Abstract
Oncolytic herpes simplex virus-1 (oHSV) vectors selectively replicate in tumor cells where they kill through oncolysis, while sparing normal cells. One of the drawbacks of oHSV vectors is their limited replication and spread to neighboring cancer cells. Here we report the outcome of a high-throughput chemical library screen to identify small molecule compounds that augment the replication of oHSV G47Δ. Of the 2640-screened bioactives, six compounds were identified and subsequently validated for enhanced G47Δ replication. Two of these compounds, dipyridamole (DP) and dilazep (DL), interfered with nucleotide metabolism by potently and directly inhibiting ENT1 (equilibrative nucleoside transporter-1). Replicative amplification promoted by DP and DL were dependent upon HSV mutations in ICP6, the large subunit of ribonucleotide reductase (RR). Our results indicate that ENT1 antagonists augment oHSV replication in tumor cells by increasing cellular ribonucleoside activity.
Keywords: Prostate cancer, HSV, oncolytic virus, virotherapy, high-throughput screen
Introduction
Viral vectors genetically engineered for cancer cell restricted replication, represent an attractive strategy for tumor therapy because these viruses can replicate and spread in situ, exhibiting oncolytic activity through direct cytopathic effects (1,2). We and others have previously demonstrated that appropriately selected pharmaceuticals can synergize with oHSV's to increase oncolytic efficacy (3-5). To identify new agents and mechanisms that would increase G47Δ replication in cancer cells, we undertook an unbiased high throughput screen (HTS) of known bioactive molecules. We have identified dipyridamole (DP) and dilazep (DL) as potent enhancers of G47Δ replication, revealing a previously unidentified function for two well-characterized inhibitors of the equilibrative nucleoside transporter-1 (ENT1).
Material and Methods
HTS screen
Piloting and primary screen was conducted at the ICCB-Longwood core facility. Z'-factors were used to normalize for plate-to-plate variation (6). PC3 cells were seeded in 384-well cell culture plates and the following day compounds were added in duplicate and incubated for 6 hrs prior to infection with G47Δ-GFP (MOI=0.05). Forty-eight hrs post-infection, PC3 cells were imaged on an automated Image-Xpress inverted fluorescent microscope (Molecular Devices) using two wavelengths, 488 nM to detect G47Δ-GFP infected cells and 350 nM for nuclear DNA bound by Hoechst-33342.
Viruses
G47Δ-GFP (G47Δ-BAC) contains a cytomegalovirus promoter–driven enhanced green fluorescent protein (GFP) in place of lacZ in G47Δ (7). R3616 contains 1-kb deletions of both copies of γ34.5 (8). G47Δ was derived from G207 by deleting α47 and the US11 (9). F△6 is a strain F–derived recombinant with an ICP6-inactivating lacZ insertion (10).
Compounds
Dipyridamole, dialazep, NBMPR, zaprinast, uridine, thymidine and adenosine were purchased from Sigma (St. Louis, MO). SB203580, GF109203X, EHNA, AG1517 (PD153035) and IBMX were purchased from Calbiochem-Novabiochem (La Jolla, CA).
Gene expression analysis
Reverse-transcription PCR was used to verify human ENT1, RR1, RR2 and GAPDH mRNA levels in tumor cells. For quantitative RT-PCR analysis, PCR was performed using the 7000 Real-Time PCR Sequence Detection System (Applied BioSystems).
Multi-step growth assays
Tumor cells were pretreated for 4-6 hrs with a dose-range of compounds followed by virus infection at an MOI of 0.05. Forty-eight hours after infection, virus titers (plaque forming units (pfu)/ml) were determined by standard plaque assay on Vero cells.
Ribonucleotide Reductase assay
RR activity was measured utilizing the CDP assay method as previously described (11).
In vivo studies
Du145 cells (5 × 106 cells) were implanted subcutaneously into the flanks of athymic male mice (NCI, Fredrick, MD). Mice were administered dipyridamole (dissolved in DMSO and diluted in 0.1N HCl plus 0.9% NaCl) or dilazep (dissolved in H20 and diluted in PBS) intraperitoneal (40 mg/kg/injection) for 14 consecutive days starting 2 days prior to the first intratumoral injection of G47Δ (2×106 pfu).
