Abstract
Compound K (20-O-(β-D-glucopyranosyl)-20(S)-protopanaxadiol) is an active metabolite of ginsenosides and induces apoptosis in various types of cancer cells. This study investigated the role of autophagy in compound K-induced cell death of human HCT-116 colon cancer cells. Compound K activated an autophagy pathway characterized by the accumulation of vesicles, the increased positive acridine orange-stained cells, the accumulation of LC3-II, and the elevation of autophagic flux. Whereas blockade of compound K-induced autophagy by 3-methyladenein and bafilomycin A1 significantly increased cell viability. In addition, compound K augmented the time-dependent expression of the autophagy-related proteins Atg5, Atg6, and Atg7. However, knockdown of Atg5, Atg6, and Atg7 markedly inhibited the detrimental impact of compound K on LC3-II accumulation and cell vitality. Compound K-provoked autophagy was also linked to the generation of intracellular reactive oxygen species (ROS); both of these processes were mitigated by the pre-treatment of cells with the antioxidant N-acetylcysteine. Moreover, compound K activated the c-Jun NH2-terminal kinase (JNK) signaling pathway, whereas downregulation of JNK by its specific inhibitor SP600125 or by small interfering RNA against JNK attenuated autophagy-mediated cell death in response to compound K. Compound K also provoked apoptosis, as evidenced by an increased number of apoptotic bodies and sub-G1 hypodiploid cells, enhanced activation of caspase-3 and caspase-9, and modulation of Bcl-2 and Bcl-2-associated X protein expression. Notably, compound K-stimulated autophagy as well as apoptosis was induced by disrupting the interaction between Atg6 and Bcl-2. Taken together, these results indicate that the induction of autophagy and apoptosis by compound K is mediated through ROS generation and JNK activation in human colon cancer cells.
Keywords: apoptosis, autophagy, compound K, reactive oxygen species, colon cancer
Autophagy is a highly conserved cellular degradation process characterized by the presence of abundant intracellular autophagic vacuoles termed autophagosomes. Autophagosomes sequester cellular proteins and organelles and then deliver them to lysosomes for degradation or recycling.1 Recently, autophagy has been recognized as a cell death mechanism that takes place in various types of cancer cells in response to anticancer therapies.2
Reactive oxygen species (ROS) have an important role in cell death, particularly in cancer cells. A ROS imbalance occurs when ROS such as the superoxide anion, the hydroxyl radical, and hydrogen peroxide are produced more rapidly than they are eliminated. Antioxidant defenses are overwhelmed under these conditions, resulting in oxidative stress.3 Oxidative stress is implicated in a number of physiological and pathological processes, including cancer, diabetes, and neurodegenerative diseases. ROS act as central mediators of cell fate decisions, depending on the extent of oxidative damage. Under physiological conditions, low levels of ROS lead to growth adaptation and survival.4 In contrast, excessive ROS production causes cellular damage and provokes apoptotic and necrotic cell death.4
Cancer cells, even at rest, have a higher metabolic rate than normal cells, resulting in comparatively higher intracellular ROS concentrations. Thus, cancer cells are under increased oxidative stress, rendering them more vulnerable to ROS-mediated insults and apoptosis. Recent investigations indicate that ROS also regulate autophagy, a catabolic progression that is involved in energy homeostasis, organelle turnover, development and differentiation, and cancer cell death.5 ROS similarly trigger autophagy during periods of starvation-provoked stress.6 Moreover, activation of c-Jun NH2-terminal kinase (JNK), which occurs downstream of ROS, induces autophagy via the upregulation of autophagy-related protein 6 (Atg6, also known as Beclin 1) expression7 and B-cell lymphoma 2 (Bcl-2) phosphorylation.8
Intestinal bacteria in humans and rats form ginsenoside metabolites after oral administration of ginseng extract.9 Compound K (20-O-(β-D-glucopyranosyl)-20(S)-protopanaxadiol) is a major ginsenoside metabolite detected in the urine and blood of rats after ginseng administration. Compound K is active in biological systems and inhibits glucose uptake in tumor cells. Compound K also possesses chemopreventive activity against chemical carcinogens, impairs metastasis in vivo, and constrains tumor growth through the inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced cyclooxygenase-2 expression.10, 11, 12, 13 We recently reported that compound K exhibits cytotoxicity against tumor cells by the induction of apoptosis and cell cycle arrest at G1 phase. These actions occur by a caspase-dependent pathway via mitochondrial disruption and inhibition of telomerase activity.14, 15 Furthermore, combination treatment with compound K and radiation enhances human lung cancer cell death16 and compound K induces apoptosis in MCF-7 human breast cancer cells by the generation of ROS and modulation of AMP-activated protein kinase signaling.17 Finally, compound K-mediated generation of ROS leads to apoptosis in HT-29 human colon cancer cells through the modulation of a mitochondria-dependent apoptotic pathway and the mitogen-activated protein kinase pathway.18
Apoptosis and autophagy are two distinct processes. Apoptosis is a tightly controlled suicide program that serves to remove defective, damaged, or otherwise superfluous cells, whereas autophagy may contribute to either cell survival or cell degradation.19 Recent investigations revealed that Bcl family proteins regulate autophagy, despite the well-established role of these proteins in apoptosis.20 In addition, increasing evidence shows an association between ROS generation and the initiation of autophagy.5, 6, 21 Several reports have indicated that compound K provokes apoptosis in colon cancer cells.18, 22 However, little is known regarding its ability to induce autophagy. Therefore, the aim of this study was to elucidate the cytotoxic mechanisms of compound K with respect to autophagy and apoptosis in human HCT-116 colon cancer cells.
