adult Leukemia Group ALL 4-2002 MRD Study. Br J Haematol. 2008;142:227–237. doi: 10.1111/j.1365-2141.2008.07185.x. [DOI] [PubMed] [Google Scholar]
- Bruggemann M, Raff T, Flohr T, Gokbuget N, Nakao M, Droese J, et al. Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood. 2006;107:1116–1123. doi: 10.1182/blood-2005-07-2708. [DOI] [PubMed] [Google Scholar]
- Bruggemann M, Raff T, Kneba M. Has MRD monitoring superseded other prognostic factors in adult ALL. Blood. 2012;120:4470–4481. doi: 10.1182/blood-2012-06-379040. [DOI] [PubMed] [Google Scholar]
- Gokbuget N, Kneba M, Raff T, Trautmann H, Bartram CR, Arnold R, et al. Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood. 2012;120:1868–1876. doi: 10.1182/blood-2011-09-377713. [DOI] [PubMed] [Google Scholar]
- Raff T, Gokbuget N, Luschen S, Reutzel R, Ritgen M, Irmer S, et al. Molecular relapse in adult standard-risk ALL patients detected by prospective MRD monitoring during and after maintenance treatment: data from the GMALL 06/99 and 07/03 trials. Blood. 2007;109:910–915. doi: 10.1182/blood-2006-07-037093. [DOI] [PubMed] [Google Scholar]
- Gokbuget N, Kneba M, Raff T, Bruggemann M, Scheuring U, Reutzel R, et al. Risk-adapted treatment according to minimal residual disease in adult ALL. Best Pract Res Clin Haematol. 2002;15:639–652. doi: 10.1053/beha.2002.0229. [DOI] [PubMed] [Google Scholar]
- Beillard E, Pallisgaard N, van der Velden VH, Bi W, Dee R, van der Schoot E, et al. Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using 'real-time' quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR)—a Europe Against Cancer program. Leukemia. 2003;17:2474–2486. doi: 10.1038/sj.leu.2403136. [DOI] [PubMed] [Google Scholar]
- Gabert J, Beillard E, van der Velden VH, Bi W, Grimwade D, Pallisgaard N, et al. Standardization and quality control studies of 'real-time' quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia—a Europe Against Cancer program. Leukemia. 2003;17:2318–2357. doi: 10.1038/sj.leu.2403135. [DOI] [PubMed] [Google Scholar]
- McKenna RW, Washington LT, Aquino DB, Picker LJ, Kroft SH. Immunophenotypic analysis of hematogones (B-lymphocyte precursors) in 662 consecutive bone marrow specimens by 4-color flow cytometry. Blood. 2001;98:2498–2507. doi: 10.1182/blood.v98.8.2498. [DOI] [PubMed] [Google Scholar]
- Dworzak MN, Froschl G, Printz D, Mann G, Potschger U, Muhlegger N, et al. Prognostic significance and modalities of flow cytometric minimal residual disease detection in childhood acute lymphoblastic leukemia. Blood. 2002;99:1952–1958. doi: 10.1182/blood.v99.6.1952. [DOI] [PubMed] [Google Scholar]
- Griesinger F, Piro-Noack M, Kaib N, Falk M, Renziehausen A, Troff C, et al. Leukaemia-associated immunophenotypes (LAIP) are observed in 90% of adult and childhood acute lymphoblastic leukaemia: detection in remission marrow predicts outcome. Br J Haematol. 1999;105:241–255. [PubMed] [Google Scholar]
- Lucio P, Parreira A, van den Beemd MW, van Lochem EG, van Wering ER, Baars E, et al. Flow cytometric analysis of normal B cell differentiation: a frame of reference for the detection of minimal residual disease in precursor-B-ALL. Leukemia. 1999;13:419–427. doi: 10.1038/sj.leu.2401279. [DOI] [PubMed] [Google Scholar]
- Schultz KR, Pullen DJ, Sather HN, Shuster JJ, Devidas M, Borowitz MJ, et al. Risk- and response-based classification of childhood B-precursor acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children's Cancer Group (CCG) Blood. 2007;109:926–935. doi: 10.1182/blood-2006-01-024729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale GA, Coustan-Smith E, Stow P, Pan Q, Chen X, Pui CH, et al. Comparative analysis of flow cytometry and polymerase chain reaction for the detection of minimal residual disease in childhood acute lymphoblastic leukemia. Leukemia. 2004;18:934–938. doi: 10.1038/sj.leu.2403348. [DOI] [PubMed] [Google Scholar]
- Malec M, van der Velden VH, Bjorklund E, Wijkhuijs JM, Soderhall S, Mazur J, et al. Analysis of minimal residual disease in childhood acute lymphoblastic leukemia: comparison between RQ-PCR analysis of Ig/TcR gene rearrangements and multicolor flow cytometric immunophenotyping. Leukemia. 2004;18:1630–1636. doi: 10.1038/sj.leu.2403444. [DOI] [PubMed] [Google Scholar]
- Cave H, van der Werff ten Bosch J, Suciu S, Guidal C, Waterkeyn C, Otten J, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia. European Organization for Research and Treatment of Cancer—Childhood Leukemia Cooperative Group. N Engl J Med. 1998;339:591–598. doi: 10.1056/NEJM199808273390904. [DOI] [PubMed] [Google Scholar]
- Liu-Dumlao T, Kantarjian H, Thomas DA, O'Brien S, Ravandi F. Philadelphia-positive acute lymphoblastic leukemia: current treatment options. Curr Oncol Rep. 2012;14:387–394. doi: 10.1007/s11912-012-0247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Shen Y, Zhu Y, Chen S. Prognostic analysis of chronic myeloid leukemia in Chinese population in an imatinib era. Front Med. 2012;6:204–211. doi: 10.1007/s11684-012-0202-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.