Organ culture assay
G47Δ titers were assessed in the presence or absence of DP or DL in prostate organ cultures as described (12).
Statistical analysis
For cell susceptibility assays, in vivo efficacy studies, Student's t test (two-tailed) was used to analyze significance between two treatment groups using GraphPad Prism v.4 (SanDiego, CA).
Results and Discussion
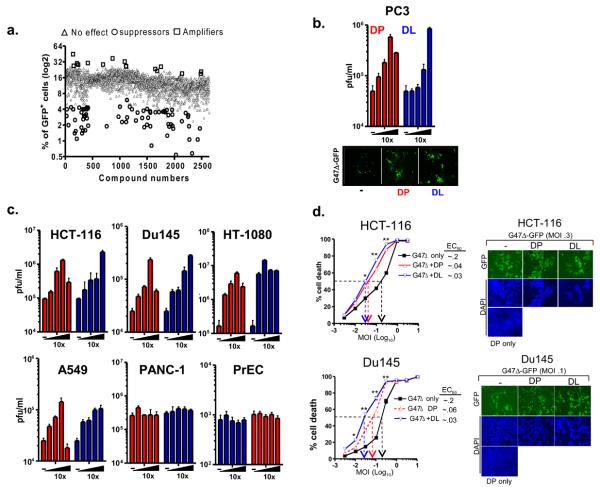
We screened 2640 compounds of known bioactives derived from three pharmacologically active libraries (NINDS, Biomol and Prestwick1-collection). PC3 prostate cancer cells were pretreated with compounds and subsequently infected with G47Δ expressing GFP at a multiplicity of infection (MOI) of 0.05 for an additional 48 hrs (Supplementary Fig. 1). Compounds that amplified G47Δ-GFP (as measured by GFP+ cells) at least 3 standard deviations (SD) above the overall plate average were considered strong “hits.” Scatter plot analysis showed that 15 (0.57%) of the library compounds reflected potential “amplifiers” of viral spread (Fig. 1a). Many of small molecule compounds that fit these criteria were anti-metabolites: two antifolates (pyrimethamine and methotrexate) and two fluoropyrimidines (fluorodeoxyuridine (FudR) and carmofur) (Table 1). FudR has been reported to enhance the spread of oncolytic G207 (13), a HSV vector related to G47Δ and therefore, its identification validated the effectiveness of the chemical library screen. DP and DL have been clinically used as vasodilators and fall into another pharmacologic group, termed ENT1 inhibitors (14). We have focused our efforts on DP and DL since they represent a class of compounds that have not been studied in the context of virotherapy and they could be readily translated to clinical trial. Multistep growth curve assays (herein referred to plaque assays) were performed in PC3 cells to validate the amplifying-promoting activities of these compounds (Fig. 1b). Pretreatment of PC3 cells with DP or DL resulted in a dose-dependent increase in G47Δ production 48 hrs post-infection. Fluorescent imaging of G47Δ-GFP infected PC3 cells showed that DP and DL increased GFP+ cell numbers (Fig. 1b).
Figure 1.
High-throughput screen of chemical amplifiers of G47Δ. (a) Scatter plot analysis of the 2640-small molecule bioactives tested that either increased (open squares), decreased (open circles), or had no effect (closed triangles) on viral replication or spread. (b) Validation of augmented G47Δ replication by DP or DL in PC3 cells. (c) DP and DL augment viral replication in a panel of tumor cell lines. Virus titers are expressed as the mean pfu/ml ± SEM and represent one of three independent experiments. (d) (Left panels) G47Δ-mediated DU145 or HCT-116 tumor cell killing is enhanced by DP or DL. * and ** indicates statistical significance at P values ≤ 0.05 and 0.01, respectively. (Right panels) Fluorescent images depicting enhanced plaque formation by G47Δ-GFP as a function of treatment with either DP or DL.
| Class/Name | Mode of action | z-score* |
|---|---|---|
| Antimetabolites: | ||
| Methotrexate | Inhibits folic acid metabolism | 3.3 |
| Pyrimethamine | Inhibits folic acid synthesis | 3.6 |
| Carmofur | Inhibits thymidylate synthase | 3.1 |
| Floxuridine | Inhibits thymidylate synthase | 4.5 |
| Vasodilators: | ||
| Dipyridamole** | Inhibits ENT's | 3.6 |
| Dilazep | Inhibits ENT's | 4.0 |
z-score value indicates SD above the plate mean for each readout value.