Results
Induction of cell death by compound K in HCT-116 colon cancer cells
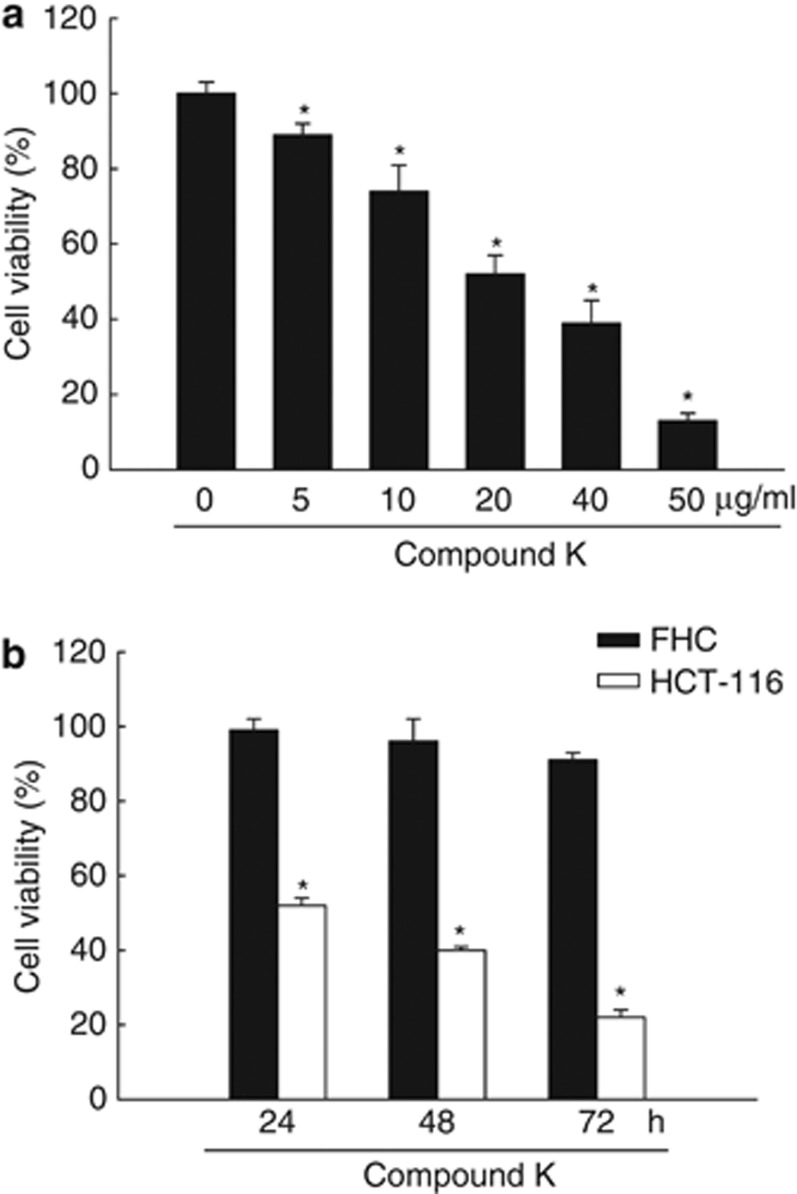
HCT-116 colon cancer cells were treated for 24 h with various concentrations of compound K to investigate the cytotoxic activity of compound K against these cells. Cell viability was then assessed using the [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium] bromide (MTT) assay. Compound K inhibited cell growth in a dose-dependent manner (Figure 1a), with 50% growth inhibition at a concentration of 20 μg/ml (the IC50). Compound K at its IC50 inhibited cell growth in a time-dependent manner; however, the growth of normal colon (fetal human colon, FHC) cells was inhibited to a lesser extent than the growth of HCT-116 colon cancer cells (Figure 1b).
Figure 1.
Compound K induces cell death in HCT-116 cells. (a) HCT-116 cell viability following treatment with the indicated concentrations of compound K for 24 h was assessed using the MTT assay. *Significantly different from untreated control (P<0.05). (b) HCT-116 and FHC cell viability at the indicated times was assessed in the presence of compound K (20 μg/ml) using the MTT assay. *Significantly different from FHC cells (P<0.05)
Compound K induces autophagy in HCT-116 cells
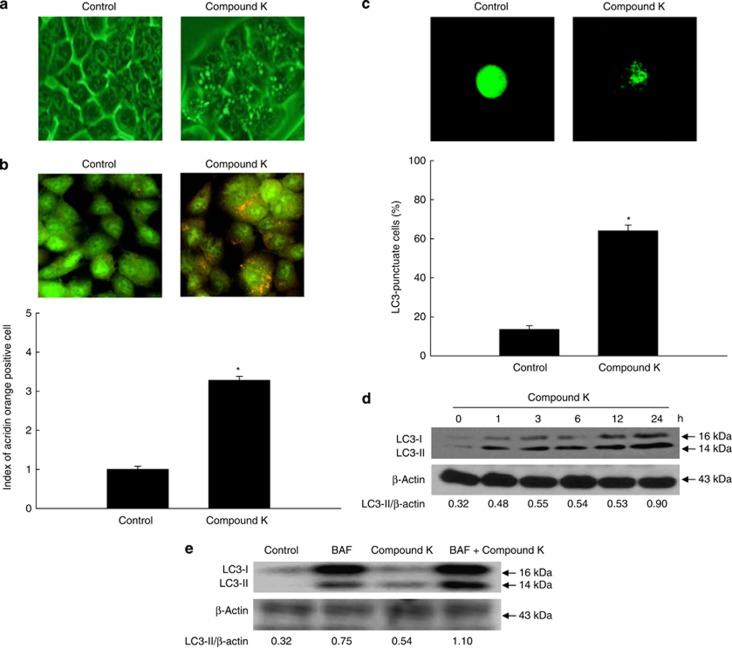
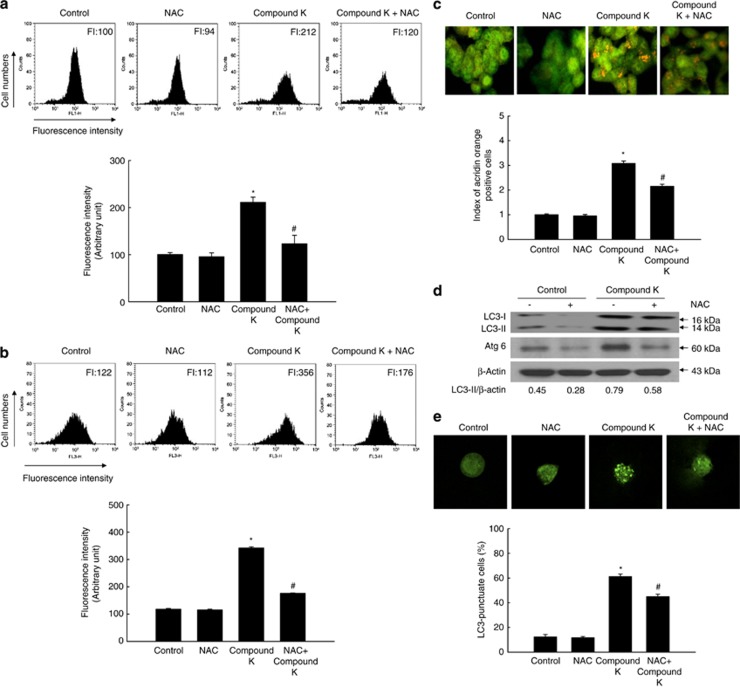
Next, we determined whether compound K-induced cell death occurred via autophagy. Autophagy-related cell death is characterized by the accumulation of vesicles.23 Compound K (20 μg/ml) triggered the accumulation of vacuolated HCT-116 cells following treatment for 24 h (Figure 2a). The vacuoles were positively stained with the lysosome marker dye, acridine orange (Figure 2b). Autophagy-related cell death is also characterized by the formation of autophagosomes. These structures, as well as autolysosomes, can be discerned by the presence of membrane-bound microtubule-associated protein 1 light chain 3 (LC3)-phospholipid conjugates.24 Transfection of cells with green fluorescent protein (GFP)-LC3 showed that the fraction of cells with a punctate GFP-LC3 localization was increased following treatment with compound K (20 μg/ml; Figure 2c). Compound K also yielded a time-dependent increase in the levels of LC3-II (the processed form of LC3; Figure 2d). It also detected autophagic flux using bafilomycin A1 (BAF), a specific autophagy inhibitor acting as autophagosomal lysosome degradation inhibition, which accumulates the LC3-II.25 Compound K treatment increased accumulation of LC3-II in BAF-treated cells (Figure 2e).
Figure 2.