Identified in all three libraries with z-score ≥ 3.0.
The effects by DP or DL on oHSV replication were also tested in other human tumor cell lines (Fig. 1c). HCT-116, Du145 and HT-1080 were particularly sensitive towards the effects of DP and DL on G47Δ replication whereas, SK-Mel-2, T98 cell lines (Supplementary Fig. 2) were less sensitive and PANC-1 appeared to be resistant. These cell lines expressed relatively similar levels of ENT1 mRNA and protein (Supplementary Fig. 3). DP and DL did not increase viral production of G47Δ in normal prostate epithelial cells (PrEC) (Fig. 2c). Cell cytotoxicity assays demonstrated that cell killing mediated by G47Δ was significantly increased by DP and DL (P < 0.05). These data were further supported by fluorescence imaging of DAPI stained nuclei, which also revealed that DP and DL enhanced tumor cell killing mediated by G47Δ (Fig. 1d). Overall, these data demonstrate that DP and DL potentiate G47Δ replication and tumor cell killing.
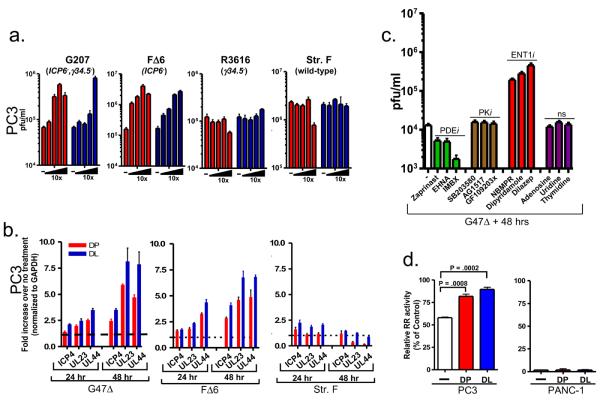
Figure 2.
HSV ICP6-negative mutants are responsive to the amplifying-activities of DP and DL. (a) Effects of DP (red bars) and DL (blue bars) on wild-type HSV-1 or oHSV's carrying deletion-mutations present in G47Δ. Values are the average ± SEM and represent three independent experiments. (b) Quantitative RT-PCR analysis of ICP4, UL23 or UL44 viral transcript levels in PC3 cells untreated or treated with DP or DL and infected with G47Δ (left panel), F△6 (middle panel) or strain F (right panel) for 24 or 48 hrs. GAPDH was used to normalize for mRNA levels. (c) Pharmacological inhibitors of PDE, PK or ENT1 and nucleosides (ns) were assessed for their ability to promote increased G47Δ replication by plaque assays. (d) Ribonucleotide reductase activity is transiently elevated by DP and DL in PC3 (left panel) but not PANC-1 (right panel) cells. Results are expressed as a percentage of the control value (relative RR activity).
To determine whether one or more of the deletion mutations within G47Δ confers augmented viral replication by DP or DL, we tested HSV-1 viruses F△6 (ICP6−), R3616 (γ34.5−), G207 (γ34.5−, ICP6−) and wild-type strain-F in the presence or absence of DP or DL. Plaque assays consistently revealed that oHSV's lacking ICP6, a gene that encodes the large subunit of ribonucleotide reductase, were associated with augmented viral replication by DP and DL (Fig. 2a). Furthermore, quantitative RT-PCR demonstrated that the viral transcripts representing immediate early (ICP4), early (UL23) or late (UL44) genes were enhanced by DP and DL from viruses lacking ICP6 (G47Δ and F△6), whereas minimal changes were seen in transcripts from ICP6-intact strain-F (Figure 2b).