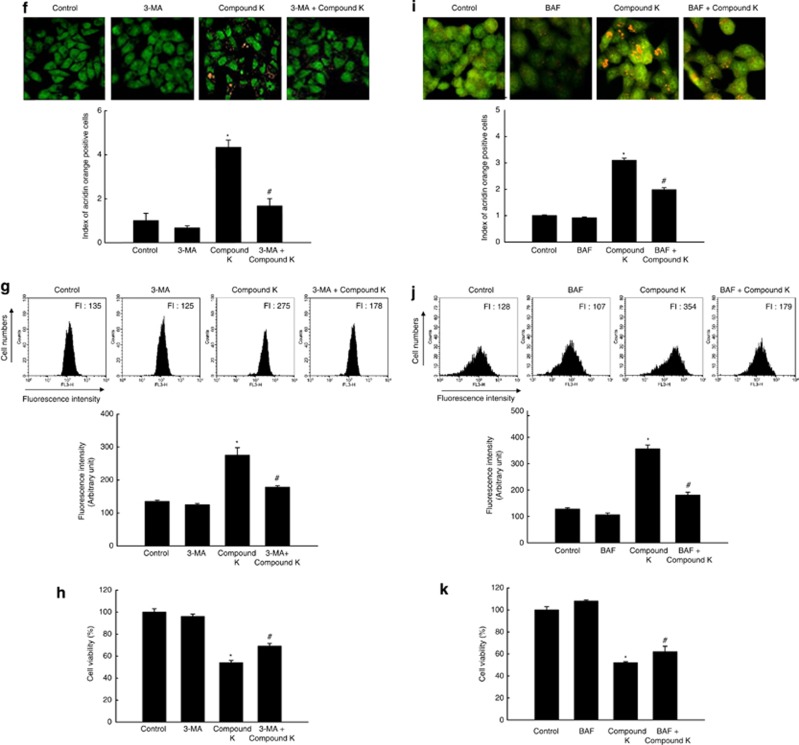
Compound K induces autophagy in HCT-116 cells. (a) HCT-116 cells were imaged with a light microscope after 24 h of compound K treatment (20 μg/ml; magnification, × 400). (b) After 24 h of compound K treatment (20 μg/ml), cells were stained with acridine orange, imaged by fluorescence microscopy, and quantified. *Significantly different from control (P<0.05). (c) After 24 h of compound K treatment (20 μg/ml), cells were transfected with GFP-LC3, imaged by fluorescence microscopy, and quantified. *Significantly different from control (P<0.05). (d) Cells were treated with compound K (20 μg/ml), and LC3-I (the unprocessed form of LC3) and LC3-II (the processed form of LC3) levels were assessed by western blot analysis. (e) Autophagy flux was detected in BAF (1 μM)-pre-treated cells with compound K (20 μg/ml) after 24 h. Cells were lysed and the level of LC3 protein was analyzed by western blot. Compound K-provoked autophagy was assessed by (f) fluorescence microscopy and (g) flow cytometry after acridine orange staining in 3-MA pre-treated cells, and (h) compound K-provoked autophagic cell death was assessed by MTT test in 3-MA pre-treated cells. *Significantly different from control (P<0.05) and #significantly different from compound K-treated cells (P<0.05). Compound K-provoked autophagic cell death was assessed by (i) fluorescence microscopy and (j) flow cytometry after acridine orange staining, and (k) by MTT test in BAF pre-treated cells. *Significantly different from control (P<0.05) and #significantly different from compound K-treated cells (P<0.05)
An autophagic inhibitor 3-methyladenine (3-MA) pre-treatment inhibited acridine orange-positive vacuole accumulation induced by compound K (Figures 2f and g) and attenuated cell death by compound K (Figure 2h). BAF pre-treatment also showed similar results of 3-MA (Figures 2i, j and k). These results suggested that compound K-triggered autophagy has a role as cell death process.
Autophagy-related (Atg) proteins are involved in autophagy induced by compound K
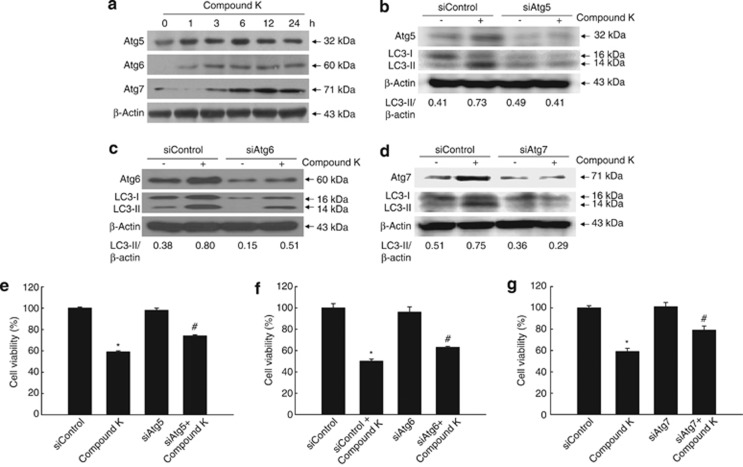
Expression patterns of Atg proteins provide key information about the autophagic state of a cell.26 Atg5 and Atg7 are ubiquitin ligase-like proteins that are specifically required for autophagy.27 The expression levels of Atg5 and Atg7 in HCT-116 cells were both markedly increased by compound K in a time-dependent manner (Figure 3a). The expression of Atg6, which initiates autophagosome formation during autophagy,28 was also increased in compound K-treated cells (Figure 3a). Moreover, siAtg5, 6, or 7 effectively attenuated the compound K-induced LC3-II (Figures 3b, c and d), as well as compound K-induced cell death (Figures 3e, f and g). These results indicate that Atg proteins are involved in autophagy provoked by compound K.
Figure 3.
Compound K upregulates the expression of Atg and LC3 proteins. (a) HCT-116 cells were treated with compound K (20 μg/ml) for the indicated times. The expression levels of the autophagy-related proteins Atg5, Atg6, and Atg7 were detected by western blot analysis. Cells were transfected with (b) siAtg5, (c) siAtg6, (d) siAtg7 and treated with compound K for 24 h. Expression levels of each Atg, and LC3 were assessed by western blot analysis. Cell viability was assessed using the MTT assay in (e) siAtg5, (f) siAtg6, and (g) siAtg7-transfected cells. *Significantly different from control (P<0.05) and #significantly different from siAtg-transfected cells (P<0.05)
Compound K induces autophagy-mediated cell death via ROS generation
ROS can reportedly mediate autophagy-mediated cell death.5, 21 Therefore, ROS generation was analyzed in compound K-treated cells by performing 2′,7′-dichlorodihydrofluorescein diacetate (DCF-DA) staining followed by flow cytometry. Compound K-stimulated ROS generation in HCT-116 cells (Figure 4a), but the enhanced ROS levels were significantly attenuated by treatment with N-acetylcysteine (NAC), a powerful antioxidant (Figure 4a). NAC also attenuated compound K-induced autophagy, as shown by a decrease in the number of acridine orange-positive cells (Figures 4b and c), LC3-II levels (Figure 4d), Atg6 expression (Figure 4d), and the fraction of cells with a punctate GFP-LC3 localization (Figure 4e). Taken together, these results suggest that ROS induced by compound K participate in the induction of autophagy.
Figure 4.