DP and DL are also known to function as phosphodiesterase (PDE) antagonists as well as protein kinase (PK) inhibitors (14,15). Therefore, we tested representative inhibitors of PDE and PK activity using virus yield assays and fluorescent imaging (Fig. 2c, Supplementary Fig. 4). Inhibitors of PDE (Zaprinast, IBMX, EHNA) or PK (SB203580, GF109203x, AG1517) had minimal effects on increasing G47Δ replication relative to virus alone. Furthermore, increasing adenosine, uridine, or thymidine concentrations had no effect. This is in contrast to the action of NBMPR, a potent ENT1 antagonist (16), which increased G47Δ virus replication to a similar degree as DP or DL (Fig. 2c). We next examined the effects on G47Δ replication of a panel of DP analogs that have been previously shown to exhibit a range of binding affinities toward ENT1 based on structure-activity relationship studies (17). A number of DP derivatives reported to exhibit strong binding affinities toward ENT1 (compounds 2, 4, 11, 15, 52, 58 and 64) also promoted notable increases in G47Δ replication in PC3 cells (Supplementary Fig. 5). These data demonstrate that DP and DL likely acts on ENT1 to augment the replication of ICP6 deficient HSV vectors.
RR, which is composed of two subunits RR1 and RR2, plays a critical role in the synthesis of DNA by reducing ribonucleotides to their corresponding deoxyribonucleotides, thereby providing an essential reservoir of precursors for DNA synthesis and repair (18). As DP, DL and NBMPR inhibit nucleoside import/export through binding to ENT1 (16), we hypothesized that the actions of these compounds could possibly affect the RR1 and RR2 mRNA levels. RT-PCR analysis indicated that RR1 and RR2 mRNA levels were indistinguishable from DP or DL-treated PC3 cells compared to untreated controls (Supplementary Fig. 6). Next, we investigated whether treatment with either DP or DL results in increased RR activity. Figure 2d shows that by 6 hrs after treatment with DP or DL, RR activity increased 30% and 36%, respectively, over baseline levels in PC3 cells. By contrast, RR activity in PANC-1 cells was not increased with treatment of either compound and moreover, was minimally detected (Fig. 2d). This observation strongly parallels the data derived in Figure 1b illustrating that neither drug augmented G47Δ in PANC-1 cells.
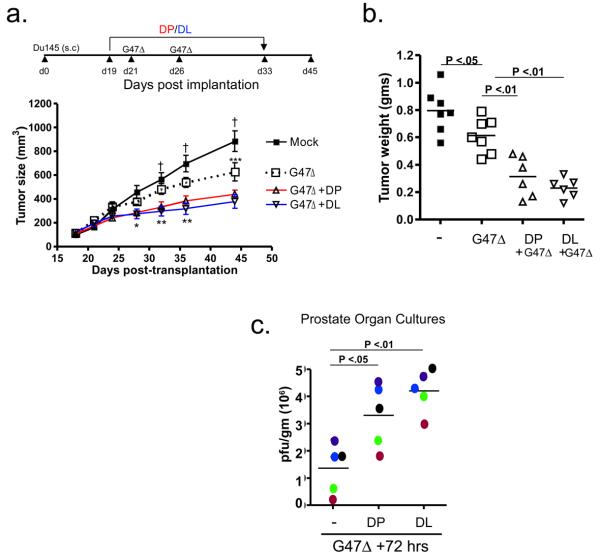
Lastly, we evaluated the in vivo antitumor efficacy of G47Δ in combination with either DP or DL in subcutaneous Du145 tumors. By day 45 after tumor implantation, a statistically significant decrease in tumor volume was observed between control (882±86 mm3, n=7) and all of the treatment groups (Fig. 3a). As compared to mock, treatment with G47Δ resulted in a notable reduction in tumor size (625±76 mm3; n=7), while the combination of G47Δ with DP (426+37 mm3; n=6) or DL (377+56 mm3; n=6) resulted in a statistically significant tumor regression compared to G47Δ alone. Neither compound by itself reduced or delayed tumor growth (data not shown). At day 45, statistically significant differences in tumor weights were also observed between untreated control and G47Δ treatment groups, as well as for G47Δ alone and in combination with DP or DL (Fig. 3b). Recently, we reported on the use of prostate organ cultures derived from radical prostatectomies to assess oHSV target specificity and replication competence (13). We have exploited this system to address whether DP and DL enhances G47Δ replication in primary human prostate cancer specimens. These results demonstrate that treatment of organ cultures with DP or DL over a 3-day period increased G47Δ titers 2-4 fold over the no treatment control (Fig. 3c).