Compound K induces autophagy-mediated cell death via ROS generation. HCT-116 cells were treated with compound K (20 μg/ml) and/or NAC (1 mM) for 18 h. (a) ROS levels were assessed by flow cytometry after DCF-DA staining. Compound K-induced autophagy was assessed by (b) flow cytometry and (c) fluorescence microscopy after acridine orange staining, (d) western blot analysis, and (e) fluorescence microscopy after transfection with GFP-LC3. *Significantly different from control (P<0.05) and #significantly different from compound K-treated cells (P<0.05)
Compound K induces apoptosis in HCT-116 colon cancer cells
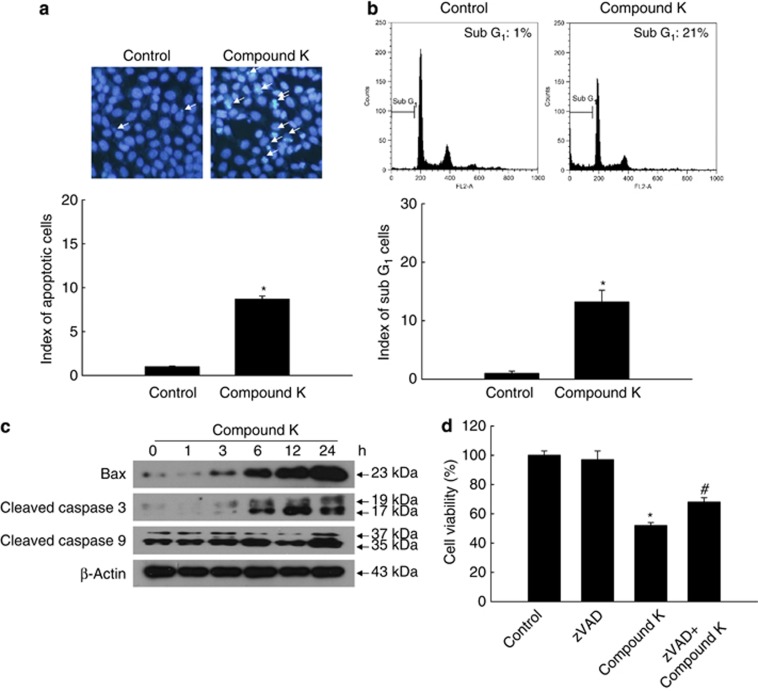
We next investigated whether compound K can induce apoptosis in HCT-116 cells. Compound K-specific induction of apoptosis was confirmed by the formation of apoptotic bodies (Figure 5a), an increase in the sub-G1 cell population from 1% of the total cell population to 21% (Figure 5b), and an increase in Bcl-2-associated X protein (Bax) expression and active (cleaved) caspase-9 and caspase-3 levels (Figure 5c). Concurrent exposure of cells to the pan-caspase inhibitor Z-VAD-FMK partially prevented compound K-provoked apoptotic cell death (Figure 5d). These results suggest that compound K engenders apoptotic cell death in addition to autophagy.
Figure 5.
Compound K induces apoptosis in HCT-116 cells. HCT-116 cells were treated with compound K (20 μg/ml) for 24 h. (a) Apoptotic body formation was observed after Hoechst 33342 staining and quantified. Apoptotic bodies are indicated by arrows. (b) The apoptotic sub-G1 DNA content was detected by flow cytometry after PI staining and quantified. (c) Bax, active (cleaved) caspase-3, and caspase-9 levels were assessed by western blot analysis. (d) Cells were treated with compound K (20 μg/ml) and/or Z-VAD-FMK (10 μM) for 24 h, and cell viability was assessed using the MTT assay. *Significantly different from control (P<0.05) and #significantly different from compound K-treated cells (P<0.05)
Activation of JNK is required for Atg5 and Atg6 upregulation and caspase activation in response to compound K
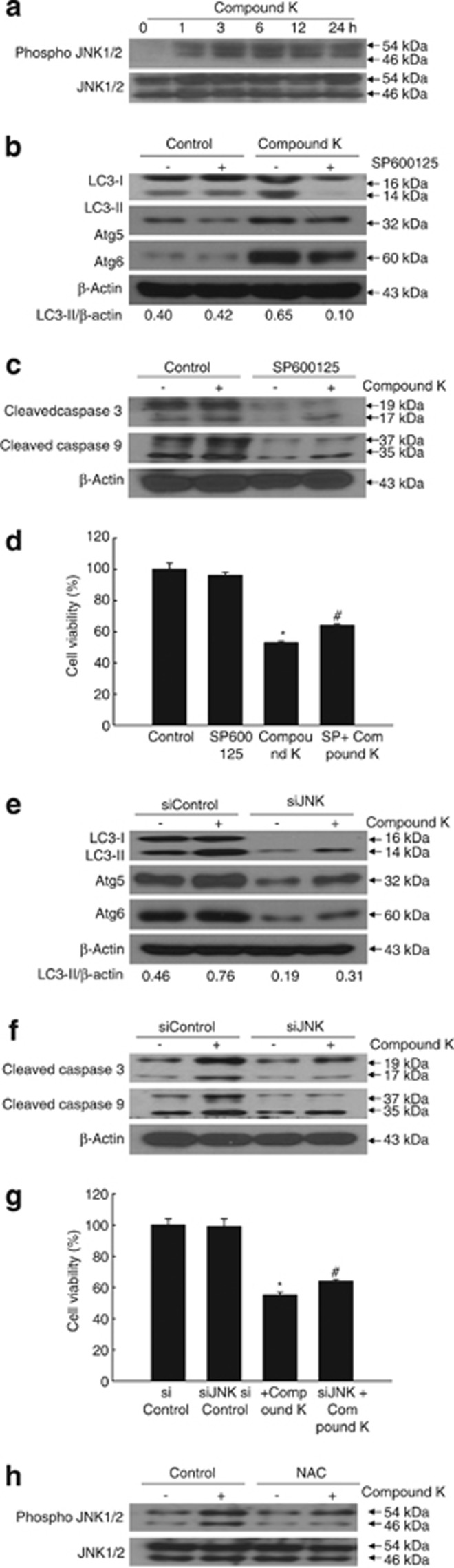
The JNK pathway is critical for the mediation of both autophagy and apoptosis.21, 29 Compound K increased the active (phosphorylated) form of JNK1/2 in a time-dependent manner (Figure 6a). JNK activation in turn increases the gene expression of Atg5 and Atg6.7, 21 Notably, the selective JNK inhibitor SP600125 significantly reduced compound K-augmented LC3-II, Atg5, and Atg6 expression (Figure 6b), levels of activated caspase-3 and caspase-9 (Figure 6c), and apoptotic cell death (Figure 6d), as did siJNK (Figures 6e, f and g). Furthermore, NAC treatment decreased compound K-induced phosphorylation of JNK, suggesting that intracellular ROS act upstream of JNK to mediate the actions of compound K (Figure 6h). Taken together, these results suggest that the activation of JNK is required for compound K-induced autophagy and apoptosis in colon cancer cells.
Figure 6.
Compound K-induced JNK activation is involved in autophagy-mediated cell death. (a) HCT-116 cells were treated with compound K (20 μg/ml) for the indicated times. Phospho-JNK and JNK levels were detected by western blot analysis. Cells were treated with compound K and/or SP600125 for 24 h. (b) LC3-II, Atg5, and Atg6 levels and (c) cleaved caspase-3 and caspase-9 levels were detected by western blot analysis. (d) Cell viability was assessed using the MTT assay. *Significantly different from control (P<0.05) and #significantly different from compound K-treated cells (P<0.05). Cells were transfected with siJNK and treated with compound K (20 μg/ml) for 24 h. (e) LC3-II, Atg5, and Atg6 levels and (f) cleaved caspase-3 and caspase-9 levels were detected by western blot analysis. (g) Cell viability was assessed using the MTT assay. (h) Cells were treated with compound K (20 μg/ml) and/or NAC (1 mM) for 18 h. Phospho-JNK1/2 and JNK1/2 levels were assessed by western blot analysis. *Significantly different from control (P<0.05) and #significantly different from compound K-treated cells (P<0.05)
Bcl family proteins regulate compound K-induced autophagy
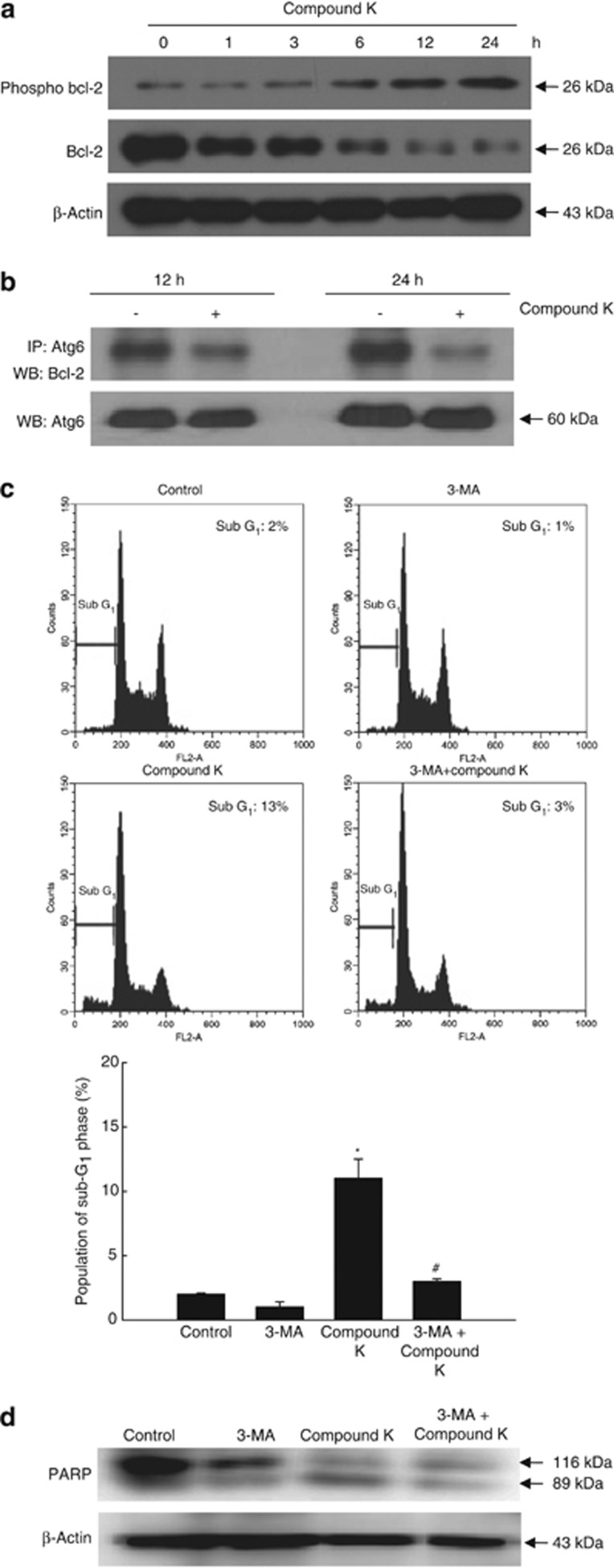
The autophagic function of Atg6 can be inhibited by interaction with Bcl family proteins (e.g., Bcl-2 and Bcl-xL) to form Atg6-Bcl-2 and Atg6-Bcl-xL complexes.20 Here, Bcl-2 functions as an oncogene by negatively regulating Atg6-dependent autophagy, as well as cell death.30 On the other hand, Bcl-2 phosphorylation disrupts the interaction between Atg6 and Bcl-2, releasing Atg6 to promote autophagy.8 Moreover, the resultant phosphorylated form of Bcl-2 can also induce apoptosis.30 Compound K increased the levels of phospho-Bcl-2 in a time-dependent manner, while concomitantly decreasing the levels of total Bcl-2 (Figure 7a). In addition, examination of the interaction between Atg6 and Bcl-2 by immune-precipitation with an anti-Atg6 antibody revealed that compound K significantly decreased the amount of Bcl-2 complexed with Atg6 (Figure 7b). In addition, 3-MA pre-treatment attenuated compound K-induced apoptotic phenomenon such as sub-G1 cell population (Figure 7c) and caspase 3 activity, which is detected by cleaved PARP level (Figure 7d).
Figure 7.
Bcl family members regulate compound K-induced autophagy. HCT-116 cells were treated with compound K (20 μg/ml) for the indicated times. (a) Bcl-2 and phospho-Bcl-2 levels were assessed by western blot analysis. (b) Cell lysates were subjected to immune-precipitation with an antibody against Atg6, and the immune-precipitates were subjected to western blot analysis with an antibody against Bcl-2. Compound K-provoked apoptosis was assessed by (c) flow cytometry and (d) western blot analysis in 3-MA pre-treated cells. *Significantly different from control (P<0.05) and #significantly different from compound K-treated cells (P<0.05)
Discussion
Cell death is a complex phenomenon that is independently or coordinately regulated by multiple cellular and molecular mechanisms.31 Programmed cell death is a genetically regulated form of cell death that is essential for various biological events and acts as an ultimate determinant of cancer cell fate.31 Indeed, therapeutic approaches designed to destroy malignant cells via programmed cell death are critical for the treatment of various forms of cancer.32
Programmed cell death can be divided into two categories, apoptosis and autophagic cell death. Apoptosis was initially described according to its morphological characteristics, including cell shrinkage, dynamic membrane blebbing, chromatin condensation, and nuclear fragmentation.33 Apoptosis involves many pathways and signaling proteins, such as death receptor family proteins, the nuclear transcription factor p53, and Bcl-2 family proteins.34 Apoptosis can be triggered through the extrinsic (or death receptor) pathway, which is initiated from outside the cell through pro-apoptotic receptors on the cell surface, or the intrinsic (or mitochondrial) pathway, which is initiated from within the cell.32, 34 Autophagic cell death, on the other hand, indicates a cell death that is mediated by autophagy, and therefore suppressed by autophagy inhibitor via chemicals and/or genetic means.35
Autophagy is an evolutionarily conserved catabolic process that begins with the formation of autophagosomes. Autophagosomes participate in the recycling of cellular components by sequestering damaged organelles and misfolded proteins, targeting them for lysosomal degradation.1, 36
The formation of a double-membraned autophagosome requires specific autophagy-associated proteins. Some of the most important evidence for the role of autophagic cell death in tumor suppression comes from studies on Atg6, a Bcl-2-interacting protein28, 30 that promotes autophagy when not complexed with Bcl-2. Furthermore, constitutive activation of the phosphoinositide 3-kinase-Akt (also known as protein kinase B)-mammalian target of rapamycin (mTOR) axis,37 a common characteristic of cancer cells, suppresses autophagy while promoting tumor cell growth, proliferation, and survival.38 In addition, recent studies have shown that LC3, an autophagy-related, ubiquitin-like modifier, is processed by a unique protein activation/conjugation system to form autophagosomal membranes in cancer cells during autophagy.39
In this study, compound K-induced autophagy in HCT-116 colon cancer cells was evidenced by an increase in the number of autophagic vesicles and the enhanced conversion of LC3-I to LC3-II. Notably, many anticancer drugs (e.g., bortezomib, sorafenib, resveratrol, chloroquine, histone deacetylase inhibitors, mTOR inhibitors, and sulforaphane) similarly engender autophagic cell death.36 The cellular redox status is among the effector mechanisms implicated in the control and regulation of cell death pathways, including autophagy and apoptosis. Recent investigations have shown that autophagic cell death is associated with alterations in ROS levels and/or the JNK signaling pathway.21, 27 Consistent with these findings, we found that ROS generation and JNK activation were both involved in compound K-stimulated autophagy in colon cancer cells.