Figure 3.
DP and DL enhances the in vivo anti-tumor efficacy of G47Δ. (a) Assessment of Du145 tumor growth. * P < 0.05 for Mock (n=7) or G47Δ only (n=7) versus G47Δ + DP (n=6) or + DL (n=6) at d28; ** P < 0.05 for G47Δ only versus G47Δ in combination with either DP or DL at d32 and d36; *** P < 0.05 for Mock versus G47Δ only; G47Δ versus G47Δ in combination with either DP or DL at d45. † P < 0.01 for Mock versus G47Δ in combination with either DP or DL at d32, d36 and d45. (b) Du145 tumor weights were assessed for the indicated treatment groups 45 days post-implantation. P values are indicated. (c) Evaluation of DP and DL augmented G47Δ replication in prostate cancer surgical specimens. Each color dot indicates a different prostate surgical specimen (n=5).
Using an unbiased chemical library screen, we have identified a novel application for the ENT1 antagonists, dipyridamole and dilazep (and NBMPR), namely to “amplify” the replication of G47Δ in cancer cells. Our results reveal that these ENT1 antagonists augment HSV vectors specifically lacking ICP6, which is the viral homolog of the human RR1 gene and that ENT1 antagonists may predispose some cancer cells to augmented oHSV replication by increasing cellular RR activity. From a translational perspective, DP has been used to potentiate the effects of chemotherapeutic agents in phase II clinical trials in solid tumors (19). While the therapeutic dosing for these studies was less than what was used in the present study, DP is well tolerated at higher doses with minimal adverse reactions in animals (20). These data suggest that the combination of DP and oHSV represents a clinically relevant treatment paradigm and should merit consideration for clinical studies. In this context, blocking nucleoside import and/or export via ENT1 antagonists represents a new strategy towards enhancing the efficacy of oHSV vectors for cancer therapy.
Supplementary Material
Acknowledgements
We thank the ICCB-L (Harvard Medical School) screening staff for their technical support: Caroline Shamu, Stewart Rudnicki, Katrina Rudnicki, Melody Tsui, David Fletcher, David Wrobel, and Sean Johnston. We also thank Dr. Donald Coen (Harvard Medical School) for his helpful advice on setting up the conditions for the HTS. We thank Ms. Melissa Marinelli for laboratory assistance. Wild-type strain F and its derivative R3616 were both obtained from Dr. B. Roizman, (The University of Chicago, Chicago, IL). Supported in part by grants; R01 CA10139 and NS032677 (to RLM), R03 MH083258 (to BJP) and P30 NS045776 (to SDR) for the real-time PCR core. RLM and SDR are consultants to MediGene Ag, which has a license from Georgetown University for G207.