In our system, compound K-induced generation of ROS occurred upstream of JNK activation. ROS generation mediates the activation of JNK in the copper compound-induced cell death pathway.40 Furthermore, tumor necrosis factor-α-dependent ROS production leads to sustained JNK activation and cell death;41 notably, ROS formation is essential for monitoring autophagy-mediated cell death in cancer cells.4, 6, 40, 41 JNK-mediated upregulation of Atg5 and Atg6 also has a causal role in autophagy-mediated cell death.7 Similarly, ROS-mediated JNK activation has a causal role in the initiation of autophagy through the upregulation of Atg5 and Atg7,28, 39 and the subsequent induction of malignant transformation by oncogenic K-Ras. Our results showed that activation of JNK by compound K is required for Atg5 and Atg6 upregulation and caspase activation.
Activated JNK can directly phosphorylate Bcl-2 and indirectly increase the expression of Atg6 through the activation of c-Jun, its target transcription factor.10, 33 In this regard, JNK-mediated phosphorylation of Bcl-2 upregulates free Atg6 levels by releasing Atg6 from the Atg6-Bcl-2 complex,8, 28, 30 thereby promoting autophagic and apoptotic cell death. Compound K significantly decreased the amount of Bcl-2 complexed with Atg6.
Apoptosis and autophagy share common molecular regulators and signaling pathways.19, 42 However, the phenotypic outcome of the interaction between these two processes is extremely complex because apoptosis and autophagy can exhibit either synergistic or antagonistic effects on each other to modulate programmed cell death.32, 43 The mechanisms that generate this phenotypic complexity have not yet been well characterized. Furthermore, autophagy induced by clinically available anticancer agents can protect cancer cells from apoptotic cell death, depending on the cell type and/or the context.44 Therefore, understanding the crosstalk between apoptosis and autophagy may be crucial for the development of therapeutic strategies to improve the efficacy of anticancer agents.
In summary, we showed that (1) compound K induces autophagy as well as apoptosis in HCT-116 colon cancer cells; (2) inhibition of apoptosis or autophagy restores cell viability in compound K-treated cells; and (3) exposure to compound K augments ROS levels and activates JNK signaling. Therefore, our findings provide a basis for future investigations aimed at elucidating the role of apoptosis and autophagy in colon cancer therapy.
Materials and Methods
Preparation of compound K
Compound K was prepared by the incubation of ginsenosides with Bacteroides JY-6, a human intestinal bacterium, which was then subcultured in a general anaerobic medium for 24 h at 37 °C. The conditioned medium was extracted with n-butanol, and the supernatant was concentrated in vacuo and processed using silica gel column chromatography with chloroform/methanol/H2O (65:33:10). Isolated compound K was characterized by mass spectroscopy and 1H and 13C nuclear magnetic resonance spectrometry.
Chemicals and antibodies
Propidium iodide (PI), DCF-DA, NAC, MTT, 3-MA, and an anti-β-actin antibody were purchased from Sigma Chemical Co. (St. Louis, MO, USA). The pan-caspase inhibitor (Z-VAD-FMK) was purchased from Tocris (Bristol, UK). BAF and JNK inhibitor (SP600125) were purchased from Calbiochem (San Diego, CA, USA). Primary antibodies against Bcl-2, Bax, phospho-Bcl-2, Atg5, Atg6, Atg7, caspase-3, caspase-9, JNK, and phospho-JNK were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The antibody against LC3 (a documented autophagy marker) was purchased from Cell Signaling Technology (Beverly, MA, USA). All other reagents were of analytical grade.
Cell culture
Human colon cancer (HCT-116) and normal FHC cells were obtained from the American Type Culture Collection (Rockville, MD, USA) and maintained at 37 °C in an incubator with a humidified atmosphere of 5% CO2 in air. HCT-116 cells were cultured in RPMI 1640 medium containing 0.1le, MD, USA) and maintained at 37er reagents were o fetal calf serum (FCS), streptomycin (100 μg/ml), and penicillin (100 units/ml). FHC cells cultured in a 1:1 mixture of Ham's F12 and Dulbecco's modified Eagle's medium containing HEPES (25 mM), cholera toxin (10 ng/ml), insulin (5 μg/ml), transferrin (5 μg/ml) and hydrocortisone (100 ng/ml), streptomycin (100 μg/ml), penicillin (100 units/ml), and 10% FCS.
Cell viability
Cell viability was determined using the MTT assay, which is based on the reduction of a tetrazolium salt by mitochondrial dehydrogenases in viable cells.45 HCT-116 and FHC cells were seeded into a 96-well plate at a density of 1 × 105 cells per well and treated with compound K or reagents. After incubating for 24 h, MTT stock solution (50 μl; 2 mg/ml) was added to each well to yield a total reaction volume of 250 μl. After incubating for a further 4 h the supernatants were aspirated, and the formazan crystals in each well were dissolved in dimethyl sulfoxide (150 μl). The absorbance at 540 nm was read on a scanning multi-well spectrophotometer.
Acridine orange staining
Acidic intracellular compartments were visualized by acridine orange staining. After seeding, HCT-116 cells were washed with phosphate-buffered saline (PBS) and stained with 10 μg/ml acridine orange (Invitrogen, Carlsbad, CA, USA) for 15 min at 37 °C. Subsequently, cells were washed with PBS and viewed under a laser scanning confocal microscope. Microscopic images were collected using the Laser Scanning Microscope 5 PASCAL program (Carl Zeiss, Jena, Germany). Depending on their acidity, autophagic lysosomes appeared as orange/red fluorescent cytoplasmic vesicles, while the nuclei were stained green. Alternatively, acridine orange-stained cells were trypsinized, washed with PBS, and analyzed with a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA, USA) using CellQuest Pro software (Becton Dickinson).
LC3 transfection and detection of punctate LC3-positive structures
Autophagy was evidenced by the formation of punctate LC3-positive structures, which are essential for the dynamic process of autophagosome formation.46 HCT-116 cells were transfected with GFP-tagged LC3 using Lipofectamine reagent (Invitrogen) according to the manufacturer's instructions. GFP-LC3 fluorescence was imaged using a confocal microscope equipped with the Laser Scanning Microscope 5 PASCAL program (Carl Zeiss).
Western blot analysis
Cell lysates were electrophoresed, and separated proteins were transferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA, USA). The membranes were incubated with primary antibodies, followed by horseradish peroxidase-conjugated immunoglobulin G secondary antibodies (Pierce, Rockford, IL, USA). Protein bands were detected with an enhanced chemiluminescence western blot detection kit (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
Measurement of intracellular ROS
The DCF-DA method was used to measure the level of intracellular ROS in HCT-116 cells.47 Image analysis of the generation of intracellular ROS was achieved by seeding the cells into a six-well plate containing coverslips at a density of 1 × 105 cells per well. Twenty-four hours after plating, cells were treated with 1 mM NAC for 1 h, at which time compound K was added. After changing the media, 100 μM DCF-DA was added to each well and incubated for an additional 30 min at 37 °C. After washing with PBS, the stained cells were mounted onto microscope slides in mounting medium (Dako, Carpinteria, CA, USA). Microscopic images of fluorescence were collected using a confocal microscope equipped with the Laser Scanning Microscope 5 PASCAL program (Carl Zeiss). Alternatively, 25 μM DCF-DA was incubated with the cells for 45 min, and the fluorescence of 2′,7′-dichlorofluorescein was detected via flow cytometry.
Small interfering RNA (siRNA) transfection
HCT-116 cells were seeded into a 24-well plate at a density of 1.5 × 105 cells per well and allowed to reach approximately 50% confluence on the day of transfection. The siRNA constructs employed herein were (1) mismatched siRNA control (siControl; Santa Cruz Biotechnology), (2) siRNA against JNK (siJNK; Bioneer Corporation, Daejeon, Republic of Korea), and (3) siRNA against Atg5, Atg6, Atg7 (siAtg5, siAtg6, siAtg7, Bioneer Corporation). Cells were transfected with 10–50 nM siRNA using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions.
Nuclear staining with Hoechst 33342
Cells were seeded into a 24-well plate, and Hoechst 33342 (1.5 μl; 10 mg/ml stock solution), a DNA-specific fluorescent dye, was added to each well and incubated with the cells for 10 min at 37 °C. The stained cells were visualized under a fluorescence microscope equipped with a CoolSNAP-Pro color digital camera (Media Cybernetics, Rockville, MD, USA).
Detection of sub-G1 hypodiploid cells
Apoptotic sub-G1 hypodiploid cells were detected via flow cytometry.48 Cells were first treated with compound K for 24 h. Harvested cells were then washed twice with PBS and fixed in 70% ethanol for 30 min at 4 °C. The cells were incubated with PI (50 mg/ml) and RNase A (50 μg/ml) in the dark for 30 min at 37 °C. Flow cytometric analysis was performed using a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA, USA). The sub-G1 hypodiploid cells were assessed based on histograms generated by the CellQuest and Mod-Fit computer programs (Becton Dickinson).
Immune-precipitation
Cells were treated with compound K for 12 and 24 h. Harvested cells were washed with PBS and lysed in radioimmune-precipitation buffer (1 ml; 50 mM Tris-HCl [pH 6.8], 0.1% sodium dodecyl sulfate, 150 mM NaCl, 1 mM EDTA, 0.1 mM Na3VO4, 1 mM sodium fluoride, 1% Triton X-100, 1% NP40, 1 mM dithiothreitol, 1 mM PMSF, 1 μg/ml aprotinin, and 1 μg/ml leupeptin). After lysis for 30 min and centrifugation at 14 000 × g for 15 min, the supernatants were collected. An aliquot of protein G agarose beads (30 μl; Sigma Chemical Co.) was added to the immune-precipitation buffer.49 Cell lysates were then subjected to immune-precipitation with an anti-Atg6 antibody, followed by an agarose-conjugated antibody. Western blot analysis of co-immune-precipitated Bcl-2 was then performed.
Statistical analysis
All measurements were made in triplicate, and all values are expressed as the mean±S.E.M. The results were subjected to an analysis of variance followed by Tukey's post hoc test to analyze differences between conditions. P<0.05 was considered significant.
Acknowledgments
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry for Health and Welfare, Republic of Korea (1120340).
Glossary
- ROS
reactive oxygen species
- JNK
c-Jun NH2-terminal kinase
- Bcl-2
B-cell lymphoma 2
- Atg
autophagy-related protein
- PI
propidium iodide
- DCF-DA
2′,7′-dichlorodihydrofluorescein diacetate
- NAC
N-acetylcysteine
- MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium] bromide
- GFP
green fluorescent protein
- 3-MA
3-methyladenine
- BAF
bafilomycin A1
- Bax
Bcl-2-associated X protein
- LC3
microtubule-associated protein 1 light chain 3
- siRNA
small interfering RNA
The authors declare no conflict of interest.
Footnotes
Edited by A Finazzi-Agró
References
- Rubinsztein DC, Gestwicki JE, Murphy LO, Klionsky DJ. Potential therapeutic applications of autophagy. Nat Rev Drug Discov. 2007;6:304–312. doi: 10.1038/nrd2272. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Li F, Shi K, Yang Y, Xu C. Sodium selenite-induced activation of DAPK promotes autophagy in human leukemia HL60 cells. BMB Rep. 2012;45:194–199. doi: 10.5483/BMBRep.2012.45.3.194. [DOI] [PubMed] [Google Scholar]
- Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol. 2007;47:143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- Azad MB, Chen Y, Gibson SB. Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid Redox Signal. 2009;11:777–790. doi: 10.1089/ars.2008.2270. [DOI] [PubMed] [Google Scholar]
- Ling LU, Tan KB, Lin H, Chiu GN. The role of reactive oxygen species and autophagy in safingol-induced cell death. Cell Death Dis. 2011;2:e129. doi: 10.1038/cddis.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DD, Wang LL, Deng R, Tang J, Shen Y, Guo JF, et al. The pivotal role of c-Jun NH2-terminal kinase-mediated Beclin 1 expression during anticancer agents induced autophagy in cancer cells. Oncogene. 2009;28:886–898. doi: 10.1038/onc.2008.441. [DOI] [PubMed] [Google Scholar]
- Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell. 2008;30:678–688. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa H, Sung JH, Matsumiya S, Uchiyama M. Main ginseng saponin metabolites formed by intestinal bacteria. Planta Med. 1996;62:453–457. doi: 10.1055/s-2006-957938. [DOI] [PubMed] [Google Scholar]
- Shin JY, Lee JM, Shin HS, Park SY, Yang JE, Cho SK, et al. Anti-cancer effect of ginsenoside F2 against glioblastoma multiforme in xenograft model in SD rats. J Ginseng Res. 2012;36:86–92. doi: 10.5142/jgr.2012.36.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen T, Lee J, Park MH, Lee YG, Rho HS, Kwak Y, et al. Ginsenoside Rp1, a ginsenoside derivative, blocks promoter activation of iNOS and COX-2 genes by suppression of an IKKb-mediated NF-kB pathway in HEK293 cells. J Ginseng Res. 2011;35:200–208. doi: 10.5142/jgr.2011.35.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Shin JW, Chun KS, Park KK, Chung WY, Bang YJ, et al. Antitumor promotional effects of a novel intestinal bacterial metabolite (IH-901) derived from the protopanaxadiol-type ginsenosides in mouse skin. Carcinogenesis. 2005;26:359–367. doi: 10.1093/carcin/bgh313. [DOI] [PubMed] [Google Scholar]