References
- 1.Martuza RL, Malick A, Markert JM, Ruffner KL, Coen DM. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252:854–56. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- 2.Liu TC, Hwang TH, Bell JC, Kirn DH. Development of targeted oncolytic virotherapeutics through translational research. Expert Opin Biol Ther. 2008;9:1381–91. doi: 10.1517/14712598.8.9.1381. [DOI] [PubMed] [Google Scholar]
- 3.Passer BJ, Castelo-Branco P, Buhrman JS, et al. Oncolytic herpes simplex virus vectors and taxanes synergize to promote killing of prostate cancer cells. Cancer Gene Ther. 2009;16:551–60. doi: 10.1038/cgt.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett JJ, Adusumilli P, Petrowsky H, et al. Up-regulation of GADD34 mediates the synergistic anticancer activity of mitomycin C and a gamma134.5 deleted oncolytic herpes virus (G207) J Faseb. 2004;18:1001–3. doi: 10.1096/fj.02-1080fje. [DOI] [PubMed] [Google Scholar]
- 5.Lin SF, Gao SP, Price DL, et al. Synergy of a herpes oncolytic virus and paclitaxel for anaplastic thyroid cancer. Clin Cancer Res. 2008;14:151928. doi: 10.1158/1078-0432.CCR-07-4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang JH, Chung TD, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 7.Fukuhara H, Ino Y, Kuroda T, Martuza RL, Todo T. Triple gene-deleted oncolytic herpes simplex virus vector double-armed with interleukin 18 and soluble B7-1 constructed by bacterial artificial chromosome-mediated system. Cancer Res. 2005;65:10663–68. doi: 10.1158/0008-5472.CAN-05-2534. [DOI] [PubMed] [Google Scholar]
- 8.Chou J, Kern ER, Whitley RJ, Roizman B. Mapping of herpes simplex virus-1 neurovirulence to γ134.5, a gene nonessential for growth in culture. Science. 1990;250:1262–66. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- 9.Todo T, Martuza RL, Rabkin SD, Johnson PA. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci USA. 2001;98:6396–401. doi: 10.1073/pnas.101136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wakimoto H, Kesari S, Farrell CJ, et al. Human glioblastoma-derived cancer stem cells: establishment of invasive glioma models and treatment with oncolytic herpes simplex virus vectors. Cancer Res. 2009;69:3472–81. doi: 10.1158/0008-5472.CAN-08-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou B, Liu X, Mo X, et al. The human ribonucleotide reductase subunit hRRM2 complements p53R2 in response to UV-induced DNA repair in cells with mutant p53. Cancer Res. 2003;63:6383–94. [PubMed] [Google Scholar]
- 12.Passer BJ, Wu CL, Wu S, Rabkin SD, Martuza RL. Analysis of genetically engineered oncolytic herpes simplex viruses in human prostate cancer organotypic cultures. Gene Ther. 2009;12:1477–82. doi: 10.1038/gt.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrowsky H, Roberts GD, Kooby DA, et al. Functional interaction between fluorodeoxyuridine-induced cellular alterations and replication of a ribonucleotide reductase-negative herpes simplex virus. J Virol. 2001;75:7050–58. doi: 10.1128/JVI.75.15.7050-7058.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King AE, Ackley MA, Cass CE, Young JD, Baldwin SA. Nucleoside transporters: from scavengers to novel therapeutic targets. Trends Pharmacol Sci. 2006;27:416–25. doi: 10.1016/j.tips.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Kim HH, Liao JK. Translational therapeutics of dipyridamole. Arterioscler Thromb Vasc Biol. 2008;28:s39–42. doi: 10.1161/ATVBAHA.107.160226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffiths M, Beaumont N, Yao SY, et al. Cloning of a human nucleoside transporter implicated in the cellular uptake of adenosine and chemotherapeutic drugs. Nat Med. 1997;3:89–93. doi: 10.1038/nm0197-89. [DOI] [PubMed] [Google Scholar]
- 17.Wenwei WL, Buolamwini JK. Synthesis, Flow cytometric evaluation, and identification of highly potent dipyridamole analogues as equilibrative nucleoside transporter 1 inhibitors. J Med Chem. 2007;50:3906–20. doi: 10.1021/jm070311l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elledge SJ, Zhou Z, Allen JB. Ribonucleotide reductase: regulation, regulation, regulation. Trends Biol Sci. 1992;17:119–23. doi: 10.1016/0968-0004(92)90249-9. [DOI] [PubMed] [Google Scholar]
- 19.Isacoff WH, Bendetti JK, Barstis JJ, Jazieh AR, Macdonald JS, Philip PA. Phase II trial of infusional fluorouracil, leucovorin, mitomycin, and dipyridamole in locally advanced unresectable pancreatic adenocarcinoma: SWOG S9700. J Clin Oncol. 2007;25:1665–9. doi: 10.1200/JCO.2006.06.7637. [DOI] [PubMed] [Google Scholar]
- 20.Venkatesh PK, Pattillo CB, Branch B, et al. Dipyridamole enhances ischaemia-induced arteriogenesis through an endocrine nitrite/nitric oxide-dependent pathway. Cardiovasc Res. 2010;85:661–70. doi: 10.1093/cvr/cvq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.