- Yim HW, Jong HS, Kim TY, Choi HH, Kim SG, Song SH, et al. Cyclooxygenase-2 inhibits novel ginseng metabolite-mediated apoptosis. Cancer Res. 2005;65:1952–1960. doi: 10.1158/0008-5472.CAN-04-1740. [DOI] [PubMed] [Google Scholar]
- Kang KA, Kim YW, Kim SU, Chae S, Koh YS, Kim HS, et al. G1 phase arrest of the cell cycle by a ginseng metabolite, compound K, in U937 human monocytic leukamia cells. Arch Pharm Res. 2005;28:685–690. doi: 10.1007/BF02969359. [DOI] [PubMed] [Google Scholar]
- Kang KA, Lim HK, Chae S, Kim JK, Seo JY, Ham YH, et al. Inhibition of telomerase activity in U937 human monocytic leukemia cells by compound K, a ginseng saponin metabolite. Biotechnol. Bioprocess Eng. 2005;11:7–12. [Google Scholar]
- Chae S, Kang KA, Chang WY, Kim MJ, Lee SJ, Lee YS, et al. Effect of compound K, a metabolite of ginseng saponin, combined with gamma-ray radiation in human lung cancer cells in vitro and in vivo. J Agric Food Chem. 2009;57:5777–5782. doi: 10.1021/jf900331g. [DOI] [PubMed] [Google Scholar]
- Kim AD, Kang KA, Zhang R, Lim CM, Kim HS, Kim DH, et al. Ginseng saponin metabolite induces apoptosis in MCF-7 breast cancer cells through the modulation of AMP-activated protein kinase. Environ. Toxicol Pharmacol. 2010;30:134–140. doi: 10.1016/j.etap.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Lee IK, Kang KA, Lim CM, Kim KC, Kim HS, Kim DH, et al. Compound K, a metabolite of ginseng saponin, induces mitochondria-dependent and caspase-dependent apoptosis via the generation of reactive oxygen species in human colon cancer cells. Int J Mol Sci. 2010;11:4916–4931. doi: 10.3390/ijms11124916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Xie CM, Chan WY, Yu S, Zhao J, Cheng CH. Bufalin induces autophagy-mediated cell death in human colon cancer cells through reactive oxygen species generation and JNK activation. Free Radic Biol Med. 2011;51:1365–1375. doi: 10.1016/j.freeradbiomed.2011.06.016. [DOI] [PubMed] [Google Scholar]
- Kim DY, Park MW, Yuan HD, Lee HJ, Kim SH, Chung SH. Compound K induces apoptosis via CAMK-IV/AMPK pathways in HT-29 colon cancer cells. J Agric Food Chem. 2009;57:10573–10578. doi: 10.1021/jf902700h. [DOI] [PubMed] [Google Scholar]
- Kim YK, Ahn JH, Lee M. Src family kinase inhibitor PP2 induces LC3 conversion in a manner that is uncoupled from autophagy and increases apoptosis in multidrug-resistant cells. Biomol Ther. 2012;20:393–398. doi: 10.4062/biomolther.2012.20.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanida I, Minematsu-Ikeguchi N, Ueno T, Kominami E. Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy. 2005;1:84–91. doi: 10.4161/auto.1.2.1697. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Tagawa Y, Yoshimori T, Moriyama Y, Masaki R, Tashiro Y. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct. 1998;23:33–42. doi: 10.1247/csf.23.33. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T, Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol. 2011;27:107–132. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- Gao M, Yeh PY, Lu YS, Hsu CH, Chen KF, Lee WC, et al. OSU-03012, a novel celecoxib derivative, induces reactive oxygen species-related autophagy in hepatocellular carcinoma. Cancer Res. 2008;68:9348–9357. doi: 10.1158/0008-5472.CAN-08-1642. [DOI] [PubMed] [Google Scholar]
- Cao Y, Klionsky DJ. Physiological functions of Atg6/Beclin 1: a unique autophagy-related protein. Cell Res. 2007;17:839–849. doi: 10.1038/cr.2007.78. [DOI] [PubMed] [Google Scholar]
- Liu J, Lin A. Role of JNK activation in apoptosis: a double-edged sword. Cell Res. 2005;15:36–42. doi: 10.1038/sj.cr.7290262. [DOI] [PubMed] [Google Scholar]
- Pattingre S, Levine B. Bcl-2 inhibition of autophagy: a new route to cancer. Cancer Res. 2006;66:2885–2888. doi: 10.1158/0008-5472.CAN-05-4412. [DOI] [PubMed] [Google Scholar]
- Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A. Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 2009;16:966–975. doi: 10.1038/cdd.2009.33. [DOI] [PubMed] [Google Scholar]
- Laubenbacher R, Hower V, Jarrah A, Torti SV, Shulaev V, Mendes P, et al. A systems biology view of cancer. Biochim Biophys Acta. 2009;1796:129–139. doi: 10.1016/j.bbcan.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73:2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT, Liu B, et al. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012;45:487–498. doi: 10.1111/j.1365-2184.2012.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periyasamy-Thandavan S, Jiang M, Schoenlein P, Dong Z. Autophagy: molecular machinery, regulation, and implications for renal pathophysiology. Am J Physiol Renal Physiol. 2009;297:F244–F256. doi: 10.1152/ajprenal.00033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updat. 2008;11:32–50. doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janku F, McConkey DJ, Hong DS, Kurzrock R. Autophagy as a target for anticancer therapy. Nat Rev Clin Oncol. 2011;8:528–539. doi: 10.1038/nrclinonc.2011.71. [DOI] [PubMed] [Google Scholar]
- Byun JY, Yoon CH, An S, Park IC, Kang CM, Kim MJ, et al. The Rac1/MKK7/JNK pathway signals upregulation of Atg5 and subsequent autophagic cell death in response to oncogenic Ras. Carcinogenesis. 2009;30:1880–1888. doi: 10.1093/carcin/bgp235. [DOI] [PubMed] [Google Scholar]
- Trejo-Solís C, Jimenez-Farfan D, Rodriguez-Enriquez S, Fernandez-Valverde F, Cruz-Salgado A, Ruiz-Azuara L, et al. Copper compound induces autophagy and apoptosis of glioma cells by reactive oxygen species and JNK activation. BMC Cancer. 2012;12:156. doi: 10.1186/1471-2407-12-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFα-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Maiuri MC, Criollo A, Kroemer G. Crosstalk between apoptosis and autophagy within the Beclin 1 interactome. EMBO J. 2010;29:515–516. doi: 10.1038/emboj.2009.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Kepp O, Michaud M, Martins I, Minoux H, Métivier D, et al. Association and dissociation of autophagy, apoptosis and necrosis by systematic chemical study. Oncogene. 2011;30:4544–4556. doi: 10.1038/onc.2011.168. [DOI] [PubMed] [Google Scholar]
- Dalby KN, Tekedereli I, Lopez-Berestein G, Ozpolat B. Targeting the prodeath and prosurvival functions of autophagy as novel therapeutic strategies in cancer. Autophagy. 2010;6:322–329. doi: 10.4161/auto.6.3.11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael J, DeGraff WG, Gazdar AF. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987;47:936–941. [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz AR, Schmaldienst S, Stuhlmeier KM, Chen W, Knapp W, Zlabinger GJ. A microplate assay for the detection of oxidative products using 2',7'dichlorofluorescein-diacetate. J Immunol Methods. 1992;156:39–45. doi: 10.1016/0022-1759(92)90008-h. [DOI] [PubMed] [Google Scholar]
- Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- Doolittle MH, Martin DC, Davis RC, Reuben MA, Elovson J. A two-cycle immunoprecipitation procedure for reducing nonspecific protein contamination. Anal Biochem. 1991;195:364–368. doi: 10.1016/0003-2697(91)90342-q. [DOI] [PubMed] [Google Scholar]